Abstract
Background
Genetic and nongenetic factors account for the association of family history with disease risk. Comparing adopted and nonadopted individuals provides an opportunity to disentangle those factors.
Methods and Results
We examined associations between family history of stroke and heart disease with incident stroke and myocardial infarction (MI) in 495 640 UK Biobank participants (mean age, 56.5 years; 55% women) stratified by childhood adoption status (5747 adoptees). We estimated hazard ratios (HRs) per affected family member, and for polygenic risk scores in Cox models adjusted for baseline age and sex. A total of 12 518 strokes and 23 923 MIs occurred over a 13‐year follow‐up. In nonadoptees, family history of stroke and heart disease was associated with increased stroke and MI risk, with the strongest association of family history of stroke for incident stroke (HR, 1.16 [95% CI, 1.12–1.19]) and family history of heart disease for incident MI (HR, 1.48 [95% CI, 1.45–1.50]). In adoptees, family history of stroke associated with incident stroke (HR, 1.41 [95% CI, 1.06–1.86]), but family history of heart disease was not associated with incident MI (P>0.5). Polygenic risk scores showed strong disease‐specific associations in both groups. In nonadoptees, the stroke polygenic risk score mediated 6% risk between family history of stroke and incident stroke, and the MI polygenic risk score mediated 13% risk between family history of heart disease and incident MI.
Conclusions
Family history of stroke and heart disease increases risk for their respective conditions. Family history of stroke contains substantial potentially modifiable nongenetic risk, indicating a need for novel prevention strategies, whereas family history of heart disease represents predominantly genetic risk.
Keywords: family history, genetic risk, heart disease, myocardial infarction, stroke
Subject Categories: Cerebrovascular Disease/Stroke, Cardiovascular Disease, Epidemiology, Risk Factors, Primary Prevention
Nonstandard Abbreviations and Acronyms
- PRS
polygenic risk score
- UKB
UK Biobank
Clinical Perspective.
What Is New?
The association between family history of stroke and heart disease with the prospective incidence of stroke and myocardial infarction in biological compared with adopted offspring reveals that family history of stroke contains substantial potentially modifiable nongenetic risk, whereas family history of heart disease represents predominantly genetic risk.
What Are the Clinical Implications?
The information that family history of stroke increases stroke risk by environmental and lifestyle factors could serve as a risk stratification tool for targeted prevention efforts.
Instead of conveying a genetic predisposition with no actionable outcomes, family history of stroke could function as a motivating factor for physicians and patients, fostering awareness and encouraging lifestyle modifications or even more aggressive risk factor targets in primary prevention.
Stroke and coronary artery disease (CAD) are the leading causes of morbidity and mortality worldwide with increasing incidence. 1 , 2 Environmental, lifestyle, and genetic factors contribute substantially to their risk, and primary prevention efforts aim to control modifiable risk factors to decrease the burden of disease. 3
The observation from epidemiologic 4 , 5 and twin studies 6 , 7 that family history of stroke and CAD predispose individuals to a higher risk of developing these diseases has led to large international collaborations to study the genetic risk factors for stroke and CAD. 8 , 9 Both stroke and CAD have been found to be highly polygenic, with heritability estimates, representing the variance explained by common variant genetics, of ≈40% for stroke and ≈60% for CAD. 10 , 11 Over time, genome‐wide association studies (GWASs) have become large enough to identify common genetic variants that causally contribute to individual genetic risk of these common diseases. Polygenic risk scores (PRSs) have been developed as a tool to capture the cumulative effect of multiple genetic variants on the risk of complex diseases and have gained association strength similar to traditional cardiovascular risk factors. 12 , 13 As such, PRSs have been investigated as potential clinical tools for risk prediction and stratification. 14 Unlike family history, which represents the cumulative effects of the developmental family environment as well as the genetics of each parent and blood relative, genetic markers are constant throughout the lifespan and specific to the individual.
Previous studies support this assertion that family history and genetic risk may not be interchangeable terms. 15 , 16 , 17 Depending on the heritability of the disease, family history might contain a substantial proportion of inherited nongenetic risk, such as smoking, dietary patterns, or general risk factor awareness, making it a partially modifiable risk factor. PRSs for many diseases mediate only a minor proportion of family history, and the association of PRS and family history on many health outcomes is largely independent. 16 , 18 Although such combined analyses of family history and PRSs suggest that family history contains a substantial proportion of nongenetic risk, they are limited by the performance of the PRS, which relies on the discovery power of the underlying GWAS and other challenges of PRSs, such as portability, 19 sensitivity to population stratification, 20 and miscalibration. 21
Individuals adopted early in childhood provide a unique opportunity to investigate the nongenetic risk contribution of family history, as they share lifestyle and environmental exposures with their adoptive families but not genetics (Figure 1). The objective of this study was to investigate whether family history of stroke and heart disease is specific for incident stroke and myocardial infarction (MI) and how much of their risk is attributable to genetics. By examining the associations between family history, PRS, and the risk of stroke and MI in both biological and adopted families in a large cohort of individuals from the UK Biobank (UKB), we aimed to gain insight into the modifiability of disease‐specific inherited risk, which could support efforts to engage in aggressive risk factor management and behavioral interventions in those with strong family history of disease.
Figure 1. Study design and hypothesis.
