Abstract
Cardiovascular disease is the leading cause of death worldwide. Its prevalence is rising due to ageing populations and the increasing incidence of diseases such as chronic kidney disease, obesity, and diabetes that are associated with elevated cardiovascular risk. Despite currently available treatments, there remains a huge burden of cardiovascular disease-associated morbidity for patients and healthcare systems, and newer treatments are needed. The apelin system, comprising the apelin receptor and its two endogenous ligands apelin and elabela, is a broad regulator of physiology that opposes the actions of the renin-angiotensin and vasopressin systems. Activation of the apelin receptor promotes endothelium-dependent vasodilatation and inotropy, lowers blood pressure, and promotes angiogenesis. The apelin system appears to protect against arrhythmias, inhibits thrombosis, and has broad anti-inflammatory and anti-fibrotic actions. It also promotes aqueous diuresis through direct and indirect (central) effects in the kidney. Thus, the apelin system offers therapeutic promise for a range of cardiovascular, kidney, and metabolic diseases. This review will discuss current cardiovascular disease targets of the apelin system and future clinical utility of apelin receptor agonism.
Keywords: Apelin, Inotrope, Vasodilator, Cardiovascular disease
1. Introduction
The global prevalence of cardiovascular disease has nearly doubled in the last 30 years, and mortality is rising. In 2019, 18.6 million deaths were attributed to cardiovascular causes, equating to around one-third of all global deaths.1 Much of this mortality is due to population growth and ageing, and the associated accumulation of risk factors, although the increase in age-standardized rates of cardiovascular disease in some regions suggests that other factors also contribute. Hypertension remains the leading risk factor for cardiovascular disease, affecting over one-quarter of the world’s population.2 Additionally, the prevalence of chronic kidney disease, type 2 diabetes mellitus, and obesity is increasing and each independently contributes to cardiovascular disease risk.1
Endothelial dysfunction is central to the development of cardiovascular disease, promoting inflammation, thrombosis, and the development of arterial stiffness.3 Current management of cardiovascular disease focuses on the use of antihypertensive medications, antiplatelet agents, and cholesterol-lowering therapies. Several of these agents improve endothelial function.3,4 However, despite these therapies, cardiovascular disease is associated with an unacceptable burden of morbidity and mortality, and there is increasing urgency to find newer treatments. An agent that could provide cardiovascular protection and benefit associated conditions would be particularly attractive.
The apelin system is a broad regulator of physiology. It consists of the apelin receptor (encoded by the APLNR gene previously known as APJ) and its two endogenous ligands, apelin and elabela (also known as Toddler).5 The system is a particularly appealing target for cardiovascular disease as it promotes endothelium-dependent vasodilatation, inotropy, lowers blood pressure, and increases aqueous diuresis. Activating the apelin system also has metabolic and renal benefits. This review will provide an overview of the role of apelin signalling in the (patho)physiology of cardiovascular disease and the potential benefits of apelin treatment. The following should be consulted for a more detailed discussion of apelin agonism in disorders of water balance,6 diabetes mellitus,7 obesity8,9 and metabolic disorders,10,11 and apelin analogues and therapeutic agents.6,12,13
2. The apelin system
2.1. Apelin
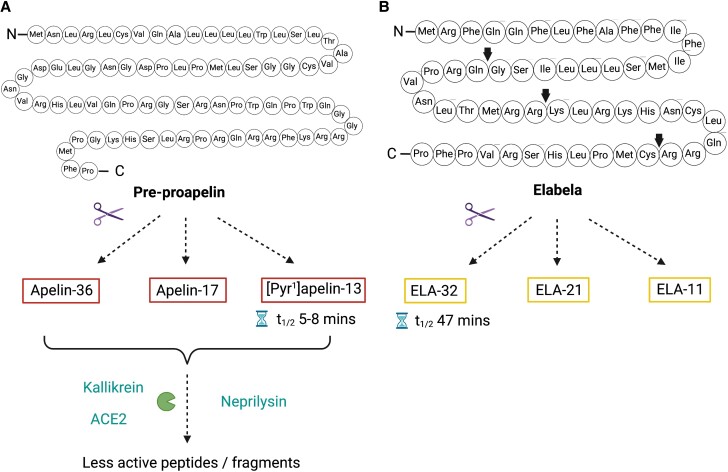
Apelin, encoded by the APLN gene on the long arm of the X-chromosome, was the first ligand identified for the apelin receptor in 1998.14 Apelin peptides are formed by cleavage of the 77-amino acid precursor, pre-proapelin (Figure 1 and Table 1). Pyroglutamated apelin-13 ([Pyr1] apelin-13) is the predominant isoform in the human cardiovascular system and plasma,15,16 with other biologically active isoforms including apelin-36, apelin-17, and apelin-13 also detectable.5 The half-life of these peptides is short (a few minutes) and may be even shorter in vivo than ex vivo, a major limitation for clinical application.17 Apelin peptides are cleaved by plasma kallikrein, neprilysin, and angiotensin-converting enzyme 2 (ACE2).18–22 Interestingly, only neprilysin has been demonstrated to inactivate apelin peptides fully, which may be of significance when considering the benefits of neprilysin inhibition in heart failure.19 In humans, apelin mRNA is expressed throughout the vasculature, the central nervous system and in many organs including the heart, lungs, and kidney.5 Apelin protein is predominantly found within endocardial and vascular endothelial cells, suggesting that circulating apelin may originate from these tissues.23 Centrally derived apelin (e.g. from magnocellular neurons) may also contribute to circulating apelin.24 However, plasma apelin concentrations are low so it does not appear to be a major circulating hormone and it may function in an autocrine/paracrine manner.
Figure 1.
(A) Apelin peptides are cleaved from the precursor pre-proapelin. Several circulating isoforms have been identified including apelin-36, apelin-17, and pyroglutamated apelin-13 ([Pyr1]apelin-13), the commonest isoform in human plasma. Apelin peptides are subsequently cleaved into less active peptides by plasma kallikrein, angiotensin-converting enzyme 2 (ACE2), and neprilysin. (B) Elabela (ELA) is a 54-amino acid peptide with 3 predicted mature isoforms, ELA-32, ELA-21, and ELA-11. Predicted cleavage sites are highlighted by arrows. The half-life of ELA-32 is 10 times that of apelin peptides. Figure created using BioRender.com.
Table 1.
Apelin receptor endogenous peptides, and synthetic peptide and small molecule agonists and antagonists
| Action | Value | Parameter | |
|---|---|---|---|
| Human endogenous peptides | |||
| apelin-13 | Full agonist | 8.8, 9.2 | pIC50 |
| [Pyr1]apelin-13 | Full agonist | 8.9 | pIC50 |
| apelin-17 | Agonist | 9.0 | pIC50 |
| apelin-36 | Full agonist | 8.6 | pIC50 |
| Elabela/Toddler-11 | Agonist | 7.2 | pIC50 |
| Elabela/Toddler-21 | Agonist | 8.7 | pIC50 |
| Elabela/Toddler-32 | Agonist | 8.7 | pIC50 |
| Radiolabelled apelin peptide analogues | |||
| [3H](Pyr1)[Met(0)11]-apelin-13 | Full agonist | 8.6 | pKd |
| [125I]apelin-13 | Full agonist | 9.2 | pKd |
| [125I](Pyr1)apelin-13 | Full agonist | 9.5 | pKd |
| [125I][Glp65Nle75,Tyr77]apelin-13 | Full agonist | 10.7 | pKd |
| Agonists: peptide analogues | |||
| cyclo apelin-12 (1–12) | Full agonist | 6.3 | pEC50 |
| cyclourea apelin-12 (1–7) | Full agonist | 6.8 | pEC50 |
| cyclo apelin-12 (7–12) | Full agonist | 7.1 | pEC50 |
| Palmitate-VTLPLWATYTYR (compound 1 [PMID: 25241924]) | Full agonist | 7.5 | pEC50 |
| LIT01–196 | Agonist | 9.1 | pKi |
| H2N-c[X-R-L-S-X]-K-G-P-(D-2Nal) (compound 40 [PMID: 34982553]) | Agonist | 8.2 | pKi |
| H2N-c[X-R-L-S-X]-K-G-P-(D-1Nal) (compound 39 [PMID: 34982553]) | Agonist | 9.2 | pKi |
| MM07 | Biased agonist | 9.5 | pEC50 |
| Agonists: small molecules | |||
| ML233 | Full agonist | 5.4 | pEC50 |
| E339-3D6 | Agonist | 6.4 | pKi |
| BMS-986224 | Agonist | 9.5 | pKd |
| Azelaprag (AMG 986; BGE-105) | Agonist | 9.5 | pEC50 |
| Compound 15a [PMID:31724863] | Agonist | 10.0 | pEC50 |
| Compound 21 [PMID: 34855405] | Agonist | 10.2 | pEC50 |
| Compound 14a [PMID: 34795866] | Agonist | 10.6 | pEC50 |
| CMF-019 | Biased agonist | 10.0 | pEC50 |
| Antagonists | |||
| MM54 peptide | Antagonist | 8.2 | pKi |
| ALX40-4C peptide | Antagonist | 5.5 | pIC50 |
| ML221 small molecule | Antagonist | 5.8 | pIC50 |
2.2. Elabela
Elabela, the second ligand for the apelin receptor from the APELA gene, is a 54-amino acid peptide that was originally identified in the human genome as a potentially secreted peptide in 2013 and provided an elegant explanation for the difference between the phenotypes of apelin and apelin receptor knockout mice.25,26 Whilst animals lacking apelin develop normally,27 those lacking the apelin receptor show significant cardiovascular developmental defects and many do not survive to birth.27,28 Similarly, mice lacking elabela show cardiovascular defects and embryologic lethality. However, there are some differences between the phenotypes early in embryogenesis with ∼10% of elabela mutants showing abnormal yolk sac vasculature with variable cardiac malformations in contrast to only ∼2% of apelin receptor mutants.29 Embryos lacking the apelin receptor also show abnormal tail bending. Therefore, it is possible that elabela may also signal through currently unidentified alternative pathways. There are several predicted mature isoforms of elabela peptides, elabela-32, elabela-21, and elabela-11 (Table 1).25,26 At present, elabela has only been detected in the vascular endothelium and the kidney in adult humans.30,31 Of note, the half-life of elabela-32 in human plasma ex vivo is 10 times longer than that of apelin.32 Intriguingly apelin and elabela interact with different amino acids in the orthosteric binding site of the apelin receptor, although impact on signalling remains to be explored.33–35
2.3. The apelin receptor
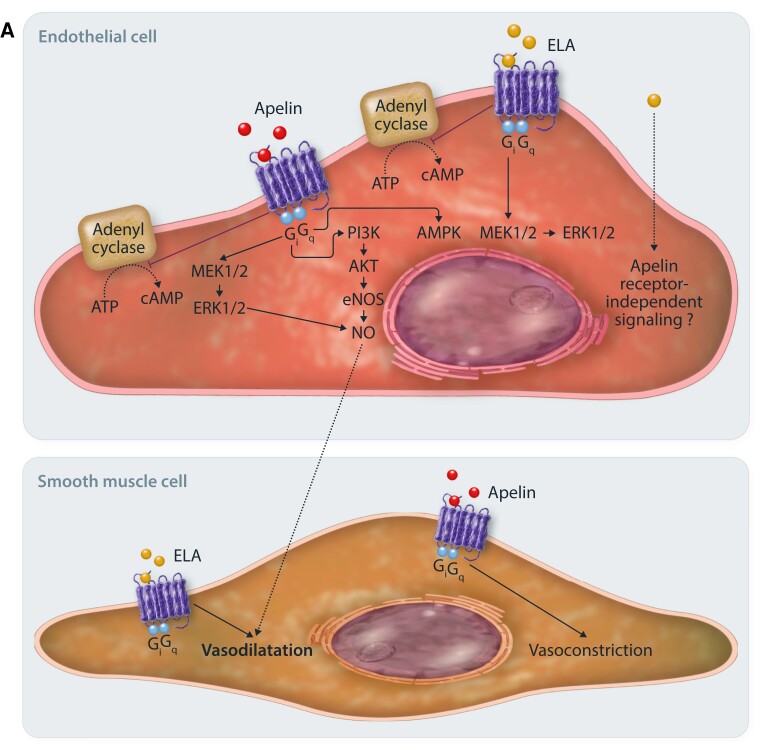
The apelin receptor was first identified in 1993.36 It is a G-protein coupled receptor that shares ∼50% homology with the angiotensin II-type 1 receptor (AT1) but is not activated by angiotensin II. In fact, the actions of the apelin system broadly oppose those of the renin-angiotensin system.37–43 The apelin receptor couples to pertussis toxin-sensitive Gi proteins, and binding of either apelin or elabela results in inhibition of adenylyl cyclase and a reduction in intracellular cyclic adenosine monophosphate.44 Downstream signalling then occurs via extracellular regulated kinases (ERKs) and phosphoinositide 3-kinase (PI3K)-AKT pathways.5,44 The resulting physiological action is dependent on the cell type activated. Proposed apelin and elabela signalling pathways are shown in Figure 2A.45–47
Figure 2.
(A) Endothelial cell. The apelin receptor couples to pertussis toxin-sensitive inhibitory G proteins (Gi) and ligand binding inhibits adenylyl cyclase and cyclic AMP production and promotes activation of extracellular signal-regulated protein kinase 1/2 (ERK1/2) pathways. Within the vasculature, apelin and elabela promote vasorelaxation through different mechanisms. Apelin promotes nitric oxide (NO) production via ERK1/2, phosphatidylinositol 3-kinase/protein kinase B (PI3K-AKT), and AMP-activated protein kinase (AMPK) pathways. However, when acting on vascular smooth muscle cells, apelin promotes vasoconstriction. Elabela activates ERK1/2 pathways however nitric oxide production is not required for vasorelaxation to occur. Additionally, elabela causes direct vasorelaxation of vascular smooth muscle cells.
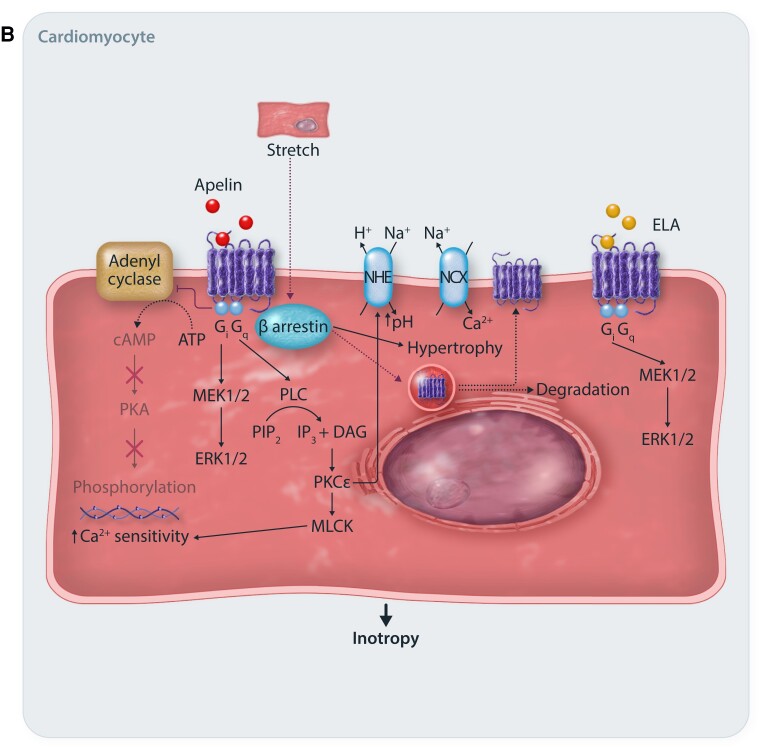
Figure 2.
