Abstract
Objectives
The aim of this study was to investigate the effect of the vibration amplitude of mechanical ultrasound waves (27 kHz) on the viability and morphology of human gingival fibroblasts (hGFs) when cultured on a biomaterial substrate.
Method
hGFs were seeded on tissue culture plates (TCPs) and an Ti6Al4V titanium alloy surface in two groups for three days and seven days of cell culture. The cells were subjected to three vibration amplitudes for 20 min each day. Scanning electron microscope (SEM) images were used to characterize cell morphology.
Results
Experiments showed that hGF cells became detached from their plates at a vibration amplitude comparable to an intensity of 260 mW/cm2. In addition, hGfs that received a vibrational amplitude comparable to an intensity of 50 mW/cm2 underwent significant proliferation proliferated significantly; however, cells receiving higher amplitudes suffered from adverse effects.
Conclusions
SEM images of hGFs on titanium disks at vibration amplitude comparable to an intensity 50 mW/cm2 showed a remarkable hexagonal architecture, which we refer to as a honeycomb pattern. On day 6 the observed hGFs on TCPs, proliferated at a higher rate and new cells attached uniformly on the existing layer of cells. These data indicate the effect of cellular tissue as a substrate on the growth of new hGFs under low-intensity ultrasound.
Keywords: Cell architecture, Honeycomb structure, Stress adaptation, Ultrasound device
المخلص
أهداف البحث
كان الهدف من هذه الدراسة هو التحقيق في تأثير سعة الاهتزاز للموجات الميكانيكية بالموجات فوق الصوتية (27 كيلو هرتز) على قدرة الخلايا اللثوية البشرية على الحياة وتشكلها التي تم زرعها على مادة حيوية.
طرق البحث
تم زرع الخلايا اللثوية البشرية على أطباق زرع الأنسجة وسطح سبائك التيتانيوم "تي آي 6 ايه 14 في" في مجموعتين تشمل ثلاثة أيام وسبعة أيام من زرع الخلايا. تعرضت الخلايا لثلاث سعات اهتزاز لمدة 20 دقيقة يوميا. تم استخدام صور مجهر المسح الإلكتروني لتحديد شكل الخلايا.
النتائج
لأول مرة، عند مستوى معين من سعة الاهتزاز المقارن بشدة 260 مللي وات / سم2، تم تحديد كيفية فقدان الخلايا الملتصقة اتصالها. تكاثرت الخلايا اللثوية البشرية التي تلقت مستوى معين من سعة الاهتزاز المقارن بشدة 50 مللي وات / سم2 بشكل كبير، في حين كانت السعات الأعلى لها آثارا سلبية.
الاستنتاجات
أظهر مسح صور المجهر الإلكتروني للأرومات الليفية اللثوية البشرية على أقراص التيتانيوم عند مستوى معين من سعة الاهتزاز مقارنة بكثافة 50 ميغاواط / سم2 بتشكل سداسي ملحوظ، والتي كانت تسمى نمط قرص العسل في هذا البحث التجريبي، وفي اليوم السادس لوحظ أن الخلايا اللثوية البشرية تكاثرت على أطباق زرع الأنسجة بمعدل أعلى وخلايا جديدة متصلة بشكل موحد على طبقة من الخلايا. يشير هذا إلى تأثير الأنسجة الخلوية كركيزة لنمو الخلايا الليفية اللثوية البشرية الجديدة تحت الموجات فوق الصوتية منخفضة الكثافة.
الكلمات المفتاحية: جهاز الموجات فوق الصوتية؛ التكيف مع الإجهاد؛ هندسة الخلية؛ هيكل قرص العسل
Introduction
It is vital to reduce complications after surgery in order to perform successful implant treatment. Not only should surgical wounds be repaired faster, but the integrity of the newly formed tissues around the implanted biomaterial should also be improved. Gingival fibroblasts play an important role in oral wound healing.1 Therefore, studying the interaction of human gingival fibroblasts (hGFs) with biomaterials will provide scientific indications for their optimal application. The first step to achieving this goal is to conduct an in vitro study to reduce the number of animal experiments (and animal sacrifice).
The study of cell-substrate and cell–cell attachment is important since morphology, proliferation, migration, and most importantly, the vitality of connective tissue cells, are all associated with the degree of cell attachment to the substrate.2 Furthermore, the state of connection between cells determines the degree of cell tissue integrity and their ability to sense external mechanical forces.3,4 If the proliferation of fibroblast cells and the formation of gingival tissue in contact with a biomaterial can be organized such that the tissue becomes more resistant to separation from the prosthesis, the chances of overcoming treatment limitations can be increased and diseases such as pre-implantitis be prevented.
The term “ultrasound” is used to describe mechanical waves in a frequency range of 20 kHz and above. How this mechanical wave is transmitted depends on the elastic properties and density of the environment. In fluids, fluid flow is proportional to the magnitude of the mechanical wave applied.5 In previous research, high-intensity ultrasound vibration was used to uniformly disperse cellulose nanofibers and improve humidity in a hydrogel.6 Low-intensity ultrasound (LIUS) vibration increases the temperature of a tissue and the exchangeability of substances between the outside and inside of a cell.7 Ultrasound wave stimulation has been shown to be effective in treating chronic, diabetic and elderly wounds.8,9 According to an ex vivo study conducted by Alshihah et al. (2020), hard and soft tissues of diabetic rats under LIUS showed an increase in the number of the cells and the thickness of mandibular tissues.10
At the cellular level, mechanical vibration causes intermittent expansion and contraction to accelerate the connection between protein transport fluids, interstitial fluids, and blood flow11; external forces have been shown to play an important role in this regard.12,13 At the molecular level, the absorbed mechanical vibration causes an increase in temperature.14, 15, 16 This increase in temperature increases Brownian motion in the blood, thus facilitating protein-transporting fluids in the cell, and interstitial fluids.17,18
Recent studies explored the effect of MHz (1–5 MHz) ultrasound vibration on cell viability.19, 20, 21 However, some other studies have considered kHz ultrasound mechanical waves (40 kHz) and showed that continuous ultrasound vibration had a positive effect on parameters associated with cell survival.7,22 Therefore, for the application of restoration, ultrasound frequency cannot be considered in a specific range and applying ultrasound mechanical waves with lower frequencies to living cells is worth investigating.