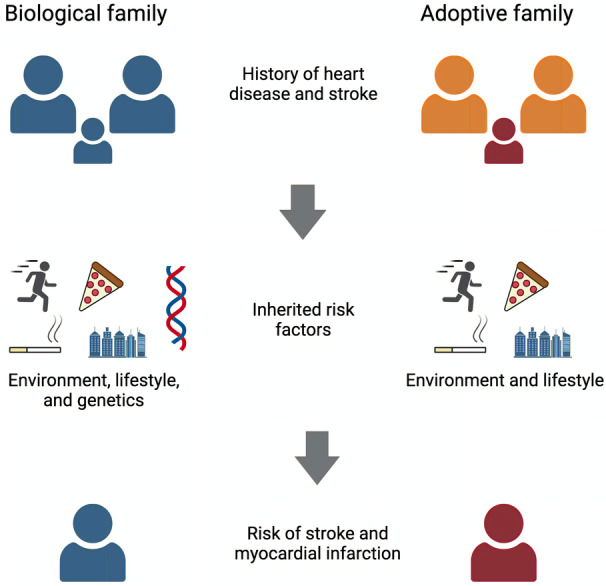
Inherited risk that is passed from biological parents to children includes environment, lifestyle, and genetic risk factors. Similarly, parents of adopted children pass environmental and lifestyle risk factors but not genetics to their offspring. This model enables us to study the genetic contribution of familial risk for stroke and myocardial infarction.
Methods
UKB participant data can be accessed by submitting an approved research proposal to UKB. 22 The GWAS summary statistics used to create genetic scores are publicly accessible. 23 , 24
Study Population
The UKB is a population‐based prospective cohort study that recruited >500 000 participants, aged 40 to 70 years, between 2006 and 2010 from across the United Kingdom. Extensive baseline data, including sociodemographic, lifestyle, and health‐related information, were collected through questionnaires and physical measurements. In addition, biological samples were obtained from each participant for genomic analyses. Genotyping was performed on 2 similar platforms, and imputation was performed on the Haplotype Reference Consortium for 488 377 participants. 25 For the current study, we stratified individuals by adoption status (as defined by field 1767 gathered from the touchscreen question “Were you adopted as a child?”). Information about adoption circumstances, such as age at adoption, was not collected from study participants.
The UKB was approved by the North West Multi‐Centre Research Ethics Committee, and all participants provided informed consent. We accessed the data following approval of an application by the UKB Ethics and Governance Council (application No. 36993).
Family History
Family history was assessed through self‐reported information from study participants at baseline. Illnesses of parents and siblings were gathered from study participants through multiple‐choice touchscreen questions. For individuals who indicated they were adopted, specific questions were used to gather information on illnesses of adoptive parents and siblings (fields 20112–20114). In contrast, for nonadopted participants, the illnesses of biological parents and siblings were collected (fields 20107, 20110, and 20111). We gathered for each individual the number of adopted or biological nuclear family members with stroke and heart disease as well as the number of total biological and adopted siblings (fields 1873, 1883, 3972, and 3982). We also gathered the number of adopted or biological parents (omitting siblings) with stroke and heart disease.
Construction of Genetic Scores
We gathered summary statistics for stroke and MI from the most recent published GWAS that did not include data from UKB participants: from MEGASTROKE 23 for stroke and from coronary artery disease genome wide replication and meta‐analysis plus the coronary artery disease genetics consortium 24 for MI. All GWASs were performed in individuals of European ancestry. We generated PRSs for those traits using PRS‐CS, an unsupervised method that uses a high‐dimensional Bayesian regression framework to derive a PRS from GWAS summary statistics without requiring an external validation cohort. 26 This method outperforms traditional PRS approaches via its use of external linkage disequilibrium reference panels. 26 PRS‐CS with default parameters generated 1 108 218 single‐nucleotide polymorphism weights for stroke and 1 106 964 single‐nucleotide polymorphism weights for MI.
Outcome Assessment
The primary outcomes of interest were incident stroke and MI events. These outcomes were ascertained through linkage to national hospital admission and mortality registries. Stroke events were identified using International Classification of Diseases, Tenth Revision (ICD‐10), codes I60 to I64, whereas MI events were identified using ICD‐10 codes I21 to I22, aligned with the diagnostic algorithm in the UKB (https://biobank.ndph.ox.ac.uk/showcase/ukb/docs/alg_outcome_main.pdf). Events that occurred after baseline were defined as incident events.
Statistical Analysis and Software
We used t test, ANOVA, and χ2 test for comparison of continuous and discrete baseline variables, respectively. We used Cox proportional hazards models to estimate hazard ratios (HRs) and 95% CIs for the associations between family history and genetic scores and the risk of developing stroke and MI. In total, we used 4 exposure variables: the number of family members affected by heart disease and stroke and the PRSs for stroke and MI.
Separate models were constructed with each exposure adjusted for age and sex, stratified by adoption status to explore potential differences in the associations. We also investigated potential effect modification by age, sex, and number of total family members by including interaction terms in the models and conducting stratified analyses by age group and sex. We conducted mediation analyses to calculate the fraction of family history risk that was mediated by the PRSs. To maximize power, we performed the association models for family history in all individuals regardless of available genotyping data. Because the GWASs for stroke and MI were performed in European ancestry individuals, association models for the PRSs were restricted to individuals with available genetic data and of European ancestry. To rule out that the associations between family history and the outcomes were driven by those with unavailable genetic data or non‐European ancestry, we performed sensitivity analyses for all models in those with available genetic data and European genetic ancestry to confirm robustness of our findings. We performed additional sensitivity analyses to rule out confounding: (1) models that were additionally adjusted for cardiovascular risk factors at baseline: presence of hypertension, hyperlipidemia, diabetes, and smoking status (current/former/never smoker); (2) models using as exposure the family histories of stroke and heart disease of parents (without siblings) only; and (3) models that were additionally adjusted for the number of biological or adopted siblings (in models of biological and adopted families, respectively).