(B) Cardiomyocyte signalling. Signalling at the apelin receptor in cardiomyocytes promotes inotropy and an increase in cardiac output through several mechanisms. Gi-protein signalling and inhibition of adenylyl cyclase and the protein kinase A (PKA) pathway, with subsequent inhibition of phosphorylation of troponin and enhanced calcium sensitivity. Apelin also signals via Gi to promote ERK1/2 signalling. Gq-mediated signalling promotes the phospholipase C (PLC)/protein kinase Cɛ (PKC) pathway, stimulating the Na+-H+ exchanger that ultimately leads to increased intracellular pH. The subsequent increase in intracellular Na+ promotes activation of the Na+-Ca2+ exchanger, increasing intracellular calcium concentration. PKCɛ also stimulates activation of myosin light chain kinase, promoting phosphorylation of myosin and again increasing calcium sensitivity. The unoccupied apelin receptor signals via β-arrestin to promote myocardial hypertrophy, which is inhibited by ligand binding. When the receptor is activated, β-arrestin promotes internalization of the receptor with subsequent recycling to the cell membrane. Elabela has been shown to promote inotropy through ERK1/2 signalling pathways but not PKC pathways.
Following receptor activation, β-arrestins are recruited and the receptor is internalized before being recycled to the cell surface.5,30,33,48 Receptor internalization and recycling appear to be dependent on the activating ligand.48,49 β-Arrestins are also implicated in ligand-independent signalling at the apelin receptor (Figure 2B). Cardiomyocytes develop markers of hypertrophy in response to stretch, and this is dependent on the presence of the apelin receptor. Knockdown of β-arrestins or administration of apelin protects against this.50 Thus, when not bound by ligand, the inactivated apelin receptor may act as a mechanosensor that promotes hypertrophy through β-arrestin signalling.
Apelin receptor message is expressed in many tissues including the adipose tissue, heart, lung, kidney, placenta, and skeletal muscle, and also throughout the central nervous system.5 Apelin receptor protein is expressed in the brain, heart, kidney, and lung and spinal cord and within the cardiovascular system, it is present throughout the vascular endothelium, in vascular smooth muscle cells of conduit arteries and veins, and in cardiomyocytes.5 Similarities in the expression patterns of apelin and the apelin receptor suggest a predominantly autocrine or paracrine mechanism of action.
3. The apelin system and the renin-angiotensin system
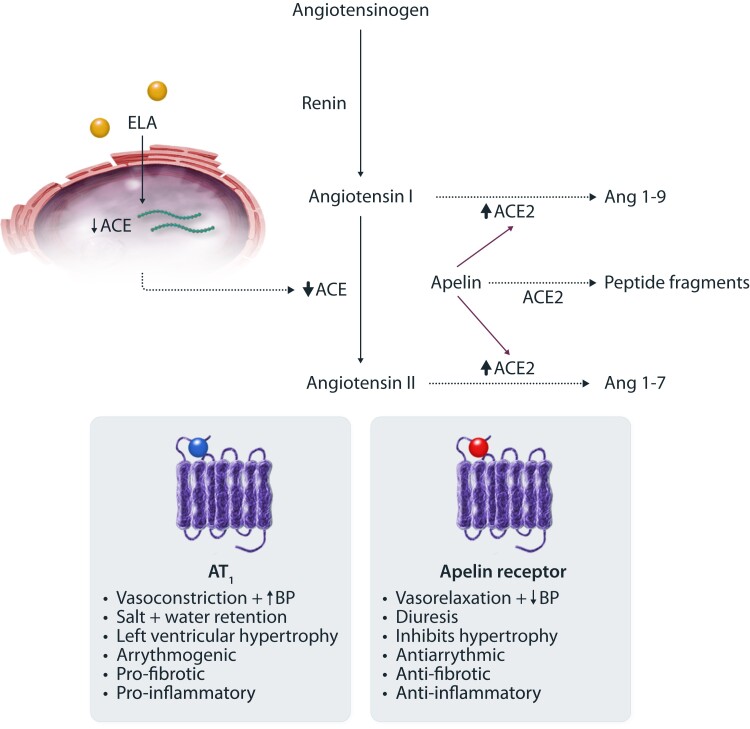
Overactivation of the renin-angiotensin system is central to the development of cardiovascular disease. Angiotensin II promotes salt retention, hypertension, and end-organ inflammation and fibrosis. Therefore, angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers are key therapeutic tools in the management of cardiovascular disease. The apelin and AT1 receptors are co-expressed throughout the cardiovascular system, and the apelin system opposes the actions of the renin-angiotensin system (Figure 3).10,37,51 The systems may also reciprocally regulate each other.52 In an animal model of heart failure, down-regulation of cardiac apelin and apelin receptor mRNA is restored by AT1 blockade. Similarly, angiotensin II infusion down-regulates cardiac apelin mRNA that is restored by an AT1 blocker.38 Loss of apelin potentiates angiotensin II-induced myocardial injury and abdominal aortic aneurysm development, and apelin treatment reverses these changes.18,42,43,53 Angiotensin-converting enzyme 2 (ACE2) is a major negative regulator of angiotensin II, converting it to angiotensin 1–7 that promotes vasodilatation.54,55 ACE2 also cleaves apelin peptides to generally less active compounds, and its production is enhanced by them.10,22,56 Elabela does not affect ACE2 but down-regulates ACE expression.41 If apelin analogues could act synergistically with renin-angiotensin system blockers to offer broad cardiovascular benefits, this would be a particularly useful therapeutic development.
Figure 3.
There is crosstalk between the apelin system and the renin-angiotensin system. Angiotensin I is converted to angiotensin II (angiotensin II) by angiotensin-converting enzyme (ACE) and then acts on the angiotensin II-type 1 receptor (AT1) to promote an increase in blood pressure (BP), left ventricular remodelling, inflammation, and fibrosis. Activation of the apelin receptor by apelin or elabela opposes the actions of angiotensin II. The apelin system also influences production of angiotensin II. Elabela reduces expression of ACE, indirectly limiting production of angiotensin II, and apelin promotes production of angiotensin-converting enzyme 2 (ACE2), enhancing breakdown of both angiotensin I and II and promoting the production of angiotensin 1–7 (angiotensin 1–7) that causes vasodilatation.
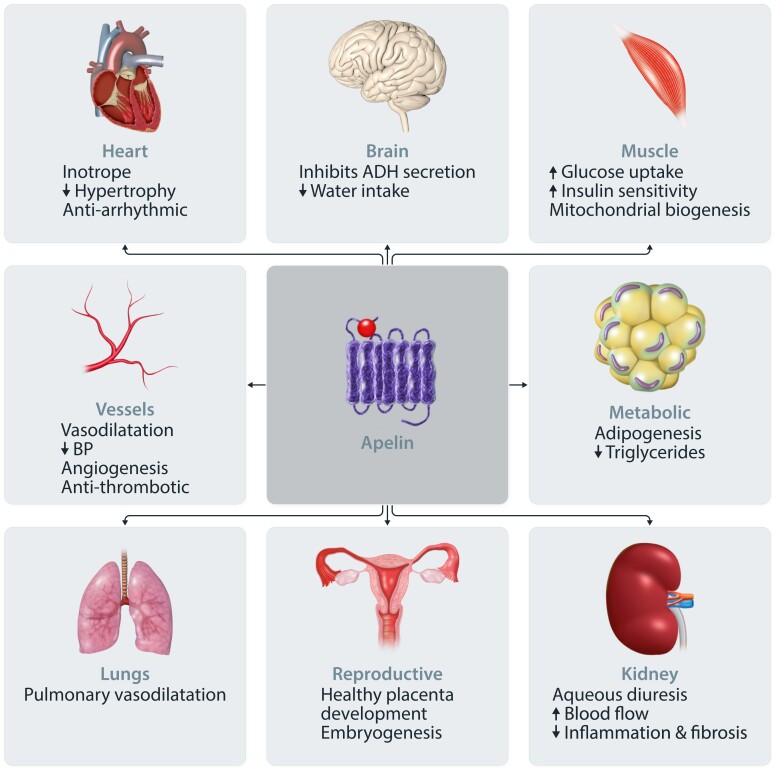
4. Clinical targeting of the apelin system
Although several clinical studies have examined the actions of the apelin system in health and disease, all have focused on apelin peptides given the relatively recent discovery of elabela. Overall, the apelin system offers exciting therapeutic potential for a range of cardiovascular diseases (Figure 4).
Figure 4.
The apelin system is a broad regulator of physiology.
4.1. Hypertension
Hypertension, recently re-defined by the American Heart Association as a systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 80 mmHg, is a leading cause of cardiovascular disease and affects almost 50% of adults in the USA.57 Many adults require a combination of antihypertensive agents, but despite this, only ∼45% adults achieve blood pressure control.58
The apelin system regulates vascular tone in vitro, ex vivo, and in vivo. Activation of the apelin receptor on vascular endothelial cells by apelin peptides promotes nitric oxide production and leads to vasodilatation, with ß-arrestin recruitment also implicated.10,37 This occurs in healthy and diseased vessels although vasodilatation may occur via prostanoids rather than nitric oxide in diseased states.59,60 In contrast, several studies do report a vasoconstrictor action of both central and peripherally administered apelin in vitro and in vivo however these involve different animal species and different vascular beds.61 Elabela causes dose-dependent vasodilatation, both in the presence and absence of endothelium, but is not dependent on nitric oxide.31,45In vivo studies find that elabela attenuates angiotensin II-induced increases in blood pressure.30,41
In hypertensive rats, apelin receptor message and protein are reduced in the aorta, heart, and kidney, and elabela mRNA is reduced in the renal medulla.62–64 The development of hypertension is accelerated by elabela deficiency, and both apelin and elabela are protective against it.37,64 Overexpression of elabela also reduced kidney injury and fibrosis through inhibition of the Nod-like receptor protein 3 inflammasome, and, intriguingly, in this study, elabela appeared to be acting independently of the apelin receptor.64 Similarly, in the Dahl salt-sensitive rat, overexpression of elabela in the heart delayed the onset of hypertension and was protective against kidney damage.65 Of interest, fractional excretion of sodium and chloride was reduced both before and after the initiation of a high-salt diet in these animals, despite no obvious change in expression of elabela within the kidney. Unexpectedly, cardiac function also deteriorated that the authors hypothesized may be due to sodium resorption.65 In the hypertensive DOCA-salt rat, the long-acting apelin analogue LIT01-196 significantly reduced blood pressure with once-daily dosing, without inducing any undesirable change in kidney function or sodium concentration.66
Clinical studies in healthy volunteers confirm apelin-induced vasodilatation to be dependent on nitric oxide.17 Systemic infusion of [Pyr1]apelin-13 leads to a ∼5% reduction in blood pressure and a fall in peripheral vascular resistance in health and heart failure.39,67 Importantly, apelin promotes vasodilatation even in the setting of renin-angiotensin system activation,39 and circulating concentrations of apelin are lower in patients with hypertension.68–70 There is no clinical evidence of apelin-induced vasoconstriction in health or disease.17,39,67,71,72
Overall, the apelin system is altered in hypertension and is a potential therapeutic target. Apelin and elabela promote vasodilatation by different mechanisms and could have varying roles in maintaining vascular health. Further pre-clinical and clinical studies are required to further define these mechanisms and determine the actions of elabela on the vasculature in humans.
4.2. Atherosclerosis
Atheromatous cardiovascular disease is the leading cause of mortality worldwide. The role apelin plays in the pathogenesis of atherosclerosis has not been fully defined. It promotes vascular smooth muscle cell proliferation,73 and loss of the apelin receptor protects against the development of atherosclerosis in the apolipoprotein E knockout mouse.74 However, loss of apelin promotes atherosclerosis in this model and apelin treatment reduces angiotensin II-induced atherosclerosis43 and enhances atherosclerotic plaque stability.75 The reasons for these discrepancies are unclear, and to date, there is no evidence for ligand-independent signalling at the apelin receptor in this setting.
In humans, apelin expression is up-regulated within atherosclerotic coronary artery, and both apelin and the apelin receptor are found in atherosclerotic plaques, co-localizing with macrophages and smooth muscle cells.76 It is unclear whether this is protective—perhaps through opposition of angiotensin II signalling—or pathological. Patients with coronary artery disease have lower circulating apelin concentrations, and these are lowest in those with symptomatic coronary artery disease.77
The relationship between statin use and apelin is intriguing. Statins lower low-density lipoprotein cholesterol but also have pleiotropic effects that contribute to their beneficial cardiovascular effects.78 Statins induce kruppel-like factor 2, an important regulator of endothelial cell homeostasis, and subsequently production of endothelial nitric oxide synthase and thrombomodulin.79 This promotes vasorelaxation alongside anti-inflammatory and antithrombotic effects, and is dependent on an intact apelin signalling pathway.80 Statins also reduce monocyte adhesion to endothelial cells, and this is impaired by loss of apelin. Additionally, statins promote endothelial expression of the apelin receptor at the mRNA and protein level,80 and increase circulating apelin peptide concentrations.81 There is, therefore, potential for synergistic vascular benefit from combined statin and apelin therapy in patients with atherosclerotic disease. However, this is yet to be conformed in clinical studies.
4.3. Myocardial infarction
Atherosclerotic plaque rupture is the first step in the cascade of events that leads to thrombotic coronary artery occlusion and myocardial infarction. Both apelin and the apelin receptor are expressed on human platelets, and apelin can inhibit platelet aggregation through the nitric oxide-cyclic guanosine monophosphate pathway.82 Apelin-deficient mice have reduced tail bleeding time and enhanced platelet aggregation and are prothrombotic, whereas apelin-13 prolongs tail bleeding in wild-type animals.82 Apelin can also influence thrombosis by regulating plasminogen activator inhibitor-1 (PAI-1) that inactivates the endothelium-derived fibrinolytic factor, tissue plasminogen activator. PAI-1 expression is induced by angiotensin II83 but inhibited by apelin.40 No clinical studies have explored whether apelin has antithrombotic actions in humans. Current medical management of myocardial infarction involves treatment with dual antiplatelet agents. The potential for apelin to offer additional antiplatelet activity and broad anticoagulant effect is an attractive prospect that deserves further examination.