A previous experimental study demonstrated that an ultrasonic wave intensity of 10–50 [mW/cm2] had an amplifying and positive effect on parameters associated with cell growth and survival while an intensity 0.075 [W/cm2] attenuated these effects.21,22 Therefore, the use of mechanical vibration for tissue repair depends on the ability to produce a waveform with a certain intensity in order to exert positive effects and prevent detrimental effects. Wave intensity can be defined by Formula 1.
| (1) |
In Formula 1, Z represents acoustic impedance and represents the amplitude of fluid velocity. According to this formula, as the mechanical wave speed increases in a given fluid, the wave intensity also increases. The speed of oscillation depends on two factors, amplitude and frequency. Therefore, in this study, by choosing a fixed frequency that has not been considered in previous research, we were able to investigate the effect of changing the vibration amplitude on the viability and morphology of cultured hGFs.
We aimed to develop an innovative solution in the field of mechanobiology by investigating the effect of ultrasound vibration on the architecture of fibroblasts. Previous studies only attempted to create a pattern on the surface of biomaterials.23 However, creating surface micropatterns is not recommended due to improvement in bacteria attachment to the surface. As an alternative, studies have reported that strained fibroblasts both aligned and proliferated in the direction of the strain.24 Accordingly, in this study, we investigated tissue patterning through a stress adaptation mechanism, which could occur separately from the creation of biomaterial surface patterns and their associated problems.
Materials and Methods
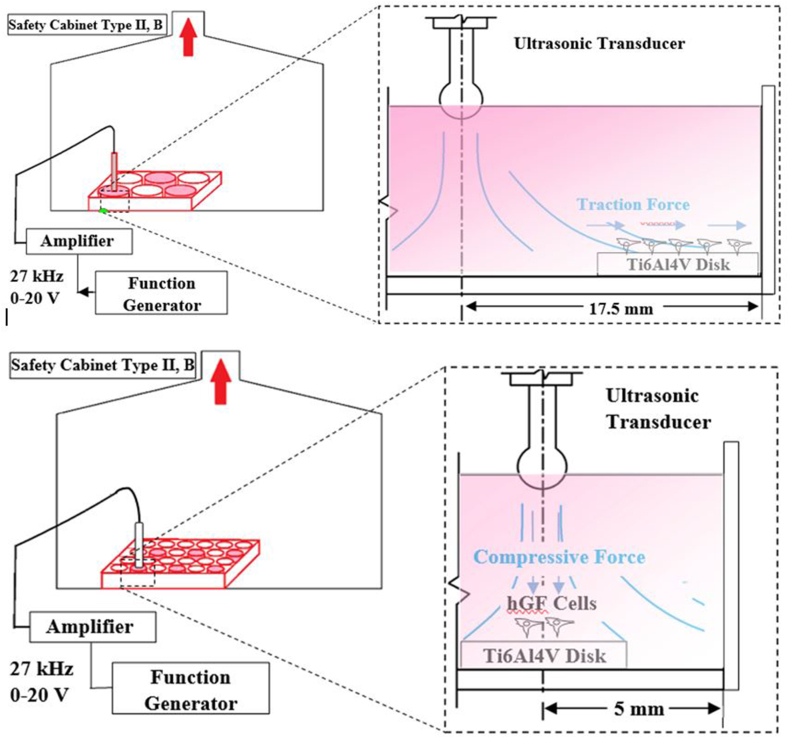
In this study, we investigated the effect of ultrasound vibration in the range of kilohertz (kHz) on human gingival fibroblast cells (hGFs). One group of hGfs was cultured on tissue culture plates (TCPs), and the other group was cultured on grade 5 titanium polished disks. To determine the effect of vibration duration, one group of cells was studied for 3 days while another group was studied for 7 days. Three specific vibration amplitudes in the range of 27 kHz were applied to the culture medium, and in one case, the tool was applied in “no flow” mode without vibration. To perform the test, an ultrasonic transducer was designed and manufactured. The form of the tooltip in contact with the culture medium had a spherical design.
A commercial function generator and an amplifier were used to generate a 27 kHz (continuous) electrical current and to amplify its voltage, respectively (Figure 1). The ultrasonic current then was converted through the custom-made transducer to mechanical vibration at the interface of the transducer and culture medium. Suitable vibration amplitudes were measured by a commercial gap sensor and considered for three vibration modes: level 1, level 2 and level 3 (Table 1). The equivalent average intensity was measured using Formula 1.
Figure 1.
Settings for the two phases of cell stimulation using ultrasonic vibration at 27 kHz: culture medium flow with sweeping cells (top) and culture medium flow by the compression and dissection of cells from each other (bottom).
Table 1.
Vibration amplification adjustment for three mechanical wave stimulation modes at a frequency of 27 kHz.
| LIPUS (Perpendicular flow) Setting | Level 1 | Level 2 | Level 3 |
|---|---|---|---|
| Vibration Amplitude (um) | 0.3 | 0.5 | 0.8 |
| Calculated Intensity (mW/cm2) | 50.7 | 72 | 261.1 |
The height of the tool was adjusted manually, and contact of the tool with the culture environment was limited to its tip. Considering that the height of all test instruments in all cell culture wells was adjusted manually, it could be concluded that this adjustment did not have an interfering effect on the validity of the results in this study. According to the purpose of this research, the Ti6Al4V alloy disks were cut and polished in equal dimensions and surface roughness (Ra 0.11 ± 0.004) and were placed flat in the culture environment (Figure 1).
Cell culture
Human gingival fibroblasts (hGFs) were purchased from the Pasteur Institute of Iran (Passage 2), and RPMI 1640 culture environment was used for the cultivation of fibroblasts Initially, cells were cultured in a cell culture flask containing a suitable culture media for fibroblasts (RPMI + 10%FBS + 1%Pen/Str). The flask containing the cells was transferred to an incubator with a humid atmosphere, containing 5% carbon dioxide and a temperature of 37 °C. After two days, the supernatant was removed, and fresh culture medium was added. Subsequently, the cell media was changed every 3–4 days for a week.
In total, 10,000 hGFs proliferating in a normal-conditioned flux were counted using a Neobar Lam according to standard procedures and seeded onto selected wells on tissue culture plates (TCPs). In this manner, the cells were seeded on the plate wells at least one well apart. This distance was performed to prevent the transmission of vibration from a well being tested to a well nearby. During the test, the plates containing the cells were kept in an incubator at 60%–80% humidity and in the presence of 2.5% CO2 gas. Each day and during ultrasonic processing, cell culture plates were placed (for a maximum duration of 60 min) in a safety cabinet suitable for laboratory-cell tests, including both ultrasonic treated and non-treated hGfs. For this purpose, we designed and built a complex structure capable of handling parallel processes utilizing 4 transducers.