Single‐nucleotide polymorphism extraction and genetic score calculation were performed with PLINK, 27 PRS‐CS, 26 and bcftools, 28 and relationship inference was performed with KING. 29 All statistical analyses were conducted using R software, and a 2‐sided P<0.05 was considered statistically significant. Mediation analyses were performed with the R package mediator (https://gerkelab.github.io/mediator/) that uses a counterfactual framework. 30 Data extraction, curation, and preparation, statistical analysis, and figure generation were done with RStudio (Posit, Boston, MA).
Results
Study Population Characteristics
A total of 495 640 individuals were included in the analysis; 5747 adoptees and 489 893 nonadoptees (Figure 2). The baseline characteristics of the study population are shown in the Table. Adoptees were more likely to be current smokers and take lipid‐lowering medications, had higher body mass index, and had a lower prevalence of self‐reported family history of stroke and heart disease; other statistically significant, but not clinically meaningful, differences in glycated hemoglobin and the PRS were found. We found sex‐specific differences in self‐reported family history: both adopted and nonadopted women were more likely to report a positive family history for heart disease than men, and nonadopted women were also more likely to report a family history of stroke than nonadopted men (Table S1). Over a median 13.4 years of follow‐up, 12 518 strokes (of those, 79% ischemic strokes) and 23 923 MI events occurred. Adoptees were at higher absolute risk for an MI event (absolute risk, 5.7% versus 4.8%; P=0.003).
Figure 2. Study overview.
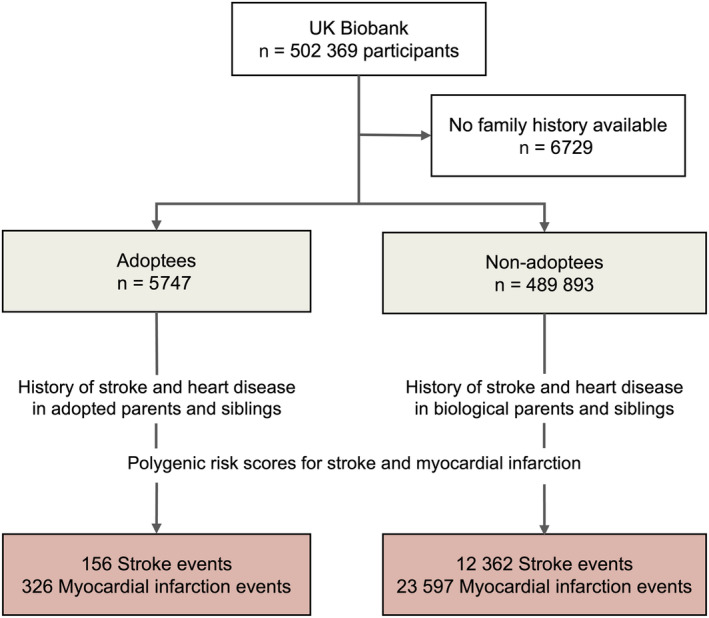
Participants from the UK Biobank were stratified on the basis of their self‐identified adoption status during childhood. The association between family history of stroke and heart disease and polygenic risk scores for stroke and myocardial infarction with the incidence of stroke and myocardial infarction over a 13‐year follow‐up period was evaluated.
Table .
Baseline Characteristics by Adoption Status
| Characteristic | Nonadoptees (N=489 893) | Adoptees (N=5747) | P value |
|---|---|---|---|
| Age at baseline, mean (SD), y | 56.5 (8.08) | 56.0 (8.60) | <0.001 |
| Female sex, n (%) | 267 550 (54.6) | 3089 (53.7) | 0.195 |
| White race, n (%) | 433 127 (88.4) | 4830 (84.0) | <0.001 |
| European genetic ancestry, n (%) | 401 378 (81.9) | 4156 (72.3) | <0.001 |
| Smoking status, n (%) | <0.001 | ||
| Never smoker | 268 229 (54.8) | 2610 (45.4) | |
| Ex‐smoker | 168 864 (34.5) | 2148 (37.4) | |
| Current smoker | 50 971 (10.4) | 961 (16.7) | |
| Systolic blood pressure, mean (SD), mm Hg | 138 (18.6) | 138 (18.8) | 0.0442 |
| Body mass index, mean (SD), kg/m2 | 27.4 (4.80) | 28.1 (5.20) | <0.001 |
| Use of lipid‐lowering medication, n (%) | 80 275 (16.4) | 998 (17.4) | 0.0482 |
| HbA1c, mean (SD), mmol/L | 36.1 (6.73) | 36.7 (7.73) | <0.001 |
| Family history of stroke, n (%) | 136 946 (28.0) | 1346 (23.4) | <0.001 |
| Family history of heart disease, n (%) | 221 991 (45.3) | 2245 (39.1) | <0.001 |
| PRS for myocardial infarction, mean (SD) | −0.441 (0.125) | −0.435 (0.124) | 0.0029 |
| PRS for stroke, mean (SD) | −0.736 (0.0723) | −0.731 (0.0721) | <0.001 |
| All‐cause stroke events, n (%) | 12 362 (2.5) | 156 (2.7) | 0.381 |
| Ischemic stroke events, n (%) | 9730 (2.0) | 123 (2.1) | 0.433 |
| Subarachnoid hemorrhage events, n (%) | 1870 (0.4) | 21 (0.4) | 0.927 |
| Intracerebral hemorrhage events, n (%) | 1056 (0.2) | 13 (0.2) | 0.976 |
| Myocardial infarction events, n (%) | 23 597 (4.8) | 326 (5.7) | 0.0029 |
The first incident stroke and myocardial infarction events after baseline were considered outcomes of interest. Stroke subtype events exceed the number of stroke events because some patients had a diagnosis of >1 stroke subtype on the same date. HbA1c indicates glycated hemoglobin; and PRS, polygenic risk score.
