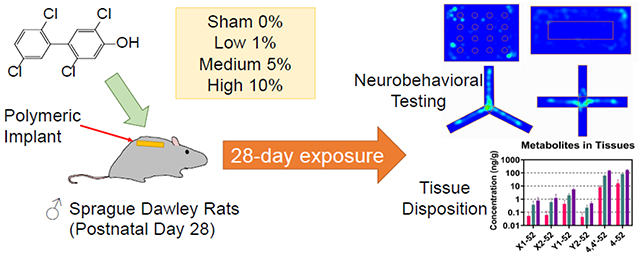
Abstract
Polychlorinated biphenyls (PCBs) are persistent organic pollutants (POPs) that ubiquitously exist in the environment. PCB exposure has been linked to cancer and multi-system toxicity, including endocrine disruption, immune inhibition, and reproductive and neurotoxicity. 2,2’,5,5’-Tetrachloro-biphenyl (PCB 52) is one of the most frequently detected congeners in the environment and human blood. The hydroxylated metabolites of PCB 52 may also be neurotoxic, especially for children whose brains are still developing. However, it is challenging to discern the contribution of these metabolites to PCB neurotoxicity because the metabolism of PCB is species-dependent. In this study, we evaluated the subacute neurotoxicity of a human-relevant metabolite, 2,2’,5,5’-tetrachlorobiphenyl-4-ol (4-52), on male adolescent Sprague Dawley rats, via a novel polymeric implant drug delivery system grafted subcutaneously, at total loading concentrations ranging from 0%, 1%, 5%, and 10% of the implant (w/w) for 28 days. Y-maze, hole board test, open field test, and elevated plus maze were performed on exposure days 24 to 28 to assess their locomotor activity, and exploratory and anxiety-like behavior. 4-52 and other possible hydroxylated metabolites in serum and vital tissues were quantified using gas chromatography with tandem mass spectrometry (GC-MS/MS). Our results demonstrate the sustained release of 4-52 from the polymeric implants into the systemic circulation in serum and tissues. Dihydroxylated and dechlorinated metabolites were detected in serum and tissues, depending on the dose and tissue type. No statistically significant changes were observed in the neurobehavioral tasks across all exposure groups. The results demonstrate that subcutaneous polymeric implants provide a straightforward method to expose rats to phenolic PCB metabolites to study neurotoxic outcomes, e.g., in memory, anxiety, and exploratory behaviors.
Keywords: adolescent rats; neurotoxicity; polymeric implant; 2,2’,5,5’-tetrachlorobiphenyl-4-ol; subacute exposure
Graphical abstract
1. Introduction
PCBs, or polychlorinated biphenyls, are environmental pollutants that can cause toxicity in multiple systems of both humans and animals, including neurological (Klocke et al., 2020), endocrine (Takaguchi et al., 2019), immune (Fair et al., 2021), developmental and reproductive toxicity (Berghuis and Roze, 2019; He et al., 2021). The International Agency for Research on Cancer (IARC) has classified PCBs as Group 1 Human Carcinogens based on evidence of PCBs-associated non-Hodgkin lymphoma and breast cancer (IARC, 2016). Although the intentional production of PCBs was banned many decades ago, they are still being ubiquitously detected in all environmental matrices, wildlife, livestock, and humans due to their chemical stability and bioaccumulative properties (Beyer and Biziuk, 2009). Emerging sources, such as the inadvertent production of PCBs during the manufacturing of paint pigment (e.g., PCB 11) (Hannah et al., 2022; Hu and Hornbuckle, 2010) or in the silicone industry (Schettgen et al., 2022), raise current and significant public health concerns.
Recent studies suggest that the general population is exposed to PCBs through diet and inhalation, specifically of lower chlorinated PCBs containing ⩽ 4 chlorine substituents (Othman et al., 2022). Previous research demonstrates that exposure to PCB during pregnancy and adolescence increases the risk of adverse neurological functions in children, including cognition (e.g., IQ, memory, and learning), exploratory behavior, social behavior, anxiety, autism spectrum disorder (ASD), and attention-deficit/hyperactivity disorder (ADHD) (Bullert et al., 2021; Klocke and Lein, 2020; Klocke et al., 2020). PCB exposure across the lifespan is also implicated in neurodegenerative diseases, such as Parkinson’s disease and Alzheimer’s disease and related dementias (Goldman, 2014; Yegambaram et al., 2015). For example, a 28-day repeat dose study of PCB 180 via gavage has reported altered open field behavior in female rats, as evidenced by increased activity and distance traveled in the inner zone, suggesting altered emotional responses to unfamiliar environments and impaired behavioral inhibition (Viluksela et al., 2014). Our previous animal studies have reported compromised spatial learning and memory and increased anxiety-like behavior in female rats after inhalation exposure to an environmental mixture of PCBs (Wang et al., 2020; Wang et al., 2022). The potential molecular mechanisms of PCB neurotoxicity involve oxidative stress, thyroid hormone disruption, and disruption of neurotransmitter or calcium homeostasis (Pessah et al., 2019; Shain et al., 1991; Tilson and Kodavanti, 1998).
2,2’,5,5’-Tetrachlorobiphenyl (PCB 52) is a frequently detected congener in the environment, including indoor air, outdoor air, human blood, and human brain (Grimm et al., 2015; Li et al., 2022; Sethi et al., 2019). PCB 52 is also one of seven indicator PCBs (along with PCB 28, 101, 118, 138, 153, and 180) used by international agencies to assess the magnitude of total PCB contamination in environmental studies (Bester et al., 2001; Megson et al., 2019). It is oxidized by human CYP2A6 to 2,2’,5,5’-tetrachlorobiphenyl-4-ol (4-52) (Shimada et al., 2016), a PCB 52 metabolite that is more toxic than the parent compound, as evidenced by increased cytotoxicity in various cell lines and more transcript changes in human preadipocytes than the parent PCB 52 (Gourronc et al., 2023; Paranjape et al., 2023). In addition to 4-52, in vitro metabolism studies with rats liver microsomes have identified 2,2’,5,5’-tetrachlorobiphenyl-3-ol (3-52) and a secondary metabolite, 2,2’,5,5’-tetrachlorobiphenyl-3,4-diol (3,4-52), as the oxidation products of PCB 52 (Borlakoglu et al., 1991; Forgue and Allen, 1982; Preston et al., 1983). Following dietary exposure, 4-52 was also detected in Drosophila melanogaster (fruit fly) (Idda et al., 2020). Hydroxylated, sulfated, and methylated PCB 52 metabolites were detected in female rats following intraperitoneal exposure to PCB 52 (Bullert et al., 2023a). There is also evidence that PCB 52 quinone-derived protein adducts are present in the rat brain and liver (Lin et al., 2000). This protein adduct is likely formed by the oxidation of a dihydroxylated PCB 52 metabolite.
There is evidence that hydroxylated and other PCB 52 metabolites may be neurotoxic (Paranjape et al., 2023; Rodriguez et al., 2018); however, it is challenging to discern the contribution of these metabolites to PCB neurotoxicity, in part because the metabolism of PCB is species-dependent (Grimm et al., 2015; Kania-Korwel and Lehmler, 2016). Studies have assessed the disposition and toxicity of synthetic PCB metabolites in rodents (Meerts et al., 2002; Meerts et al., 2004a; Meerts et al., 2004b). There are several ways to administer the desired PCB metabolite, which include oral intake, inhalation, and intraperitoneal or intravenous injection. However, these routes of exposure may not be relevant, for example, because metabolites are subject to hepatic first-pass metabolism. Oral gavage, inhalation exposures, and injections are invasive and cause stress to the animals, which can affect neurotoxic outcomes (Balcombe et al., 2004; Stuart and Robinson, 2015). Polymeric implants are an alternative approach for the sustained delivery of PCB metabolites over a more extended period to mimic human exposure (Gupta et al., 2012).
Here we investigate the release of 4-52, a human metabolite of PCB 52, from polymeric implants grafted on the back of the subcutaneous cavity of male rats and associated effects on behavioral outcomes. Our results demonstrate the sustained release of 4-52 from the implants into the systemic circulation. No statistically significant changes were observed in the neurobehavioral outcomes across all exposure groups. However, 4-52 exposure seemed to reduce exploratory and anxiety-like behavior in a dose-dependent manner, an observation that warrants further investigation.
2. Materials and Methods
2.1. Chemicals and Reagents
2,2’,3,4,4’,5,6,6’-octachlorobiphenyl (PCB 204, internal standard), 3,3’,4,5’-tetrachlorobiphenyl-4’-ol (4’-79, surrogate standard) and 2,3,3’,4,5,5’-hexachlorobiphenyl-4’-ol (4’-159, surrogate standard) were purchased from Accustandard (New Haven, CT, USA). 2,2’,5,5’-tetrachlorobiphenyl-4-ol (4-52) and 4,4’-dihydroxy-2,2’,5,5’-tetrachlorobiphenyl (4,4’-52) were synthesized (Lehmler and Robertson, 2001) and authenticated as described previously (Li et al., 2018; Li and Lehmler, 2022). Biphenyl-4-ol, polycaprolactone P-80 (average Mn 80,000), and Pluronic F-127 were obtained from Sigma-Aldrich (St. Louis, MO, USA). All solvents (pesticide grade), concentrated sulfuric acid, concentrated hydrochloric acid, potassium chloride, sodium chloride, silica gel, and sodium sulfite were purchased from Fisher Scientific (Pittsburg, PA, USA). Silicone tubing (internal diameter 2.6 mm) was provided by VWR International (Radnor, PA, USA). Bovine calf serum was obtained from Hyclone (Logan, UT, USA). Penicillin-streptomycin (Pen Strep) and phosphate-buffered saline (PBS) were purchased from Gibco (Grand Island, NY, USA). Tetrabutylammonium hydrogen sulfate was obtained from J.T. Baker (Phillipsburg, NJ, USA). Diazomethane was synthesized as previously described (Black, 1983).
2.2. Preparation of Polymeric Implants Containing 4-52
Implants were initially prepared using a published dipping/coating method (Aqil et al., 2012); however, in our hands, this method did not generate implants with a homogenous coating containing the test compound. Subsequently, polycaprolactone P-80-based implants containing the test compound were prepared using an extrusion method (Aqil et al., 2014). P-80 is a polymer approved by FDA for human use for drug delivery devices due to its biodegradable and non-toxic properties (Woodruff and Hutmacher, 2010). The preparation of the implants was optimized using biphenyl-4-ol as a test compound, as outlined in a published protocol (Bullert et al., 2023c) and described in the Supplementary Material. Subsequently, implants containing 4-52 were prepared as follows: 4-52 (0, 1, 5, or 10% by weight) was mixed with the lipophilic polymer P-80 and the hydrophilic polymer F-127 (10:1, w/w) in dichloromethane (DCM). The resulting homogenous solution was placed in an oil bath at 70 °C with continuous stirring to remove DCM. The viscous mixture was loaded into a 5 mL plastic syringe with about 8 cm of silicone tubing attached and placed in a 70 °C oven for 5 mins. The viscous material was then extruded through silicone tubing and allowed to harden at room temperature for 24 h. The implant was removed from the tubing and cut to desired sizes (~2 cm length, 2.3 mm diameter; see Fig. S1 for representative implants). The residual DCM in the implants was removed under reduced pressure using a Speed-Vac (Thermo-Savant, Holbrook, NY, USA) for 2 h at 35 °C. Implants were stored individually in 2 mL amber vials at −80 °C until use.
2.3. In Vitro Release Experiment
The release of the 4-52 from the implants was assessed over 28 days in vitro. In short, implants (n=3) were placed in separate 20 mL amber vials with 10 mL medium containing 10% bovine calf serum in PBS and 1% Pen Strep (v/v). Vials were incubated in a shaking water bath at 37 °C, agitating at 80 rpm. The medium was collected and replaced with fresh medium daily for 28 days. The collected medium (3 mL) was extracted using hexane and MTBE (9:1, v/v) after acidification with 1 mL 2 M hydrochloric acid and measured spectrophotometrically using UV/Vis (Cary 50, Varian, Inc., Walnut Creek, CA, USA) at 285 nm (see Fig. S2. showing the maximum absorption at 285 nm using a full scan from 400 to 200 nm). Concentrations of the test compound in the medium were determined with a matrix-matched calibration curve generated by spiking known concentrations of the target compound into fresh medium, which underwent the same extraction process. A detailed protocol describing the in vitro release experiment is available online (Bullert et al., 2023b).
