Abstract
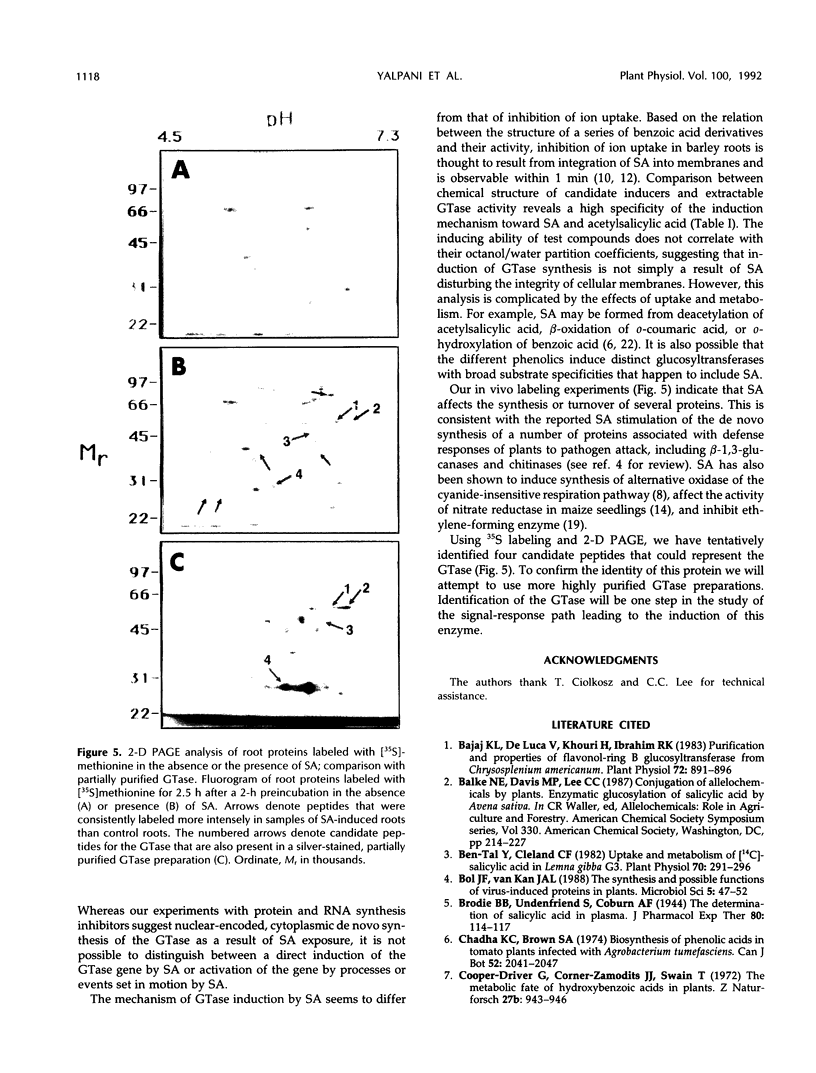
A UDP-glucose:salicylic acid 3-O-glucosyltransferase (EC 2.4.1.35) (GTase) from oat (Avena sativa L. cv Dal) root extracts was assayed in vitro using [14C]salicylic acid (SA) and an ion exchange column to separate SA from β-glucosylsalicylic acid. The GTase, present at a very low constitutive level, was inducible to 23 times the constitutive level. When excised roots were exposed to SA at pH 6.5, the specific activity of the enzyme increased within 1.5 h, peaked after 8 to 10 h, and then declined. The increase in specific activity depended on the concentration of SA in the induction medium. Among 16 phenolics and phenolic derivatives tested, GTase induction showed high specificity toward SA and acetylsalicylic acid. Specific activity of the enzyme was induced to higher levels in roots from 7-d-old seedlings than roots from younger plants. GTase activity was less inducible in basal compared with median or apical root sections. Induction of GTase activity was a result of de novo RNA and protein synthesis. Candidate peptides for the GTase were identified by comparison of two-dimensional electrophoresis gels of proteins labeled with [35S]methionine during incubation of roots in the presence or the absence of SA and a gel of a partially purified GTase preparation.