Following thrombotic occlusion of the coronary artery, tissue ischaemia and myocardial cell death occur, and ultimately an area of scar tissue forms that can lead to left ventricular impairment. The apelin system promotes new vessel formation and can minimize ischaemic injury.31,84,85 Hypoxia induces apelin expression in vitro, ex vivo, and in vivo, and this is driven by hypoxia inducible factor 1α.86–88 Ischaemia-reperfusion injury is a widely used model of myocardial infarction, and in this model, animals lacking apelin show enhanced susceptibility to injury with larger infarct sizes, more significant left ventricular impairment and an increase in mortality.89 Following injury, apelin and apelin receptor mRNA and protein are up-regulated initially, then down-regulated as early as 24 h on. Apelin and elabela both protect against ischaemia-reperfusion injury in models, including when given at the time of reperfusion mimicking potential clinical utility.86,89,90 Their beneficial effects are likely due to the promotion of angiogenesis, activation of the PI3K-AKT and p44/42 mitogen-activated protein kinase components of the reperfusion injury salvage kinase pathway and, with respect to apelin, nitric oxide production.89–93
Following myocardial infarction, the renin-angiotensin system is activated, and this propagates myocardial injury. Thus, renin-angiotensin system inhibition is part of current standard of care treatment. Pre-clinical studies show that pre-treatment with a combination of apelin and the AT1 blocker, losartan, results in synergistic benefit following ischaemia-reperfusion injury, reducing infarct size by ∼50% and improving left ventricular function.94
No clinical studies have examined the apelin system around the time of myocardial infarction. The available pre-clinical data suggest that apelin receptor agonism could limit myocardial injury and left ventricular impairment and offer broad anticoagulant effects, with additive benefits to current standard of care. Clinical trials of apelin or elabela treatment post-myocardial infarction are needed but are currently impractical due to the lack of oral preparations.
4.4. Heart failure
Heart failure is common, with a prevalence of ∼2% in many parts of the Western World.95 It is characterized by the progressive loss of cardiac systolic and diastolic function, often with acute exacerbations requiring hospitalization, and is associated with severe morbidity and mortality.95 The apelin system has an important role in maintaining cardiac function in health, and is altered in heart failure and may therefore have potential as a beneficial treatment in this setting.
Apelin is the most potent inotrope in isolated human heart tissue discovered to date.59 Mice lacking apelin or the apelin receptor show impaired myocardial contractility at baseline, and apelin-deficient animals are more sensitive to the negative effects of ageing and pressure overload.27,96 Traditional inotropes increase intracellular calcium concentrations and subsequently myocardial oxygen demand, promote hypertrophy and arrhythmias, and are associated with increased mortality.97 The mechanisms of apelin-induced inotropy are not fully understood. There is evidence both for and against apelin increasing intracellular calcium transients,98–100 although apelin’s inotropic effect may be mediated by enhanced calcium sensitivity rather than a change in total intracellular calcium. This would suggest that apelin may not be associated with the same adverse effect profile as other inotropes.
Apelin improves contractility via phospholipase C, protein kinase C, and ERK1/2 pathways.46,47 It promotes downstream activation of myosin light chain kinase and inhibits protein kinase A-induced phosphorylation of troponin I, both of which enhance myofilament calcium sensitivity (Figure 2B).47,100 Elabela also promotes dose-dependent inotropy in isolated perfused rat hearts, and this is partly via ERK1/2 pathways.34,45In vivo, chronic apelin infusion increased cardiac output without causing myocardial hypertrophy.101 Similarly, clinical studies show that intracoronary apelin-36 increased left ventricular contractility, and both short and prolonged systemic infusions of [Pyr1]apelin-13 increased cardiac index whilst lowering blood pressure and peripheral vascular resistance.39,67
The apelin system is implicated in the pathogenesis of heart failure, with expression of apelin and its receptor up-regulated early in the disease and down-regulated as it progresses.102–104 Apelin protein has been detected in myocardial cells from patients with severe heart failure, yet is not found in these cells in health.103 Importantly, in patients with heart failure, APLNR was the most up-regulated gene in the left ventricle following placement of a left ventricular assist device and was associated with up-regulated tissue levels of apelin protein.103 Plasma apelin concentration increases in early heart failure, falls with disease progression, and is restored by cardiac resynchronization therapy.102,105 Overall, it may be that early on in disease, the apelin system is attempting to support the failing heart but is less able to do so as cardiac function deteriorates.
Pre-clinical studies find that apelin and elabela are protective in models of heart failure, improving cardiac contractility, preventing hypertrophy, and reducing mortality.41,89,106,107 Their actions may be mediated through regulation of the renin-angiotensin system. Apelin acts to inhibit the detrimental actions of angiotensin II in vitro and in vivo.53 Additionally, treatment with ACE inhibitors restores cardiac function in the apelin knockout mouse,53 and in a rodent model of chronic heart failure treatment with an AT1 blocker restored cardiac apelin and apelin receptor expression.38 Elabela also prevents the pressure overload-induced increase in ACE mRNA and protein, restoring the balance of ACE and ACE2.41
Both brief and prolonged infusions of [Pyr1]apelin-13 lead to a sustained ∼10% increase in cardiac index, with increased ejection fraction seen on echocardiography, and a reduction in systemic vascular resistance and blood pressure in patients with chronic heart failure.39,67 Currently standard of care treatment for heart failure includes combination therapy with a renin-angiotensin system inhibitor, beta blocker, and mineralocorticoid receptor antagonist. Recently, a combination of an angiotensin receptor antagonist and neprilysin inhibitor was shown to improve patient outcomes in patients with reduced ejection fraction and these agents are now recommended for patients who remain symptomatic despite ACE inhibitor therapy.108 As neprilysin fully inactivates apelin peptides, the success of these agents might be partly due to an increase in apelin peptide levels and this warrants further investigation in clinical studies.
In end-stage heart failure, patients may undergo heart transplantation. The longevity of the transplant is limited by the development of immune-mediated vascular injury in the graft, and apelin may act to mitigate this. Apelin receptor agonism protects against immune-mediated vascular injury both in vitro and in a mouse model of heart transplantation, and apelin is up-regulated in myocardial microvasculature and arteries within human failing heart grafts.109 Overall, this may be an attempt to reduce vascular injury and apelin treatment may offer therapeutic promise in the transplant setting.
Overall, a combination of inotropy and vasodilatation has been shown to improve haemodynamics in acute heart failure,110 and targeting the apelin system in heart failure could therefore offer benefit over and above that from currently available treatments. Clinical trials of apelin receptor agonism are now needed in both acute and chronic heart failure.
4.5. Atrial fibrillation
Atrial fibrillation is the commonest sustained arrhythmia in the general population, and is associated with other cardiovascular diseases and morbidity.111 Apelin is involved in regulation of cardiomyocyte electrophysiology, acting on several ion channels to shorten the action potential and increase conduction velocity.99,112 Slowed myocardial conduction velocity is associated with an increased risk of arrhythmia,113 and apelin knockout mice have reduced atrial conduction velocities.114
Apelin protects against the development of atrial fibrillation in pre-clinical studies by prolonging the atrial refractory period and inhibiting the actions of angiotensin II.114,115 The apelin system may also contribute to thrombotic risk. In patients with atrial fibrillation and thrombosis, expression of apelin and the apelin receptor was reduced and expression of AT1 receptors and PAI-1 was increased in the left atrial appendage compared to those in sinus rhythm or atrial fibrillation without thrombosis.116
Both atrial and plasma apelin and plasma elabela concentrations are reduced in patients with atrial fibrillation, even in the presence of other cardiovascular comorbidities.114,117–119 Plasma apelin also independently predicts the risk of atrial fibrillation and its recurrence following successful cardioversion or pulmonary vein isolation.119–121
Taken together, the apelin system protects against the development of atrial fibrillation by influencing electrical conduction and there is further evidence of its potential antithrombotic actions. Increasing circulating apelin in patients with atrial fibrillation could be a future therapeutic strategy, and apelin may be useful biomarker for atrial fibrillation.
4.6. Pulmonary arterial hypertension
Pulmonary arterial hypertension is a rare, chronic, and progressive disorder characterized by pulmonary vascular remodelling with smooth muscle cell hypertrophy, increasing vascular resistance and ultimately right ventricular failure.122 It has an estimated incidence of 15–50 cases per million and predominantly affects women.122 Pulmonary arterial hypertension may be idiopathic, genetic, or secondary to a variety of causes including drugs (e.g. the appetite suppressant aminorex), infection (e.g. schistosomiasis), or multi-system disease (e.g. connective tissue diseases).122 Survival has increased dramatically in the last two decades from a 5-year survival of 34% due to newer therapies becoming available. However, there is still substantial associated morbidity and mortality, and prognosis is dependent on many factors including aetiology and the severity of disease.122 Despite current treatments, patients have an overall 3-year survival of only 83%.123
Apelin, elabela, and the apelin receptor are expressed throughout the pulmonary vasculature.5 A small interfering RNA approach to induce apelin deficiency in pulmonary artery endothelial cells impaired cell survival and promoted pulmonary artery smooth muscle cell hypertrophy. Conversely, apelin treatment of pulmonary artery endothelial cells protected against cell death and promoted pulmonary artery smooth muscle cell apoptosis.124 Expression of the system is altered in models of pulmonary arterial hypertension, with reduced apelin, elabela, and apelin receptor expression in the right ventricle, and this may contribute to its pathogenesis.30,125 Mice lacking the apelin gene develop worse pulmonary arterial hypertension in response to hypoxia than wild-type animals, with reduced endothelial nitric oxide synthase and more pronounced vascular remodelling.126 Importantly, animal models of pulmonary arterial hypertension show that treatment with apelin, the G-protein-biased apelin receptor agonist MM07, or elabela can improve pulmonary haemodynamics and even reverse pulmonary arterial hypertension.30,124,125,127
Patients with pulmonary arterial hypertension have dysfunctional pulmonary artery endothelial cells, and these exhibit reduced apelin and elabela expression.30,124,128 Plasma apelin concentrations are reduced in patients with pulmonary arterial hypertension,126 and short infusions of [Pyr1]apelin-13 led to additional favourable changes in pulmonary haemodynamics, with a fall in pulmonary vascular resistance and a rise in cardiac output.72 Interestingly, although this study was not designed to explore this effect, patients on phosphodiesterase-5 inhibitors with more severe disease showed a greater improvement in pulmonary vascular resistance, stroke volume, and cardiac output than those patients not on this treatment.72 Phosphodiesterase-5 inhibitors act on the nitric oxide pathway, preventing the degradation of cyclic guanosine monophosphate and thereby promoting vasodilatation. Apelin may therefore offer synergistic benefit in this setting by promoting upstream nitric oxide production.
Mutations in the bone morphogenic protein receptor type 2 (BMPR2) gene and alterations in BMPR2 signalling can predispose to pulmonary vascular remodelling and the development of pulmonary arterial hypertension.129 This may be partly due to regulation of the apelin system. A mouse model of hypoxia-induced pulmonary arterial hypertension found that loss of bone morphogenic protein 9 (BMP9, a ligand of the Activin Receptor-like Kinase 1 receptor that heterocomplexes with BMPR2) increased apelin expression and reduced susceptibility to pulmonary arterial hypertension.130 Similarly, application of BMP9 to pulmonary artery endothelial cells from patients with pulmonary arterial hypertension and healthy controls led to reduced apelin expression.130 Notably, a pre-clinical model has recently shown that oestradiol is protective against right ventricular failure by up-regulating apelin via oestrogen receptor alpha/BMPR2 signalling.131
Taken together, these data suggest that loss of apelin may contribute to the pathogenesis of pulmonary arterial hypertension and apelin treatment may provide additional benefits on top of standard of care. Clinical studies with prolonged apelin treatment and apelin analogues with longer half-lives are needed.
4.7. Pre-eclampsia
Pre-eclampsia is a multi-system disorder that affects ∼5% of pregnancies.132 It is characterized by the development of gestational hypertension with additional evidence of end-organ dysfunction, such as proteinuria.133 It can occur early (<34 weeks’ gestation) or late in pregnancy, and ranges in severity. Once established, there is no curative treatment other than delivery of the foetus, and it remains a major cause of maternal and infant morbidity and mortality.133 The pathogenesis of pre-eclampsia is not fully understood, but poor development of the placental vascular network is key, resulting in placental ischaemia.133
Apelin and elabela contribute to placental development and embryogenesis, and are implicated in the pathogenesis of pre-eclampsia.134 Apelin promotes small vessel angiogenesis and elabela is essential for endoderm differentiation and heart development, and also increases trophoblast invasion into the maternal uterine wall seemingly independent of the apelin receptor.26,135 Apelin and pre-proapelin are abundantly expressed in the healthy human placenta,136 with [Pyr1]apelin-13 the commonest isoform.137 Changes in the placental expression of the apelin system in humans with pre-eclampsia are not fully defined. Apelin mRNA and protein and apelin receptor protein are down-regulated in severe disease, but it is unclear whether changes occur earlier.138,139 The relationship with the renin-angiotensin system is again implicated. In pre-eclampsia, there is up-regulation of angiotensin II and the AT1 receptor.140 Angiotensin II reduces apelin release from healthy human chorionic villus explants, but this is prevented and apelin release is in fact enhanced by the AT1 receptor blocker, losartan.137 Elabela expression has only been explored in the early stages of pre-eclampsia, and no changes are seen.139 Interestingly, elabela-deficient, but not apelin-deficient, mice develop a pre-eclampsia phenotype with small, poorly vascularized placentas, hypertension, proteinuria and glomerular endotheliosis (a hallmark feature of pre-eclampsia), and treatment with elabela prevented these changes.141 However, apelin treatment does lower blood pressure and proteinuria in models of pre-eclampsia.142–144
Published data describing plasma concentrations of apelin and elabela in pre-eclampsia are conflicting and derive from studies with heterogeneous designs and populations that are difficult to compare. On balance, plasma apelin appears to increase in early-onset and severe pre-eclampsia, but whether this is the case in late-onset or mild disease is unclear.138,139,145 However, the placenta may not be the source of circulating apelin, so any rise in plasma concentrations may reflect a compensatory increase in production from other tissues due to hypertension. Data on plasma elabela in pre-eclampsia are inconsistent, with both increased and decreased concentrations found.146–148 Overall, further studies are required to develop understanding of the apelin system in pre-eclampsia and establish whether it could be a therapeutic target for this disease.