2.4. Animal and In Vivo Exposure
All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Iowa and conform with the National Research Council’s Guide for the Care and Use of Laboratory Animals. Furthermore, the reporting of the animal experiments is in accordance with the ARRIVE guidelines. Sixty male juvenile Sprague-Dawley rats (n=15 for each exposure group, 21 days of age, 52±8 g) were ordered in 4 cohorts 3 weeks apart from Envigo (Indianapolis, IN, USA). Group sizes were chosen to detect differences of 20% between groups, depending on the parameters under investigation (e.g., PCB metabolite levels and behavioral outcomes). At least six biological replicates per experimental group were be needed to provide 0.8 power to detect a difference of 1.8 standard deviation at a significance level of 0.05. Multiple comparisons required 15 animals per experimental group. Rats were housed in polypropylene, fiber-covered cages in an AAALAC-accredited animal facility at 20-26 °C and 55% relative humidity with a 12 h light:dark cycle. Food (sterile Teklad 5% stock diet, Harlan, Madison, Wisconsin, USA) and water via an automated watering system were available ad libitum. After 7 days of acclimation, rats in each cohort were randomly assigned to four groups (sham, low (1%), medium (5%), or high (10%); 3 or 4 rats in each exposure group) using a random number generator (https://www.random.org/) and grafted with implants (1.3 ± 0.1 mg/g body weight) containing 0, 1, 5 or 10% of 4-52 on the back of the subcutaneous cavity on PND 28. We did not add a surgery control group (surgery performed but no implant grafted) considering the biodegradable and non-toxic properties of the implants (Woodruff and Hutmacher, 2010). No adverse outcomes were observed throughout the study.
Rats were anesthetized with 4% isoflurane for 2 min until they no longer responded to firm pressure applied to forepaw. One drop of eye lube (Optixcare, Ontario, Canada) was applied to both eyes, and 2% isoflurane was continuously supplied during the surgery. Next, rats were subcutaneously injected with 1 mg/kg meloxicam (Boehringer Ingelheim Animal Health USA Inc., Duluth, GA). The back of the rats was shaved and sanitized with a Povidone-iodine swab. A sharp needle (12-gauge, AVID Identification Systems Inc., Norco, CA, USA) was used to make an incision through the skin, creating a pocket between the dermal layers and muscle. A blunt needle (10-gauge, Hamilton Co., Reno, NV, USA) loaded with the implant was used to push the implant into this pocket using a syringe-style microchip implanter (AVID Identification Systems Inc., Norco, CA, USA). The incision was closed with AutoClips (Alzet, Cupertino, CA, USA). Animals were allowed to recover from anesthesia on a heating pad with continuous monitoring until animals were awake and ambulant. The surgical site and animal behavior were monitored daily for 5 consecutive days, including holidays and weekends. Clips were removed after 7 days using an AutoClip remover (Alzet, Cupertino, CA, USA).
After 28 days of exposure (PND 56), rats were anesthetized by inhalation of 5% isoflurane. With a continuous supply of isoflurane, blood was collected via cardiac puncture. The remaining blood in the circulatory system was immediately flushed out by whole-body perfusion with cold PBS. The vital organs were harvested and aliquoted. Some aliquots were snap-frozen or placed in RNAlater (Fisher Scientific, Pittsburg, PA, USA) and stored at −80 °C. One aliquot was placed in 4% formalin for pathological evaluation. The implants were retrieved from the back of rats to quantify the amount of 4-52 remaining in the implant. The exposure timeline is illustrated in Fig. 1.
Fig. 1.
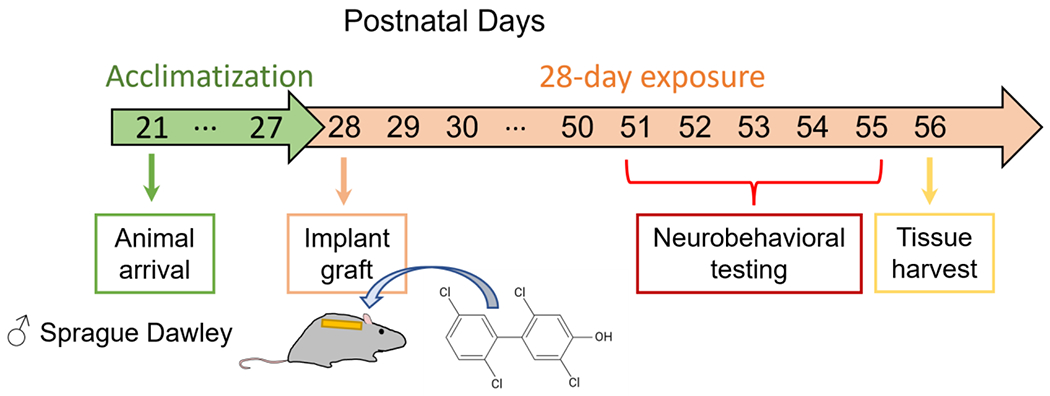
Schematic of the timeline for the implant exposure. Numbers in the arrows indicate the postnatal days.
2.5. Dosimetry Determination
The internal dose of 4-52 was estimated by subtracting the 4-52 remaining in the implants from the total load, assuming 100% bioavailability via subcutaneous exposure. On the necropsy day, implants were retrieved and dissolved in 10 mL DCM. The solution was diluted 3-fold and analyzed by UV/Vis (Cary 50, Varian, Inc., Walnut Creek, CA, USA) at 285 nm. The 4-52 concentration in the DCM solution was determined by a matrix-matched calibration curve prepared in parallel.
2.6. Histopathology
The brain, the accessory lobe of the right lung, and the left lateral lobe of the liver were excised and kept in formalin. Two animals from each cohort were random selected (total of 8 animals per exposure group) and samples were immediately sent to the Comparative Pathology Laboratory at the University of Iowa (Iowa City, IA, USA) for hematoxylin and eosin (H&E) staining. The slides were evaluated by a board-certified veterinary pathologist from Comparative Pathology Core Service, Iowa State University (Ames, IA, USA). The pathologist evaluated brain regions (including the prefrontal cortex, striatum, hippocampus, and cerebellum) for inflammation, gliosis, edema, hemorrhage, necrosis, apoptosis, and morphologic changes. The lung and liver were assessed for inflammation, hemorrhage, edema, necrosis, apoptosis, extramedullary hematopoiesis, and morphologic changes. Inflammation was evaluated by the presence of any type of immune cells (lymphocytes, macrophages, neutrophils, mast cells, eosinophils, etc.).
2.7. Neurobehavioral Testing
All behavioral tests were performed with all animals (n=15) on exposure days 24 to 28, with only one test conducted daily (Fig. 1). Rats were brought during the light cycle to the dedicated testing room 60 min before the tests. Rats were assessed randomly, with the persons carrying out the tests blind to the animal sequence and exposure information (Landis et al., 2012). The person assessing, measuring, or quantifying the experiment outcomes was also blind to the animal exposure information. Each testing apparatus was cleaned with 75% ethanol between animals to remove excrement and scent. The movement of the animals was recorded by an overhead camera and analyzed by ANY-maze video tracking system Version 7.1 (Stoelting Co., Wood Dale, IL, USA). All zone entries were manually scored in addition to ANY-maze software measurements to determine consistency.
2.7.1. Y-maze
Y-maze is a measure of short-term spatial working memory driven by the innate curiosity of rodents (Kraeuter et al., 2019). Rodents tend to explore a new arm instead of returning to one visited recently, a behavior called spontaneous alternation (Hughes, 2004). The maze comprised three enclosed arms (50 cm long, 10 cm wide, and 30 cm high) at a 120° angle from each other. Rats were put in the central intersection and had 5 min to explore. The ANY-maze video tracking system recorded the entry of each arm and travel distance. The percentage of spontaneous alternation was calculated as triads through three arms (e.g., arm A to B to C back to A but not arm A to B to A) over a total of three-entry sets.
2.7.2. Open Field Test (OFT)
The OFT is widely used for measuring exploratory, anxiety-like behavior and locomotor activity in rodents (Gould et al., 2009). The OFT was performed in an enclosed opaque rectangular box (~5000 cm2) with surrounding walls (50 cm high). The space was divided into a central zone and an outer zone. Each rat was placed in the center of the box and explored freely for 30 min on two consecutive days. An overhead camera recorded the number of central zone crossings and the duration and distance traveled in each zone.
2.7.3. Hole Board Test (HBT)
The HBT is a behavioral test for evaluating anxiety and exploratory behavior (Takeda et al., 1998). The hole board apparatus is an enclosed opaque square box (66 cm × 55 cm) with surrounding walls (50 cm high). The box was raised 50 cm above the floor. Sixteen holes (4 × 4, 4 cm diameter) were evenly distributed on the board. One rat at a time was released in the center of the board, and each rat had 5 minutes to explore the arena freely. The distance traveled and the total number of head dipping were recorded with the ANY-maze video tracking system. The head dipping was counted when the ears of the rat passed through the hole.
2.7.4. Elevated Plus Maze (EPM)
The EPM assesses anxiety-related behavior (Walf and Frye, 2007). The maze is made of black plexiglass and comprises four arms (two open arms and two enclosed wide arms, 40 cm long and 13 cm wide, with 30 cm high walls) elevated 60 cm above the floor. One rat at a time was placed on the central platform, facing a closed arm, and allowed 5 min to explore the maze freely. The number of arm entries and duration in each arm were recorded by the ANY-maze video tracking system.
2.8. Extraction of hydroxylated PCB 52 metabolites from the Tissues and Serum
A liquid-liquid extraction method was used to extract hydroxylated PCB 52 metabolites (Li et al., 2019; Wu et al., 2020). Briefly, tissues (0.31±0.02 mg for the brain, 0.21±0.02 mg for the liver, and 0.22±0.02 mg for the lung, n=8 per exposure group for each tissue type, two animals from each cohort were random selected) were homogenized with 3 mL 2-propanol using a TissueRuptor (QIAGEN, Hilden, Germany). After spiking 4’-79 and 4’-159 (10 ng each in 100 ng/mL in methanol) as surrogate standards to all samples, PCB 52 metabolites were extracted with hexane and diethyl ether (9:1, v/v), followed by washing the organic extracts with 5 mL of 0.1 M phosphoric acid in 0.9% sodium chloride solution. The extracts were concentrated under a gentle stream of nitrogen and derivatized with 0.1 mmol of diazomethane (200 mM in diethyl ether) at 4 °C overnight (Kania-Korwel et al., 2008). The extracts were passed through a sulfuric acid and silica gel (1:2, w/w) cartridge for further clean-up. Finally, the extracts were concentrated under gentle nitrogen flow and spiked with PCB 204 (10 ng in isooctane) as the internal standard for gas chromatography with tandem mass spectrometry (GC-MS/MS) analysis.
Serum (0.53±0.01 g, n=8 per exposure group, two animals from each cohort were random selected) samples were extracted analogously but with slight modifications (Li et al., 2019). Briefly, 1 mL of 6 M HCl was added to the serum samples. Next, the surrogate standards (4’-79 and 4’-159, 10 ng each in 100 ng/mL in methanol) and 5 mL of 2-propanol were added, and samples were extracted with 5 mL of hexane: methyl tert-butyl ether (MTBE) (1:1, v/v). After washing the organic phase with 3 mL of a 1% aqueous KCl solution, the extracts were concentrated and derivatized as described above. After further clean-up using 2-propanol and tetrabutylammonium hydrogen sulfate (TBA) (Hu et al., 2013), the extracts were subjected to the same clean-up steps described for tissue extraction. All metabolites were analyzed under the multiple reaction monitoring (MRM) mode on an Agilent 7890 A GC system equipped with an Agilent 7000 Triple Quad and Agilent 7693 autosampler (Agilent Technologies, Santa Clara, CA). All equipment parameters are described in the Supplementary Material. Precursor and product ions of all analytical standards and the corresponding collision energy are summarized in Table S2.
