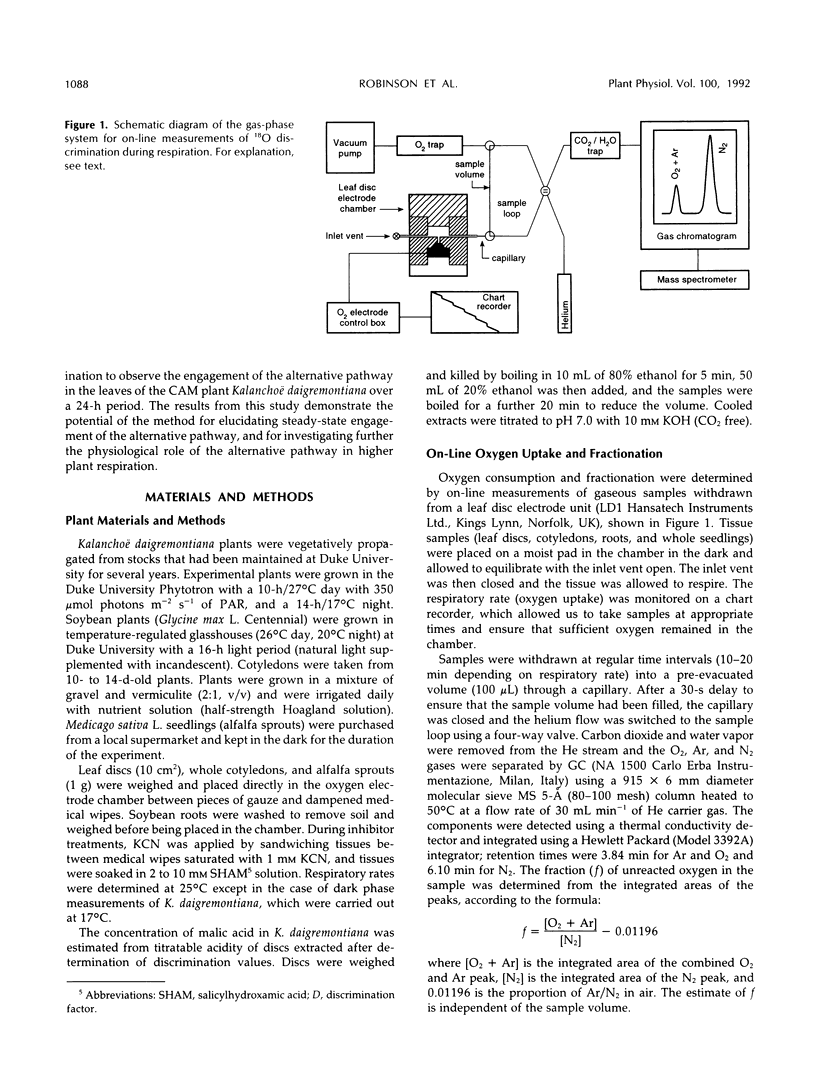
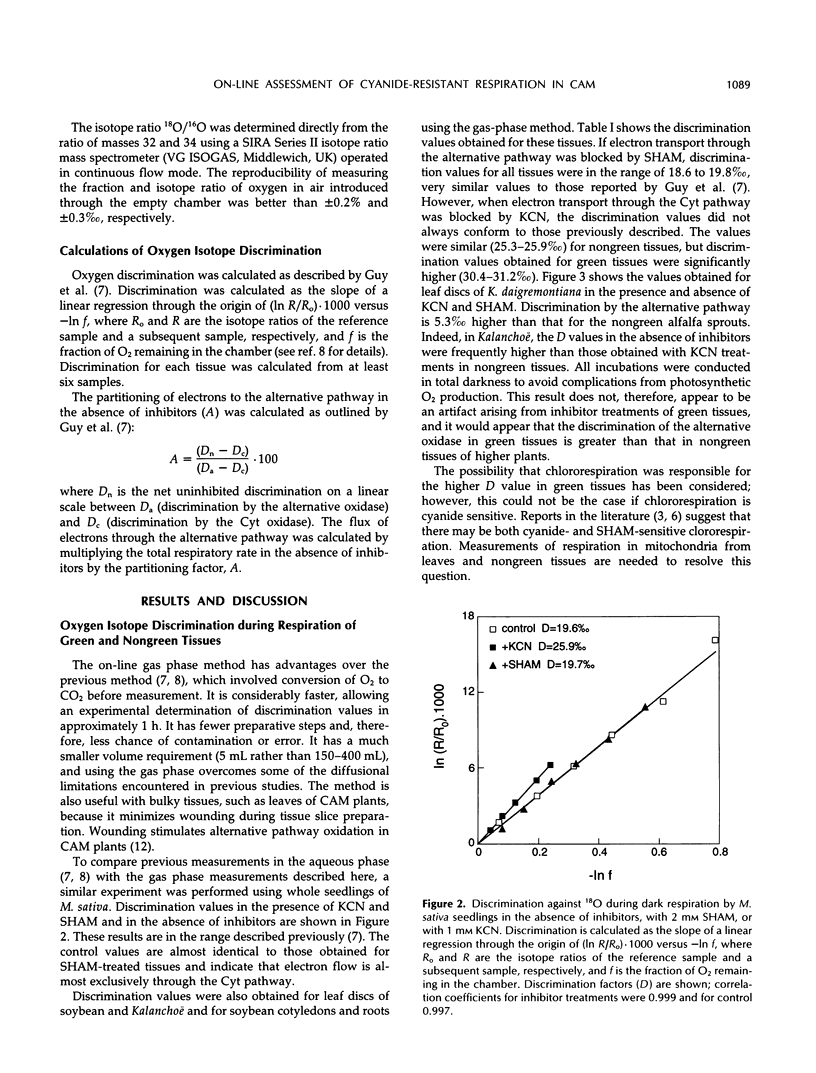
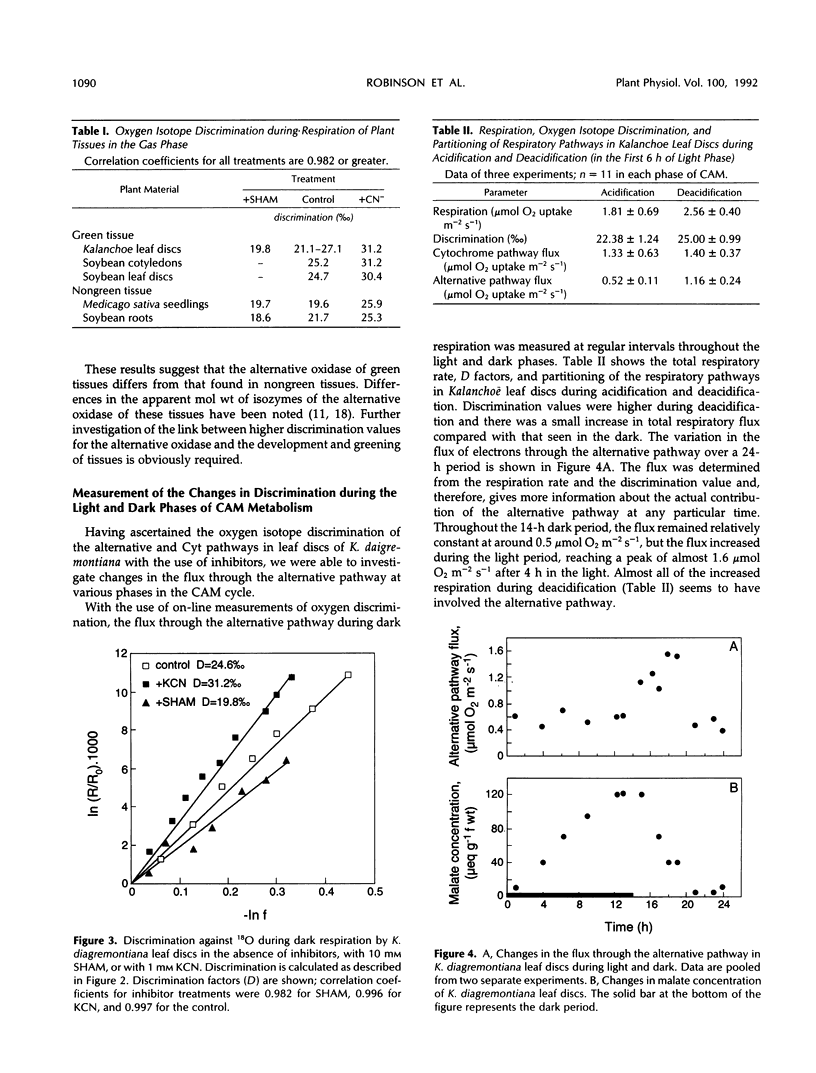
Abstract
Discrimination against 18O during dark respiration in tissues of Kalanchoë daigremontiana, Medicago sativa, and Glycine max was measured using an on-line system that enabled direct measurements of the oxygen fractionation of samples in a gas-phase leaf disk electrode unit. Discrimination factors for cytochrome pathway respiration were 18.6 to 19.8%o for all tissues. However, discrimination in cyanide-resistant respiration was significantly higher in green tissues (30.4-31.2%o) compared with nongreen tissues (25.3-25.9%o). Using these discrimination factors, the partitioning of electron transport to these pathways was calculated from measurements of discrimination in the absence of inhibitors. Changes in flux through the alternative pathway were measured during the light and dark phases of Crassulacean acid metabolism in leaf disks of K. daigremontiana. The flux of electrons through the alternative pathway was higher during deacidification than during the other phases of Crassulacean acid metabolism. The increase in alternative pathway electron flux accounted for all of the increased respiration in the light phase. Despite this increase, simultaneous measurements of malate concentration and respiratory flux confirm that only a small proportion of the total malate decarboxylation occurs in the mitochondria.