The cells were returned to the incubator after receiving 20 min of ultrasound stimulation or no stimulation. Furthermore, the status of cell growth was monitored daily under an inverted microscope prior to testing. The complex structure included a portable digital microscope, such that it was possible to perform live observation of ultrasonic flow in selected wells.
Exerting a shear force on fibroblasts
The first phase of the study involved investigating the flow parallel to the surface of the grade 5 titanium disks and the effect of sweeping such flow on hGFs on the disk. The surfaces of the disks were polished according to the conditions of the apical aspect of dental implants.
Cell culture on the surface of tissue culture plates was also taken into account to evaluate the effect of the presence of implant material in comparison with its absence. For this purpose, 6-well plates and 24-well plates were used so that the position between the source of ultrasonic vibration and the titanium disk could be adjusted.
Exerting a compressive force on fibroblasts
In the second phase, hGFs were placed under a perpendicular force. The onset of ultrasound vibration in this phase occurred 24 h after cell seeding on the surface. To study the effect of vibration amplitude, three values were selected: level 1, level 2 and level 3. The titanium substrates at this stage were smoother.
Preparing samples for imaging
Qualitative interpretation of scanning electron microscope (SEM) images provided us with a tool to enhance our observations. The ideal goal of this study, if plausible, was to link the results of cell culture with the amplitude and intensity of vibration corresponding to the desired biological function; this provided us with the ability to study specific and desirable parameters in a direct manner.
The disks containing the hGFs were fixed in glutaraldehyde (Merck Germany) and dehydrated with a series of alcohols (70, 80, 90 and 100% ethanol). The fixed and dehydrated samples were then coated with gold by sputtering for 50 s and then placed in the SEM system. SEM images were magnified and the specific morphology of cells was observed.
MTT assays
The standard optical density method was used to measure cellular metabolic activity. In the MTT assays, the supernatant was removed and then washed twice with PBS solution. Then, 800 μl of culture media was added to each well, followed by MTT solution (80 μl, Sigma). The plates were then placed in an incubator for 3 h of culture. After following all of the required steps of the test, we added DMSO solution (400 μl, Sigma) and analyzed data with an ELISA system at a wavelength of 540 nm based on revived formazan. Finally, the intensity of light absorption was read on a plate reader.
According to our hypothesis, it was necessary to control fluid flow in the culture environment by determining the position of the ultrasonic vibration source relative to the position of the cells on the titanium disk. In the first case, the radial distance of the titanium disk inside the cell culture plate with the vibration source was more than three-fold greater than the diameter of the vibration source (Figure 1).
Statistical analysis
Based on the statistically acceptable number of replicates in cell research, we performed three replicates for each sample that represented one of the conditions. For example, in an ultrasound setting, three replicates were cultured for 3 days and three replications for 7 days. In addition, for each test condition, in two replicates, hGFs were seeded on polished titanium disks.
After reviewing the MTT results, the data were analyzed according to their control sample. Data were analyzed by analysis of variance (ANOVA) after confirming the conditions for using this test. All independent variables were tested and examined as a sub-formula to demonstrate the relationship between the independent variables and their mutual impacts on the dependent variable.
Results
This multidisciplinary research adopted a mechano-biological approach to interpret the results of a cell culture experiment in which hGFs were subjected to ultrasonic fluid microflow. We showed that in the compressive direction, the protection limit was higher than the tensile direction and that the fluid velocity corresponding to level 3 ultrasound vibration protection was excessive. In that case, the strain applied to the cell was proportional to the reduction of cell attachment to its substrate surface. The mechanism of “reduction” can be described as tilting the cells in the direction of flow, removing cell junctions under tension while maintaining the junction on the compressed side through the acceptance process, ultimately losing all junctions (Supplementary Material 1).
MTT assay
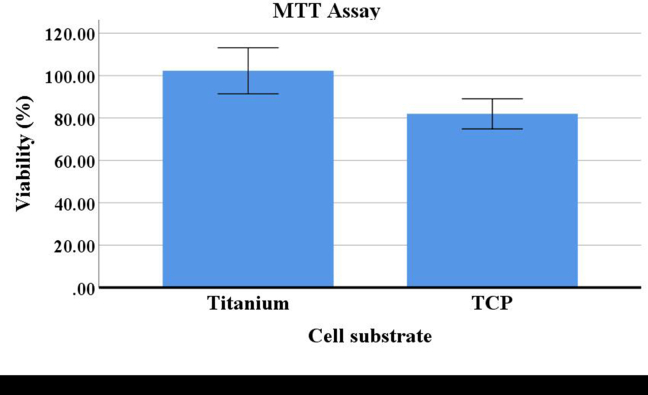
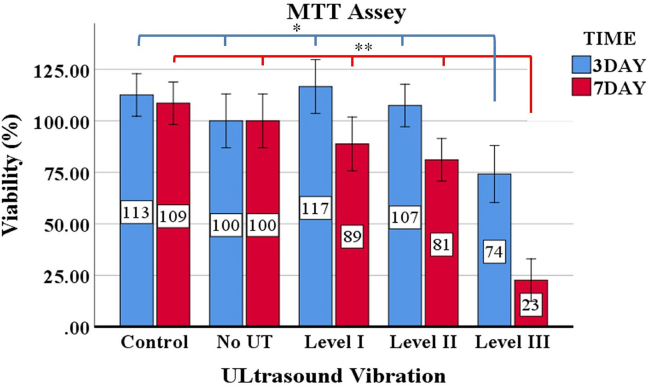
In this study, the MTT results are presented according to the cell substrate as an independent variable (Figure 2). In addition, Figure 3 demonstrates the interaction effects between the number of days of vibration and its magnitude on cell survival (Figure 3).
Figure 2.
Mean and standard deviation of hGF viability (%) on Ti6Al4V disks or tissue culture plates (TCPs).
Figure 3.
Mean and standard deviation of the effect of ultrasound vibration on the viability of hGFs over three or seven days of vibration. The viability of hGFs under the highest level of vibration amplitude (level 3) was significantly reduced in both of the time periods (three days and seven days) when compared to other vibration setups (p < 0.05).