2.9. Quality Assurance and Quality Control (QA/QC)
Solvent blanks, method blank samples, vehicle exposed animal tissues, and an ongoing precision recovery standard were analyzed in parallel with all samples. Each sample was spiked with the surrogate standards, and the mass of each analyte was corrected for the recoveries of the appropriate surrogate standard. Internal standards were added for volume correction. Method detection limits (MDL) were calculated using the formula:
where meanblank is the mean of method blanks, t0.01, n-1 is the Student’s t-value for n – 1 degrees of freedom at the 99% confidence level, and SDblank is the standard deviation of the method blanks. Similarly, the limits of detection (LOD) were calculated with the formula:
where meansham is the mean of sham tissue measures, t0.01, n-1 is the Student’s t-value for n–1 degrees of freedom at the 99% confidence level, and SDsham is the standard deviation of the sham tissue measurements.
The recoveries of the surrogate standards and the Ongoing Precision and Recovery standard are listed in Table S3 and averaged 82-108%. The method detection limits and limits of detection for all analytes are summarized in Table S4.
2.10. Statistical Analysis
Data are presented as the mean ± standard deviation. Statistical differences were compared by one-way analysis of variance (ANOVA) with Fisher’s LSD test for multiple comparisons. Two-way ANOVA was used for comparing behavioral endpoints over 5-minute intervals in the Open Field Test. Skewed data were log-transformed to conform to normality. The animal was considered the statistical unit in this study and no animals were excluded from the analysis. Dose-response effects were explored using simple linear regression. A p-value < 0.05 was considered to be statistically significant. All statistical analyses were carried out in GraphPad Prism Version 9 (La Jolla, CA, USA). The original data of this study are openly available through the Iowa Research Online repository at https://doi.org/10.25820/data.006796 (Wang et al., 2023).
3. Results and Discussion
3.1. Formulation of Polymeric Implants
Different formulation methods, including dip-coating and extrusion approaches, were explored to prepare implants for the sustained release of 4-52, a human-relevant hydroxylated PCB metabolite. Biphenyl-4-ol, which has a chemical structure similar to 4-52, was used as an inexpensive model compound to optimize the preparation of implants suitable for animal studies. We first prepared implants using the dip-coating approach which was used previously to prepare implants for PCB exposure in rats (Aqil et al., 2014). Briefly, blank core implants generated by an extrusion method were coated by dipping them 25 to 30 times into a solution of 10% (w/v) P-80 and 1% biphenyl-4-ol in DCM. The coated implants were allowed to dry completely before the next coating step. The implants generated using this method were not homogeneous or reproducible in our hands. Importantly, this approach also requires a large amount of the test compound to generate enough solution for dip coating and, thus, is not the method of choice for preparing implants containing valuable, custom-synthesized test compounds, such as 4-52.
Subsequent experiments used an extrusion approach where a molten mixture of the polymers (P-80 and F-127 at 10:1, w/w) and the desired amount of biphenyl-4-ol (0%, 1%, 5%, and 10% of total load) is extruded into a silastic tubing mold. Water-soluble F-127 was added to aid in extrusion and formation of micro-pores once it dissipates a few days after implantation (Gilbert et al., 1987). The resulting implants can be cut to a desired length (about 20 mm) and have a highly reproducible shape (Fig. S1; average diameter 2.34 ± 0.06 mm). Using this approach, we prepared implants containing 0, 1, 5, and 10% of 4-52 for the animal studies described below. Overall, the extrusion approach is a straightforward and inexpensive approach to prepare implants for the sustained release of lipophilic phenolic chemicals, such as 4-52, that are heat resistant and not volatile.
3.2. In Vitro Release
We conducted an in vitro experiment investigating the release of biphenyl-4-ol from implants into PBS containing 10% bovine serum albumin (Gupta et al., 2012). The implant consistently released biphenyl-4-ol into the medium, peaking at 12 hours and day 2 (Fig. S3). Over 28 days, the release gradually decreased, resulting in a cumulative release of 77% to 84% of biphenyl-4-ol. The in vitro release of 4-52 from implants was subsequently assessed with PBS containing 10% bovine calf serum. Like the initial test using biphenyl-4-ol, 4-52 was released into the medium over 28 days, with 78-91% of the total 4-52 released from the implant after 28 days (Table 1 and Fig. 2). The release of 4-52 showed a spike around day 2 and subsequently decreased gradually. Similarly, the release of PCB 153 from polymeric implants prepared with a dip-coating approach spiked at around 4 days and continuously decreased until day 21 (Aqil et al., 2014). Considering the relatively low release of 4-52 after 2 weeks, a secondary implant may be grafted in future studies to provide a more consistent dose throughout 28-day studies.
Table 1.
The estimated dosimetry from in vitro release and in vivo animal exposure.
| Groups | Total 4-52 load in the implant (%) | In vitro release | In vivo exposure | |||||
|---|---|---|---|---|---|---|---|---|
| Total 4-52 released into the medium (%) | 4-52 remaining in the implant (%) | Tota1 (%) | Total 4-52 load in the implant (mg) | 4-52 remaining in the implant (mg) | 4-52 remaining in the implant (%) | Dose (mg/kg)a | ||
| Sham | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Low | 1 | 91.4 ± 2.5 | 3.4 ± 0.9 | 94.8 ± 1.7 | 1.1 ± 0.1 | 0.06 ± 0.02 | 5.8 ± 2.3 | 5.8 ± 0.4 |
| Medium | 5 | 82.3 ± 8.6 | 2.9 ± 1.8 | 85.3 ± 6.9 | 5.5 ± 0.1 | 0.40 ± 0.20 | 6.3 ± 2.8 | 29.5 ± 2.5 |
| High | 10 | 77.5 ± 3.9 | 5.2 ± 1.1 | 82.8 ± 3.1 | 11.6 ± 0.3 | 0.80 ± 0.40 | 6.9 ± 3.1 | 62.2 ± 4.2 |
The body weight was based on the time-weighted average over 28 days. Data are shown as mean ± standard deviation (n=15).
Fig. 2.
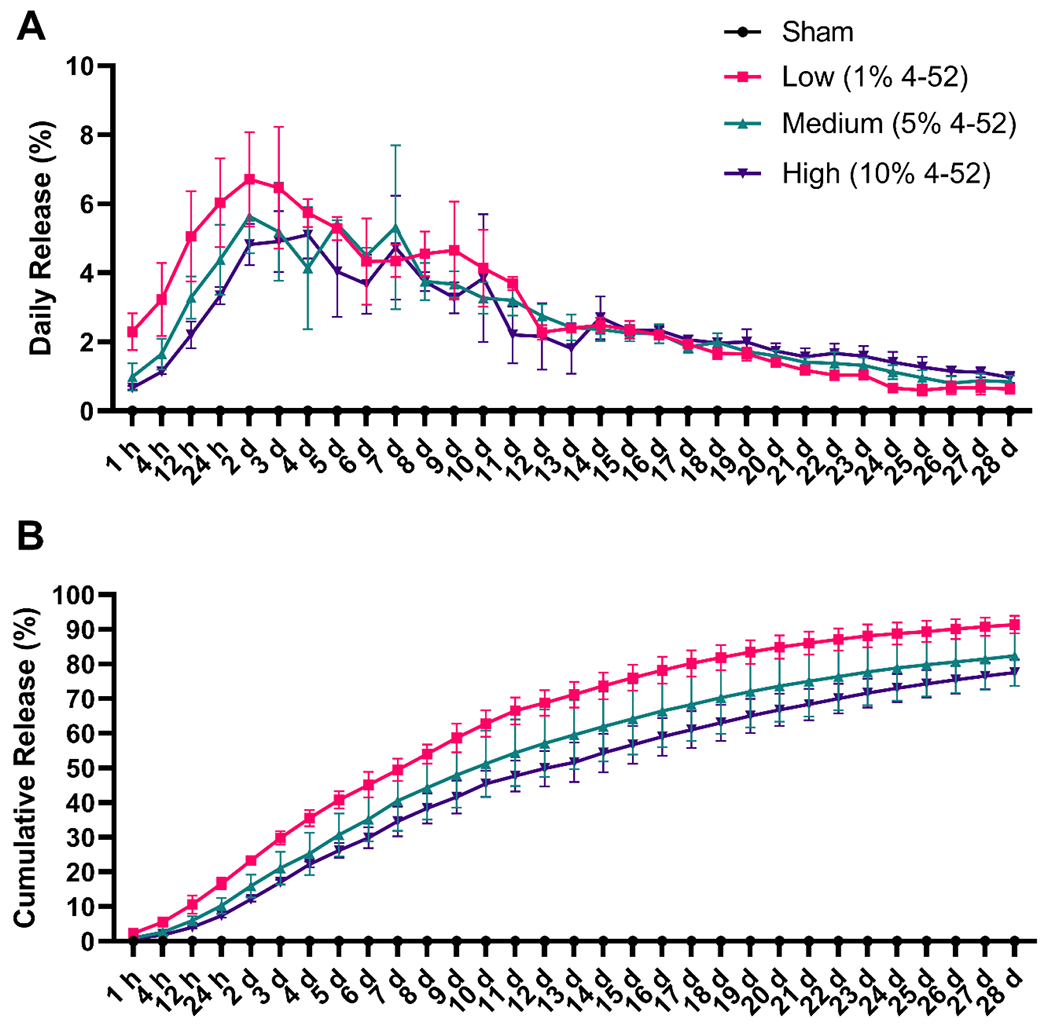
4-52, a human-relevant PCB 52 metabolite (Shimada et al., 2016), was continuously released in vitro from implants containing 0 (sham), 1, 5, or 10% of 4-52 over 28 days. The percentage of 4-52 released is shown for both (A) daily and (B) cumulative release from the implants. These implants were incubated in PBS with 10% bovine calf serum at 37 °C. Data are expressed as mean ± standard deviation (n=3).
At the end of the release experiment, the implants were dissolved in DCM to determine the amount of 4-52 remaining in the implants. Based on these measurements, 2.9 to 5.2% of 4-52 remained in the implants (Table 1). For comparisons, polycaprolactone-coated polymeric implants containing 5% of PCB 153 released 45% of the total PCB 153 after three weeks under comparable experimental conditions (Aqil et al., 2014). Together with the amount of 4-52 released into the medium, we recovered 83 to 95% of 4-52, likely due to the inevitable loss of 4-52 from heating and repeated transfer processes (Table 1). These in vitro release experiments demonstrate that the polymeric implants are a suitable delivery system for the sustained and controlled release of 4-52 and other phenolic PCB metabolites.
3.3. In Vivo Exposure and Dosimetry
Male Sprague-Dawley rats were exposed to 4-52 using subcutaneous polymeric implants containing 0, 1, 5, and 10% of 4-52. The implants were removed at the end of the study. The implantation site in the animals showed no signs of adverse effects, and the appearance of the implants was unchanged. The amount of 4-52 remaining in the implants was determined spectrophotometrically. The internal 4-52 dose was estimated by subtracting the amount of 4-52 remaining in the implants from the total load. As shown in Table 1, about 5.8-6.9% were left in the implants after 28 days of exposure, compared to 2.9-5.2% from the in vitro release experiment. For comparison, 49% of PCB126 and 73% of PCB 153 were released from polymeric implants in a 45-day study of the toxicity of PCBs in female rats (Aqil et al., 2014). The total internal doses of 4-52 were calculated as 0, 5.8, 29.5, and 62.2 mg/kg body weight (bw) for sham, low, medium, and high groups, respectively, assuming a 100% bioavailability from subcutaneous exposure. The administration of 4-52 using a polymeric implant has several advantages. It avoids the intestinal and hepatic first-pass metabolites while minimizing stress from repeated dosing and handling, which may compromise behavior and other outcomes in rats (Balcombe et al., 2004).