Stroke Risk in Biological and Adopted Families
In nonadoptees, both family history of stroke and heart disease were significantly associated with stroke risk, with a much stronger association for family history of stroke than for family history of heart disease (HR, 1.16 [95% CI, 1.12–1.19] versus 1.08 [95% CI, 1.06–1.11] per 1 affected family member; Figure 3A). A 1‐SD increase in the stroke PRS increased the risk for stroke by an HR of 1.19 (95% CI, 1.17–1.21). Family history of stroke was significantly associated with the stroke PRS (0.052 [95% CI, 0.046–0.058] SD increase in the stroke PRS per family member with a stroke).
Figure 3. Comparison of association between family history of stroke and incident stroke among nonadoptees and adoptees.
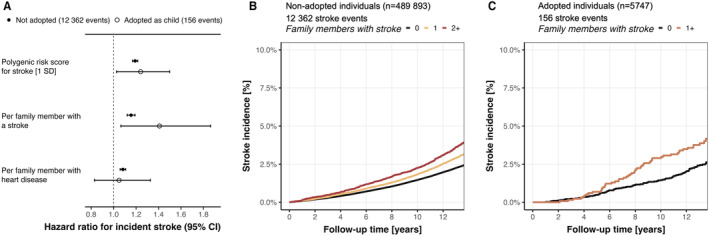
A, Family history for stroke increased risk for incident stroke among both nonadoptees and adoptees. Absolute incidence curves for stroke in nonadoptees (B) and adoptees (C) across strata of family members affected by a stroke. In adoptees, groups were collapsed because of the low number of people with ≥2 family members with a stroke. Family history was self‐reported at baseline for biological and adopted father, mother, and siblings.
In the cohort of 5747 adoptees, positive family history of stroke revealed a similar yet higher point estimate of association (HR, 1.41 [95% CI, 1.06–1.86] per affected family member) with incident stroke compared with nonadoptees, considering overlapping CIs. The stroke PRS had a similar association with stroke risk as in nonadoptees, with an HR of 1.24 (95% CI, 1.03–1.50). When comparing absolute risk, we found a clear separation of the incidence curves in both nonadoptees (Figure 3B) and adoptees (Figure 3C). We found no association between family history of stroke and the stroke PRS in adoptees (P=0.49).
We found a stronger association between family history of stroke and incident stroke among younger nonadoptees, with a similar, yet statistically insignificant, trend observed in adoptees (Figures S1 and S2). There was a stronger association of family history of heart disease with incident stroke in nonadopted women (P interaction=0.0037; Figure S3), but no sex‐specific effects were found in adoptees (all P interaction>0.27; Figure S4). The PRS mediated 6% of the association between family history of stroke on incident stroke in nonadoptees, and 0% in adoptees. Sensitivity analyses confirmed the findings in fully adjusted models for cardiovascular risk factors, in the subgroup of individuals with available genetic data and European ancestry, and in the models that only considered the family history of the parents (Figures S5–S7). We found no interaction between family history of stroke and the number of biological (P=0.89) or adopted (P=0.62) siblings in the cohort of nonadoptees and adoptees, respectively, and models adjusted for number of siblings yielded almost identical results (Figure S8).
MI Risk in Biological and Adopted Families
In the cohort of 489 893 nonadoptees, both family history of heart disease and stroke were significantly associated with incident MI risk, with a much stronger association for family history of heart disease than for stroke (HR, 1.48 [95% CI, 1.45–1.50] versus HR, 1.05 [95% CI, 1.03–1.08] per 1 affected family member; Figure 4A). A 1‐SD increase in the MI PRS increased the risk for MI by an HR of 1.43 (95% CI, 1.41–1.46). Family history of heart disease was significantly associated with the MI PRS (0.119 [95% CI, 0.115–0.124] SD increase in the MI PRS per family member with heart disease).
Figure 4. Comparison of association between family history of illnesses related to cardiovascular disease and incident myocardial infarction among nonadoptees and adoptees.
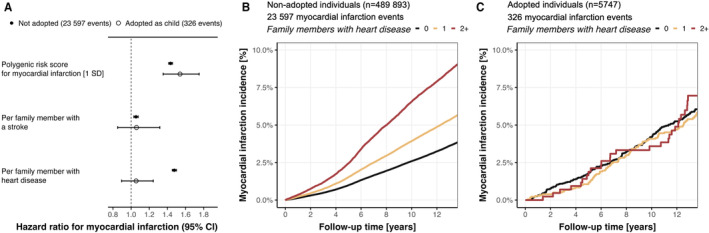
A, Family history for cardiovascular‐related traits was associated with incident stroke only among nonadoptees, not among adoptees. Absolute incidence curves for stroke in nonadoptees (B) and adoptees (C) across strata of family members affected by heart disease. Family history was self‐reported at baseline for biological and adopted father, mother, and siblings.
In the cohort of 5747 adoptees, neither positive family history of heart disease nor stroke was associated with MI (P=0.52 and P=0.61, respectively; Figure 4A). The MI PRS had a similar association with MI risk as in nonadoptees, with an HR of 1.54 (95% CI, 1.35–1.75). When comparing absolute risk across strata of family history of heart disease, we found a clear separation of the incidence curves in nonadoptees (Figure 4B), but not in adoptees (Figure 4C). We found no association between family history of heart disease and the MI PRS in adoptees (P=0.65).