According to the results, the presence or absence of the titanium disks did not significantly affect the survival of hGFs (Figure 2). Furthermore, the interaction effects between vibration and cell substrate was not significant (p > 0.05). In other words, applying level 3 ultrasound vibration reduced cell viability, and the presence or absence of the disks (as a substrate) did not result in a significant difference.
Online observations of fibroblasts on TCPs
Under the in vitro conditions used in this study, we were able to clearly observe the effect of ultrasound vibration on the viability of hGFs. Two adjustments of ultrasound vibration, including moderate and high intensity, led to the reduction of cell viability. This effect was much more evident at high intensity vibrations. Live imaging recorded cell detachment during high vibration applications. During this vibration, a constant velocity was created in the culture medium, and the cells that adhered to the plate surface were displaced by this velocity, as they separated and rotated. Furthermore, we found that a few cells that did not detach from the surface were not elongated and were widely established.
At vibration level 1, the cell viability was comparable to the no-flow condition (Figure 3), and the reduction in cell viability in response to low vibration was not significant. However, in the vibration group for 7 days and over the last two days, live observations showed an increase in the number of new cells.
Fibroblasts under ultrasonic fluid flow
Studying cell morphology is one of the criteria for investigating the effect of ultrasound vibration. For this purpose, we used ultrasonic vibration to create two force direction adjustments via the culture medium to the hGFs.
Effect of shear force on the morphology of fibroblasts
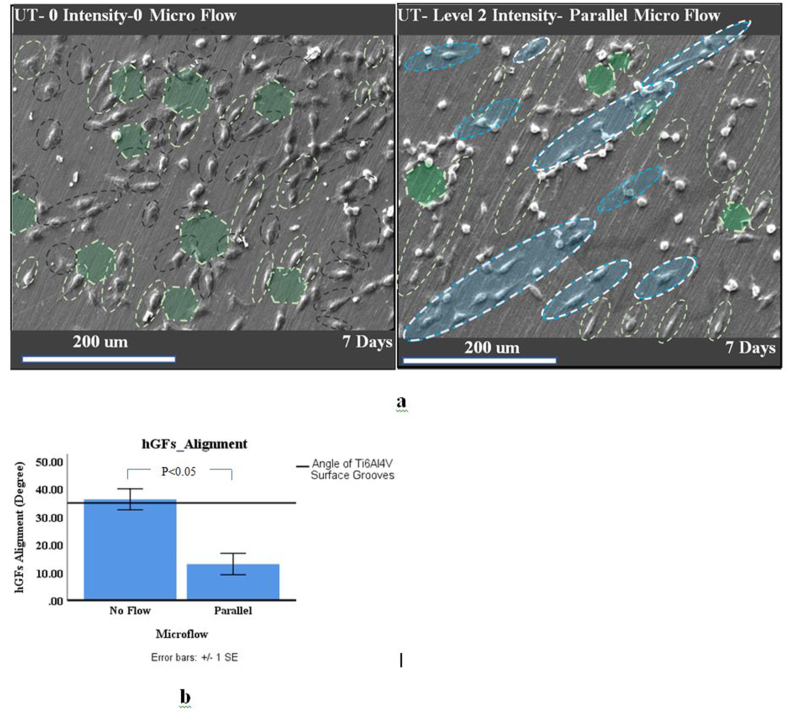
Scanning electron microscope (SEM) images of hGFs under parallel microflow and “no-flow” conditions were comparable in terms of how cells attached to the substrate and whether surface topography or microflow represents the dominant parameter in determining cell morphology (Figure 4a).
Figure 4.
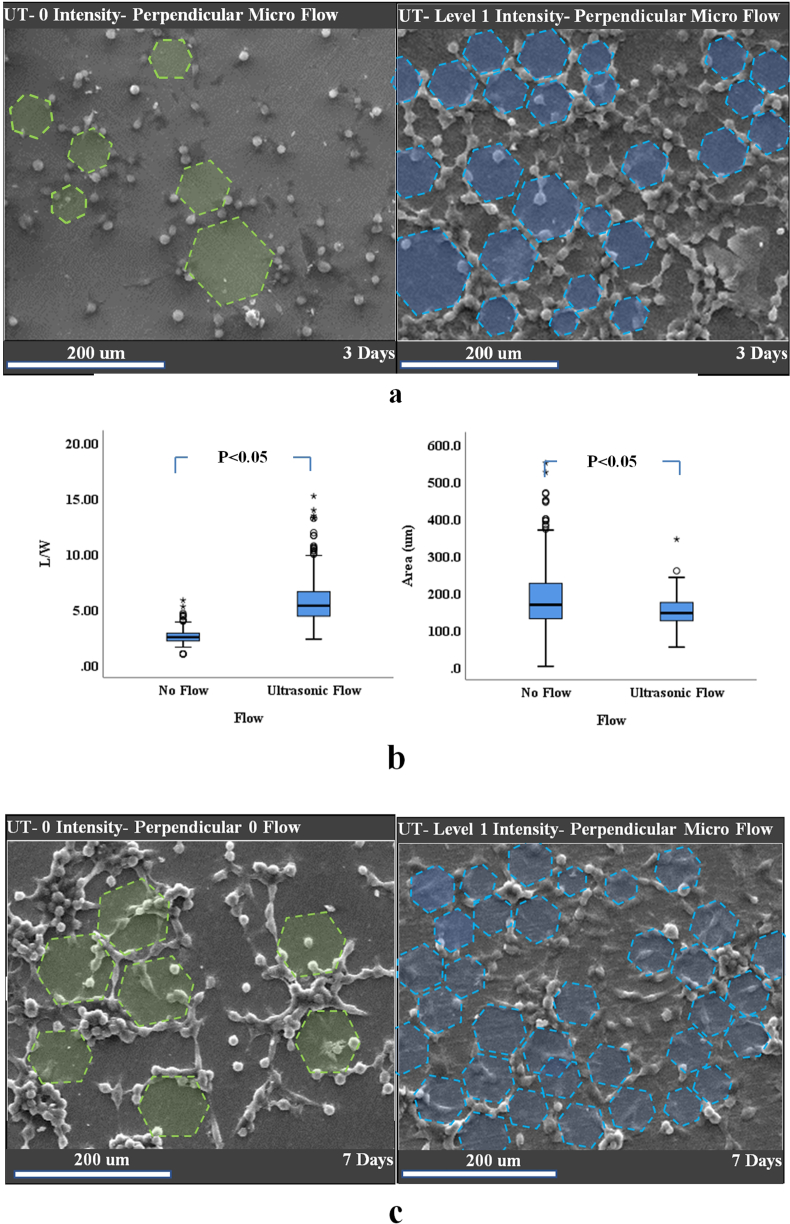
SEM images (a) of fibroblast cell morphology on the surface of the titanium disk over seven days of vibration. hGFs under no microflow were either aligned to the titanium substrate surface grooves (light green dashed lines) or randomly (black dashed lines). hGFs under ultrasonic vibration were either directed to the substrate surface (light green dashed lines), inclined (blue lines) or aligned (dashed white lines and highlighted) to a certain direction. Diagram of (b) the mean and standard error of quantified hGF alignment (in degrees). Few honeycomb patterns were evident in both hGFs treated with fluid flow and those without on the titanium disks (no flow) (a).