The doses used in this study were extrapolated to human lifetime inhalation exposure, assuming 100% conversion of PCB 52 to 4-52, using the equation:
where i is breath volume per minute (m3/min), T is the duration of exposure (min), α is the uptake rate from the lung of 99.8% (Hu et al., 2014), bw is the body weight (kg), and Cair is the PCB concentration in air (μg/m3), with an average bw of 80 kg and 70 years lifetime exposure, 0.5 L/breath, 12 breaths/min. To reach the low, medium, and high doses, the PCB concentration in the air a person breathes should be 2.1, 10.7, and 22.6 μg/m3. These concentrations are higher than most indoor (e.g., concentration ranging from 0.5 to 194 ng/m3 in schools in Indiana and Iowa, United States) (Marek et al., 2017) or outdoor environments (e.g., concentrations up to 94 ng/m3) (Othman et al., 2022), but comparable with some contaminated indoor air in Germany, which is as much as 2.1 μg/m3 (Gabrio et al., 2000).
Equivalent human oral exposure can be estimated similarly with the following equation:
where T is the duration of exposure (days), F is the oral bioavailability rate (52.9% for PCB 52 administered orally with corn oil) (Rostami and Juhasz, 2011), with an average bw of 80 kg and 70 years of lifetime exposure. Assuming 100% conversion of PCB 52 to 4-52, the daily oral PCB intake in humans corresponding to the low, medium, and high 4-52 doses in this animal study was estimated to be 35 μg, 170 μg, and 370 μg, respectively. For comparison, the Tolerable Daily Intake (TDI) of PCBs for humans has been set at 1.6 μg per day for an 80 kg individual and, thus, is lower than the doses used in this study (Faroon et al., 2003).
3.4. General toxicity Assessment
No significant differences in body weight gain were observed in rats exposed to 4-52 with a polymeric implant (Fig. S4). The relative liver weight decreased dose-dependently following subcutaneous 4-52 exposure (p < 0.001). In contrast, no significant effects on other organ weights were observed (Fig. 3). Earlier studies show increased liver weights due to liver hypertrophy after oral exposure to the parent PCBs (Chu et al., 1995; Wahlang et al., 2019). Consistent with these studies, Aqil et al. (2014) reported significantly increased liver weights following exposure of rats to PCB 126 but not PCB 153 compared to sham controls using dip-coated implants. Meerts et al. (2004b) reported liver weights from male and female offspring at PND 4 were significantly increased after in-utero exposure to Aroclor 1254, but not 4-OH-PCB107. Histopathological evaluation showed no overt treatment-specific lesions in the brain and lung in either exposed or sham rats. However, we observed a higher prevalence of minimal liver inflammation in the high dose (75% of evaluated slides), compared to 12.5% in the low, 25% in the medium, and 37.5% in the sham group.
Fig. 3.
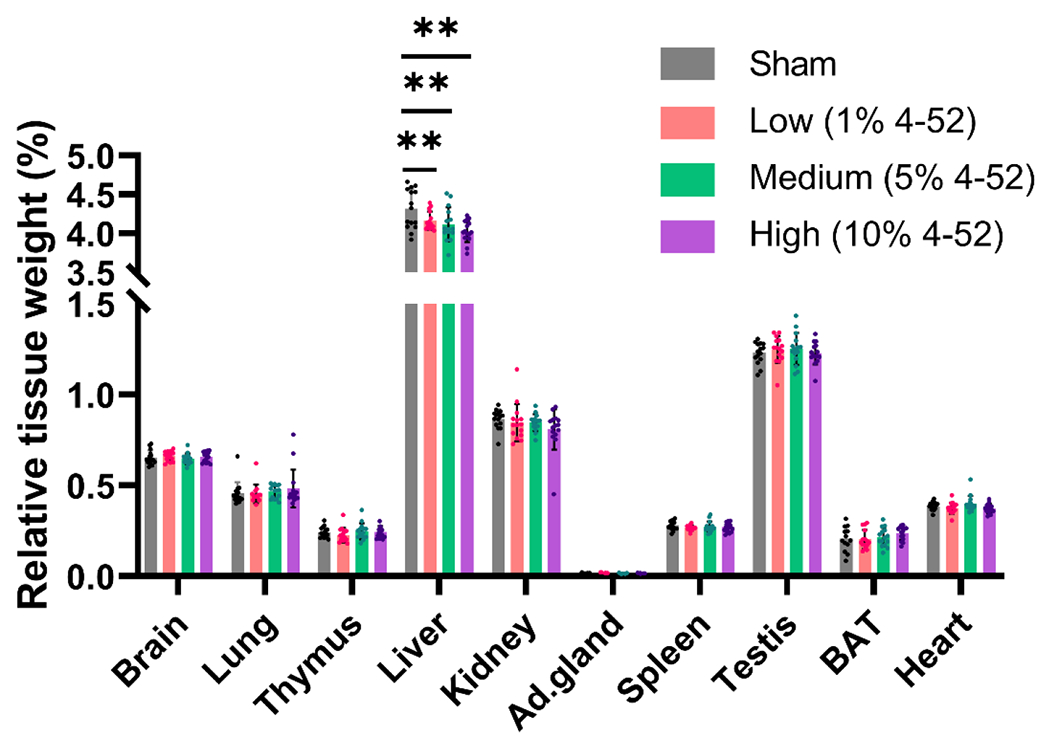
Male rats exposed to 4-52 via polymeric implants containing 0 (sham), 1, 5, or 10% of 4-52 for 28 days showed a significant decrease in liver weight as a percentage of their body weight, but no other organ weights were affected. Ad.gland: adrenal gland, BAT: brown adipose tissue. Data are expressed as mean ± standard deviation (n=15). ** p < 0.001. Statistical differences were compared by one-way analysis of variance (ANOVA) with Fisher’s LSD test for multiple comparisons.
3.5. Neurobehavioral Testing
The Y-maze demonstrated no significant changes in the total distance traveled (F(3, 56) = 1.25, p = 0.300), the number of total entries (F(3, 56) = 1.14, p = 0.342), and the percentage of spontaneous alternation (F(3, 56) = 1.37, p = 0.262) via one-way ANOVA, indicating the spatial working memory was not affected by subcutaneous 4-52 exposure (Fig. 4). However, the total distance traveled (p = 0.067) and the total number of entries (p = 0.087) showed a trend to decrease in the medium-exposed group compared to sham in the post hoc Fisher’s LSD test for multiple comparisons. This observation suggests a reduction in exploratory behavior and activity. A developmental study of mice using Aroclor 1254 (6 mg/kg/day and 18 mg/kg/day), a commercial PCB mixture containing PCB 52, also reported no changes in the percentage of alternation behavior in the Y-maze test (Tian et al., 2011). Similarly, another study reported that developmental PCB 52 exposure via the maternal diet impaired motor coordination but did not affect Y-maze performance in rats (Boix et al., 2010).
Fig. 4.
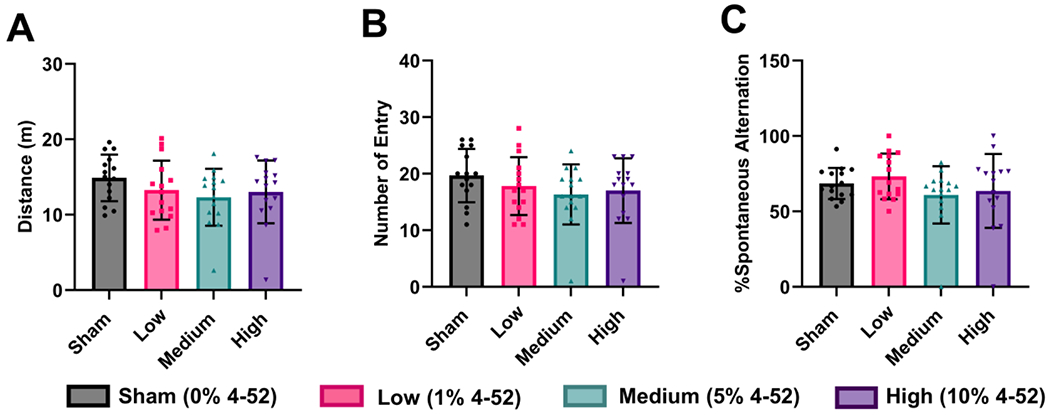
Subacute exposure to 4-52 via polymeric implants containing 0 (sham), 1, 5, or 10% of 4-52 showed a trend of a decrease in (A) the total distance traveled and (B) the total number of arm entries in the medium dose exposure group but (C) did not affect the percentage of spontaneous alternation in the Y-maze, a behavioral test for spatial working memory (Kraeuter et al., 2019). Data are expressed as mean ± standard deviation with individual dots representing different animals (n=15). Statistical differences were compared by one-way analysis of variance (ANOVA) with Fisher’s LSD test for multiple comparisons.
The Open Field Test was performed for 30 min for 2 consecutive days. The center entry, center time, and center distance for the first 5 min and total travel distance (Fig. 5A–5F, 5I–5J) were compared. No significant changes in either parameter were observed in 4-52 exposed rats by one-way ANOVA. The locomotor activity was gradually reduced when rats were familiar with the environment over 30 min (Fig. 5G and 5H) but with no significant differences between the groups at any time epoch. Developmental exposure to Aroclor 1254 significantly increased central distance, time duration, and entry into the central zone in female but not male mice (Tian et al., 2011).
Fig. 5.
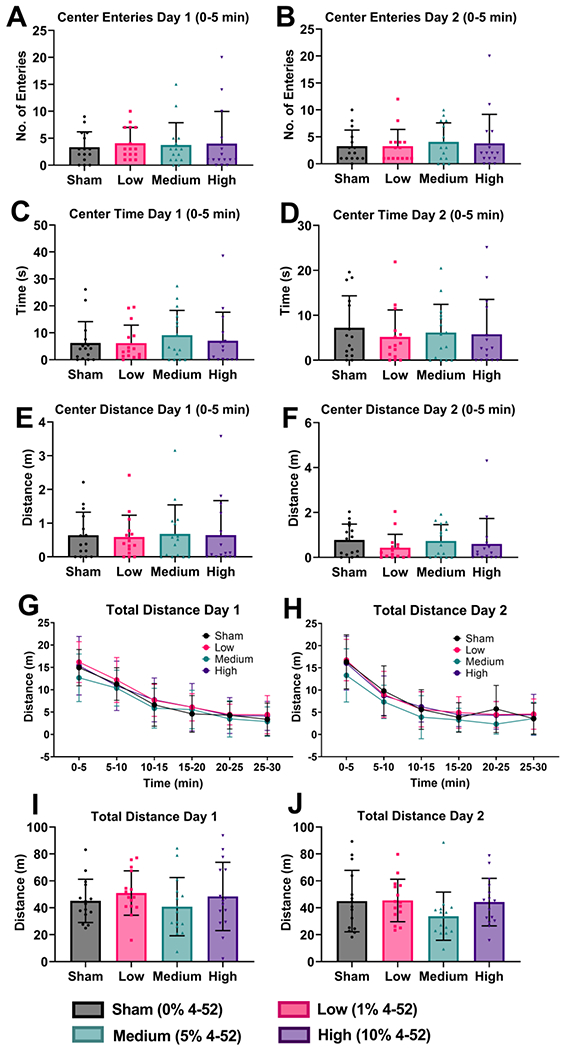
Subacute exposure to 4-52 via polymeric implants containing 0 (sham), 1, 5, or 10% of 4-52 did not alter (A) 5 min center zone entries of day 1, (B) 5 min center zone entries of day 2, (C) time in the center zone during the first 5 min on day 1, (D) time in the center zone during the first 5 min on day 2, (E) travel distance in the center zone during the first 5 min on day 1, (F) travel distance in the center zone during the first 5 min on day 2, (I) total distance on day1, and (J) total distance on day 2 in the Open Field Test. The travel distance was also not altered by 4-52 exposure over time, illustrated in 5 min epochs for 30 min on (G) day 1 and (H) day 2 of the Open Field Test. Data are expressed as mean ± standard deviation with individual dots representing different animals (n=15). Statistical differences were compared by one-way analysis of variance (ANOVA) using Fisher’s LSD test for multiple comparisons. Two-way ANOVA was used to compare the distance traveled over 5-minute intervals.