We found stronger effect sizes for family history for stroke and heart disease and the MI PRS in younger nonadoptees (all P interaction<0.001; Figure S9), but no age‐dependent association in adoptees (Figure S10). Sex‐specific analyses yielded a better MI PRS performance in nonadopted men (P interaction=0.021; Figure S11), but no interactions with sex in adoptees (all P interaction>0.09; Figure S12). The MI PRS mediated 13% of the association between family history of heart disease on incident MI in nonadoptees, and 0% in adoptees. Sensitivity analyses confirmed the findings in fully adjusted models for cardiovascular risk factors in the subgroup of individuals with available genetic data and of European ancestry, and in the models that only considered the family history of the parents (Figures S13–S15). We found no interaction between family history of heart disease and the number of biological (P=0.87) or adopted (P=0.27) siblings in the cohort of nonadoptees and adoptees, respectively, and models adjusted for number of siblings yielded almost identical results (Figure S16).
Discussion
In this study, we investigated the association between family history of stroke and heart disease, PRS for each, and the incidence of stroke and MI in the UKB among individuals with biological and adopted parents. We found that self‐reported family histories for stroke and heart disease are disease‐specific risk factors for incident stroke and MI in biological families. Family history of stroke was also a strong risk factor for incident stroke among adoptees, suggesting that it contains a substantial proportion of nongenetic risk. In contrast, family history of heart disease followed primarily genetic risk, as evidenced by a much larger mediation by the MI PRS and no association with MI risk in adoptees, despite greater statistical power for MI outcomes. Both family history and genetic risk showed a stronger association among younger individuals, suggesting that inherited risk factors might indicate risk earlier in life.
The disease‐specific associations between family history of stroke and CAD are potentially attributable to the diverse causes of stroke compared with the relatively more homogeneous pathogenesis of CAD. Stroke has multiple causes, each with distinct risk factors and underlying pathophysiological features 31 , 32 ; in contrast, CAD is primarily driven by atherosclerosis. 33 Although risk factors for CAD and stroke overlap, they are different in magnitude. 34 These differences could explain the variation in genetic and environmental contributions observed in our study when comparing family history of stroke and heart disease. Furthermore, the diversity in stroke subtypes (ischemic versus hemorrhagic, but also across ischemic stroke subtypes) may also account for the stronger disease‐specific association observed for CAD family history compared with stroke family history, as the latter may encompass a wider range of causal factors.
Although family history of stroke is associated with genetic risk for stroke among biological families, it contains a substantial proportion of nongenetic and thus potentially modifiable risk, as it increases the risk for stroke in adoptees by a similar magnitude as in biological offspring. No such observation was observed for CAD, confirming results from previous studies with similar design. 35 , 36 Our results carry practical implications, as they indicate that family history of stroke is an indicator of a higher burden of modifiable risk factors rather than predominantly genetic risk, offering insights for primary prevention strategies. Inquiring about family history is a rapid, straightforward approach to stratify individuals for targeted primary prevention interventions. Our results show that depending on the answer to a few simple questions, absolute stroke risk differs substantially over the subsequent 13 years, with implications on potential risk factor interventions, especially for stroke. On the basis of our data, this information could serve as a foundation for determining which patients may benefit from more aggressive risk factor management. Instead of conveying a genetic predisposition with no actionable outcomes, family history of stroke could function as a motivating factor, fostering awareness and encouraging lifestyle modifications or even more aggressive risk factor targets in primary prevention.
Because of the low number of nonischemic stroke outcomes among adoptees, we were unable to disentangle the genetic and nongenetic contributions across different stroke subtypes, but observed associations are most likely driven by ischemic strokes (79% of all stroke events). Given the shared modifiable risk factors, such as hypertension, smoking, and alcohol consumption, and similar pathophysiological features, particularly cerebral small‐vessel disease, our findings could extend to both ischemic stroke and intracerebral hemorrhage, which also have similar heritability estimates of 38% 10 and 44%, 37 respectively. The potential applicability extends more cautiously to subarachnoid hemorrhage because of some shared risk factors and a heritability estimate of 30%. 38
In our study, we observed that adoptees had a higher burden of cardiovascular risk factors and related outcomes. There is evidence for a higher burden of behavioral and psychological health problems, leading to elevated rates of alcohol, smoking, and substance abuse in adoptees, 39 as well as increased all‐cause mortality in adoptees compared with biological offspring, 40 linked to age at adoption. 41 Because we stratified our analyses by adoption status, the overall elevated risk among adoptees should not have biased our results. Although we did not specifically focus on this aspect, our results highlight the importance of tailored risk factor management strategies targeting adoptees for prevention efforts to reduce their elevated risk and improve overall cardiovascular health.
Age‐stratified analyses revealed that inherited risk factors might exert a stronger influence on stroke and MI risk in younger individuals. Because we used self‐reported family history at inclusion, younger participants had presumably younger parents and thus a positive family history in those individuals may be indicative of a high burden of genetic or environmental risk factors. This observation implies that early identification of individuals with a family history of stroke or heart disease, particularly among younger populations, could facilitate more effective targeting of preventive interventions. In addition, we found that women were more likely to report family history than men. Previous studies have found similar sex‐specific behavioral bias, 42 , 43 indicating that family history questions should be tailored according to sex and might be more useful for risk stratification for women than men because of elevated response rates.
Our study has several limitations. First, the PRSs were derived from European populations, potentially limiting the generalizability of the associations between the PRSs and outcomes to other populations. However, the concept of PRS is not limited by genetic ancestry but rather by the available GWAS data. Furthermore, by using a PRS based on common genetic variants, we might have underestimated genetic risk for stroke and MI transmitted by other genetic and epigenetic mechanisms, such as rare variants, 44 methylation patterns, 45 and genetic imprinting, 46 among others. But because our primary focus was not the actual performance of the PRSs, but rather on using them as tools to compare family history and markers of inherited risk, this should not have biased our findings. In addition, because we were unable to use the most up‐to‐date GWAS because of its overlap with UKB participants, our PRSs might have been underpowered compared with performance of most recent PRSs and thus the mediation estimates of PRS on family history may have been underestimated. 8 , 47 , 48 Second, our analysis relied on self‐reported family history, introducing recall and reporting biases. Nonetheless, this reflects real‐world conditions, and there is evidence that self‐reported family history for cardiovascular‐related traits is reliable compared with ascertainment through disease registries or relative's self‐report. 49 , 50 Third, mediation estimation in Cox models remains an area of active development and may warrant further methodological investigations. 51 Finally, although we adjusted for potential confounders, residual confounding remains a possibility.