The average angle (degree) of hGFs in the no-flow condition was almost parallel to the direction of the grooves on the surface of the titanium substrate. Furthermore, under parallel microflow, a number of hGFs were formed parallel to the microflow direction, and the average angle of the cells was different from the substrate groove angle (Figure 4b). Interestingly, the standard deviation (16.4°) of the angle of hGFs was reduced (by 1.5-fold) in the parallel flow group (compared to the standard deviation of cells under no-flow conditions). Therefore, the angles of hGFs had a preferred direction.
Effect of compressive force on fibroblast morphology
According to the results shown in Figure 5, cells under a perpendicular flow formed regular hexagonal patterns, while in those in the “no flow” condition formed several irregular hexagons. There was one common feature between these two patterns; both formed a honeycomb-like array of hexagons. Thus, this pattern of cell arrangement was referred to as hGF honeycomb architecture (Figure 5).
Figure 5.
SEM images of (a) fibroblast cell morphology on the surface of the titanium disk over the three days of vibration. hGFs under no microflow were aligned randomly since the titanium substrates were highly polished and the surface grooves were not detectable by the cells. (b) Statistical analysis of the mean values of cellular length to width ratio and surface area (μm) showing a significant difference between the no flow and ultrasonic flow conditions. Hexagonal (honeycomb) architecture of the hGFs are highlighted (light green) and the pattern of hGFs under level 1 microflow was finer and in larger numbers (compared to the no flow condition). (c) SEM images of fibroblast cell morphology on the surface of the titanium disk over the seven days of vibration. Differences between the honeycomb patterns under level 1 microflow and no flow were also evident.
Discussion
For the purpose of physiotherapy, most of the ultrasonic devices used at present operate in the frequency range of 500 kHz and above.25 In this research, we innovatively set the frequency at 27 kHz. To the best of our knowledge, this frequency has never been applied to human gingival fibroblasts (hGFs).
In this study, a vibration amplitude of level 1 to 3 was selected as the lowest to highest amplitude, respectively. According to our results, levels 2 and 3 caused significant cell death, as reported in previous similar studies.21,22 In the case of level 3 ultrasound vibration, hGFs were observed online using a mobile digital microscope at a magnification of 200×. Although this magnitude of vibration was considered undesirable in terms of cell viability, our observations provided valuable information in terms of understanding the structure and mechanisms of cell adhesion and transportation. “Tension buffering” refers to the mechanism used by cells to protect their structure against external forces (after the perturbation process). According to previous in vitro research, depending on the strain rate exerted by an external force on a cell, this active mechanism can minimize the tension created in the cell by adapting to it26 (Webster et al., 2014).
A review of the literature showed that the optimal intensity for cell proliferation is 50 mW/cm2, while an adverse effect was observed at 70 mW/cm2 and above. Cased on a specific formula for calculating sound intensity (Formula 1), the wave intensity in the culture medium was calculated at the interface of the ultrasonic instrument and the fluid (Table 1). Interestingly, level 1 ultrasound resulted in the formation of the honeycomb architecture, as characterized by an intensity of 50 mW/cm2.
The focus of this paper was to create a reactive architecture in fibroblast cells, once under an induced shear fluid flow and then under a compressive flow (in a different set-up). In this study, the alignment of hGFs exhibited a preferred orientation and differed significantly from those under no flow (p < 0.05). In other words, during the hGF cell cycle, a number of cells proliferated and expanded parallel to the direction of flow to reduce the effect of the drag force of the fluid flow and maintain their homeostasis. Consequently, these cells had a stretched shape (high length-to-width ratio) and were significantly different from those under no flow (p < 0.05). Aktas et al. (2019) previously investigated orienting live cells in the direction of machined grooves (on the surface of biocompatible materials) in terms of shape, cell health, and density by using grade 5 titanium surface engineering and creating grooves on a nanoscale.27 One problem was that surface patterning was not significant when the surface was characterized by nano-grooves. Several studies reported that cell patterning could be achieved if the mechanical work on a surface was in the form of a micron-sized architecture. Nevertheless, the absorption of bacteria due to the pattern roughness of the biomaterial surface creates a significant weakness.
The honeycomb networks described herein were simulations created by the authors from the community structures of fibroblasts cells under level 1 ultrasound vibration. In other words, the mechanism of tension compensation in fibroblasts, in response to the creation of a flow that acted as pressure and then the dissection of cells from each other (tension), formed rings of chains in the form of beehives (Figure 3). Interestingly, in the field of mechanobiology, recent studies have shown that cell-to-cell connections not only maintain tissue integrity but also develop a mechanical-to-biological transducing structure. This “adhesion/cytoskeleton system” reportedly controls cell proliferation and cell migration.4,28 Thus, the honeycomb networks of hGf connections described herein are the result of cells detecting mechanical force. The network created improved both the sensory mechanism and also the integrity of the cells and tissues.
At the molecular level, the clustering of integrin in the form of hexagonal patterns (the same pattern as that adopted by fibroblasts in the current study) resulted in optimized integrin functionality in terms of receiving signals from the environment surrounding fibroblasts.29 Integrin is a protein that plays a key role in the focal adhesion of fibroblasts responsible for cell attachment to its substrate. Integrin clustering in such a structure naturally provides each fibroblast with the optimum conditions for sensing external mechanical cues (receiving signals) and cell shape formation and migration (actuating).