Full text
PDF
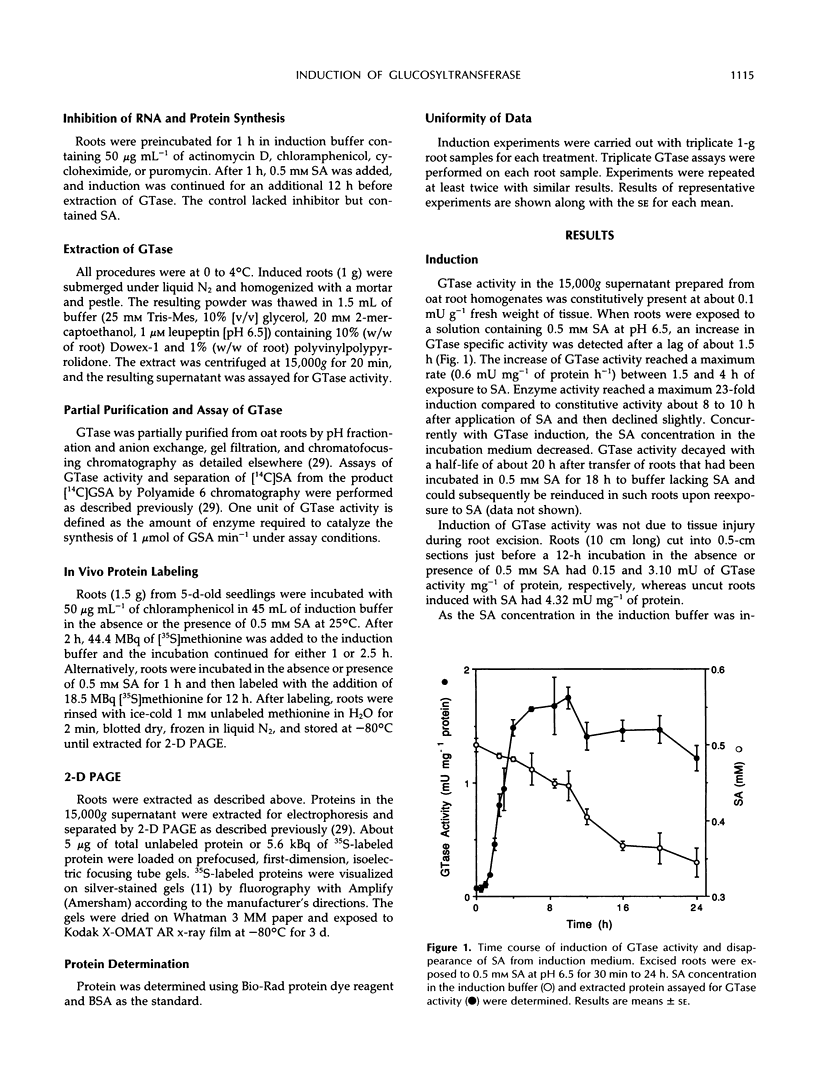
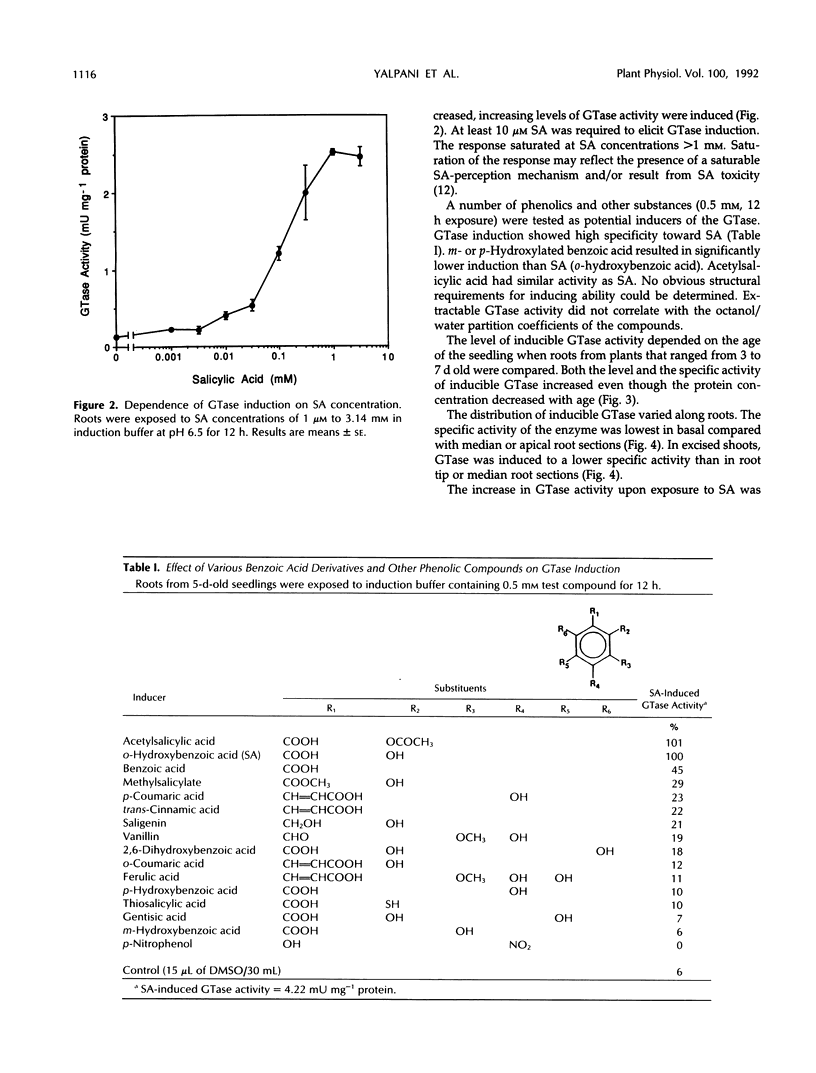
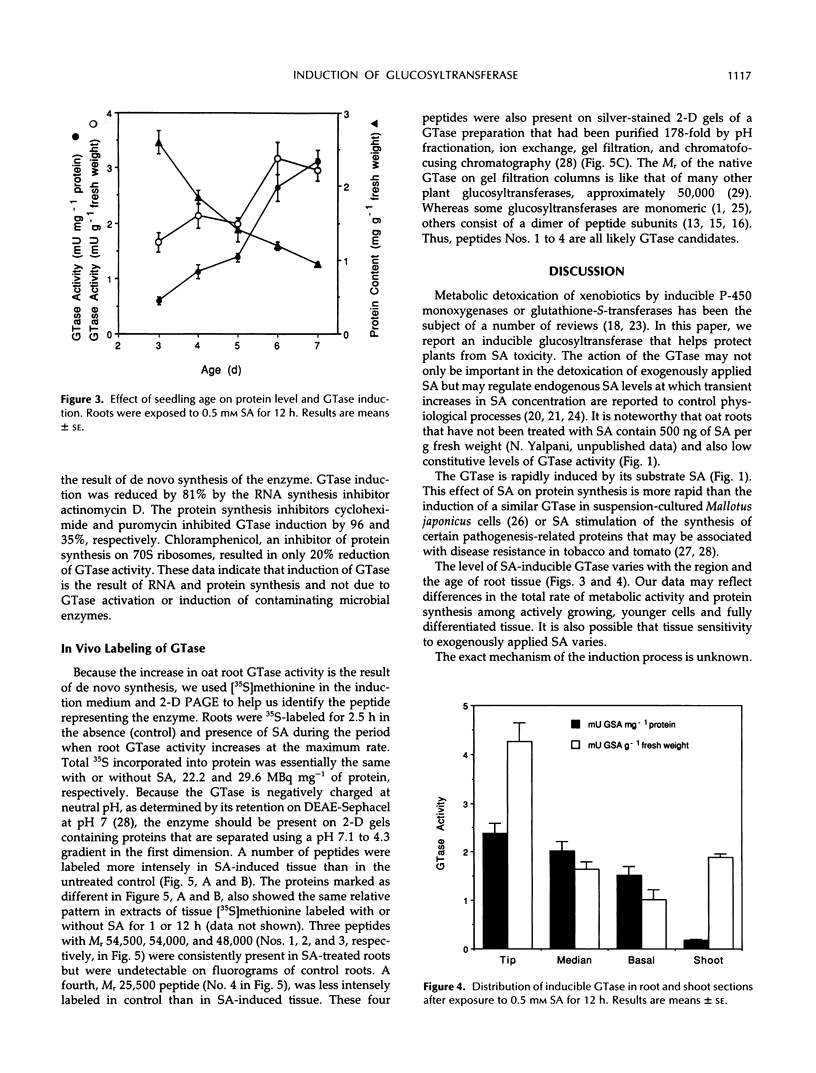

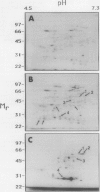
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajaj K. L., de Luca V., Khouri H., Ibrahim R. K. Purification and Properties of Flavonol-Ring B Glucosyltransferase from Chrysosplenium americanum. Plant Physiol. 1983 Jul;72(3):891–896. doi: 10.1104/pp.72.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Tal Y., Cleland C. F. Uptake and Metabolism of [C]Salicylic Acid in Lemna gibba G3. Plant Physiol. 1982 Jul;70(1):291–296. doi: 10.1104/pp.70.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bol J. F., van Kan J. A. The synthesis and possible functions of virus-induced proteins in plants. Microbiol Sci. 1988 Feb;5(2):47–52. [PubMed] [Google Scholar]