To evaluate the exploratory behavior of rats exposed to 4-52, the Hole Board Test was conducted. Fig. 6 illustrates no significant differences in the total distance traveled (F(3, 56) = 1.26, p = 0.297) or the number of head dipping (F(3, 56) = 0.93, p = 0.433) between the exposed and sham groups. A trend of slightly reduced travel was observed in medium-exposed rats (p = 0.071, Fig. 6A) in the post hoc Fisher’s LSD test for multiple comparisons, analogous to the result displayed in Y-Maze (Fig. 4A). The effect of PCB exposure on the exploratory behavior of rats in the Hole Board Test has not been reported previously. However, a study by Fanini et al. (1990) reported less head dipping in mice after intraperitoneal injection of Fenclor 54, a technical PCB mixture, at 150 mg/kg and 300 mg/kg.
Fig. 6.
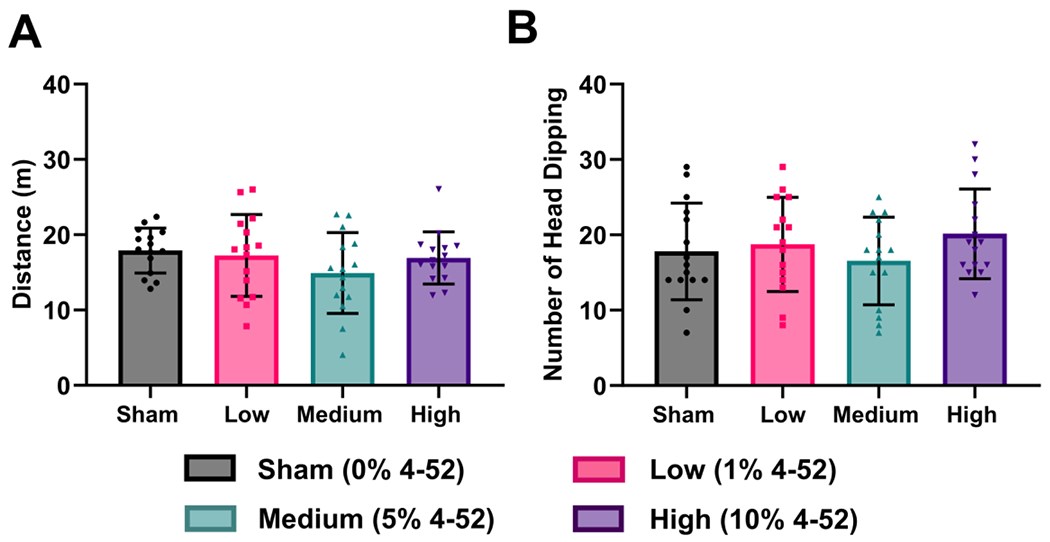
Subacute exposure to 4-52 via polymeric implants containing 0 (sham), 1, 5, or 10% of 4-52 showed (A) a trend of a decrease in the total distance traveled in the medium exposure group but (B) did not affect the number of head dipping in the Hole Board Test. Data are expressed as mean ± standard deviation with individual dots representing different animals (n=15). Statistical differences were compared by one-way analysis of variance (ANOVA) using Fisher’s LSD test for multiple comparisons.
The Elevated Plus Maze was used to evaluate anxiety-like behavior. No parameters were significantly changed by one-way ANOVA (Fig. 7) (Fig. 7A: F(3,56) = 0.70, p = 0.556; Fig. 7B: F(3,56) = 1.76, p = 0.165; Fig. 7C: F(3,56) = 1.57, p = 0.207; Fig. 7D: F(3,56) = 1.09, p = 0.361; Fig. 7E: F(3,56) = 1.20, p = 0.318; Fig. 7F: F(3,56) = 1.59, p = 0.203; ). However, post hoc Fisher’s LSD test for multiple comparisons indicated that the time in the enclosed arm was significantly decreased in the high-exposed group in comparison to the sham group (p = 0.043, Fig. 7F). Similarly, the medium exposed group showed a trend of reduction of the distance traveled in the enclosed arm (p = 0.097, Fig. 7D). Additionally, the time in the open arm for the high group was increased. However, this effect did not reach statistical significance (p = 0.076, Fig. 7E). Collectively, PCB-exposed rats, especially in the high-dose group, exhibited less anxious-like behavior. Similarly, one study reported increased time spent and entries in the open arms of the Elevated Plus maze for female but not male rats following prenatal and juvenile exposure to Aroclor 1221, suggesting less anxiety (Bell et al., 2016). Conversely, a previous study demonstrated increased anxiety-like behavior in female rats exposed to an environmentally relevant PCB mixture (Aroclor 1254 and Aroclor 1221) via inhalation (Wang et al., 2022). Simple linear regression was conducted to explore the dose-response effects by assigning 0, 1, 2, 3 to sham, low, medium, and high group, respectively. None of the behavioral parameters showed significant differences in the linear regression analysis.
Fig. 7.
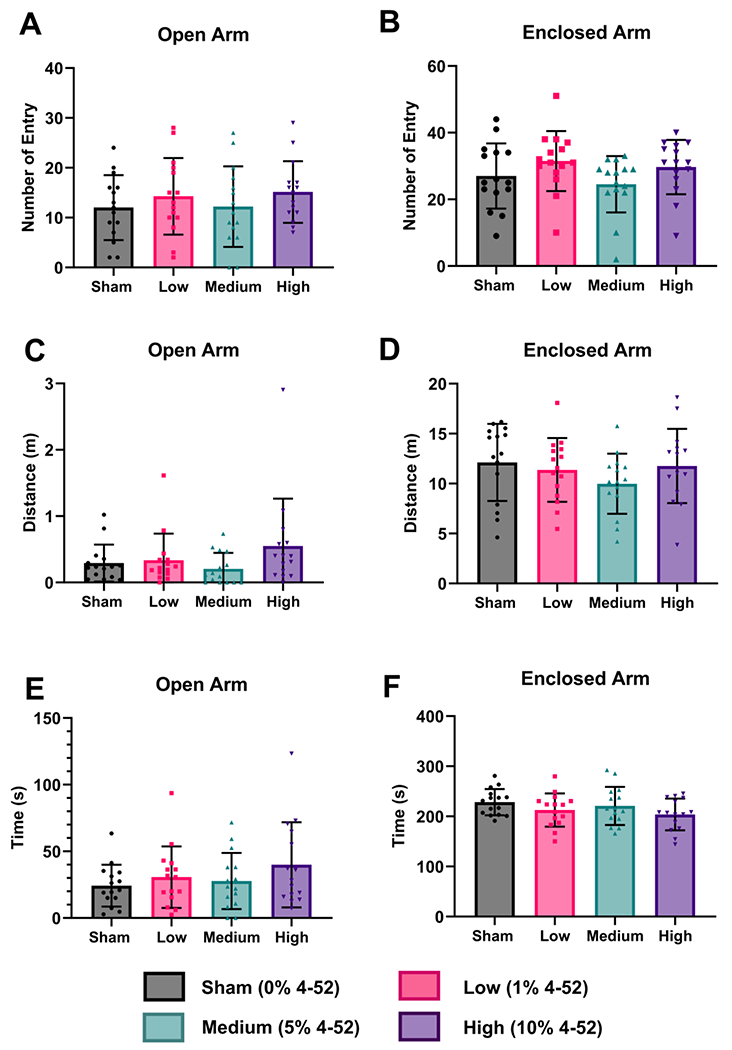
Elevated Plus Maze after subacute exposure to 4-52 via polymeric implants containing 0 (sham), 1, 5, or 10% of 4-52 did not alter the (A) number of open and (B) closed arm entries and (C) the open arm travel distance, but (D) decreased the closed arm travel distance in the medium exposure group, (E) marginally increased the time in the open arm in the high exposure group, and (F) decreased the time in the closed arm, also in the high exposure group. Data are expressed as mean ± standard deviation with individual dots representing different animals (n=15). Statistical tests were carried out by one-way analysis of variance (ANOVA) using Fisher’s LSD test for multiple comparisons.
3.6. OH-PCB Disposition in Serum and Tissues
The release of 4-52 from the implants into the systemic circulation and its distribution into target tissues were assessed after liquid-liquid extraction using GC-MS/MS. 4-52 was detected in serum, brain, liver, and lung (Fig. 8). Its levels increased with increasing 4-52 doses in all compartments. Across tissues, levels of 4-52 decreased in the order of serum >> liver > lung > brain. The fact that 4-52 was preferentially retained in the serum is consistent with earlier studies demonstrating that para-hydroxylated PCBs are retained in human and rat blood (Bergman et al., 1994). The retention of these OH-PCB congeners in the blood is due to several factors. OH-PCBs have a high binding affinity to transthyretin (Purkey et al., 2004) and other transport proteins in the blood, including albumin (Rodriguez et al., 2016). The affinity of OH-PCBs for transthyretin is stronger for congeners with a para-substituted hydroxyl group with adjacent chlorine on the meta position of the biphenyl rings (Rickenbacher et al., 1986). Moreover, the sulfation and glucuronidation of OH-PCBs depend on the degree of chlorination and the substitution pattern, which contributes to the persistence of certain OH-PCBs in blood, as suggested for the glucuronidation of OH-PCBs (Tampal et al., 2002).
Fig. 8.
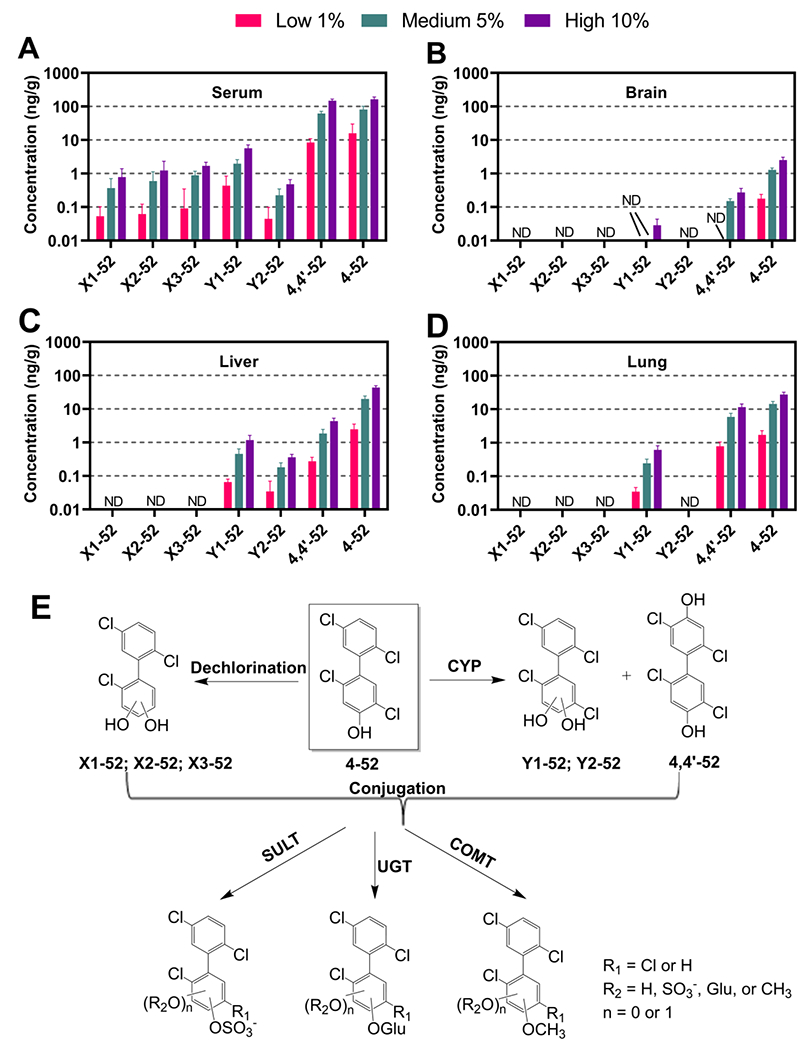
4-52 and six dihydroxylated metabolites, including three dechlorinated metabolites X1-52 X2-52,and X3-52 were detected in (A) serum, (B) brain, (C) liver, and (D) lung of male rats exposed for 28 days to 4-52 via polymeric implants containing 0 (sham), 1, 5, or 10% of 4-52. (E) illustrated the possible metabolic pathway of 4-52. Data are the mean ± standard deviation (n = 8). X indicates a dihydroxylated trichlorobiphenyl metabolite; Y indicates a dihydroxylated tetrachlorobiphenyl metabolite. ND: not detected. SULT: sulfotransferase. UGT: UDP-glucuronosyltransferase. COMT: Catechol-O-methyltransferase The y-axis is in a log scale to facilitate a comparison between tissue levels. The concentrations are normalized by tissue wet weight (ng/g).