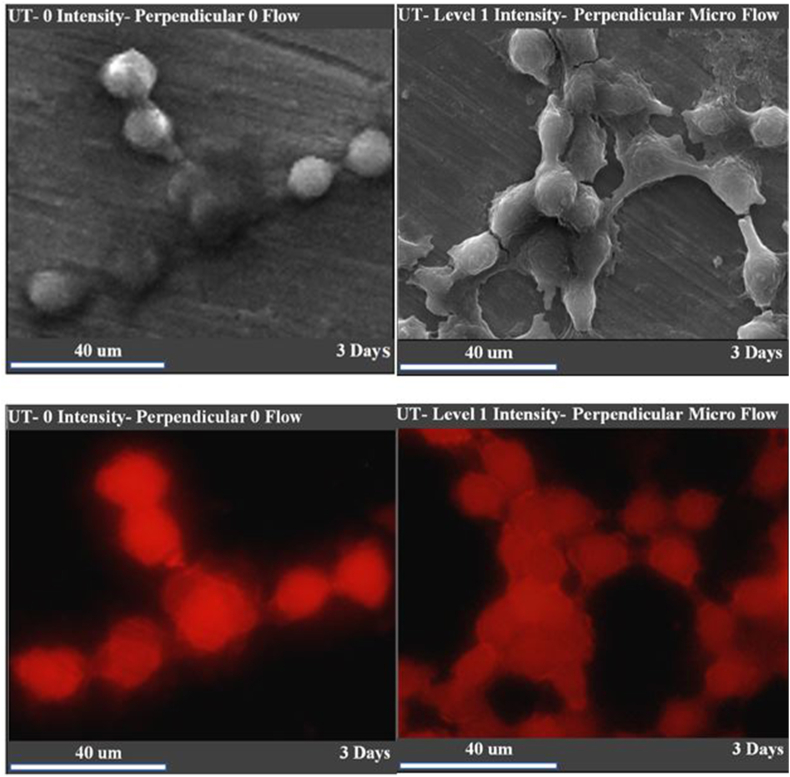
Statistical analysis (Figure 5) also revealed a significant (P < 0.05) difference between both the surface area and the ratio of length to width of hGFs under LIUS and those under no flow conditions. Moreover, hGFs on the titanium disks that were prepared and coated for SEM imaging were visualized under a fluorescent microscope after taking SEM images, while the titanium disks (prior to the cell culture test) could not be detected by microscopy. The fluorescent images at the junctions of teo cells revealed a change in the cytoskeletal structure inside the cells as a result of ultrasonic microflow (Figure 6).
Figure 6.
SEM (above) and fluorescent images (below) of hGFs on the surface of the titanium disks in the 3 day group. In the ultrasonic microflow fluorescent images, the region of interest in the hGFs were the connections of cell–cell junctions where the red fluorescence was clearly lighter in the ultrasonic microflow condition than in the no flow condition. SEM images of the same sites (for the two conditions) is also provided to allow comparison and permit tracing of cell–cell contacts
To obtain a promising outcome from dental implants, secondary stability is a key factor to consider.30 The Ti6Al4V alloy used in our study is a commercial biomaterial that is used to fabricate dental and orthopedic implants. Improving the adsorption of molecules to achieve secondary stability is of vital importance. Furthermore, the availability of proteins at the healing site as well as implant surface treatment is important.31 We found that microwaves produced a constant flow as well as heat. Thus, diffusion and flow could be considered as two separate parameters that can affect adsorption, and hence, the morphology and proliferation of hGFs.
According to the dispersion theory, the diffusion rate of living cells on a surface depends on the flow rate of the medium containing the cells32,33; as the rate increases, the rate of cellular expansion improves. Diffusion is described by Formula 2.
| (2) |
In Formula 2, D represents the diffusion coefficient and n represents the surface concentration of proteins; t represents time. According to Formula 2, an increase in the flow rate results in the improved accumulation of molecules at the biomaterial surface.
With regards to the architecture and proliferation of hGFs, the increase of flow rate should be limited to 0.025 m/s which is equivalent to the level 1 setup in the current study. In other words, the relationship between fluid flow and adsorption should follow a different pattern (in comparison to Formula 2) and be described by a parabolic equation in which the maximum point is located at the optimum flow rate.
There are two explanations for the observed honeycomb structure as a result of level 1 flow. First, according to the buffering process, the architecture of the tissue adapts to the mechanical force of the flow; this is because this type of structure can bear higher loads than random structures. In a previous study, Mostafa et al. (2009) observed fibroblast differentiation under low intensity pulsed ultrasound.34 Thus, the manner by which hGFs strengthened cell–cell attachments or aligned to the direction of flow must be investigated further, especially with regards to whether this attachment was achieved through cell differentiation or not. The second explanation could be related to the repulse force within the protein molecules of the hGFs. As the surface of the biomaterial was occupied by hGFs and there was a reduction in the space available for protein bonding, molecules adhered to the surface in a random and heterogeneous direction to avoid a repulsive force under no flow conditions. On the surface of the biomaterial, the resultant flow provided a transportation mechanism by which the cells migrated in the direction of flow to less populated areas. Subsequently, the cells positioned and aligned to create and maintain a balance between repulsive and attractive forces between the proteins. Integrin clustering in the form of hexagons,29 as reported by Vanamee et al. (2022), provides strength to this explanation in that the honeycomb structure of hGFs observed in this study represents the intrinsic ability of the cells generated from the nano-scale protein molecules up to fibroblasts at the micro-scale. The attractive force can be traced by the direction in which one hGF extended and connected to an adjacent hGF.
Due to the limitations of this study, it was not possible to study the genome of the cultured cells; therefore, it was not possible to study fibroblast changes exposed to mechanical stimulation at the genomic level. Thus, in future research, cells exposed to ultrasound should be examined for variability in myofibroblasts to determine the relationship between the amplitude of ultrasound fluctuations and the degree of cellular differentiation. In fact, it is necessary to investigate vibration amplitude values that can cause the permanent differentiation of myofibroblasts into fibroblasts. On one hand, this possible differentiation is favorable in terms of tissue strength and resistance to functional loads. On the other hand, the persistence of myofibroblasts in tissue can be unfavorable in terms of fibrosis and cancerous growth. Therefore, we also need to identify the optimal range of ultrasound waves that could reduce the risk of myofibroblast stability. In addition, we suggest that this hypothesis should be tested by investigating if the ultrasound stimulating effect will be amplified when a modified biomaterial surface, for example an oxidized titanium disk,35 is used as a substrate for cell culture. It will also be important to investigate whether the morphology of cellular adhesion and the proliferation rate would differ when low intensity ultrasound is applied.