- Glass A. D. Influence of phenolic acids on ion uptake: I. Inhibition of phosphate uptake. Plant Physiol. 1973 Jun;51(6):1037–1041. doi: 10.1104/pp.51.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. R., Balke N. E. Characterization of the inhibition of k absorption in oat roots by salicylic Acid. Plant Physiol. 1981 Dec;68(6):1349–1353. doi: 10.1104/pp.68.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie C. A., Romani R. J. Inhibition of ethylene biosynthesis by salicylic Acid. Plant Physiol. 1988 Nov;88(3):833–837. doi: 10.1104/pp.88.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy J., Carr J. P., Klessig D. F., Raskin I. Salicylic Acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science. 1990 Nov 16;250(4983):1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- Métraux J. P., Signer H., Ryals J., Ward E., Wyss-Benz M., Gaudin J., Raschdorf K., Schmid E., Blum W., Inverardi B. Increase in salicylic Acid at the onset of systemic acquired resistance in cucumber. Science. 1990 Nov 16;250(4983):1004–1006. doi: 10.1126/science.250.4983.1004. [DOI] [PubMed] [Google Scholar]
- Raskin I., Ehmann A., Melander W. R., Meeuse B. J. Salicylic Acid: a natural inducer of heat production in arum lilies. Science. 1987 Sep 25;237(4822):1601–1602. doi: 10.1126/science.237.4822.1601. [DOI] [PubMed] [Google Scholar]
- Sandermann H., Jr, Schmitt R., Eckey H., Bauknecht T. Plant biochemistry of xenobiotics: isolation and properties of soybean O- and N-glucosyl and O- and N-malonyltransferases for chlorinated phenols and anilines. Arch Biochem Biophys. 1991 Jun;287(2):341–350. doi: 10.1016/0003-9861(91)90488-5. [DOI] [PubMed] [Google Scholar]
- Yalpani N., Schulz M., Davis M. P., Balke N. E. Partial purification and properties of an inducible uridine 5'-diphosphate-glucose-salicylic Acid glucosyltransferase from oat roots. Plant Physiol. 1992 Sep;100(1):457–463. doi: 10.1104/pp.100.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]