Abstract
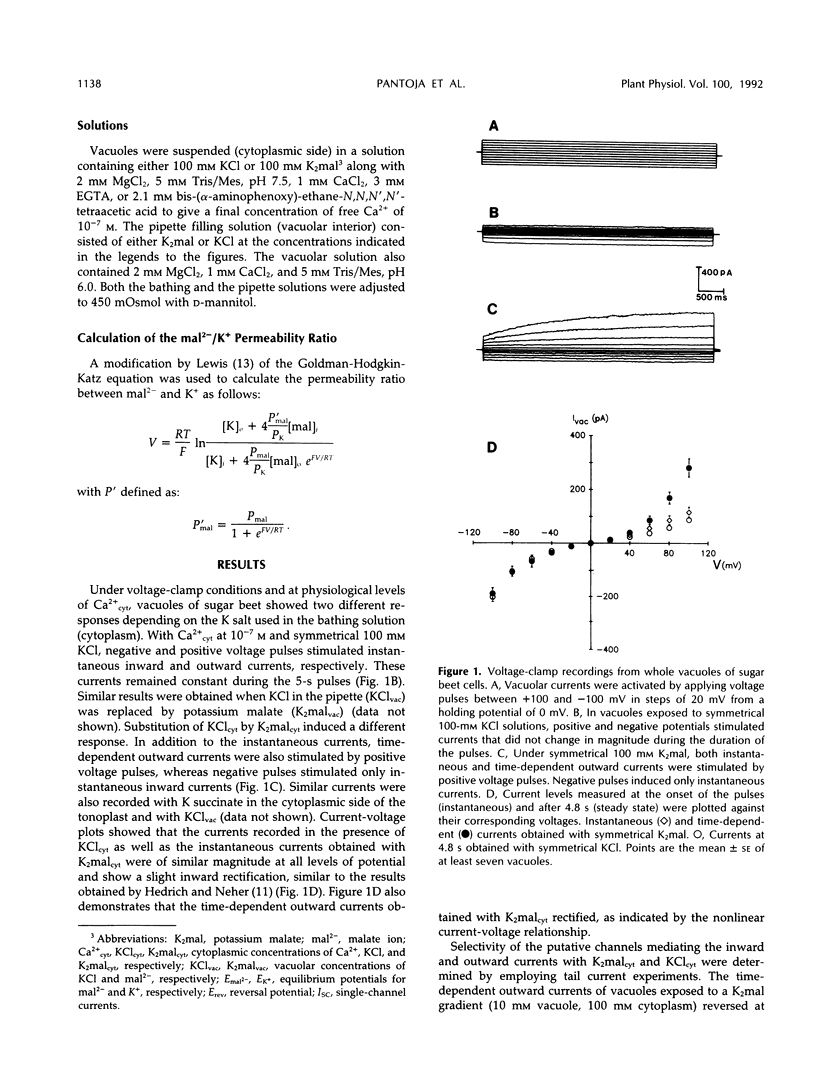
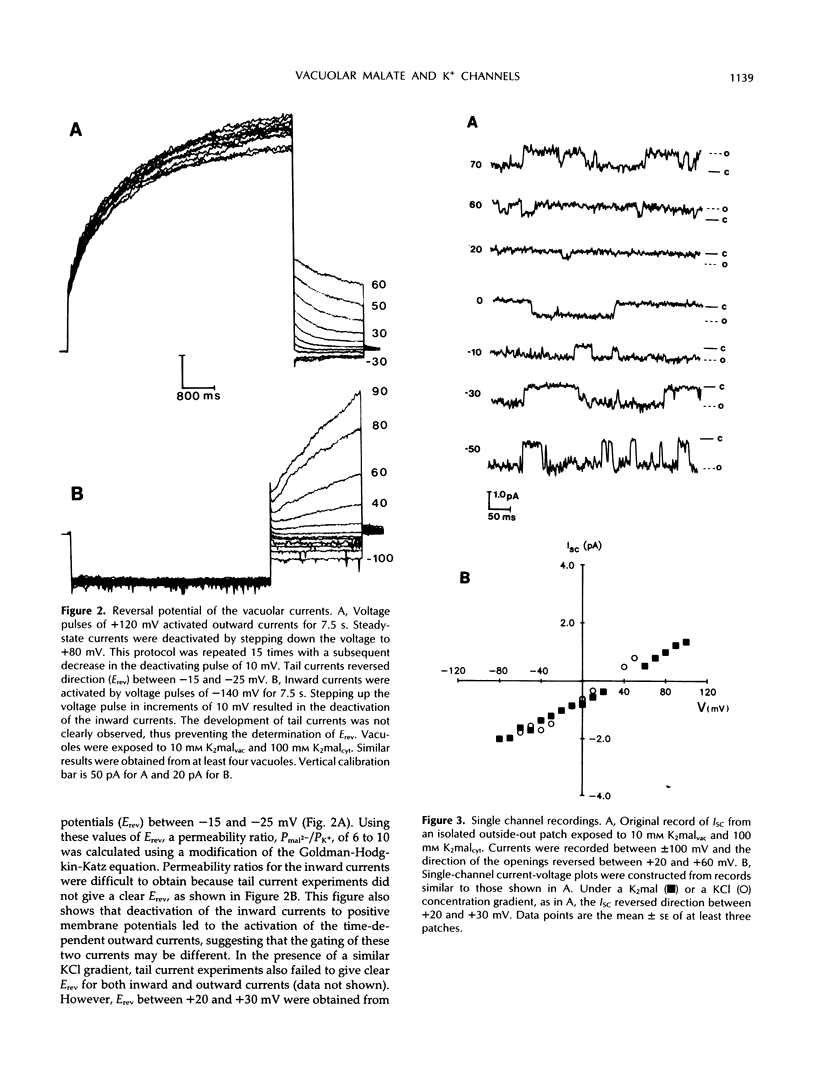
Patch-clamp techniques were employed to study the electrical properties of vacuoles from sugar beet (Beta vulgaris) cell suspensions at physiological concentrations of cytoplasmic Ca2+. Vacuoles exposed to K+ malate revealed the activation of instantaneous and time-dependent outward currents by positive membrane potentials. Negative potentials induced only instantaneous inward currents. The time-dependent outward currents were 10 times more selective for malate than for K+ and were completely blocked by zinc. Vacuoles exposed to KCl developed instantaneous currents when polarized to positive or negative membrane potentials. The time-dependent outward channels could serve as the route for the movement of malate into the vacuole, whereas K+ could move through the time-independent inward and outward channels.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumwald E., Poole R. J. Salt tolerance in suspension cultures of sugar beet : induction of na/h antiport activity at the tonoplast by growth in salt. Plant Physiol. 1987 Apr;83(4):884–887. doi: 10.1104/pp.83.4.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz K. J., Jäger R., Kaiser G., Martinoia E. Amino Acid Transport across the Tonoplast of Vacuoles Isolated from Barley Mesophyll Protoplasts : Uptake of Alanine, Leucine, and Glutamine. Plant Physiol. 1990 Jan;92(1):123–129. doi: 10.1104/pp.92.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt R., Heldt H. W. Measurement of subcellular metabolite levels in leaves by fractionation of freeze-stopped material in nonaqueous media. Plant Physiol. 1984 Jul;75(3):542–547. doi: 10.1104/pp.75.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hedrich R., Kurkdjian A. Characterization of an anion-permeable channel from sugar beet vacuoles: effect of inhibitors. EMBO J. 1988 Dec 1;7(12):3661–3666. doi: 10.1002/j.1460-2075.1988.tb03247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C. A. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979 Jan;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoja O., Dainty J., Blumwald E. Tonoplast ion channels from sugar beet cell suspensions : inhibition by amiloride and its analogs. Plant Physiol. 1990 Dec;94(4):1788–1794. doi: 10.1104/pp.94.4.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]