4.8. Metabolic disease
The apelin system has a role in glucose and lipid metabolism and may be a therapeutic target for obesity and type 2 diabetes mellitus. A full review of apelin’s metabolic effects has been described recently (see tan-Laurell et al.149). Briefly, apelin and the apelin receptor are present on adipocytes and pancreatic islet cells.5,150 Expression of apelin by these cells is regulated by both insulin and glucocorticoids.150,151 Apelin also has a biphasic effect on insulin, with lower concentrations inhibiting and higher concentrations stimulating insulin secretion.150,152 Animals lacking apelin are hyperinsulinaemic and show reduced insulin sensitivity.153
In healthy mice, apelin infusion lowers plasma glucose by promoting glucose uptake in skeletal muscle and adipose tissue via endothelial nitric oxide synthase, AMPK, and Akt-dependent pathways. This glucose-lowering effect is also seen in obese and insulin-resistant mice,154 and is maintained with chronic apelin treatment where these animals showed reduced fat mass, triglycerides, and lower insulin levels than controls.155,156 Chronic apelin treatment also inhibits hepatic steatosis.157 Apelin analogues also promote glucose uptake and inhibit food intake in healthy and obese mice.158 Chronic treatment with apelin-13 analogues improved glycaemia, increased plasma insulin, and improved response to glucose tolerance tests as effectively as the established incretin therapies liraglutide and exendin-4. Interestingly, the apelin analogue (pGlu)apelin-13 amide was more effective at lowering triglyceride levels than the incretin mimetics.159 Clinical studies in healthy overweight men also find that apelin enhances insulin sensitivity.160 Studies exploring the ability of apelin to influence glycaemia and vascular health in subjects with increased weight and type 2 diabetes mellitus are in progress (see clinicaltrials.gov: NCT03449251).161
4.9. Kidney disease
Chronic kidney disease is increasing common worldwide and is independently associated with cardiovascular disease.162 Indeed, cardiovascular disease is the commonest complication of chronic kidney disease.162 The apelin system is a promising therapeutic target in a range of kidney diseases.6,163 Apelin regulates glomerular haemodynamics, opposing the actions of angiotensin II at the afferent and efferent glomerular arterioles through nitric oxide production.52 The apelin system also contributes to the regulation of fluid balance, acting in opposition to the vasopressin system.52,164–166 Within the central nervous system, apelin and the apelin receptor colocalize with vasopressin and the vasopressin 1 receptor within the magnocellular neurons of the hypothalamus.6 Apelin promotes aquaresis both by central actions and by directly inhibiting vasopressin-induced insertion of aquaporin 2 channels in the principal cells of the collecting duct.165,167,168 Changes in plasma osmolality cause opposing effects on the regulation of vasopressin and apelin release,16 and the apelin system may therefore represent a target in disorders of water balance such as syndrome of inappropriate antidiuretic hormone. In a model of vasopressin-induced hyponatraemia, a peptide agonist with a longer half-life than apelin (LIT01-196) blocks the antidiuretic effect of vasopressin and the vasopressin-induced increase in urinary osmolality.168 Elabela has also been shown to have an aquaretic effect.34,164
Both apelin and elabela are protective in models of acute kidney injury, with anti-inflammatory, anti-apoptotic, and anti-fibrotic effects,169–171 that may be synergistic.171 At present, there are no published data on the renal actions of the apelin system in humans, but studies are underway (see clnicaltrials.gov: NCT03956576).172 A detailed review of the apelin system in kidney disease may be found at Chapman et al.163
5. Therapeutic targeting of the apelin system
Whilst the apelin system offers exciting therapeutic potential for many cardiovascular diseases, future clinical studies are limited by the lack of orally available long-acting compounds. As such, development of apelin analogues resistant to peptidases and small molecule apelin receptor agonists has been a priority over the last decade, with variable success. Apelin peptide modification has been performed by PEGylation, cyclization, the addition of unnatural amino acids and conjugation to domain antibodies that then bind to albumin in vivo. The benefits and limitations of currently available agents have been discussed.10,11 Some analogues have prolonged half-lives and preserved activity in vivo.66,158,168,173–176 Several have shown beneficial effects in disease models. One apelin analogue was protective against myocardial injury.89 Others promote insulin-dependent glucose lowering in diet-induced-obese mice.158,177 As discussed, long-term treatment of diabetic db/db mice with two the apelin analogues ((pGlu)apelin-13 amide or pGlu(Lys8GluPAL)apelin-13 amide) was in some respects more effective than established incretin therapies at improving metabolic dysfunction.177
Given the current understanding of apelin receptor signalling, the ideal agent would be a biased agonist that promotes signalling through G-protein pathways and limits β-arrestin signalling. MM07, a cyclic apelin analogue, is one such biased agonist with a half-life ex vivo that is ∼seven-fold greater than [Pyr1]apelin-13. Studies in humans have demonstrated it to be a more potent vasodilator than [Pyr1]apelin-13 with no evidence of desensitization, and it improved haemodynamics and vascular remodelling in a pre-clinical model of pulmonary arterial hypertension.71,127 The small molecule compound CMF-019 also offers biased agonism at the apelin receptor, which is preserved in vivo.178 Encouragingly, pre-clinical studies not only confirm similar vasodilator and inotropic actions to [Pyr1]apelin-13 but it also has potential as a disease-modifying agent in pulmonary arterial hypertension.178
Significant effort has been devoted to developing alternative oral small molecule apelin agonists. Two such long-acting agents that have shown promise in pre-clinical studies have undergone phase I trials.179–181 Whilst data are as yet unpublished regarding BMS-986224, AMG 986 was shown to be safe and well tolerated in healthy humans and those with heart failure, although data were inconsistent regarding its clinical efficacy.179,182 So far, neither agent has progressed to further studies. The search for other small molecule agonists continues, and recently a small molecule known as compound 47 has been shown to be a potent and selective apelin receptor agonist.183
6. Conclusions
The apelin system is a promising therapeutic target for a range of cardiovascular diseases. There is strong evidence for benefit of apelin receptor agonists in pulmonary arterial hypertension and chronic kidney disease with further potential in heart failure as apelin promotes inotropy and has anti-fibrotic and antiplatelet actions. The precise role of elabela in relation to the apelin signalling pathway in cardiovascular disease remains to be elucidated, particularly whether elabela has distinct physiological, pathophysiological, and pharmacological actions from apelin. Elabela has a longer half-life than apelin and may activate different downstream pathways. Small molecule apelin compounds, including biased agonists such as CMF-019,178 are being developed that will enable meaningful clinical studies in this space in the future.
Contributor Information
Fiona A Chapman, BHF/University of Edinburgh Centre for Cardiovascular Science, Queen’s Medical Research Institute, Edinburgh, UK; Department of Renal Medicine, Royal Infirmary of Edinburgh, Edinburgh, UK.
Janet J Maguire, Division of Experimental Medicine and Immunotherapeutics, Addenbrooke’s Centre for Clinical Investigation, University of Cambridge, Cambridge, UK.
David E Newby, BHF/University of Edinburgh Centre for Cardiovascular Science, Queen’s Medical Research Institute, Edinburgh, UK.
Anthony P Davenport, Department of Renal Medicine, Royal Infirmary of Edinburgh, Edinburgh, UK.
Neeraj Dhaun, BHF/University of Edinburgh Centre for Cardiovascular Science, Queen’s Medical Research Institute, Edinburgh, UK; Department of Renal Medicine, Royal Infirmary of Edinburgh, Edinburgh, UK.
Funding
This work was supported by Kidney Research UK (TF_006_20171124 to F.A.C.); British Heart Foundation (TG/18/4/33770 to A.P.D. and J.J.M., FS/06/064, FS/09/019, CH09/002, RG/16/10/32375, RE/18/5/34216 to D.E.N.); NIHR Cambridge Biomedical Research Centre Biomedical Resources Grant (A.P.D. and J.J.M.); Wellcome Trust (WT103782AIA to D.E.N.); and Chief Scientist Office (SCAF/19/02 to N.D.). The views expressed are those of the authors and not necessarily those of the National Institute for Health and Care Research or the Department of Health and Social Care.
References
- 1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis J, Catapano AL, Chugh SS, Cooper LT, Coresh J, Criqui M, DeCleene N, Eagle KA, Emmons-Bell S, Feigin VL, Fernández-Solà J, Fowkes G, Gakidou E, Grundy SM, He FJ, Howard G, Hu F, Inker L, Karthikeyan G, Kassebaum N, Koroshetz W, Lavie C, Lloyd-Jones D, Lu HS, Mirijello A, Temesgen AM, Mokdad A, Moran AE, Muntner P, Narula J, Neal B, Ntsekhe M, Moraes de Oliveira G, Otto C, Owolabi M, Pratt M, Rajagopalan S, Reitsma M, Ribeiro ALP, Rigotti N, Rodgers A, Sable C, Shakil S, Sliwa-Hahnle K, Stark B, Sundström J, Timpel P, Tleyjeh IM, Valgimigli M, Vos T, Whelton PK, Yacoub M, Zuhlke L, Murray C, Fuster V, Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton A, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis JR, Catapano AL, Chugh S, Cooper LT, Coresh J, Criqui MH, DeCleene NK, Eagle KA, Emmons-Bell S, Feigin VL, Fernández-Sola J, Fowkes FGR, Gakidou E, Grundy SM, He FJ, Howard G, Hu F, Inker L, Karthikeyan G, Kassebaum NJ, Koroshetz WJ, Lavie C, Lloyd-Jones D, Lu HS, Mirijello A, Misganaw AT, Mokdad AH, Moran AE, Muntner P, Narula J, Neal B, Ntsekhe M, Oliveira GMM, Otto CM, Owolabi MO, Pratt M, Rajagopalan S, Reitsma MB, Ribeiro ALP, Rigotti NA, Rodgers A, Sable CA, Shakil SS, Sliwa K, Stark BA, Sundström J, Timpel P, Tleyjeh II, Valgimigli M, Vos T, Whelton PK, Yacoub M, Zuhlke LJ, Abbasi-Kangevari M, Abdi A, Abedi A, Aboyans V, Abrha WA, Abu-Gharbieh E, Abushouk AI, Acharya D, Adair T, Adebayo OM, Ademi Z, Advani SM, Afshari K, Afshin A, Agarwal G, Agasthi P, Ahmad S, Ahmadi S, Ahmed MB, Aji B, Akalu Y, Akande-Sholabi W, Aklilu A, Akunna CJ, Alahdab F, Al-Eyadhy A, Alhabib KF, Alif SM, Alipour V, Aljunid SM, Alla F, Almasi-Hashiani A, Almustanyir S, Al-Raddadi RM, Amegah AK, Amini S, Aminorroaya A, Amu H, Amugsi DA, Ancuceanu R, Anderlini D, Andrei T, Andrei CL, Ansari-Moghaddam A, Anteneh ZA, Antonazzo IC, Antony B, Anwer R, Appiah LT, Arabloo J, Ärnlöv J, Artanti KD, Ataro Z, Ausloos M, Avila-Burgos L, Awan AT, Awoke MA, Ayele HT, Ayza MA, Azari S, B DB, Baheiraei N, Baig AA, Bakhtiari A, Banach M, Banik PC, Baptista EA, Barboza MA, Barua L, Basu S, Bedi N, Béjot Y, Bennett DA, Bensenor IM, Berman AE, Bezabih YM, Bhagavathula AS, Bhaskar S, Bhattacharyya K, Bijani A, Bikbov B, Birhanu MM, Boloor A, Brant LC, Brenner H, Briko NI, Butt ZA, Caetano dos Santos FL, Cahill LE, Cahuana-Hurtado L, Cámera LA, Campos-Nonato IR, Cantu-Brito C, Car J, Carrero JJ, Carvalho F, Castañeda-Orjuela CA, Catalá-López F, Cerin E, Charan J, Chattu VK, Chen S, Chin KL, Choi J-YJ, Chu D-T, Chung S-C, Cirillo M, Coffey S, Conti S, Costa VM, Cundiff DK, Dadras O, Dagnew B, Dai X, Damasceno AAM, Dandona L, Dandona R, Davletov K, De la Cruz-Góngora V, De la Hoz FP, De Neve J-W, Denova-Gutiérrez E, Derbew Molla M, Derseh BT, Desai R, Deuschl G, Dharmaratne SD, Dhimal M, Dhungana RR, Dianatinasab M, Diaz D, Djalalinia S, Dokova K, Douiri A, Duncan RB, Duraes AR, Eagan AW, Ebtehaj S, Eftekhari A, Eftekharzadeh S, Ekholuenetale M, El Nahas N, Elgendy IY, Elhadi M, El-Jaafary SI, Esteghamati S, Etisso AE, Eyawo O, Fadhil I, Faraon EJA, Faris PS, Farwati M, Farzadfar F, Fernandes E, Fernandez Prendes C, Ferrara P, Filip I, Fischer F, Flood D, Fukumoto T, Gad MM, Gaidhane S, Ganji M, Garg J, Gebre AK, Gebregiorgis BG, Gebregzabiher KZ, Gebremeskel GG, Getacher L, Obsa AG, Ghajar A, Ghashghaee A, Ghith N, Giampaoli S, Gilani SA, Gill PS, Gillum RF, Glushkova EV, Gnedovskaya EV, Golechha M, Gonfa KB, Goudarzian AH, Goulart AC, Guadamuz JS, Guha A, Guo Y, Gupta R, Hachinski V, Hafezi-Nejad N, Haile TG, Hamadeh RR, Hamidi S, Hankey GJ, Hargono A, Hartono RK, Hashemian M, Hashi A, Hassan S, Hassen HY, Havmoeller RJ, Hay SI, Hayat K, Heidari G, Herteliu C, Holla R, Hosseini M, Hosseinzadeh M, Hostiuc M, Hostiuc S, Househ M, Huang J, Humayun A, Iavicoli I, Ibeneme CU, Ibitoye SE, Ilesanmi OS, Ilic IM, Ilic MD, Iqbal U, Irvani SSN, Islam SMS, Islam RM, Iso H, Iwagami M, Jain V, Javaheri T, Jayapal SK, Jayaram