Conclusions
Our study demonstrates the importance of distinguishing between family history of stroke and heart disease in the context of incident stroke and MI risk assessment. Our findings further show that family history and genetic risk are not interchangeable terms as only a small part of family history is mediated by common variant genetic risk. They further demonstrate different subtypes of vascular risk carried by family history of stroke and MI, with different genetic and environmental contributions. Our study underscores the potential value of family history of stroke as a partially modifiable risk factor, and highlights the need for targeted primary prevention efforts, particularly in younger high‐risk populations and adoptees. Although our study has limitations, it provides valuable insights into the complex interplay between genetic risk, family history, and the incidence of stroke and MI. Future studies should evaluate the feasibility and the benefit of intensified risk factor management in individuals with positive family history of stroke.
Sources of Funding
Dr Anderson is supported by National Institutes of Health (NIH) R01NS103924 and U01NS069673, American Heart Association 18SFRN34250007 and 21SFRN812095, and the Massachusetts General Hospital McCance Center for Brain Health for this work. Dr Georgakis is supported from the German Research Foundation (Deutsche Forschungsgemeinschaft) with a Walter‐Benjamin Fellowship (GZ: GE 3461/1‐1; ID: 466957018), within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy; ID: 390857198), and with an Emmy Noether grant (GZ: GE 3461/2‐1; ID: 512461526), as well as grants from the Fritz‐Thyssen Foundation (reference 10.22.2.024MN) and the Hertie Foundation (Hertie Network of Excellence in Clinical Neuroscience; ID: P1230035). Dr Rosand receives research grants from NIH and the American Heart Association–Bugher Foundation. Dr Harloff is supported by the Berta‐Ottenstein Program for Advanced Clinician Scientists, Medical Faculty, University of Freiburg, Germany.
Disclosures
Dr Anderson has received sponsored research support from Bayer AG and has consulted for ApoPharma, unrelated to this work. Dr Rosand reports compensation from the National Football League and Takeda Development Center Americas for consultant services, unrelated to this work. As of August 2023, Dr Mayerhofer is a full‐time employee of Regeneron Pharmaceuticals. The remaining authors have no disclosures to report.
Supporting information
Table S1
Figures S1–S16
Acknowledgments
This research has been conducted using the UK Biobank Resource under application No. 36993. Figure 1 was created with Biorender.com.
This article was sent to Ajay K. Gupta, MD, MSc, PhD, Senior Associate Editor, for review by expert referees, editorial decision, and final disposition.
Preprint posted on MedRxiv, June 3, 2023. doi: https://doi.org/10.1101/2023.05.28.23290649.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.031566
For Sources of Funding and Disclosures, see page 8.
References
- 1. GBD 2019 Stroke Collaborators . Global, regional, and national burden of stroke and its risk factors, 1990‐2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795–820. doi: 10.1016/S1474-4422(21)00252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dai H, Much AA, Maor E, Asher E, Younis A, Xu Y, Lu Y, Liu X, Shu J, Bragazzi NL. Global, regional, and national burden of ischaemic heart disease and its attributable risk factors, 1990–2017: results from the Global Burden of Disease Study 2017. Eur Heart J Qual Care Clin Outcomes. 2022;8:50–60. doi: 10.1093/ehjqcco/qcaa076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lloyd‐Jones DM, Nam BH, D'Agostino RB Sr, Levy D, Murabito JM, Wang TJ, Wilson PW, O'Donnell CJ. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle‐aged adults: a prospective study of parents and offspring. JAMA. 2004;291:2204–2211. doi: 10.1001/jama.291.18.2204 [DOI] [PubMed] [Google Scholar]
- 5. Seshadri S, Beiser A, Pikula A, Himali JJ, Kelly‐Hayes M, Debette S, DeStefano AL, Romero JR, Kase CS, Wolf PA. Parental occurrence of stroke and risk of stroke in their children: the Framingham study. Circulation. 2010;121:1304–1312. doi: 10.1161/CIRCULATIONAHA.109.854240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marenberg ME, Risch N, Berkman LF, Floderus B, de Faire U. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. 1994;330:1041–1046. doi: 10.1056/NEJM199404143301503 [DOI] [PubMed] [Google Scholar]
- 7. Brass LM, Isaacsohn JL, Merikangas KR, Robinette CD. A study of twins and stroke. Stroke. 1992;23:221–223. doi: 10.1161/01.str.23.2.221 [DOI] [PubMed] [Google Scholar]
- 8. Mishra A, Malik R, Hachiya T, Jurgenson T, Namba S, Posner DC, Kamanu FK, Koido M, Le Grand Q, Shi M, et al. Stroke genetics informs drug discovery and risk prediction across ancestries. Nature. 2022;611:115–123. doi: 10.1038/s41586-022-05165-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tcheandjieu C, Zhu X, Hilliard AT, Clarke SL, Napolioni V, Ma S, Lee KM, Fang H, Chen F, Lu Y, et al. Large‐scale genome‐wide association study of coronary artery disease in genetically diverse populations. Nat Med. 2022;28:1679–1692. doi: 10.1038/s41591-022-01891-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bevan S, Traylor M, Adib‐Samii P, Malik R, Paul NL, Jackson C, Farrall M, Rothwell PM, Sudlow C, Dichgans M, et al. Genetic heritability of ischemic stroke and the contribution of previously reported candidate gene and genomewide associations. Stroke. 2012;43:3161–3167. doi: 10.1161/STROKEAHA.112.665760 [DOI] [PubMed] [Google Scholar]