Conclusion
We found that low intensity ultrasound stimulation could improve the sensor-actuator mechanism and result in the integrated connection of human gingival fibroblasts in a honeycomb structure. This structure is the result of natural processes at the cell and tissue levels and was sensitive to external mechanical stress. A maximum vibration amplitude of 0.4 microns at a frequency of 27 kHz was identified as the critical limit for the safety of ultrasonic vibration to maintain cellular proliferation and architectural strengthening.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not for profit sector.
Conflict of interest
The authors have no conflict of interest to declare.
Authors' contributions
Mojtaba Afshari: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Writing - Original Draft, Visualization, Project administration, final approval of the version to be submitted; Saeid Amini: Resources, Supervision, Conceptualization, Review & Editing, final approval of the version to be submitted; Batool Hashemibeni: Methodology, Validation, Resources, Writing - Review & Editing, Visualization, Supervision, Project administration, final approval of the version to be submitted.
Acknowledgement
We would like to express our sincere thanks to Professor Khadijeh Karbalaei, Royan Institute for Biotechnology, Isfahan, Iran, who analyzed fluorescent images and contributed knowledge in the field of cell culture. This research received no specific grant from any funding agency.
Footnotes
Peer review under responsibility of Taibah University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtumed.2023.05.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ahangar P., Mills S.J., Smith L.E., Gronthos S., Cowin A.J. Human gingival fibroblast secretome accelerates wound healing through anti-inflammatory and pro-angiogenic mechanisms. NPJ Regen Med. 2020;5(1):24. doi: 10.1038/s41536-020-00109-9. PMID: 33303754; PMCID: PMC7728777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao L. The morphology and growth behavior of human gingival fibroblasts on three different titanium alloy surfaces (Doctoral dissertation, lmu) https://edoc.ub.uni-muenchen.de/
- 3.Ingber D.E. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20(7):811–827. doi: 10.1096/fj.05-5424rev. PMID: 16675838. [DOI] [PubMed] [Google Scholar]
- 4.Yang R., Broussard J.A., Green K.J., Espinosa H.D. Techniques to stimulate and interrogate cell-cell adhesion mechanics. Extreme Mech Lett. 2018;20:125–139. doi: 10.1016/j.eml.2017.12.002. Epub 2017 Dec 7. PMID: 30320194; PMCID: PMC6181239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laugier P., Haïat G., editors. Bone quantitative ultrasound. Springer; Dordrecht: 2011. pp. 29–45. [DOI] [Google Scholar]
- 6.Huerta R.R., Silva E.K., Ekaette I., El-Bialy T., Saldaña M.D.A. High-intensity ultrasound-assisted formation of cellulose nanofiber scaffold with low and high lignin content and their cytocompatibility with gingival fibroblast cells. Ultrason Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2019.104759. Epub 2019 Aug 28. PMID: 31948850. [DOI] [PubMed] [Google Scholar]
- 7.Hormozi-Moghaddam Z., Mokhtari-Dizaji M., Nilforoshzadeh M.A., Bakhshandeh M. Low-intensity ultrasound to induce proliferation and collagen Ι expression of adipose-derived mesenchymal stem cells and fibroblast cells in co-culture. Measurement. 2021;167 [Google Scholar]
- 8.Cao H., Feng L., Wu Z., Hou W., Li S., Hao Y., Wu L. Corrigendum to "Effect of low-intensity pulsed ultrasound on the biological behavior of osteoblasts on porous titanium alloy scaffolds: an in vitro and in vivo study" [Mater. Sci. Eng. C 80 (2017) 7-17] Mater Sci Eng C Mater Biol Appl. 2018;82:384. doi: 10.1016/j.msec.2017.08.078. Epub 2017 Sep 6. Erratum for: Mater Sci Eng C Mater Biol Appl. 2017 Nov 1;80:7-17. PMID: 29025672. [DOI] [PubMed] [Google Scholar]
- 9.Roper J.A., Williamson R.C., Bally B., Cowell C.A.M., Brooks R., Stephens P., Harrison A.J., Bass M.D. Ultrasonic stimulation of mouse skin reverses the healing delays in diabetes and aging by activation of Rac1. J Invest Dermatol. 2015;135(11):2842–2851. doi: 10.1038/jid.2015.224. Epub 2015 Jun 16. PMID: 26079528; PMCID: PMC4902130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alshihah N., Alhadlaq A., El-Bialy T., Aldahmash A., Bello I.O. The effect of low intensity pulsed ultrasound on dentoalveolar structures during orthodontic force application in diabetic ex-vivo model. Arch Oral Biol. 2020;119 doi: 10.1016/j.archoralbio.2020.104883. Epub 2020 Sep 12. PMID: 32932147. [DOI] [PubMed] [Google Scholar]
- 11.Pounder N.M., Harrison A.J. Low intensity pulsed ultrasound for fracture healing: a review of the clinical evidence and the associated biological mechanism of action. Ultrasonics. 2008;48(4):330–338. doi: 10.1016/j.ultras.2008.02.005. Epub 2008 Mar 27. PMID: 18486959. [DOI] [PubMed] [Google Scholar]
- 12.Rawool N.M., Goldberg B.B., Forsberg F., Winder A.A., Hume E. Power Doppler assessment of vascular changes during fracture treatment with low-intensity ultrasound. J Ultrasound Med. 2003;22(2):145–153. doi: 10.7863/jum.2003.22.2.145. PMID: 12562119. [DOI] [PubMed] [Google Scholar]
- 13.Ross T.D., Coon B.G., Yun S., Baeyens N., Tanaka K., Ouyang M., Schwartz M.A. Integrins in mechanotransduction. Curr Opin Cell Biol. 2013;25(5):613–618. doi: 10.1016/j.ceb.2013.05.006. Epub 2013 Jun 21. PMID: 23797029; PMCID: PMC3757118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balleri P., Cozzolino A., Ghelli L., Momicchioli G., Varriale A. Stability measurements of osseointegrated implants using Osstell in partially edentulous jaws after 1 year of loading: a pilot study. Clin Implant Dent Relat Res. 2002;4(3):128–132. doi: 10.1111/j.1708-8208.2002.tb00162.x. PMID: 12516644. [DOI] [PubMed] [Google Scholar]