S, Jayawardena R, Jeemon P, Jha RP, Jonas JB, Jonnagaddala J, Joukar F, Jozwiak JJ, Jürisson M, Kabir A, Kahlon T, Kalani R, Kalhor R, Kamath A, Kamel I, Kandel H, Kandel A, Karch A, Kasa AS, Katoto PDMC, Kayode GA, Khader YS, Khammarnia M, Khan MS, Khan MN, Khan M, Khan EA, Khatab K, Kibria GMA, Kim YJ, Kim GR, Kimokoti RW, Kisa S, Kisa A, Kivimäki M, Kolte D, Koolivand A, Korshunov VA, Koulmane Laxminarayana SL, Koyanagi A, Krishan K, Krishnamoorthy V, Kuate Defo B, Kucuk Bicer B, Kulkarni V, Kumar GA, Kumar N, Kurmi OP, Kusuma D, Kwan GF, La Vecchia C, Lacey B, Lallukka T, Lan Q, Lasrado S, Lassi ZS, Lauriola P, Lawrence WR, Laxmaiah A, LeGrand KE, Li M-C, Li B, Li S, Lim SS, Lim L-L, Lin H, Lin Z, Lin R-T, Liu X, Lopez AD, Lorkowski S, Lotufo PA, Lugo A, M NK, Madotto F, Mahmoudi M, Majeed A, Malekzadeh R, Malik AA, Mamun AA, Manafi N, Mansournia MA, Mantovani LG, Martini S, Mathur MR, Mazzaglia G, Mehata S, Mehndiratta MM, Meier T, Menezes RG, Meretoja A, Mestrovic T, Miazgowski B, Miazgowski T, Michalek IM, Miller TR, Mirrakhimov EM, Mirzaei H, Moazen B, Moghadaszadeh M, Mohammad Y, Mohammad DK, Mohammed S, Mohammed MA, Mokhayeri Y, Molokhia M, Montasir AA, Moradi G, Moradzadeh R, Moraga P, Morawska L, Moreno Velásquez I, Morze J, Mubarik S, Muruet W, Musa KI, Nagarajan AJ, Nalini M, Nangia V, Naqvi AA, Narasimha Swamy S, Nascimento BR, Nayak VC, Nazari J, Nazarzadeh M, Negoi RI, Neupane Kandel S, Nguyen HLT, Nixon MR, Norrving B, Noubiap JJ, Nouthe BE, Nowak C, Odukoya OO, Ogbo FA, Olagunju AT, Orru H, Ortiz A, Ostroff SM, Padubidri JR, Palladino R, Pana A, Panda-Jonas S, Parekh U, Park E-C, Parvizi M, Pashazadeh Kan F, Patel UK, Pathak M, Paudel R, Pepito VCF, Perianayagam A, Perico N, Pham HQ, Pilgrim T, Piradov MA, Pishgar F, Podder V, Polibin RV, Pourshams A, Pribadi DRA, Rabiee N, Rabiee M, Radfar A, Rafiei A, Rahim F, Rahimi-Movaghar V, Ur Rahman MH, Rahman MA, Rahmani AM, Rakovac I, Ram P, Ramalingam S, Rana J, Ranasinghe P, Rao SJ, Rathi P, Rawal L, Rawasia WF, Rawassizadeh R, Remuzzi G, Renzaho AMN, Rezapour A, Riahi SM, Roberts-Thomson RL, Roever L, Rohloff P, Romoli M, Roshandel G, Rwegerera GM, Saadatagah S, Saber-Ayad MM, Sabour S, Sacco S, Sadeghi M, Saeedi Moghaddam S, Safari S, Sahebkar A, Salehi S, Salimzadeh H, Samaei M, Samy AM, Santos IS, Santric-Milicevic MM, Sarrafzadegan N, Sarveazad A, Sathish T, Sawhney M, Saylan M, Schmidt MI, Schutte AE, Senthilkumaran S, Sepanlou SG, Sha F, Shahabi S, Shahid I, Shaikh MA, Shamali M, Shamsizadeh M, Shawon MSR, Sheikh A, Shigematsu M, Shin M-J, Shin JI, Shiri R, Shiue I, Shuval K, Siabani S, Siddiqi TJ, Silva DAS, Singh JA, Mtech AS, Skryabin VY, Skryabina AA, Soheili A, Spurlock EE, Stockfelt L, Stortecky S, Stranges S, Suliankatchi Abdulkader R, Tadbiri H, Tadesse EG, Tadesse DB, Tajdini M, Tariqujjaman M, Teklehaimanot BF, Temsah M-H, Tesema AK, Thakur B, Thankappan KR, Thapar R, Thrift AG, Timalsina B, Tonelli M, Touvier M, Tovani-Palone MR, Tripathi A, Tripathy JP, Truelsen TC, Tsegay GM, Tsegaye GW, Tsilimparis N, Tusa BS, Tyrovolas S, Umapathi KK, Unim B, Unnikrishnan B, Usman MS, Vaduganathan M, Valdez PR, Vasankari TJ, Velazquez DZ, Venketasubramanian N, Vu GT, Vujcic IS, Waheed Y, Wang Y, Wang F, Wei J, Weintraub RG, Weldemariam AH, Westerman R, Winkler AS, Wiysonge CS, Wolfe CDA, Wubishet BL, Xu G, Yadollahpour A, Yamagishi K, Yan LL, Yandrapalli S, Yano Y, Yatsuya H, Yeheyis TY, Yeshaw Y, Yilgwan CS, Yonemoto N, Yu C, Yusefzadeh H, Zachariah G, Zaman SB, Zaman MS, Zamanian M, Zand R, Zandifar A, Zarghi A, Zastrozhin MS, Zastrozhina A, Zhang Z-J, Zhang Y, Zhang W, Zhong C, Zou Z, Zuniga YMH, Murray CJL, Fuster V. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol 2020;76:2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 3. Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol 2004;15:1983–1992. [DOI] [PubMed] [Google Scholar]
- 4. Tousoulis D, Simopoulou C, Papageorgiou N, Oikonomou E, Hatzis G, Siasos G, Tsiamis E, Stefanadis C. Endothelial dysfunction in conduit arteries and in microcirculation. Novel therapeutic approaches. Pharmacol Ther 2014;144:253–267. [DOI] [PubMed] [Google Scholar]
- 5. Read C, Nyimanu D, Williams TL, Huggins DJ, Sulentic P, Macrae RGC, Yang P, Glen RC, Maguire JJ, Davenport AP. International Union of Basic and Clinical Pharmacology. CVII. Structure and pharmacology of the apelin receptor with a recommendation that elabela/toddler is a second endogenous peptide ligand. Pharmacol Rev 2019;71:467–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Girault-Sotias PE, Gerbier R, Flahault A, de Mota N, Llorens-Cortes C. Apelin and vasopressin: the yin and yang of water balance. Front Endocrinol (Lausanne) 2021;12:735515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palmer ES, Irwin N, O'Harte FP. Potential therapeutic role for apelin and related peptides in diabetes: an update. Clin Med Insights Endocrinol Diabetes 2022;15:11795514221074679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li C, Cheng H, Adhikari BK, Wang S, Yang N, Liu W, Sun J, Wang Y. The role of apelin-APJ system in diabetes and obesity. Front Endocrinol (Lausanne) 2022;13:820002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Castan-Laurell I, Dray C, Valet P. The therapeutic potentials of apelin in obesity-associated diseases. Mol Cell Endocrinol 2021;529:111278. [DOI] [PubMed] [Google Scholar]
- 10. Marsault E, Llorens-Cortes C, Iturrioz X, Chun HJ, Lesur O, Oudit GY, Auger-Messier M. The apelinergic system: a perspective on challenges and opportunities in cardiovascular and metabolic disorders. Ann N Y Acad Sci 2019;1455:12–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Oliveira AA, Vergara A, Wang X, Vederas JC, Oudit GY. Apelin pathway in cardiovascular, kidney, and metabolic diseases: therapeutic role of apelin analogs and apelin receptor agonists. Peptides 2022;147:170697. [DOI] [PubMed] [Google Scholar]
- 12. Narayanan S, Harris DL, Maitra R, Runyon SP. Regulation of the apelinergic system and its potential in cardiovascular disease: peptides and small molecules as tools for discovery. J Med Chem 2015;58:7913–7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fischer C. A patent review of apelin receptor (APJR) modulators (2014–2019). Expert Opin Ther Pat 2020;30:251–261. [DOI] [PubMed] [Google Scholar]
- 14. Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun 1998;251:471–476. [DOI] [PubMed] [Google Scholar]
- 15. Zhen EY, Higgs RE, Gutierrez JA. Pyroglutamyl apelin-13 identified as the major apelin isoform in human plasma. Anal Biochem 2013;442:1–9. [DOI] [PubMed] [Google Scholar]
- 16. Azizi M, Iturrioz X, Blanchard A, Peyrard S, De Mota N, Chartrel N, Vaudry H, Corvol P, Llorens-Cortes C. Reciprocal regulation of plasma apelin and vasopressin by osmotic stimuli. J Am Soc Nephrol 2008;19:1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Japp AG, Cruden NL, Amer DA, Li VK, Goudie EB, Johnston NR, Sharma S, Neilson I, Webb DJ, Megson IL, Flapan AD, Newby DE. Vascular effects of apelin in vivo in man. J Am Coll Cardiol 2008;52:908–913. [DOI] [PubMed] [Google Scholar]
- 18. Wang W, Shen M, Fischer C, Basu R, Hazra S, Couvineau P, Paul M, Wang F, Toth S, Mix DS, Poglitsch M, Gerard NP, Bouvier M, Vederas JC, Penninger JM, Kassiri Z, Oudit GY. Apelin protects against abdominal aortic aneurysm and the therapeutic role of neutral endopeptidase resistant apelin analogs. Proc Natl Acad Sci U S A 2019;116:13006–13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McKinnie SM, Fischer C, Tran KM, Wang W, Mosquera F, Oudit GY, Vederas JC. The metalloprotease neprilysin degrades and inactivates apelin peptides. Chembiochem 2016;17:1495–1498. [DOI] [PubMed] [Google Scholar]
- 20. Fischer C, Lamer T, Wang W, McKinnie SMK, Iturrioz X, Llorens-Cortes C, Oudit GY, Vederas JC. Plasma kallikrein cleaves and inactivates apelin-17: palmitoyl- and PEG-extended apelin-17 analogs as metabolically stable blood pressure-lowering agents. Eur J Med Chem 2019;166:119–124. [DOI] [PubMed] [Google Scholar]
- 21. Murza A, Belleville K, Longpre JM, Sarret P, Marsault E. Stability and degradation patterns of chemically modified analogs of apelin-13 in plasma and cerebrospinal fluid. Biopolymers 2014;102:297–303. [DOI] [PubMed] [Google Scholar]
- 22. Wang W, McKinnie SM, Farhan M, Paul M, McDonald T, McLean B, Llorens-Cortes C, Hazra S, Murray AG, Vederas JC, Oudit GY. Angiotensin-converting enzyme 2 metabolizes and partially inactivates pyr-apelin-13 and apelin-17: physiological effects in the cardiovascular system. Hypertension 2016;68:365–377. [DOI] [PubMed] [Google Scholar]
- 23. Kleinz MJ, Davenport AP. Immunocytochemical localization of the endogenous vasoactive peptide apelin to human vascular and endocardial endothelial cells. Regul Pept 2004;118:119–125. [DOI] [PubMed] [Google Scholar]
- 24. Reaux-Le Goazigo A, Morinville A, Burlet A, Llorens-Cortes C, Beaudet A. Dehydration-induced cross-regulation of apelin and vasopressin immunoreactivity levels in magnocellular hypothalamic neurons. Endocrinology 2004;145:4392–4400. [DOI] [PubMed] [Google Scholar]
- 25. Pauli A, Norris ML, Valen E, Chew GL, Gagnon JA, Zimmerman S, Mitchell A, Ma J, Dubrulle J, Reyon D, Tsai SQ, Joung JK, Saghatelian A, Schier AF. Toddler: an embryonic signal that promotes cell movement via Apelin receptors. Science 2014;343:1248636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chng SC, Ho L, Tian J, Reversade B. ELABELA: a hormone essential for heart development signals via the apelin receptor. Dev Cell 2013;27:672–680. [DOI] [PubMed] [Google Scholar]
- 27. Charo DN, Ho M, Fajardo G, Kawana M, Kundu RK, Sheikh AY, Finsterbach TP, Leeper NJ, Ernst KV, Chen MM, Ho YD, Chun HJ, Bernstein D, Ashley EA, Quertermous T. Endogenous regulation of cardiovascular function by apelin-APJ. Am J Physiol Heart Circ Physiol 2009;297:H1904–H1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kang Y, Kim J, Anderson JP, Wu J, Gleim SR, Kundu RK, McLean DL, Kim JD, Park H, Jin SW, Hwa J, Quertermous T, Chun HJ. Apelin-APJ signaling is a critical regulator of endothelial MEF2 activation in cardiovascular development. Circ Res 2013;113:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Freyer L, Hsu CW, Nowotschin S, Pauli A, Ishida J, Kuba K, Fukamizu A, Schier AF, Hoodless PA, Dickinson ME, Hadjantonakis A-K. Loss of apela peptide in mice causes low penetrance embryonic lethality and defects in early mesodermal derivatives. Cell Rep 2017;20:2116–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang P, Read C, Kuc RE, Buonincontri G, Southwood M, Torella R, Upton PD, Crosby A, Sawiak SJ, Carpenter TA, Glen RC, Morrell NW, Maguire JJ, Davenport AP. Elabela/toddler is an endogenous agonist of the apelin APJ receptor in the adult cardiovascular system, and exogenous administration of the peptide compensates for the downregulation of its expression in pulmonary arterial hypertension. Circulation 2017;135:1160–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang Z, Yu D, Wang M, Wang Q, Kouznetsova J, Yang R, Qian K, Wu W, Shuldiner A, Sztalryd C, Zou M, Zheng W, Gong D-W. Elabela-apelin receptor signaling pathway is functional in mammalian systems. Sci Rep 2015;5:8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nyimanu D, Kay RG, Kuc RE, Brown AJH, Gribble FM, Maguire JJ, Davenport AP. In vitro metabolism of synthetic Elabela/Toddler (ELA-32) peptide in human plasma and kidney homogenates analyzed with mass spectrometry and validation of endogenous peptide quantification in tissues by ELISA. Peptides 2021;145:170642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Couvineau P, Llorens-Cortes C, Iturrioz X. Elabela/Toddler and apelin bind differently to the apelin receptor. Faseb j 2020;34:7989–8000. [DOI] [PubMed] [Google Scholar]
- 34. Murza A, Sainsily X, Coquerel D, Cote J, Marx P, Besserer-Offroy E, Longpre JM, Laine J, Reversade B, Salvail D, Leduc R, Dumaine R, Lesur O, Auger-Messier M, Sarret P, Marsault É. Discovery and structure-activity relationship of a bioactive fragment of ELABELA that modulates vascular and cardiac functions. J Med Chem 2016;59:2962–2972. [DOI] [PubMed] [Google Scholar]
- 35. Soulet F, Bodineau C, Hooks KB, Descarpentrie J, Alves I, Dubreuil M, Mouchard A, Eugenie M, Hoepffner JL, Lopez JJ, Rosado JA, Soubeyran I, Tomé M, Durán RV, Nikolski M, Villoutreix BO, Evrard S, Siegfried G, Khatib A-M. ELA/APELA precursor cleaved by furin displays tumor suppressor function in renal cell carcinoma through mTORC1 activation. JCI Insight 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O’Dowd BF, Heiber M, Chan A, Heng HHQ, Tsui LC, Kennedy JL, Shi X, Petronis A, George SR, Nguyen T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 1993;136:355–360. [DOI] [PubMed] [Google Scholar]