- 11. Zdravkovic S, Wienke A, Pedersen NL, Marenberg ME, Yashin AI, De Faire U. Heritability of death from coronary heart disease: a 36‐year follow‐up of 20 966 Swedish twins. J Intern Med. 2002;252:247–254. doi: 10.1046/j.1365-2796.2002.01029.x [DOI] [PubMed] [Google Scholar]
- 12. Inouye M, Abraham G, Nelson CP, Wood AM, Sweeting MJ, Dudbridge F, Lai FY, Kaptoge S, Brozynska M, Wang T, et al. Genomic risk prediction of coronary artery disease in 480,000 adults: implications for primary prevention. J Am Coll Cardiol. 2018;72:1883–1893. doi: 10.1016/j.jacc.2018.07.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abraham G, Malik R, Yonova‐Doing E, Salim A, Wang T, Danesh J, Butterworth AS, Howson JMM, Inouye M, Dichgans M. Genomic risk score offers predictive performance comparable to clinical risk factors for ischaemic stroke. Nat Commun. 2019;10:5819. doi: 10.1038/s41467-019-13848-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abraham G, Rutten‐Jacobs L, Inouye M. Risk prediction using polygenic risk scores for prevention of stroke and other cardiovascular diseases. Stroke. 2021;52:2983–2991. doi: 10.1161/STROKEAHA.120.032619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tada H, Melander O, Louie JZ, Catanese JJ, Rowland CM, Devlin JJ, Kathiresan S, Shiffman D. Risk prediction by genetic risk scores for coronary heart disease is independent of self‐reported family history. Eur Heart J. 2016;37:561–567. doi: 10.1093/eurheartj/ehv462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mars N, Lindbohm JV, Della Briotta Parolo P, Widen E, Kaprio J, Palotie A; FinnGen , Ripatti S. Systematic comparison of family history and polygenic risk across 24 common diseases. Am J Hum Genet. 2022;109:2152–2162. doi: 10.1016/j.ajhg.2022.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rutten‐Jacobs LC, Larsson SC, Malik R, Rannikmae K; MEGASTROKE consortium; International Stroke Genetics Consortium; Sudlow CL, Dichgans M, Markus HS, Traylor M. Genetic risk, incident stroke, and the benefits of adhering to a healthy lifestyle: cohort study of 306 473 UK biobank participants. BMJ. 2018;363:k4168. doi: 10.1136/bmj.k4168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schnitzer F, Forer L, Schonherr S, Gieger C, Grallert H, Kronenberg F, Peters A, Lamina C. Association between a polygenic and family risk score on the prevalence and incidence of myocardial infarction in the KORA‐F3 study. Atherosclerosis. 2022;352:10–17. doi: 10.1016/j.atherosclerosis.2022.05.014 [DOI] [PubMed] [Google Scholar]
- 19. Martin AR, Gignoux CR, Walters RK, Wojcik GL, Neale BM, Gravel S, Daly MJ, Bustamante CD, Kenny EE. Human demographic history impacts genetic risk prediction across diverse populations. Am J Hum Genet. 2017;100:635–649. doi: 10.1016/j.ajhg.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sohail M, Maier RM, Ganna A, Bloemendal A, Martin AR, Turchin MC, Chiang CWK, Hirschhorn J, Daly MJ, Patterson N, et al. Polygenic adaptation on height is overestimated due to uncorrected stratification in genome‐wide association studies. elife. 2019;8:e39702. doi: 10.7554/eLife.39702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wei J, Shi Z, Na R, Resurreccion WK, Wang CH, Duggan D, Zheng SL, Hulick PJ, Helfand BT, Xu J. Calibration of polygenic risk scores is required prior to clinical implementation: results of three common cancers in UKB. J Med Genet. 2022;59:243–247. doi: 10.1136/jmedgenet-2020-107286 [DOI] [PubMed] [Google Scholar]
- 22. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, Rutten‐Jacobs L, Giese AK, van der Laan SW, Gretarsdottir S, et al. Multiancestry genome‐wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. 2018;50:524–537. doi: 10.1038/s41588-018-0058-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, Saleheen D, Kyriakou T, Nelson CP, Hopewell JC, et al. A comprehensive 1,000 genomes‐based genome‐wide association meta‐analysis of coronary artery disease. Nat Genet. 2015;47:1121–1130. doi: 10.1038/ng.3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O'Connell J, et al. The UK biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ge T, Chen CY, Ni Y, Feng YA, Smoller JW. Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat Commun. 2019;10:1776. doi: 10.1038/s41467-019-09718-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second‐generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. doi: 10.1186/s13742-015-0047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 2011;27:2987–2993. doi: 10.1093/bioinformatics/btr509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, Chen WM. Robust relationship inference in genome‐wide association studies. Bioinformatics. 2010;26:2867–2873. doi: 10.1093/bioinformatics/btq559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure‐mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18:137–150. doi: 10.1037/a0031034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ohira T, Shahar E, Chambless LE, Rosamond WD, Mosley TH Jr, Folsom AR. Risk factors for ischemic stroke subtypes: the Atherosclerosis Risk in Communities study. Stroke. 2006;37:2493–2498. doi: 10.1161/01.STR.0000239694.19359.88 [DOI] [PubMed] [Google Scholar]