- 15.Lee J.H., Herzog V.W., Rigby J.H. Rate of temperature rise and decay with 3-MHz therapeutic ultrasound using different intensities. Athl Train Sports Health Care. 2019;11(6):258–263. doi: 10.3928/19425864-20190122-01. [DOI] [Google Scholar]
- 16.Petterson S., Plancher K., Klyve D., Draper D., Ortiz R. Low-intensity continuous ultrasound for the symptomatic treatment of upper shoulder and neck pain: a randomized, double-blind placebo-controlled clinical trial. J Pain Res. 2020;13:1277. doi: 10.2147/JPR.S247463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson B.A., Schwane J.A., Starcher B.C. Effect of ultrasound therapy on the repair of Achilles tendon injuries in rats. Med Sci Sports Exerc. 1991;23(2):171–176. PMID: 2017013. [PubMed] [Google Scholar]
- 18.Le Bihan D., Delannoy J., Levin R.L. Temperature mapping with MR imaging of molecular diffusion: application to hyperthermia. Radiology. 1989;171(3):853–857. doi: 10.1148/radiology.171.3.2717764. PMID: 2717764. [DOI] [PubMed] [Google Scholar]
- 19.Miller A.D., Chama A., Louw T.M., Subramanian A., Viljoen H.J. Frequency sensitive mechanism in low-intensity ultrasound enhanced bioeffects. PLoS One. 2017;12(8) doi: 10.1371/journal.pone.0181717. PMID: 28763448; PMCID: PMC5538718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louw T.M., Budhiraja G., Viljoen H.J., Subramanian A. Mechanotransduction of ultrasound is frequency dependent below the cavitation threshold. Ultrasound Med Biol. 2013;39(7):1303–1319. doi: 10.1016/j.ultrasmedbio.2013.01.015. Epub 2013 Apr 3. PMID: 23562015; PMCID: PMC4183372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi Y., Sakai D., Iwashina T., Iwabuchi S., Mochida J. Low-intensity pulsed ultrasound stimulates cell proliferation, proteoglycan synthesis and expression of growth factor-related genes in human nucleus pulposus cell line. Eur Cell Mater. 2009;17:15–22. PMID: 19598131. [PubMed] [Google Scholar]
- 22.Patel U.S., Ghorayeb S.R., Yamashita Y., Atanda F., Walmsley A.D., Scheven B.A. Ultrasound field characterization and bioeffects in multiwell culture plates. J Ther Ultrasound. 2015;3:8. doi: 10.1186/s40349-015-0028-5. PMID: 26146556; PMCID: PMC4490766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vignesh, Nayar S., Bhuminathan, Mahadevan, Santhosh S. Comparative evaluation of the three different surface treatments - conventional, laser and Nano technology methods in enhancing the surface characteristics of commercially pure titanium discs and their effects on cell adhesion: an in vitro study. J Pharm BioAllied Sci. 2015;7(Suppl 1):S87–S91. doi: 10.4103/0975-7406.155817. PMID: 26015762; PMCID: PMC4439722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wahlsten A., Rütsche D., Nanni M., Giampietro C., Biedermann T., Reichmann E., Mazza E. Mechanical stimulation induces rapid fibroblast proliferation and accelerates the early maturation of human skin substitutes. Biomaterials. 2021;273 doi: 10.1016/j.biomaterials.2021.120779. Epub 2021 Mar 27. PMID: 33932701. [DOI] [PubMed] [Google Scholar]
- 25.Rubin D.M., Anderton N., Smalberger C., Polliack J., Nathan M., Postema M. On the behaviour of living cells under the influence of ultrasound. Fluids. 2018;3(4):82. [Google Scholar]
- 26.Webster K.D., Ng W.P., Fletcher D.A. Tensional homeostasis in single fibroblasts. Biophys J. 2014;107(1):146–155. doi: 10.1016/j.bpj.2014.04.051. PMID: 24988349; PMCID: PMC4119263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aktas O.C., Metzger W., Haidar A., Açil Y., Gülses A., Wiltfang J., Sacramento C.M., Nothdurft F.P. Enhancing adhesion and alignment of human gingival fibroblasts on dental implants. J Cranio-Maxillo-Fac Surg. 2019;47(4):661–667. doi: 10.1016/j.jcms.2019.02.004. Epub 2019 Feb 21. PMID: 30846326. [DOI] [PubMed] [Google Scholar]
- 28.DuFort C.C., Paszek M.J., Weaver V.M. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol. 2011;12(5):308–319. doi: 10.1038/nrm3112. PMID: 21508987; PMCID: PMC3564968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanamee É.S., Lippner G., Faustman D.L. Signal amplification in highly ordered networks is driven by geometry. Cells. 2022;11(2):272. doi: 10.3390/cells11020272. PMID: 35053388; PMCID: PMC8773832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rismanchian M., Afshari M. The effects of implant design, bone quality, and insertion process on primary stability of dental implants: an in-vitro study. Int J Biomed Eng Technol. 2020;34(4):319–333. [Google Scholar]
- 31.Dee K.C., Puleo D.A., Bizios R. In: An introduction to tissue-biomaterial interactions. Dee K.C., Puleo D.A., Bizios R., editors. Wiley; 2002. Protein-surface interactions; pp. 37–52. [DOI] [Google Scholar]
- 32.Ganadhiepan G., Zhang L., Miramini S., Mendis P., Patel M., Ebeling P., Wang Y. The effects of dynamic loading on bone fracture healing under ilizarov circular fixators. J Biomech Eng. 2019;141(5) doi: 10.1115/1.4043037. PMID: 30835278. [DOI] [PubMed] [Google Scholar]
- 33.Walters R.R., Graham J.F., Moore R.M., Anderson D.J. Protein diffusion coefficient measurements by laminar flow analysis: method and applications. Anal Biochem. 1984;140(1):190–195. doi: 10.1016/0003-2697(84)90152-0. PMID: 6486405. [DOI] [PubMed] [Google Scholar]
- 34.Mostafa N.Z., Uludağ H., Dederich D.N., Doschak M.R., El-Bialy T.H. Anabolic effects of low-intensity pulsed ultrasound on human gingival fibroblasts. Arch Oral Biol. 2009;54(8):743–748. doi: 10.1016/j.archoralbio.2009.04.012. Epub 2009 Jun 3. PMID: 19493525. [DOI] [PubMed] [Google Scholar]
- 35.Guida L., Oliva A., Basile M.A., Giordano M., Nastri L., Annunziata M. Human gingival fibroblast functions are stimulated by oxidized nano-structured titanium surfaces. J Dent. 2013;41(10):900–907. doi: 10.1016/j.jdent.2013.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.