We also detected and quantified 4,4’-52, for which an authentic analytical standard was available, in serum, brain, liver, and lung (Fig. 8). In addition, we observed two unidentified tetra-chlorinated dihydroxylated metabolites of 4-52, Y1-52 and Y2-52, based on their transitions in the GC-MS/MS analysis. The levels of both metabolites were estimated with the relative response factor of 4,4’-52. The dihydroxylated metabolites are likely formed via the oxidation of 4-52 by cytochrome P450 enzymes, as shown in the metabolism pathway in Fig. 8E. Similarly, a study that intraperitoneally exposed PCB 52 to female rats had discovered mono- (4-52 and one unknown metabolite), and dihydroxylated metabolites in the liver (Bullert et al., 2023a). Disposition studies with PCB 95, which is structurally related to PCB 52, also report the presence of a 4,4’-dihydroxylated metabolite in the feces of rodents (Sundström and Jansson, 1975). There is also evidence from metabolism studies using recombinant enzymes or liver microsomes that CYP2B1 oxidized para-hydroxylated PCBs to PCB catechol metabolites; however, these studies did not report the formation of metabolites with hydroxy groups in separate phenyl rings (Borlakoglu et al., 1991; McLean et al., 1996; Preston et al., 1983).
Y1-52, Y2-52, and 4,4’-52 were present in the serum and liver from all exposure groups (Fig. 8A and 8C). Y1-52 was only detectable in the brain in the high-dose group, and 4,4’-52 was only found in the medium and high-dose groups (Fig. 8B). The low detection frequencies and low levels of OH-PCBs detected in the brain likely occurred because the blood-brain barrier, to some extent, prevented OH-PCBs from partitioning from the blood into the brain. Only Y1-52 and 4,4’-52 were detected in the lung (Fig. 8D). The levels of 4,4’-52 followed the rank order serum > lung > liver > brain, whereas the levels of Y1-52 decrease in the order serum > liver > lung > brain. The levels of Y2-52 were also higher in serum than in the liver. These results demonstrate that dihydroxylated PCB 52 metabolites, like 4,4’-52, are preferentially retained in the blood in rats.
Three possible dechlorinated dihydroxylated metabolites of 4-52, X1-52, X2-52, and X3-52 were found in the serum but not in the brain, liver, and lung in the GC-MS/MS analysis, as shown in Fig. 8A–D. Several animal studies have reported the formation of dechlorinated OH-PCB metabolites (Bullert et al., 2023a; Hutzinger et al., 1974; Sundström and Jansson, 1975; Tulp et al., 1977). We recently showed that 3,3’-dichlorobiphenyl-4-ol, an OH-PCB with the OH group in the ortho position of a chlorine substituent, undergoes chlorine displacement in experiments with human liver microsomes and HepG2 cells in culture, forming a dechlorinated PCB catechol metabolite (Zhang et al., 2022). The formation of PCB catechols (and hydroquinones), either by oxidation of an OH-PCB or a chlorine displacement reaction, is a toxicological concern because these metabolites are redox active and can be oxidized to reactive quinone metabolites (Liu et al., 2020). Indeed, PCB 52 quinone-derived protein adducts have been detected in vivo (Lin et al., 2000). Therefore, additional studies with authentic standards are needed to confirm the structure of the dihydroxylated metabolites detected in this study and other metabolites, including the corresponding conjugates (Fig. 8E).
Human population studies have reported OH-PCB levels, primarily in serum. One study quantified OH-PCB in serum from children and their mothers from urban and rural U.S. communities, with ΣOH-PCBs ranging from non-detectable to 1.2 ng/g fresh weight (fw) (median = 0.07 ng/g) in year 1 and from 0.04 to 0.27 ng/g fw in year 2 (median = 0.09 ng/g) (Marek et al., 2014; Marek et al., 2013). In the Canadian Inuit, higher OH-PCB concentrations have been reported in the blood, ranging from 0.117 to 11.6 ng/g fw. The high OH-PCB levels in this population are believed to be due to the high levels of PCBs found in their diet, which consists of fish and sea mammals (Sandau et al., 2000). The OH-PCB levels in the present study are higher than this highly PCB-exposed population. For example, the ΣOH-PCB levels in serum in our animal study were 25±14, 146±31, and 322±33 ng/g fw in low, medium, and high dose groups, respectively. The 4-52 levels in the serum were 16±14, 81±21, and 164±27 ng/g fw in low, medium, and high dose groups, respectively.
4. Conclusions
Polychlorinated biphenyls (PCBs) and their metabolites are implicated in adverse health outcomes, including neurotoxicity. However, it is challenging to assess the role of metabolism in PCB neurotoxicity in animal studies because of species differences in PCB metabolism. Although there are several routes to administer human-relevant PCB metabolite in animal studies, these routes of exposure may not be toxicologically relevant. For example, PCB metabolites are subject to hepatic first-pass metabolism following oral exposure. The present study demonstrates that a polymeric implant system provides sustained release of 4-52, a phenolic, human-relevant PCB 52 metabolite, both in an in vitro release experiment and a 28-day subcutaneous in vivo exposure study. 4-52 and its di-hydroxylated and dechlorinated metabolites were also detected in serum and vital tissues following 28 days of exposure via an implant. Importantly, our 28-day subacute toxicity study revealed that 4-52 had no statistically significant impacts on spatial memory, and exploratory and anxiety-like behavior in male juvenile rats across all exposed groups. However, 4-52 exposure tended to reduce exploratory and anxiety-like behavior at some doses. This finding warrants further investigation considering the well documented non-monotonic dose response curves of PCB developmental neurotoxicity. We acknowledge that the short 28-day exposure does not reflect real-life exposure during adolescence, which may partially explain negative effects in the neurological tasks. Moreover, the metabolism of 4-52 and its neurological effects could be sex-dependent. A study using age-matched female rats should be conducted to address this limitation. Despite these limitations, the results from this study indicate that subcutaneous polymeric implants are a straightforward method to expose rats to phenolic PCB metabolites during studies of their neurotoxicity. This route of PCB metabolite exposure avoids the disadvantages of other routes of exposure (e.g., oral exposure) that are more stressful for the animals and potential confounders in behavioral tests.
Supplementary Material
Highlights.
2,2’,5,5’-Tetrachlorobiphenyl-4-ol (4-52) is released from polymeric implants in vitro
Subcutaneous polymeric implants released 4-52 in male rats over 28 days in vivo
Hydroxylated and dechlorinated products were detected in vital tissues in male rats
4-52 did not significantly affect neurobehavioral tasks
Acknowledgments
The authors thank Dr. Keri C Hornbuckle and Dr. Rachel F Marek for supporting the GC-MS/MS analyses, Dr. Kai Wang for statistical support, Brian Westra for data management, and Katelin E J Scott for assisting in the measurement of in vitro release. The authors also thank Dr. Laura E Dean, Dr. Xuefang Jing, Kyleakin K Helm-Kwasny, Riley M Behan-Bush, Jesse N Liszewski, Vanessa Livania, and Mackenzie J Pattridge for their valuable assistance with the animal study.
Funding sources
This research was supported by the Iowa Superfund Research Program grant NIH P42 ES013661 and conducted in laboratory facilities supported by the Environmental Health Sciences Research Center, grant NIH P30 ES005605. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interests: The authors declare no competing financial interests.
CRediT authorship contribution statement
Wang: Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Roles/Writing - original draft; Writing - review & editing.
Bullert: Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Writing - review & editing.
Li: Data curation; Formal analysis; Investigation; Resources; Methodology; Validation; Writing - review & editing.
Stevens: Formal analysis; Resources; Methodology; Visualization; Writing - review & editing.
Klingelhutz: Conceptualization; Funding acquisition; Resources; Methodology; Writing - review & editing.
Ankrum: Conceptualization; Funding acquisition; Resources; Methodology; Writing - review & editing.
Adamcakova-Dodd: Conceptualization; Investigation; Methodology; Writing - review & editing.
Thorne: Conceptualization; Funding acquisition; Resources; Writing - review & editing.