- 37. Ishida J, Hashimoto T, Hashimoto Y, Nishiwaki S, Iguchi T, Harada S, Sugaya T, Matsuzaki H, Yamamoto R, Shiota N, Okunishi H, Kihara M, Umemura S, Sugiyama F, Yagami K-i, Kasuya Y, Mochizuki N, Fukamizu A. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J Biol Chem 2004;279:26274–26279. [DOI] [PubMed] [Google Scholar]
- 38. Iwanaga Y, Kihara Y, Takenaka H, Kita T. Down-regulation of cardiac apelin system in hypertrophied and failing hearts: possible role of angiotensin II-angiotensin type 1 receptor system. J Mol Cell Cardiol 2006;41:798–806. [DOI] [PubMed] [Google Scholar]
- 39. Barnes GD, Alam S, Carter G, Pedersen CM, Lee KM, Hubbard TJ, Veitch S, Jeong H, White A, Cruden NL, Huson L, Japp AG, Newby DE. Sustained cardiovascular actions of APJ agonism during renin-angiotensin system activation and in patients with heart failure. Circ Heart Fail 2013;6:482–491. [DOI] [PubMed] [Google Scholar]
- 40. Siddiquee K, Hampton J, Khan S, Zadory D, Gleaves L, Vaughan DE, Smith LH. Apelin protects against angiotensin II-induced cardiovascular fibrosis and decreases plasminogen activator inhibitor type-1 production. J Hypertens 2011;29:724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sato T, Sato C, Kadowaki A, Watanabe H, Ho L, Ishida J, Yamaguchi T, Kimura A, Fukamizu A, Penninger JM, Reversade B, Ito H, Imai Y, Kuba K. ELABELA-APJ axis protects from pressure overload heart failure and angiotensin II-induced cardiac damage. Cardiovasc Res 2017;113:760–769. [DOI] [PubMed] [Google Scholar]
- 42. Zhang ZZ, Wang W, Jin HY, Chen X, Cheng YW, Xu YL, Song B, Penninger JM, Oudit GY, Zhong JC. Apelin is a negative regulator of angiotensin II-mediated adverse myocardial remodeling and dysfunction. Hypertension 2017;70:1165–1175. [DOI] [PubMed] [Google Scholar]
- 43. Chun HJ, Ali ZA, Kojima Y, Kundu RK, Sheikh AY, Agrawal R, Zheng L, Leeper NJ, Pearl NE, Patterson AJ, Anderson JP, Tsao PS, Lenardo MJ, Ashley EA, Quertermous T. Apelin signaling antagonizes Ang II effects in mouse models of atherosclerosis. J Clin Invest 2008;118:3343–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. O'Carroll AM, Lolait SJ, Harris LE, Pope GR. The apelin receptor APJ: journey from an orphan to a multifaceted regulator of homeostasis. J Endocrinol 2013;219:R13–R35. [DOI] [PubMed] [Google Scholar]
- 45. Perjes A, Kilpio T, Ulvila J, Magga J, Alakoski T, Szabo Z, Vainio L, Halmetoja E, Vuolteenaho O, Petaja-Repo U, Szokodi I, Kerkelä R. Characterization of apela, a novel endogenous ligand of apelin receptor, in the adult heart. Basic Res Cardiol 2016;111:2. [DOI] [PubMed] [Google Scholar]
- 46. Szokodi I, Tavi P, Foldes G, Voutilainen-Myllyla S, Ilves M, Tokola H, Pikkarainen S, Piuhola J, Rysa J, Toth M, Ruskoaho H. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ Res 2002;91:434–440. [DOI] [PubMed] [Google Scholar]
- 47. Perjes A, Skoumal R, Tenhunen O, Konyi A, Simon M, Horvath IG, Kerkela R, Ruskoaho H, Szokodi I. Apelin increases cardiac contractility via protein kinase cepsilon- and extracellular signal-regulated kinase-dependent mechanisms. PLoS One 2014;9:e93473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhou N, Fan X, Mukhtar M, Fang J, Patel CA, DuBois GC, Pomerantz RJ. Cell–cell fusion and internalization of the CNS-based, HIV-1 co-receptor, APJ. Virology 2003;307:22–36. [DOI] [PubMed] [Google Scholar]
- 49. Lee DK, Ferguson SS, George SR, O'Dowd BF. The fate of the internalized apelin receptor is determined by different isoforms of apelin mediating differential interaction with beta-arrestin. Biochem Biophys Res Commun 2010;395:185–189. [DOI] [PubMed] [Google Scholar]
- 50. Scimia MC, Hurtado C, Ray S, Metzler S, Wei K, Wang J, Woods CE, Purcell NH, Catalucci D, Akasaka T, Bueno OF, Vlasuk GP, Kaliman P, Bodmer R, Smith LH, Ashley E, Mercola M, Brown JH, Ruiz-Lozano P. APJ acts as a dual receptor in cardiac hypertrophy. Nature 2012;488:394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ashley E, Chun HJ, Quertermous T. Opposing cardiovascular roles for the angiotensin and apelin signaling pathways. J Mol Cell Cardiol 2006;41:778–781. [DOI] [PubMed] [Google Scholar]
- 52. Hus-Citharel A, Bouby N, Frugiere A, Bodineau L, Gasc JM, Llorens-Cortes C. Effect of apelin on glomerular hemodynamic function in the rat kidney. Kidney Int 2008;74:486–494. [DOI] [PubMed] [Google Scholar]
- 53. Sato T, Kadowaki A, Suzuki T, Ito H, Watanabe H, Imai Y, Kuba K. Loss of apelin augments angiotensin II-induced cardiac dysfunction and pathological remodeling. Int J Mol Sci 2019;20:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Patel VB, Zhong JC, Fan D, Basu R, Morton JS, Parajuli N, McMurtry MS, Davidge ST, Kassiri Z, Oudit GY. Angiotensin-converting enzyme 2 is a critical determinant of angiotensin II-induced loss of vascular smooth muscle cells and adverse vascular remodeling. Hypertension 2014;64:157–164. [DOI] [PubMed] [Google Scholar]
- 55. Zhong J, Guo D, Chen CB, Wang W, Schuster M, Loibner H, Penninger JM, Scholey JW, Kassiri Z, Oudit GY. Prevention of angiotensin II-mediated renal oxidative stress, inflammation, and fibrosis by angiotensin-converting enzyme 2. Hypertension 2011;57:314–322. [DOI] [PubMed] [Google Scholar]
- 56. Sato T, Suzuki T, Watanabe H, Kadowaki A, Fukamizu A, Liu PP, Kimura A, Ito H, Penninger JM, Imai Y, Kuba K. Apelin is a positive regulator of ACE2 in failing hearts. J Clin Invest 2013;123:5203–5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension 2018;71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 58. Muntner P, Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB, Colantonio LD. Trends in blood pressure control among US adults with hypertension, 1999–2000 to 2017–2018. JAMA 2020;324:1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Maguire JJ, Kleinz MJ, Pitkin SL, Davenport AP. [Pyr1]apelin-13 identified as the predominant apelin isoform in the human heart: vasoactive mechanisms and inotropic action in disease. Hypertension 2009;54:598–604. [DOI] [PubMed] [Google Scholar]
- 60. Salcedo A, Garijo J, Monge L, Fernandez N, Luis Garcia-Villalon A, Sanchez Turrion V, Cuervas-Mons V, Dieguez G. Apelin effects in human splanchnic arteries. Role of nitric oxide and prostanoids. Regul Pept 2007;144:50–55. [DOI] [PubMed] [Google Scholar]
- 61. Rikitake Y. The apelin/APJ system in the regulation of vascular tone: friend or foe? J Biochem 2020;169:383–386. [DOI] [PubMed] [Google Scholar]
- 62. Najafipour H, Vakili A, Shahouzehi B, Soltani Hekmat A, Masoomi Y, Yeganeh Hajahmadi M, Esmaeli-Mahani S. Investigation of changes in apelin receptor mRNA and protein expression in the myocardium and aorta of rats with two-kidney, one-clip (2K1C) Goldblatt hypertension. J Physiol Biochem 2015;71:165–175. [DOI] [PubMed] [Google Scholar]
- 63. Najafipour H, Soltani Hekmat A, Nekooian AA, Esmaeili-Mahani S. Apelin receptor expression in ischemic and non- ischemic kidneys and cardiovascular responses to apelin in chronic two-kidney-one-clip hypertension in rats. Regul Pept 2012;178:43–50. [DOI] [PubMed] [Google Scholar]
- 64. Chen Z, Wu C, Liu Y, Li H, Zhu Y, Huang C, Lin H, Qiao Q, Huang M, Zhu Q, Wang L. ELABELA attenuates deoxycorticosterone acetate/salt-induced hypertension and renal injury by inhibition of NADPH oxidase/ROS/NLRP3 inflammasome pathway. Cell Death Dis 2020;11:698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schreiber CA, Holditch SJ, Generous A, Ikeda Y. Sustained ELABELA gene therapy in high-salt diet-induced hypertensive rats. Curr Gene Ther 2016;16:349–360. [DOI] [PubMed] [Google Scholar]
- 66. Flahault A, Keck M, Girault-Sotias PE, Esteoulle L, De Mota N, Bonnet D, Llorens-Cortes C. LIT01-196, a metabolically stable apelin-17 analog, normalizes blood pressure in hypertensive DOCA-salt rats via a NO synthase-dependent mechanism. Front Pharmacol 2021;12:715095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Japp AG, Cruden NL, Barnes G, van Gemeren N, Mathews J, Adamson J, Johnston NR, Denvir MA, Megson IL, Flapan AD, Newby DE. Acute cardiovascular effects of apelin in humans: potential role in patients with chronic heart failure. Circulation 2010;121:1818–1827. [DOI] [PubMed] [Google Scholar]
- 68. Gupta MD, Girish MP, Shah D, Rain M, Mehta V, Tyagi S, Trehan V, Pasha Q. Biochemical and genetic role of apelin in essential hypertension and acute coronary syndrome. Int J Cardiol 2016;223:374–378. [DOI] [PubMed] [Google Scholar]
- 69. Sonmez A, Celebi G, Erdem G, Tapan S, Genc H, Tasci I, Ercin CN, Dogru T, Kilic S, Uckaya G, Yilmaz MI, Erbil MK, Kutlu M. Plasma apelin and ADMA levels in patients with essential hypertension. Clin Exp Hypertens 2010;32:179–183. [DOI] [PubMed] [Google Scholar]
- 70. Przewlocka-Kosmala M, Kotwica T, Mysiak A, Kosmala W. Reduced circulating apelin in essential hypertension and its association with cardiac dysfunction. J Hypertens 2011;29:971–979. [DOI] [PubMed] [Google Scholar]
- 71. Brame AL, Maguire JJ, Yang P, Dyson A, Torella R, Cheriyan J, Singer M, Glen RC, Wilkinson IB, Davenport AP. Design, characterization, and first-in-human study of the vascular actions of a novel biased apelin receptor agonist. Hypertension 2015;65:834–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Brash L, Barnes GD, Brewis MJ, Church AC, Gibbs SJ, Howard L, Jayasekera G, Johnson MK, McGlinchey N, Onorato J, Simpson J, Stirrat C, Thomson S, Watson G, Wilkins MR, Xu C, Welsh DJ, Newby DE, Peacock AJ. Short-term hemodynamic effects of apelin in patients with pulmonary arterial hypertension. JACC Basic Transl Sci 2018;3:176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. He L, Zhou Q, Huang Z, Xu J, Zhou H, Lv D, Lu L, Huang S, Tang M, Zhong J, Chen J, Luo X, Li L, Chen L. PINK1/Parkin-mediated mitophagy promotes apelin-13-induced vascular smooth muscle cell proliferation by AMPKalpha and exacerbates atherosclerotic lesions. J Cell Physiol 2019;234:8668–8682. [DOI] [PubMed] [Google Scholar]
- 74. Hashimoto T, Kihara M, Imai N, Yoshida S, Shimoyamada H, Yasuzaki H, Ishida J, Toya Y, Kiuchi Y, Hirawa N, Tamura K, Yazawa T, Kitamura H, Fukamizu A, Umemura S. Requirement of apelin-apelin receptor system for oxidative stress-linked atherosclerosis. Am J Pathol 2007;171:1705–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fraga-Silva RA, Seeman H, Montecucco F, da Silva AR, Burger F, Costa-Fraga FP, Anguenot L, Mach F, Dos Santos RAS, Stergiopulos N, da Silva RF. Apelin-13 treatment enhances the stability of atherosclerotic plaques. Eur J Clin Invest 2018;48. [DOI] [PubMed] [Google Scholar]
- 76. Pitkin SL, Maguire JJ, Kuc RE, Davenport AP. Modulation of the apelin/APJ system in heart failure and atherosclerosis in man. Br J Pharmacol 2010;160:1785–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kadoglou NP, Lampropoulos S, Kapelouzou A, Gkontopoulos A, Theofilogiannakos EK, Fotiadis G, Kottas G. Serum levels of apelin and ghrelin in patients with acute coronary syndromes and established coronary artery disease—KOZANI STUDY. Transl Res 2010;155:238–246. [DOI] [PubMed] [Google Scholar]
- 78. Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res 2017;120:229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sen-Banerjee S, Mir S, Lin Z, Hamik A, Atkins GB, Das H, Banerjee P, Kumar A, Jain MK. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation 2005;112:720–726. [DOI] [PubMed] [Google Scholar]
- 80. McLean DL, Kim J, Kang Y, Shi H, Atkins GB, Jain MK, Chun HJ. Apelin/APJ signaling is a critical regulator of statin effects in vascular endothelial cells—brief report. Arterioscler Thromb Vasc Biol 2012;32:2640–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kadoglou NP, Sailer N, Kapelouzou A, Lampropoulos S, Vitta I, Kostakis A, Liapis CD. Effects of atorvastatin on apelin, visfatin (nampt), ghrelin and early carotid atherosclerosis in patients with type 2 diabetes. Acta Diabetol 2012;49:269–276. [DOI] [PubMed] [Google Scholar]
- 82. Adam F, Khatib AM, Lopez JJ, Vatier C, Turpin S, Muscat A, Soulet F, Aries A, Jardin I, Bobe R, Stepanian A, de Prost D, Dray C, Rosado JA, Valet P, Feve B, Siegfried G. Apelin: an antithrombotic factor that inhibits platelet function. Blood 2016;127:908–920. [DOI] [PubMed] [Google Scholar]
- 83. Feener EP, Northrup JM, Aiello LP, King GL. Angiotensin II induces plasminogen activator inhibitor-1 and -2 expression in vascular endothelial and smooth muscle cells. J Clin Invest 1995;95:1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kasai A, Ishimaru Y, Kinjo T, Satooka T, Matsumoto N, Yoshioka Y, Yamamuro A, Gomi F, Shintani N, Baba A, Maeda S. Apelin is a crucial factor for hypoxia-induced retinal angiogenesis. Arterioscler Thromb Vasc Biol 2010;30:2182–2187. [DOI] [PubMed] [Google Scholar]
- 85. Sharma B, Ho L, Ford GH, Chen HI, Goldstone AB, Woo YJ, Quertermous T, Reversade B, Red-Horse K. Alternative progenitor cells compensate to rebuild the coronary vasculature in elabela- and Apj-deficient hearts. Dev Cell 2017;42:655–666.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zeng XJ, Zhang LK, Wang HX, Lu LQ, Ma LQ, Tang CS. Apelin protects heart against ischemia/reperfusion injury in rat. Peptides 2009;30:1144–1152. [DOI] [PubMed] [Google Scholar]