- 32. O'Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao‐Melacini P, Zhang X, Pais P, Agapay S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case‐control study. Lancet. 2016;388:761–775. doi: 10.1016/S0140-6736(16)30506-2 [DOI] [PubMed] [Google Scholar]
- 33. Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878 [DOI] [PubMed] [Google Scholar]
- 34. Leening MJG, Cook NR, Franco OH, Manson JE, Lakshminarayan K, LaMonte MJ, Leira EC, Robinson JG, Ridker PM, Paynter NP. Comparison of cardiovascular risk factors for coronary heart disease and stroke type in women. J Am Heart Assoc. 2018;7:e007514. doi: 10.1161/JAHA.117.007514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lindgren MP, PirouziFard M, Smith JG, Sundquist J, Sundquist K, Zoller B. A Swedish nationwide adoption study of the heritability of heart failure. JAMA Cardiol. 2018;3:703–710. doi: 10.1001/jamacardio.2018.1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sundquist K, Winkleby M, Li X, Ji J, Hemminki K, Sundquist J. Familial [corrected] transmission of coronary heart disease: a cohort study of 80,214 Swedish adoptees linked to their biological and adoptive parents. Am Heart J. 2011;162:317–323. doi: 10.1016/j.ahj.2011.05.013 [DOI] [PubMed] [Google Scholar]
- 37. Devan WJ, Falcone GJ, Anderson CD, Jagiella JM, Schmidt H, Hansen BM, Jimenez‐Conde J, Giralt‐Steinhauer E, Cuadrado‐Godia E, Soriano C, et al. Heritability estimates identify a substantial genetic contribution to risk and outcome of intracerebral hemorrhage. Stroke. 2013;44:1578–1583. doi: 10.1161/STROKEAHA.111.000089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bakker MK, van der Spek RAA, van Rheenen W, Morel S, Bourcier R, Hostettler IC, Alg VS, van Eijk KR, Koido M, Akiyama M, et al. Genome‐wide association study of intracranial aneurysms identifies 17 risk loci and genetic overlap with clinical risk factors. Nat Genet. 2020;52:1303–1313. doi: 10.1038/s41588-020-00725-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miller BC, Fan X, Christensen M, Grotevant HD, van Dulmen M. Comparisons of adopted and nonadopted adolescents in a large, nationally representative sample. Child Dev. 2000;71:1458–1473. doi: 10.1111/1467-8624.00239 [DOI] [PubMed] [Google Scholar]
- 40. Petersen L, Sorensen TI, Mortensen EL, Andersen PK. Excess mortality rate during adulthood among Danish adoptees. PLoS One. 2010;5:e14365. doi: 10.1371/journal.pone.0014365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Petersen L, Andersen PK, Sorensen TIA, Mortensen EL. Delayed age at transfer of adoptees to adoptive parents is associated with increased mortality irrespective of social class of the adoptive parents: a cohort study. BMC Public Health. 2018;18:435. doi: 10.1186/s12889-018-5338-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sieverding M, Arbogast AL, Zintel S, von Wagner C. Gender differences in self‐reported family history of cancer: a review and secondary data analysis. Cancer Med. 2020;9:7772–7780. doi: 10.1002/cam4.3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pinsky PF, Kramer BS, Reding D, Buys S; PLCO Project Team . Reported family history of cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Am J Epidemiol. 2003;157:792–799. doi: 10.1093/aje/kwg043 [DOI] [PubMed] [Google Scholar]
- 44. Meschia JF. Effects of genetic variants on stroke risk. Stroke. 2020;51:736–741. doi: 10.1161/STROKEAHA.119.024158 [DOI] [PubMed] [Google Scholar]
- 45. Zeng M, Zhen J, Zheng X, Qiu H, Xu X, Wu J, Lin Z, Hu J. The role of DNA methylation in ischemic stroke: a systematic review. Front Neurol. 2020;11:566124. doi: 10.3389/fneur.2020.566124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Qureshi IA, Mehler MF. Emerging role of epigenetics in stroke: part 1: DNA methylation and chromatin modifications. Arch Neurol. 2010;67:1316–1322. doi: 10.1001/archneurol.2010.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lu X, Liu Z, Cui Q, Liu F, Li J, Niu X, Shen C, Hu D, Huang K, Chen J, et al. A polygenic risk score improves risk stratification of coronary artery disease: a large‐scale prospective Chinese cohort study. Eur Heart J. 2022;43:1702–1711. doi: 10.1093/eurheartj/ehac093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hasbani NR, Ligthart S, Brown MR, Heath AS, Bebo A, Ashley KE, Boerwinkle E, Morrison AC, Folsom AR, Aguilar D, et al. American Heart Association's Life's Simple 7: lifestyle recommendations, polygenic risk, and lifetime risk of coronary heart disease. Circulation. 2022;145:808–818. doi: 10.1161/CIRCULATIONAHA.121.053730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bensen JT, Liese AD, Rushing JT, Province M, Folsom AR, Rich SS, Higgins M. Accuracy of proband reported family history: the NHLBI Family Heart Study (FHS). Genet Epidemiol. 1999;17:141–150. doi: [DOI] [PubMed] [Google Scholar]
- 50. Murabito JM, Nam BH, D'Agostino RB Sr, Lloyd‐Jones DM, O'Donnell CJ, Wilson PW. Accuracy of offspring reports of parental cardiovascular disease history: the Framingham Offspring Study. Ann Intern Med. 2004;140:434–440. doi: 10.7326/0003-4819-140-6-200403160-00010 [DOI] [PubMed] [Google Scholar]
- 51. Lapointe‐Shaw L, Bouck Z, Howell NA, Lange T, Orchanian‐Cheff A, Austin PC, Ivers NM, Redelmeier DA, Bell CM. Mediation analysis with a time‐to‐event outcome: a review of use and reporting in healthcare research. BMC Med Res Methodol. 2018;18:118. doi: 10.1186/s12874-018-0578-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figures S1–S16