Lehmler: Conceptualization; Funding acquisition; Resources; Project administration; Supervision; Validation; Visualization; Roles/Writing - original draft; Writing - review & editing.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
References
- Aqil F, Jeyabalan J, Kausar H, Bansal SS, Sharma RJ, Singh IP, Vadhanam MV and Gupta RC 2012. Multi-layer polymeric implants for sustained release of chemopreventives. Cancer Lett 326, 33–40, 10.1016/j.canlet.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aqil F, Shen H, Jeyabalan J, Xin X, Lehmler HJ, Ludewig G, Robertson LW and Gupta RC 2014. Sustained expression of CYPs and DNA adduct accumulation with continuous exposure to PCB126 and PCB153 through a new delivery method: Polymeric implants. Toxicol Rep 1, 820–833, 10.1016/j.toxrep.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcombe JP, Barnard ND and Sandusky C 2004. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci 43, 42–51. [PubMed] [Google Scholar]
- Bell MR, Thompson LM, Rodriguez K and Gore AC 2016. Two-hit exposure to polychlorinated biphenyls at gestational and juvenile life stages: 1. Sexually dimorphic effects on social and anxiety-like behaviors. Horm Behav 78, 168–177, 10.1016/j.yhbeh.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghuis SA and Roze E 2019. Prenatal exposure to PCBs and neurological and sexual/pubertal development from birth to adolescence. Curr Probl Pediatr Adolesc Health Care 49, 133–159, 10.1016/j.cppeds.2019.04.006. [DOI] [PubMed] [Google Scholar]
- Bergman A, Klasson-Wehler E and Kuroki H 1994. Selective retention of hydroxylated PCB metabolites in blood. Environ Health Perspect 102, 464–469, 10.1289/ehp.94102464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bester K, de Vos P, Le Guern L, Harbeck S, Hendrickx F, Kramer GN, Linsinger T, Mertens I, Schimmel H, Sejeroe-Olsen B, Pauwels J, De Poorter G, Rimkus GG and Schlabach M 2001. Preparation and certification of a reference material on PCBs in pig fat and its application in quality control in monitoring laboratories during the Belgian “PCB-crisis”. Chemosphere 44, 529–537, 10.1016/s0045-6535(00)00476-8. [DOI] [PubMed] [Google Scholar]
- Beyer A and Biziuk M 2009. Environmental fate and global distribution of polychlorinated biphenyls. Rev Environ Contam Toxicol 201, 137–158, 10.1007/978-1-4419-0032-6_5. [DOI] [PubMed] [Google Scholar]
- Black TH 1983. The preparation and reactions of diazomethane. Chemischer Informationsdienst 14. [Google Scholar]
- Boix J, Cauli O and Felipo V 2010. Developmental exposure to polychlorinated biphenyls 52, 138 or 180 affects differentially learning or motor coordination in adult rats. Mechanisms involved. Neuroscience 167, 994–1003, 10.1016/j.neuroscience.2010.02.068. [DOI] [PubMed] [Google Scholar]
- Borlakoglu JT, Haegele KD, Reich HJ, Dils RR and Wilkins JP 1991. In vitro metabolism of [14C]4-chlorobiphenyl and [14C]2,2’,5,5’-tetrachlorobiphenyl by hepatic microsomes from rats and pigeons. Evidence against an obligatory arene oxide in aromatic hydroxylation reactions. Int J Biochem 23, 1427–1437, 10.1016/0020-711x(91)90286-v. [DOI] [PubMed] [Google Scholar]
- Bullert A, Li X, Chunyun Z, Lee K, Pulliam CF, Cagle BS, Doorn JA, Klingelhutz AJ, Robertson LW and Lehmler HJ 2023a. Disposition and metabolomic effects of 2,2’,5,5’-tetrachlorobiphenyl in female rats following intraperitoneal exposure. Environ Toxicol Pharmacol 102, 104245, 10.1016/j.etap.2023.104245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullert AJ, Doorn JA, Stevens HE and Lehmler HJ 2021. The Effects of Polychlorinated Biphenyl Exposure During Adolescence on the Nervous System: A Comprehensive Review. Chem Res Toxicol 34, 1948–1952, 10.1021/acs.chemrestox.1c00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullert AJ, Wang H and Lehmler H 2023b. In vitro release, extraction, and analysis of PCBs and metabolites from polymeric implants. 10.17504/protocols.io.kqdg3ppj1l25/v1 [DOI] [Google Scholar]
- Bullert AJ, Wang H and Lehmler H 2023c. Preparation of Polymeric Implants for the Sustained Release of Polychlorinated Biphenyls (PCBs) and Their Derivatives. 10.17504/protocols.io.n2bvj667plk5/v1 [DOI] [Google Scholar]
- Chu I, Villeneuve DC, Yagminas A, Lecavalier P, Hakansson H, Ahlborg UG, Valli VE, Kennedy SW, Bergman A, Seegal RF and et al. 1995. Toxicity of PCB 77 (3,3’,4,4’-tetrachlorobiphenyl) and PCB 118 (2,3’,4,4’5-pentachlorobiphenyl) in the rat following subchronic dietary exposure. Fundam Appl Toxicol 26, 282–292, 10.1006/faat.1995.1099. [DOI] [PubMed] [Google Scholar]
- Fair PA, Peden-Adams MM, Mollenhauer MAM, Bossart GD, Keil DE and White ND 2021. Effects of an environmentally relevant PCB-mixture on immune function, clinical chemistry, and thyroid hormone levels in adult female B(6)C(3)F(1) mice. J Toxicol Environ Health A 84, 279–297, 10.1080/15287394.2020.1863887. [DOI] [PubMed] [Google Scholar]
- Fanini D, Palumbo G, Giorgi R and Pantaleoni G 1990. Behavioral effects of PCBs in mice. Behav Pharmacol 1, 505–510. [PubMed] [Google Scholar]
- Faroon OM, Samuel Keith L, Smith-Simon C, De Rosa CT and Organization WH 2003. Polychlorinated biphenyls: human health aspects, World Health Organization. [Google Scholar]
- Forgue ST and Allen JR 1982. Identification of an arene oxide metabolite of 2,2’,5-5’-tetrachlorobiphenyl by gas chromatography-mass spectroscopy. Chem Biol Interact 40, 233–245, 10.1016/0009-2797(82)90103-x. [DOI] [PubMed] [Google Scholar]
- Gabrio T, Piechotowski I, Wallenhorst T, Klett M, Cott L, Friebel P, Link B and Schwenk M 2000. PCB-blood levels in teachers, working in PCB-contaminated schools. Chemosphere 40, 1055–1062, 10.1016/S0045-6535(99)00353-7. [DOI] [PubMed] [Google Scholar]
- Gilbert JC, Richardson JL, Davies MC, Palin KJ and Hadgraft J 1987. The effect of solutes and polymers on the gelation properties of pluronic F-127 solutions for controlled drug delivery. Journal of Controlled Release 5, 113–118, 10.1016/0168-3659(87)90002-2. [DOI] [Google Scholar]
- Goldman SM 2014. Environmental toxins and Parkinson’s disease. Annu Rev Pharmacol Toxicol 54, 141–164, 10.1146/annurev-pharmtox-011613-135937. [DOI] [PubMed] [Google Scholar]
- Gould TD, Dao DT and Kovacsics CE 2009. The Open Field Test. In: Gould TD (Ed), Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests, Humana Press, Totowa, NJ, pp. 1–20. [Google Scholar]
- Gourronc FA, Chimenti MS, Lehmler HJ, Ankrum JA and Klingelhutz AJ 2023. Hydroxylation markedly alters how the polychlorinated biphenyl (PCB) congener, PCB52, affects gene expression in human preadipocytes. Toxicol In Vitro 89, 105568, 10.1016/j.tiv.2023.105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm FA, Hu D, Kania-Korwel I, Lehmler HJ, Ludewig G, Hornbuckle KC, Duffel MW, Bergman A and Robertson LW 2015. Metabolism and metabolites of polychlorinated biphenyls. Crit Rev Toxicol 45, 245–272, 10.3109/10408444.2014.999365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RC, Bansal SS, Aqil F, Jeyabalan J, Cao P, Kausar H, Russell GK, Munagala R, Ravoori S and Vadhanam MV 2012. Controlled-release systemic delivery - a new concept in cancer chemoprevention. Carcinogenesis 33, 1608–1615, 10.1093/carcin/bgs209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah TJ, Megson D and Sandau CD 2022. A review of the mechanisms of by-product PCB formation in pigments, dyes and paints. Sci Total Environ 852, 158529, 10.1016/j.scitotenv.2022.158529. [DOI] [PubMed] [Google Scholar]
- He QL, Zhang L and Liu SZ 2021. Effects of Polychlorinated Biphenyls on Animal Reproductive Systems and Epigenetic Modifications. Bull Environ Contam Toxicol 107, 398–405, 10.1007/s00128-021-03285-6. [DOI] [PubMed] [Google Scholar]
- Hu D and Hornbuckle KC 2010. Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ Sci Technol 44, 2822–2827, 10.1021/es902413k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Adamcakova-Dodd A and Thorne PS 2014. The fate of inhaled (14)C-labeled PCB11 and its metabolites in vivo. Environ Int 63, 92–100, 10.1016/j.envint.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Lehmler HJ, Adamcakova-Dodd A and Thorne PS 2013. Elimination of inhaled 3,3’-dichlorobiphenyl and the formation of the 4-hydroxylated metabolite. Environ Sci Technol 47, 4743–4751, 10.1021/es3049114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RN 2004. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neuroscience & Biobehavioral Reviews 28, 497–505. [DOI] [PubMed] [Google Scholar]
- Hutzinger O, Jamieson WD, Safe S, Paulmann L and Ammon R 1974. Identification of metabolic dechlorination of highly chlorinated biphenyl in rabbit. Nature 252, 698–699, 10.1038/252698a0. [DOI] [PubMed] [Google Scholar]
- IARC. 2016. IARC monographs on the evaluation of carcinogenic risks to humans. Lyon, France [Google Scholar]
- Idda T, Bonas C, Hoffmann J, Bertram J, Quinete N, Schettgen T, Fietkau K, Esser A, Stope MB, Leijs MM, Baron JM, Kraus T, Voigt A and Ziegler P 2020. Metabolic activation and toxicological evaluation of polychlorinated biphenyls in Drosophila melanogaster. Sci Rep 10, 21587, 10.1038/s41598-020-78405-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I and Lehmler HJ 2016. Chiral polychlorinated biphenyls: absorption, metabolism and excretion--a review. Environ Sci Pollut Res Int 23, 2042–2057, 10.1007/s11356-015-4150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I, Zhao H, Norstrom K, Li X, Hornbuckle KC and Lehmler H-J 2008. Simultaneous extraction and clean-up of PCBs and their metabolites from small tissue samples using pressurized liquid extraction. J. Chromatogr. A 1214, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klocke C and Lein PJ 2020. Evidence Implicating Non-Dioxin-Like Congeners as the Key Mediators of Polychlorinated Biphenyl (PCB) Developmental Neurotoxicity. Int J Mol Sci 21, 10.3390/ijms21031013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klocke C, Sethi S and Lein PJ 2020. The developmental neurotoxicity of legacy vs. contemporary polychlorinated biphenyls (PCBs): similarities and differences. Environ Sci Pollut Res Int 27, 8885–8896, 10.1007/s11356-019-06723-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraeuter AK, Guest PC and Sarnyai Z 2019. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Methods Mol Biol 1916, 105–111, 10.1007/978-1-4939-8994-2_10. [DOI] [PubMed] [Google Scholar]
- Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, Crystal RG, Darnell RB, Ferrante RJ, Fillit H, Finkelstein R, Fisher M, Gendelman HE, Golub RM, Goudreau JL, Gross RA, Gubitz AK, Hesterlee SE, Howells DW, Huguenard J, Kelner K, Koroshetz W, Krainc D, Lazic SE, Levine MS, Macleod MR, McCall JM, Moxley RT 3rd, Narasimhan K, Noble LJ, Perrin S, Porter JD, Steward O, Unger E, Utz U and Silberberg SD 2012. A call for transparent reporting to optimize the predictive value of preclinical research. Nature 490, 187–191, 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmler HJ and Robertson LW 2001. Synthesis of hydroxylated PCB metabolites with the Suzuki-coupling. Chemosphere 45, 1119–1127, 10.1016/s0045-6535(01)00052-2. [DOI] [PubMed] [Google Scholar]
- Li X, Hefti MM, Marek RF, Hornbuckle KC, Wang K and Lehmler HJ 2022. Assessment of polychlorinated biphenyls and their hydroxylated metabolites in postmortem human brain samples: age and brain region differences. Environ Sci Technol 56, 9515–9526, 10.1021/acs.est.2c00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Holland EB, Feng W, Zheng J, Dong Y, Pessah IN, Duffel MW, Robertson LW and Lehmler HJ 2018. Authentication of synthetic environmental contaminants and their (bio)transformation products in toxicology: polychlorinated biphenyls as an example. Environ Sci Pollut Res Int 25, 16508–16521, 10.1007/s11356-017-1162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X and Lehmler H-J 2022. Dataset for synthesis and authentication of 2, 2’, 5, 5’-tetrachlorobiphenyl-4-ol (4-OH-PCB 52).DOI: 10.25820/data.006178 [DOI] [Google Scholar]