- 87. Eyries M, Siegfried G, Ciumas M, Montagne K, Agrapart M, Lebrin F, Soubrier F. Hypoxia-induced apelin expression regulates endothelial cell proliferation and regenerative angiogenesis. Circ Res 2008;103:432–440. [DOI] [PubMed] [Google Scholar]
- 88. Ronkainen VP, Ronkainen JJ, Hanninen SL, Leskinen H, Ruas JL, Pereira T, Poellinger L, Vuolteenaho O, Tavi P. Hypoxia inducible factor regulates the cardiac expression and secretion of apelin. FASEB J 2007;21:1821–1830. [DOI] [PubMed] [Google Scholar]
- 89. Wang W, McKinnie SM, Patel VB, Haddad G, Wang Z, Zhabyeyev P, Das SK, Basu R, McLean B, Kandalam V, Penninger JM, Kassiri Z, Vederas JC, Murray AG, Oudit GY. Loss of Apelin exacerbates myocardial infarction adverse remodeling and ischemia-reperfusion injury: therapeutic potential of synthetic Apelin analogues. J Am Heart Assoc 2013;2:e000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pan Y, Li Q, Yan H, Huang J, Wang Z. Apela improves cardiac and renal function in mice with acute myocardial infarction. J Cell Mol Med 2020;24:10382–10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Simpkin JC, Yellon DM, Davidson SM, Lim SY, Wynne AM, Smith CC. Apelin-13 and apelin-36 exhibit direct cardioprotective activity against ischemia-reperfusion injury. Basic Res Cardiol 2007;102:518–528. [DOI] [PubMed] [Google Scholar]
- 92. Azizi Y, Faghihi M, Imani A, Roghani M, Nazari A. Post-infarct treatment with [Pyr1]-apelin-13 reduces myocardial damage through reduction of oxidative injury and nitric oxide enhancement in the rat model of myocardial infarction. Peptides 2013;46:76–82. [DOI] [PubMed] [Google Scholar]
- 93. Yu P, Ma S, Dai X, Cao F. Elabela alleviates myocardial ischemia reperfusion-induced apoptosis, fibrosis and mitochondrial dysfunctino through PI3K/AKT signalling. Am J Transl Res 2020;12:4467–4477. [PMC free article] [PubMed] [Google Scholar]
- 94. Abbasloo E, Najafipour H, Vakili A. Chronic treatment with apelin, losartan and their combination reduces myocardial infarct size and improves cardiac mechanical function. Clin Exp Pharmacol Physiol 2020;47:393–402. [DOI] [PubMed] [Google Scholar]
- 95. Roger VL. Epidemiology of heart failure: a contemporary perspective. Circ Res 2021;128:1421–1434. [DOI] [PubMed] [Google Scholar]
- 96. Kuba K, Zhang L, Imai Y, Arab S, Chen M, Maekawa Y, Leschnik M, Leibbrandt A, Markovic M, Schwaighofer J, Beetz N, Musialek R, Neely GG, Komnenovic V, Kolm U, Metzler B, Ricci R, Hara H, Meixner A, Nghiem M, Chen X, Dawood F, Wong KM, Sarao R, Cukerman E, Kimura A, Hein L, Thalhammer J, Liu PP, Penninger JM. Impaired heart contractility in Apelin gene-deficient mice associated with aging and pressure overload. Circ Res 2007;101:e32–e42. [DOI] [PubMed] [Google Scholar]
- 97. Psotka MA, Gottlieb SS, Francis GS, Allen LA, Teerlink JR, Adams KF Jr, Rosano GMC, Lancellotti P. Cardiac calcitropes, myotropes, and mitotropes: JACC review topic of the week. J Am Coll Cardiol 2019;73:2345–2353. [DOI] [PubMed] [Google Scholar]
- 98. Wang C, Du JF, Wu F, Wang HC. Apelin decreases the SR Ca2+ content but enhances the amplitude of [Ca2+]i transient and contractions during twitches in isolated rat cardiac myocytes. Am J Physiol Heart Circ Physiol 2008;294:H2540–H2546. [DOI] [PubMed] [Google Scholar]
- 99. Farkasfalvi K, Stagg MA, Coppen SR, Siedlecka U, Lee J, Soppa GK, Marczin N, Szokodi I, Yacoub MH, Terracciano CM. Direct effects of apelin on cardiomyocyte contractility and electrophysiology. Biochem Biophys Res Commun 2007;357:889–895. [DOI] [PubMed] [Google Scholar]
- 100. Parikh VN, Liu J, Shang C, Woods C, Chang AC, Zhao M, Charo DN, Grunwald Z, Huang Y, Seo K, Tsao PS, Bernstein D, Ruiz-Lozano P, Quertermous T, Ashley EA. Apelin and APJ orchestrate complex tissue-specific control of cardiomyocyte hypertrophy and contractility in the hypertrophy-heart failure transition. Am J Physiol Heart Circ Physiol 2018;315:H348–H356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ashley EA, Powers J, Chen M, Kundu R, Finsterbach T, Caffarelli A, Deng A, Eichhorn J, Mahajan R, Agrawal R. The endogenous peptide apelin potently improves cardiac contractility and reduces cardiac loading in vivo. Cardiovasc Res 2005;65:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Japp AG, Newby DE. The apelin-APJ system in heart failure: pathophysiologic relevance and therapeutic potential. Biochem Pharmacol 2008;75:1882–1892. [DOI] [PubMed] [Google Scholar]
- 103. Chen MM, Ashley EA, Deng DX, Tsalenko A, Deng A, Tabibiazar R, Ben-Dor A, Fenster B, Yang E, King JY, Fowler M, Robbins R, Johnson FL, Bruhn L, McDonagh T, Dargie H, Yakhini Z, Tsao PS, Quertermous T. Novel role for the potent endogenous inotrope apelin in human cardiac dysfunction. Circulation 2003;108:1432–1439. [DOI] [PubMed] [Google Scholar]
- 104. Foldes G, Horkay F, Szokodi I, Vuolteenaho O, Ilves M, Lindstedt KA, Mayranpaa M, Sarman B, Seres L, Skoumal R, Lakó-Futó Z, deChâtel R, Ruskoaho H, Tóth M. Circulating and cardiac levels of apelin, the novel ligand of the orphan receptor APJ, in patients with heart failure. Biochem Biophys Res Commun 2003;308:480–485. [DOI] [PubMed] [Google Scholar]
- 105. Francia P, Salvati A, Balla C, De Paolis P, Pagannone E, Borro M, Gentile G, Simmaco M, De Biase L, Volpe M. Cardiac resynchronization therapy increases plasma levels of the endogenous inotrope apelin. Eur J Heart Fail 2007;9:306–309. [DOI] [PubMed] [Google Scholar]
- 106. Berry MF, Pirolli TJ, Jayasankar V, Burdick J, Morine KJ, Gardner TJ, Woo YJ. Apelin has in vivo inotropic effects on normal and failing hearts. Circulation 2004;110:II187–II193. [DOI] [PubMed] [Google Scholar]
- 107. Chagnon F, Coquerel D, Salvail D, Marsault E, Dumaine R, Auger-Messier M, Sarret P, Lesur O. Apelin compared with dobutamine exerts cardioprotection and extends survival in a rat model of endotoxin-induced myocardial dysfunction. Crit Care Med 2017;45:e391–e398. [DOI] [PubMed] [Google Scholar]
- 108. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 109. Masoud AG, Lin J, Azad AK, Farhan MA, Fischer C, Zhu LF, Zhang H, Sis B, Kassiri Z, Moore RB, Kim D, Anderson CC, Vederas JC, Adam BA, Oudit GY, Murray AG. Apelin directs endothelial cell differentiation and vascular repair following immune-mediated injury. J Clin Invest 2020;130:94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Verma SP, Silke B, Reynolds GW, Richmond A, Taylor SH. Modulation of inotropic therapy by venodilation in acute heart failure: a randomised comparison of four inotropic agents, alone and combined with isosorbide dinitrate. J Cardiovasc Pharmacol 1992;19:24–33. [DOI] [PubMed] [Google Scholar]
- 111. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults. National implications for rhythm management and stroke prevention: the AnTicoagulation and Risk factors In Atrial fibrillation (ATRIA) study. JAMA 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 112. Cheng CC, Weerateerangkul P, Lu YY, Chen YC, Lin YK, Chen SA, Chen YJ. Apelin regulates the electrophysiological characteristics of atrial myocytes. Eur J Clin Invest 2013;43:34–40. [DOI] [PubMed] [Google Scholar]
- 113. King JH, Huang CL, Fraser JA. Determinants of myocardial conduction velocity: implications for arrhythmogenesis. Front Physiol 2013;4:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kim YM, Lakin R, Zhang H, Liu J, Sachedina A, Singh M, Wilson E, Perez M, Verma S, Quertermous T, Olgin J, Backx PH, Ashley EA. Apelin increases atrial conduction velocity, refractoriness, and prevents inducibility of atrial fibrillation. JCI Insight 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lv W, Zhang L, Cheng X, Wang H, Qin W, Zhou X, Tang B. Apelin inhibits angiotensin II-induced atrial fibrosis and atrial fibrillation via TGF-beta1/Smad2/alpha-SMA pathway. Front Physiol 2020;11:583570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cheng H, Chen Y, Li X, Chen F, Zhao J, Hu J, Shan A, Qiao S, Wei Z, He G, Xu B. Involvement of apelin/APJ axis in thrombogenesis in valve heart disease patients with atrial fibrillation. Int Heart J 2019;60:145–150. [DOI] [PubMed] [Google Scholar]
- 117. Ellinor PT, Low AF, Macrae CA. Reduced apelin levels in lone atrial fibrillation. Eur Heart J 2006;27:222–226. [DOI] [PubMed] [Google Scholar]
- 118. Ma Z, Zhao L, Zhang YP, Zhong JC, Yang XC. Declined ELABELA plasma levels in hypertension patients with atrial fibrillation: a case control study. BMC Cardiovasc Disord 2021;21:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bohm A, Snopek P, Tothova L, Bezak B, Jajcay N, Vachalcova M, Uher T, Kurecko M, Kissova V, Danova K, Olejnik P, Michalek P, Hlavata T, Petrikova K, Mojto V, Kyselovic J, Farsky S. Association between apelin and atrial fibrillation in patients with high risk of ischemic stroke. Front Cardiovasc Med 2021;8:742601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Falcone C, Buzzi MP, D’Angelo A, Schirinzi S, Falcone R, Rordorf R, Capettini AC, Landolina M, Storti C, Pelissero G. Apelin plasma levels predict arrhythmia recurrence in patients with persistent atrial fibrillation. Int J Immunopathol Pharmacol 2010;23:917–925. [DOI] [PubMed] [Google Scholar]
- 121. Wang YZ, Fan J, Zhong B, Xu Q. Apelin: a novel prognostic predictor for atrial fibrillation recurrence after pulmonary vein isolation. Medicine (Baltimore) 2018;97:e12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lau EMT, Giannoulatou E, Celermajer DS, Humbert M. Epidemiology and treatment of pulmonary arterial hypertension. Nat Rev Cardiol 2017;14:603–614. [DOI] [PubMed] [Google Scholar]
- 123. Sitbon O, Sattler C, Bertoletti L, Savale L, Cottin V, Jais X, De Groote P, Chaouat A, Chabannes C, Bergot E, Bouvaist H, Dauphin C, Bourdin A, Bauer F, Montani D, Humbert M, Simonneau G. Initial dual oral combination therapy in pulmonary arterial hypertension. Eur Respir J 2016;47:1727–1736. [DOI] [PubMed] [Google Scholar]
- 124. Alastalo TP, Li M, Perez Vde J, Pham D, Sawada H, Wang JK, Koskenvuo M, Wang L, Freeman BA, Chang HY, Rabinovitch M. Disruption of PPARgamma/beta-catenin-mediated regulation of apelin impairs BMP-induced mouse and human pulmonary arterial EC survival. J Clin Invest 2011;121:3735–3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Falcao-Pires I, Goncalves N, Henriques-Coelho T, Moreira-Goncalves D, Roncon-Albuquerque R Jr, Leite-Moreira AF. Apelin decreases myocardial injury and improves right ventricular function in monocrotaline-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 2009;296:H2007–H2014. [DOI] [PubMed] [Google Scholar]
- 126. Chandra SM, Razavi H, Kim J, Agrawal R, Kundu RK, de Jesus Perez V, Zamanian RT, Quertermous T, Chun HJ. Disruption of the apelin-APJ system worsens hypoxia-induced pulmonary hypertension. Arterioscler Thromb Vasc Biol 2011;31:814–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Yang P, Read C, Kuc RE, Nyimanu D, Williams TL, Crosby A, Buonincontri G, Southwood M, Sawiak SJ, Glen RC, Morrell NW, Davenport AP, Maguire JJ. A novel cyclic biased agonist of the apelin receptor, MM07, is disease modifying in the rat monocrotaline model of pulmonary arterial hypertension. Br J Pharmacol 2019;176:1206–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kim J, Kang Y, Kojima Y, Lighthouse JK, Hu X, Aldred MA, McLean DL, Park H, Comhair SA, Greif DM, Erzurum SC, Chun HJ. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat Med 2013;19:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Orriols M, Gomez-Puerto MC, Ten Dijke P. BMP type II receptor as a therapeutic target in pulmonary arterial hypertension. Cell Mol Life Sci 2017;74:2979–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Tu L, Desroches-Castan A, Mallet C, Guyon L, Cumont A, Phan C, Robert F, Thuillet R, Bordenave J, Sekine A, Huertas A, Ritvos O, Savale L, Feige J-J, Humbert M, Bailly S, Guignabert C. Selective BMP-9 inhibition partially protects against experimental pulmonary hypertension. Circ Res 2019;124:846–855. [DOI] [PubMed] [Google Scholar]
- 131. Frump AL, Albrecht M, Yakubov B, Breuils-Bonnet S, Nadeau V, Tremblay E, Potus F, Omura J, Cook T, Fisher A, Rodriguez B, Brown RD, Stenmark KR, Rubinstein CD, Krentz K, Tabima DM, Li R, Sun X, Chesler NC, Provencher S, Bonnet S, Lahm T. 17beta-Estradiol and estrogen receptor alpha protect right ventricular function in pulmonary hypertension via BMPR2 and apelin. J Clin Invest 2021;131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol 2013;170:1–7. [DOI] [PubMed] [Google Scholar]
- 133. Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet 2021;398:341–354. [DOI] [PubMed] [Google Scholar]
- 134. Eberlé D, Marousez L, Hanssens S, Knauf C, Breton C, Deruelle P, Lesage J. Elabela and apelin actions in healthy and pathological pregnancies. Cytokine Growth Factor Rev 2019;46:45–53. [DOI] [PubMed] [Google Scholar]