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams W. W., Nishida K., Osmond C. B. Quantum Yields of CAM Plants Measured by Photosynthetic O(2) Exchange. Plant Physiol. 1986 May;81(1):297–300. doi: 10.1104/pp.81.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennoun P. Evidence for a respiratory chain in the chloroplast. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4352–4356. doi: 10.1073/pnas.79.14.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalt W., Osmond C. B., Siedow J. N. Malate Metabolism in the Dark After CO(2) Fixation in the Crassulacean Plant Kalanchoë tubiflora. Plant Physiol. 1990 Oct;94(2):826–832. doi: 10.1104/pp.94.2.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns A., Whelan J., Young S., Elthon T. E., Day D. A. Tissue-specific expression of the alternative oxidase in soybean and siratro. Plant Physiol. 1992 Jun;99(2):712–717. doi: 10.1104/pp.99.2.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide T. B., Marek L. F. Wounding Stimulates Cyanide-sensitive Respiration in the Highly Cyanide-resistant Leaves of Bryophyllum tubiflorum Harv. Plant Physiol. 1980 Feb;65(2):409–410. doi: 10.1104/pp.65.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weger H. G., Guy R. D., Turpin D. H. Cytochrome and alternative pathway respiration in green algae : measurements using inhibitors and o(2) discrimination. Plant Physiol. 1990 May;93(1):356–360. doi: 10.1104/pp.93.1.356. [DOI] [PMC free article] [PubMed] [Google Scholar]