- Li X, Wu X, Kelly KM, Veng-Pedersen P and Lehmler HJ 2019. Toxicokinetics of Chiral PCB 136 and Its Hydroxylated Metabolites in Mice with a Liver-Specific Deletion of Cytochrome P450 Reductase. Chem Res Toxicol 32, 727–736, 10.1021/acs.chemrestox.8b00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PH, Sangaiah R, Ranasinghe A, Upton PB, La DK, Gold A and Swenberg JA 2000. Formation of quinonoid-derived protein adducts in the liver and brain of Sprague-Dawley rats treated with 2,2’,5, 5’-tetrachlorobiphenyl. Chem Res Toxicol 13, 710–718, 10.1021/tx000030f. [DOI] [PubMed] [Google Scholar]
- Liu J, Tan Y, Song E and Song Y 2020. A critical review of polychlorinated biphenyls metabolism, metabolites, and their correlation with oxidative stress. Chem Res Toxicol 33, 2022–2042, 10.1021/acs.chemrestox.0c00078. [DOI] [PubMed] [Google Scholar]
- Marek RF, Thorne PS, DeWall J and Hornbuckle KC 2014. Variability in PCB and OH-PCB serum levels in children and their mothers in urban and rural U.S. communities. Environ Sci Technol 48, 13459–13467, 10.1021/es502490w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek RF, Thorne PS, Herkert NJ, Awad AM and Hornbuckle KC 2017. Airborne PCBs and OH-PCBs inside and outside urban and rural U.S. schools. Environ Sci Technol 51, 7853–7860, 10.1021/acs.est.7b01910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek RF, Thorne PS, Wang K, Dewall J and Hornbuckle KC 2013. PCBs and OH-PCBs in serum from children and mothers in urban and rural U.S. communities. Environ Sci Technol 47, 3353–3361, 10.1021/es304455k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean MR, Bauer U, Amaro AR and Robertson LW 1996. Identification of catechol and hydroquinone metabolites of 4-monochlorobiphenyl. Chem Res Toxicol 9, 158–164, 10.1021/tx950083a. [DOI] [PubMed] [Google Scholar]
- Meerts IA, Assink Y, Cenijn PH, Van Den Berg JH, Weijers BM, Bergman A, Koeman JH and Brouwer A 2002. Placental transfer of a hydroxylated polychlorinated biphenyl and effects on fetal and maternal thyroid hormone homeostasis in the rat. Toxicol Sci 68, 361–371, 10.1093/toxsci/68.2.361. [DOI] [PubMed] [Google Scholar]
- Meerts IA, Hoving S, van den Berg JH, Weijers BM, Swarts HJ, van der Beek EM, Bergman A, Koeman JH and Brouwer A 2004a. Effects of in utero exposure to 4-hydroxy-2,3,3’,4’,5-pentachlorobiphenyl (4-OH-CB107) on developmental landmarks, steroid hormone levels, and female estrous cyclicity in rats. Toxicol Sci 82, 259–267, 10.1093/toxsci/kfh251. [DOI] [PubMed] [Google Scholar]
- Meerts IA, Lilienthal H, Hoving S, van den Berg JH, Weijers BM, Bergman A, Koeman JH and Brouwer A 2004b. Developmental exposure to 4-hydroxy-2,3,3’,4’,5-pentachlorobiphenyl (4-OH-CB107): long-term effects on brain development, behavior, and brain stem auditory evoked potentials in rats. Toxicol Sci 82, 207–218, 10.1093/toxsci/kfh252. [DOI] [PubMed] [Google Scholar]
- Megson D, Benoit NB, Sandau CD, Chaudhuri SR, Long T, Coulthard E and Johnson GW 2019. Evaluation of the effectiveness of different indicator PCBs to estimating total PCB concentrations in environmental investigations. Chemosphere 237, 124429, 10.1016/j.chemosphere.2019.124429. [DOI] [PubMed] [Google Scholar]
- Othman N, Ismail Z, Selamat MI, Sheikh Abdul Kadir SH and Shibraumalisi NA 2022. A review of polychlorinated biphenyls (PCBs) pollution in the air: where and how much are we exposed to? Int J Environ Res Public Health 19, 10.3390/ijerph192113923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjape N, Dean LE, Martinez A, Tjalkens RB, Lehmler HJ and Doorn JA 2023. Structure-Activity Relationship of Lower Chlorinated Biphenyls and Their Human-Relevant Metabolites for Astrocyte Toxicity. Chem Res Toxicol, 10.1021/acs.chemrestox.3c00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Lein PJ, Seegal RF and Sagiv SK 2019. Neurotoxicity of polychlorinated biphenyls and related organohalogens. Acta Neuropathol 138, 363–387, 10.1007/s00401-019-01978-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston BD, Miller JA and Miller EC 1983. Non-arene oxide aromatic ring hydroxylation of 2,2’,5,5’-tetrachlorobiphenyl as the major metabolic pathway catalyzed by phenobarbital-induced rat liver microsomes. J Biol Chem 258, 8304–8311. [PubMed] [Google Scholar]
- Purkey HE, Palaninathan SK, Kent KC, Smith C, Safe SH, Sacchettini JC and Kelly JW 2004. Hydroxylated polychlorinated biphenyls selectively bind transthyretin in blood and inhibit amyloidogenesis: rationalizing rodent PCB toxicity. Chem Biol 11, 1719–1728, 10.1016/j.chembiol.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Rickenbacher U, McKinney JD, Oatley SJ and Blake CC 1986. Structurally specific binding of halogenated biphenyls to thyroxine transport protein. J Med Chem 29, 641–648, 10.1021/jm00155a010. [DOI] [PubMed] [Google Scholar]
- Rodriguez EA, Li X, Lehmler HJ, Robertson LW and Duffel MW 2016. Sulfation of lower chlorinated polychlorinated biphenyls increases their affinity for the major drug-binding sites of human serum albumin. Environ Sci Technol 50, 5320–5327, 10.1021/acs.est.6b00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez EA, Vanle BC, Doorn JA, Lehmler HJ, Robertson LW and Duffel MW 2018. Hydroxylated and sulfated metabolites of commonly observed airborne polychlorinated biphenyls display selective uptake and toxicity in N27, SH-SY5Y, and HepG2 cells. Environ Toxicol Pharmacol 62, 69–78, 10.1016/j.etap.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami I and Juhasz AL 2011. Assessment of persistent organic pollutant (POP) bioavailability and bioaccessibility for human health exposure assessment: a critical review. Critical Reviews in Environmental Science and Technology 41, 623–656, 10.1080/10643380903044178. [DOI] [Google Scholar]
- Sandau CD, Ayotte P, Dewailly E, Duffe J and Norstrom RJ 2000. Analysis of hydroxylated metabolites of PCBs (OH-PCBs) and other chlorinated phenolic compounds in whole blood from Canadian inuit. Environ Health Perspect 108, 611–616, 10.1289/ehp.00108611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schettgen T, Esser A, Alt A, Randerath I, Kraus T and Ziegler P 2022. Decomposition products of the initiator bis (2,4-dichlorobenzoyl) peroxide in the silicone industry: human biomonitoring in plasma and urine of workers. Environ Sci Technol 56, 8518–8527, 10.1021/acs.est.2c01530. [DOI] [PubMed] [Google Scholar]
- Sethi S, Morgan RK, Feng W, Lin Y, Li X, Luna C, Koch M, Bansal R, Duffel MW, Puschner B, Zoeller RT, Lehmler HJ, Pessah IN and Lein PJ 2019. Comparative Analyses of the 12 Most Abundant PCB Congeners Detected in Human Maternal Serum for Activity at the Thyroid Hormone Receptor and Ryanodine Receptor. Environ Sci Technol 53, 3948–3958, 10.1021/acs.est.9b00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shain W, Bush B and Seegal R 1991. Neurotoxicity of polychlorinated biphenyls: structure-activity relationship of individual congeners. Toxicol Appl Pharmacol 111, 33–42, 10.1016/0041-008x(91)90131-w. [DOI] [PubMed] [Google Scholar]
- Shimada T, Kakimoto K, Takenaka S, Koga N, Uehara S, Murayama N, Yamazaki H, Kim D, Guengerich FP and Komori M 2016. Roles of human CYP2A6 and monkey CYP2A24 and 2A26 cytochrome P450 enzymes in the oxidation of 2,5,2’,5’-tetrachlorobiphenyl. Drug Metab Dispos 44, 1899–1909, 10.1124/dmd.116.072991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart SA and Robinson ES 2015. Reducing the stress of drug administration: implications for the 3Rs. Sci Rep 5, 14288, 10.1038/srepl4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström G and Jansson B 1975. The metabolism of 2,2’,3,5’,6-pentachlorobiphenyl in rats, mice and quails. Chemosphere 4, 361–370, 10.1016/0045-6535(75)90032-6. [DOI] [Google Scholar]
- Takaguchi K, Nishikawa H, Mizukawa H, Tanoue R, Yokoyama N, Ichii O, Takiguchi M, Nakayama SMM, Ikenaka Y, Kunisue T, Ishizuka M, Tanabe S, Iwata H and Nomiyama K 2019. Effects of PCB exposure on serum thyroid hormone levels in dogs and cats. Sci Total Environ 688, 1172–1183, 10.1016/j.scitotenv.2019.06.300. [DOI] [PubMed] [Google Scholar]
- Takeda H, Tsuji M and Matsumiya T 1998. Changes in head-dipping behavior in the hole-board test reflect the anxiogenic and/or anxiolytic state in mice. Eur J Pharmacol 350, 21–29, 10.1016/s0014-2999(98)00223-4. [DOI] [PubMed] [Google Scholar]
- Tampal N, Lehmler HJ, Espandiari P, Malmberg T and Robertson LW 2002. Glucuronidation of hydroxylated polychlorinated biphenyls (PCBs). Chem Res Toxicol 15, 1259–1266, 10.1021/tx0200212. [DOI] [PubMed] [Google Scholar]
- Tian YH, Hwan Kim S, Lee SY and Jang CG 2011. Lactational and postnatal exposure to polychlorinated biphenyls induces sex-specific anxiolytic behavior and cognitive deficit in mice offspring. Synapse 65, 1032–1041, 10.1002/syn.20934. [DOI] [PubMed] [Google Scholar]
- Tilson HA and Kodavanti PR 1998. The neurotoxicity of polychlorinated biphenyls. Neurotoxicology 19, 517–525. [PubMed] [Google Scholar]
- Tulp MT, Bruggeman WA and Hutzinger O 1977. Reductive dechlorination of chlorobiphenylols by rats. Experientia 33, 1134–1136, 10.1007/BF01922284. [DOI] [PubMed] [Google Scholar]
- Viluksela M, Heikkinen P, van der Ven LT, Rendel F, Roos R, Esteban J, Korkalainen M, Lensu S, Miettinen HM, Savolainen K, Sankari S, Lilienthal H, Adamsson A, Toppari J, Herlin M, Finnila M, Tuukkanen J, Leslie HA, Hamers T, Hamscher G, Al-Anati L, Stenius U, Dervola KS, Bogen IL, Fonnum F, Andersson PL, Schrenk D, Halldin K and Hakansson H 2014. Toxicological profile of ultrapure 2,2’,3,4,4’,5,5’-heptachlorbiphenyl (PCB 180) in adult rats. PLoS One 9, e104639, 10.1371/journal.pone.0104639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlang B, Jin J, Hardesty JE, Head KZ, Shi H, Falkner KC, Prough RA, Klinge CM and Cave MC 2019. Identifying sex differences arising from polychlorinated biphenyl exposures in toxicant-associated liver disease. Food Chem Toxicol 129, 64–76, 10.1016/j.fct.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA and Frye CA 2007. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2, 322–328, 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Adamcakova-Dodd A, Flor S, Gosse L, Klenov VE, Stolwijk JM, Lehmler HJ, Hornbuckle KC, Ludewig G, Robertson LW and Thorne PS 2020. Comprehensive Subchronic Inhalation Toxicity Assessment of an Indoor School Air Mixture of PCBs. Environ Sci Technol 54, 15976–15985, 10.1021/acs.est.0c04470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Adamcakova-Dodd A, Lehmler HJ, Hornbuckle KC and Thorne PS 2022. Toxicity Assessment of 91-Day Repeated Inhalation Exposure to an Indoor School Air Mixture of PCBs. Environ Sci Technol 56, 1780–1790, 10.1021/acs.est.lc05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bullert AJ, Li X, Stevens H, Klingelhutz AJ, Ankrum JA, Adamcakova-Dodd A, Thorne PS and Lehmler HJ 2023. Dataset: Polymeric Implant to Assess the Neurotoxicity of Subacute Exposure to 2,2’,5,5’-Tetrachlorobiphenyl-4-ol in Male Rats. University of Iowa (dataset). 10.25820/data.006796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff MA and Hutmacher DW 2010. The return of a forgotten polymer—Polycaprolactone in the 21st century. Progress in Polymer Science 35, 1217–1256, 10.1016/j.progpolymsci.2010.04.002. [DOI] [Google Scholar]
- Wu X, Zhai G, Schnoor JL and Lehmler HJ 2020. Atropselective Disposition of 2,2’,3,4’,6-Pentachlorobiphenyl (PCB 91) and Identification of Its Metabolites in Mice with Liver-Specific Deletion of Cytochrome P450 Reductase. Chem Res Toxicol 33, 1328–1338, 10.1021/acs.chemrestox.9b00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegambaram M, Manivannan B, Beach TG and Halden RU 2015. Role of environmental contaminants in the etiology of Alzheimer’s disease: a review. Curr Alzheimer Res 12, 116–146, 10.2174/1567205012666150204121719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CY, Li X, Flor S, Ruiz P, Kruve A, Ludewig G and Lehmler HJ 2022. Metabolism of 3-chlorobiphenyl (PCB 2) in a human-relevant cell line: evidence of dechlorinated metabolites. Environ Sci Technol 56, 12460–12472, 10.1021/acs.est.2c03687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