- 135. Georgiadou D, Boussata S, Ranzijn WHM, Root LEA, Hillenius S, Bij de Weg JM, Abheiden CNH, de Boer MA, de Vries JIP, Vrijkotte TGM, Lambalk CB, Kuijper EAM, Afink GB, van Dijk M. Peptide hormone ELABELA enhances extravillous trophoblast differentiation, but placenta is not the major source of circulating ELABELA in pregnancy. Sci Rep 2019;9:19077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Medhurst AD, Jennings CA, Robbins MJ, Davis RP, Ellis C, Winborn KY, Lawrie KW, Hervieu G, Riley G, Bolaky JE, Herrity NC, Murdock P, Darker JG. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J Neurochem 2003;84:1162–1172. [DOI] [PubMed] [Google Scholar]
- 137. Yamaleyeva LM, Chappell MC, Brosnihan KB, Anton L, Caudell DL, Shi S, McGee C, Pirro N, Gallagher PE, Taylor RN, Merrill DC, Mertz HL. Downregulation of apelin in the human placental chorionic villi from preeclamptic pregnancies. Am J Physiol Endocrinol Metab 2015;309:E852–E860. [DOI] [PubMed] [Google Scholar]
- 138. Inuzuka H, Nishizawa H, Inagaki A, Suzuki M, Ota S, Miyamura H, Miyazaki J, Sekiya T, Kurahashi H, Udagawa Y. Decreased expression of apelin in placentas from severe pre-eclampsia patients. Hypertens Pregnancy 2013;32:410–421. [DOI] [PubMed] [Google Scholar]
- 139. Georgiadou D, Afink GB, van Dijk M. The apelinergic-axis in human preeclamptic pregnancies: a systematic review. Pregnancy Hypertens 2019;17:148–157. [DOI] [PubMed] [Google Scholar]
- 140. Anton L, Brosnihan KB. Systemic and uteroplacental renin–angiotensin system in normal and pre-eclamptic pregnancies. Ther Adv Cardiovasc Dis 2008;2:349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ho L, van Dijk M, Chye STJ, Messerchmidt DM, Chng SC, Ong S, Yi LK, Boussata S, Goh GH, Afink GB, Lim CY, Dunn NR, Solter D, Knowles BB, Reversade B. ELABELA deficiency promotes preeclampsia and cardiovascular malformations in mice. Science 2017;357:707–713. [DOI] [PubMed] [Google Scholar]
- 142. Wang C, Liu X, Kong D, Qin X, Li Y, Teng X, Huang X. Apelin as a novel drug for treating preeclampsia. Exp Ther Med 2017;14:5917–5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Yamaleyeva LM, Brosnihan KB, Elsangeedy E, McGee C, Shi S, Caudell D, Miller C, Varagic J, Bader M, Dechend R, Shaltout HA. Systemic outcomes of (Pyr(1))-Apelin-13 infusion at mid-late pregnancy in a rat model with preeclamptic features. Sci Rep 2019;9:8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Hamza RZ, Diab AAA, Zahra MH, Asalah AK, Moursi SMM, Al-Baqami NM, Al-Salmi FA, Attia MS. Correlation between apelin and some angiogenic factors in the pathogenesis of preeclampsia: apelin-13 as novel drug for treating preeclampsia and its physiological effects on placenta. Int J Endocrinol 2021;2021:5017362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Kucur M, Tuten A, Oncul M, Acikgoz AS, Yuksel MA, Imamoglu M, Balci Ekmekci O, Yilmaz N, Madazli R. Maternal serum apelin and YKL-40 levels in early and late-onset pre-eclampsia. Hypertens Pregnancy 2014;33:467–475. [DOI] [PubMed] [Google Scholar]
- 146. Pritchard N, Kaitu'u-Lino TJ, Gong S, Dopierala J, Smith GCS, Charnock-Jones DS, Tong S. ELABELA/APELA levels are not decreased in the maternal circulation or placenta among women with preeclampsia. Am J Pathol 2018;188:1749–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Panaitescu B, Romero R, Gomez-Lopez N, Pacora P, Erez O, Vadillo-Ortega F, Yeo L, Hassan SS, Hsu CD. ELABELA plasma concentrations are increased in women with late-onset preeclampsia. J Matern Fetal Neonatal Med 2020;33:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Zhou L, Sun H, Cheng R, Fan X, Lai S, Deng C. ELABELA, as a potential diagnostic biomarker of preeclampsia, regulates abnormally shallow placentation via APJ. Am J Physiol Endocrinol Metab 2019;316:E773–E781. [DOI] [PubMed] [Google Scholar]
- 149. Castan-Laurell I, Masri B, Valet P. The apelin/APJ system as a therapeutic target in metabolic diseases. Expert Opin Ther Targets 2019;23:215–225. [DOI] [PubMed] [Google Scholar]
- 150. Ringstrom C, Nitert MD, Bennet H, Fex M, Valet P, Rehfeld JF, Friis-Hansen L, Wierup N. Apelin is a novel islet peptide. Regul Pept 2010;162:44–51. [DOI] [PubMed] [Google Scholar]
- 151. Boucher J, Masri B, Daviaud D, Gesta S, Guigne C, Mazzucotelli A, Castan-Laurell I, Tack I, Knibiehler B, Carpene C, Audigier Y, Saulnier-Blache J-S, Valet P. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 2005;146:1764–1771. [DOI] [PubMed] [Google Scholar]
- 152. Guo L, Li Q, Wang W, Yu P, Pan H, Li P, Sun Y, Zhang J. Apelin inhibits insulin secretion in pancreatic beta-cells by activation of PI3-kinase-phosphodiesterase 3B. Endocr Res 2009;34:142–154. [DOI] [PubMed] [Google Scholar]
- 153. Yue P, Jin H, Aillaud M, Deng AC, Azuma J, Asagami T, Kundu RK, Reaven GM, Quertermous T, Tsao PS. Apelin is necessary for the maintenance of insulin sensitivity. Am J Physiol Endocrinol Metab 2010;298:E59–E67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Dray C, Knauf C, Daviaud D, Waget A, Boucher J, Buleon M, Cani PD, Attane C, Guigne C, Carpene C, Burcelin R, Castan-Laurell I, Valet P. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab 2008;8:437–445. [DOI] [PubMed] [Google Scholar]
- 155. Higuchi K, Masaki T, Gotoh K, Chiba S, Katsuragi I, Tanaka K, Kakuma T, Yoshimatsu H. Apelin, an APJ receptor ligand, regulates body adiposity and favors the messenger ribonucleic acid expression of uncoupling proteins in mice. Endocrinology 2007;148:2690–2697. [DOI] [PubMed] [Google Scholar]
- 156. Attane C, Foussal C, Le Gonidec S, Benani A, Daviaud D, Wanecq E, Guzman-Ruiz R, Dray C, Bezaire V, Rancoule C, Kuba K, Ruiz-Gayo M, Levade T, Penninger J, Burcelin R, Pénicaud L, Valet P, Castan-Laurell I. Apelin treatment increases complete fatty acid oxidation, mitochondrial oxidative capacity, and biogenesis in muscle of insulin-resistant mice. Diabetes 2012;61:310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Bertrand C, Pradere JP, Geoffre N, Deleruyelle S, Masri B, Personnaz J, Le Gonidec S, Batut A, Louche K, Moro C, Valet P, Castan-Laurell I. Chronic apelin treatment improves hepatic lipid metabolism in obese and insulin-resistant mice by an indirect mechanism. Endocrine 2018;60:112–121. [DOI] [PubMed] [Google Scholar]
- 158. O'Harte FPM, Parthsarathy V, Hogg C, Flatt PR. Apelin-13 analogues show potent in vitro and in vivo insulinotropic and glucose lowering actions. Peptides 2018;100:219–228. [DOI] [PubMed] [Google Scholar]
- 159. O'Harte FPM, Parthsarathy V, Flatt PR. Chronic apelin analogue administration is more effective than established incretin therapies for alleviating metabolic dysfunction in diabetic db/db mice. Mol Cell Endocrinol 2020;504:110695. [DOI] [PubMed] [Google Scholar]
- 160. Gourdy P, Cazals L, Thalamas C, Sommet A, Calvas F, Galitzky M, Vinel C, Dray C, Hanaire H, Castan-Laurell I, Valet P. Apelin administration improves insulin sensitivity in overweight men during hyperinsulinaemic-euglycaemic clamp. Diabetes Obes Metab 2018;20:157–164. [DOI] [PubMed] [Google Scholar]
- 161. US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03449251. 2018.
- 162. Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet 2017;389:1238–1252. [DOI] [PubMed] [Google Scholar]
- 163. Chapman FA, Nyimanu D, Maguire JJ, Davenport AP, Newby DE, Dhaun N. The therapeutic potential of apelin in kidney disease. Nat Rev Nephrol 2021;17:840–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Deng C, Chen H, Yang N, Feng Y, Hsueh AJ. Apela regulates fluid homeostasis by binding to the APJ receptor to activate Gi signaling. J Biol Chem 2015;290:18261–18268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Hus-Citharel A, Bodineau L, Frugiere A, Joubert F, Bouby N, Llorens-Cortes C. Apelin counteracts vasopressin-induced water reabsorption via cross talk between apelin and vasopressin receptor signaling pathways in the rat collecting duct. Endocrinology 2014;155:4483–4493. [DOI] [PubMed] [Google Scholar]
- 166. De Mota N, Reaux-Le Goazigo A, El Messari S, Chartrel N, Roesch D, Dujardin C, Kordon C, Vaudry H, Moos F, Llorens-Cortes C. Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release. Proc Natl Acad Sci U S A 2004;101:10464–10469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Boulkeroua C, Ayari H, Khalfaoui T, Lafrance M, Besserer-Offroy E, Ekindi N, Sabbagh R, Dumaine R, Lesur O, Sarret P, Chraibi A. Apelin-13 regulates vasopressin-induced aquaporin-2 expression and trafficking in kidney collecting duct cells. Cell Physiol Biochem 2019;53:687–700. [DOI] [PubMed] [Google Scholar]
- 168. Flahault A, Girault-Sotias PE, Keck M, Alvear-Perez R, De Mota N, Esteoulle L, Ramanoudjame SM, Iturrioz X, Bonnet D, Llorens-Cortes C. A metabolically stable apelin-17 analog decreases AVP-induced antidiuresis and improves hyponatremia. Nat Commun 2021;12:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Chen H, Wan D, Wang L, Peng A, Xiao H, Petersen RB, Liu C, Zheng L, Huang K. Apelin protects against acute renal injury by inhibiting TGF-beta1. Biochim Biophys Acta 2015;1852:1278–1287. [DOI] [PubMed] [Google Scholar]
- 170. Sagiroglu T, Torun N, Yagci M, Yalta T, Sagiroglu G, Oguz S. Effects of apelin and leptin on renal functions following renal ischemia/reperfusion: an experimental study. Exp Ther Med 2012;3:908–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Chen H, Wang L, Wang W, Cheng C, Zhang Y, Zhou Y, Wang C, Miao X, Wang J, Wang C, Li J, Zheng L, Huang K. ELABELA and an ELABELA fragment protect against AKI. J Am Soc Nephrol 2017;28:2694–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03956576. 2018.
- 173. Murza A, Parent A, Besserer-Offroy E, Tremblay H, Karadereye F, Beaudet N, Leduc R, Sarret P, Marsault É. Elucidation of the structure-activity relationships of apelin: influence of unnatural amino acids on binding, signaling, and plasma stability. ChemMedChem 2012;7:318–325. [DOI] [PubMed] [Google Scholar]
- 174. Jia ZQ, Hou L, Leger A, Wu I, Kudej AB, Stefano J, Jiang C, Pan CQ, Akita GY. Cardiovascular effects of a PEGylated apelin. Peptides 2012;38:181–188. [DOI] [PubMed] [Google Scholar]
- 175. Read C, Yang P, Kuc RE, Nyimanu D, Williams TL, Glen RC, Holt LJ, Arulanantham H, Smart A, Davenport AP, Maguire JJ. Apelin peptides linked to anti-serum albumin domain antibodies retain affinity in vitro and are efficacious receptor agonists in vivo. Basic Clin Pharmacol Toxicol 2020;126(Suppl 6):96–103. [DOI] [PubMed] [Google Scholar]
- 176. Gerbier R, Alvear-Perez R, Margathe JF, Flahault A, Couvineau P, Gao J, De Mota N, Dabire H, Li B, Ceraudo E, Hus-Citharel A, Esteoulle L, Bisoo C, Hibert M, Berdeaux A, Iturrioz X, Bonnet D, Llorens-Cortes C. Development of original metabolically stable apelin-17 analogs with diuretic and cardiovascular effects. FASEB J 2017;31:687–700. [DOI] [PubMed] [Google Scholar]
- 177. O'Harte FPM, Parthsarathy V, Hogg C, Flatt PR. Long-term treatment with acylated analogues of apelin-13 amide ameliorates diabetes and improves lipid profile of high-fat fed mice. PLoS One 2018;13:e0202350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Read C, Nyimanu D, Yang P, Kuc RE, Williams TL, Fitzpatrick CM, Foster R, Glen RC, Maguire JJ, Davenport AP. The G protein biased small molecule apelin agonist CMF-019 is disease modifying in endothelial cell apoptosis in vitro and induces vasodilatation without desensitisation in vivo. Front Pharmacol 2020;11:588669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Winkle P, Goldsmith S, Koren MJ, Lepage S, Hellawell J, Trivedi A, Tsirtsonis K, Abbasi SA, Kaufman A, Troughton R, Voors A, Hulot J-S, Donal E, Kazemi N, Neutel J. A first-in-human study of AMG 986, a novel apelin receptor agonist, in healthy subjects and heart failure patients. Cardiovasc Drugs Ther 2023;37:743–755. [DOI] [PubMed] [Google Scholar]
- 180. US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/study/NCT03281122. 2017.
- 181. Gargalovic P, Wong P, Onorato J, Finlay H, Wang T, Yan M, Crain E, St-Onge S, Heroux M, Bouvier M, Xu C, Chen X-Q, Generaux C, Lawrence M, Wexler R, Gordon D. In vitro and in vivo evaluation of a small-molecule APJ (apelin receptor) agonist, BMS-986224, as a potential treatment for heart failure. Circ Heart Fail 2021;14:e007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Trivedi A, Mather O, Vega S, Hutton S, Hellawell J, Lee E. A phase I, open-label, single-dose study to evaluate the pharmacokinetics, safety, and tolerability of AMG 986 in healthy Japanese subjects. Drugs R D 2022;22:141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Narayanan S, Dai D, Vyas Devambatla RK, Albert V, Bruneau-Latour N, Vasukuttan V, Ciblat S, Rehder K, Runyon SP, Maitra R. Synthesis and characterization of an orally bioavailable small molecule agonist of the apelin receptor. Bioorg Med Chem 2022;66:116789. [DOI] [PubMed] [Google Scholar]