Abstract
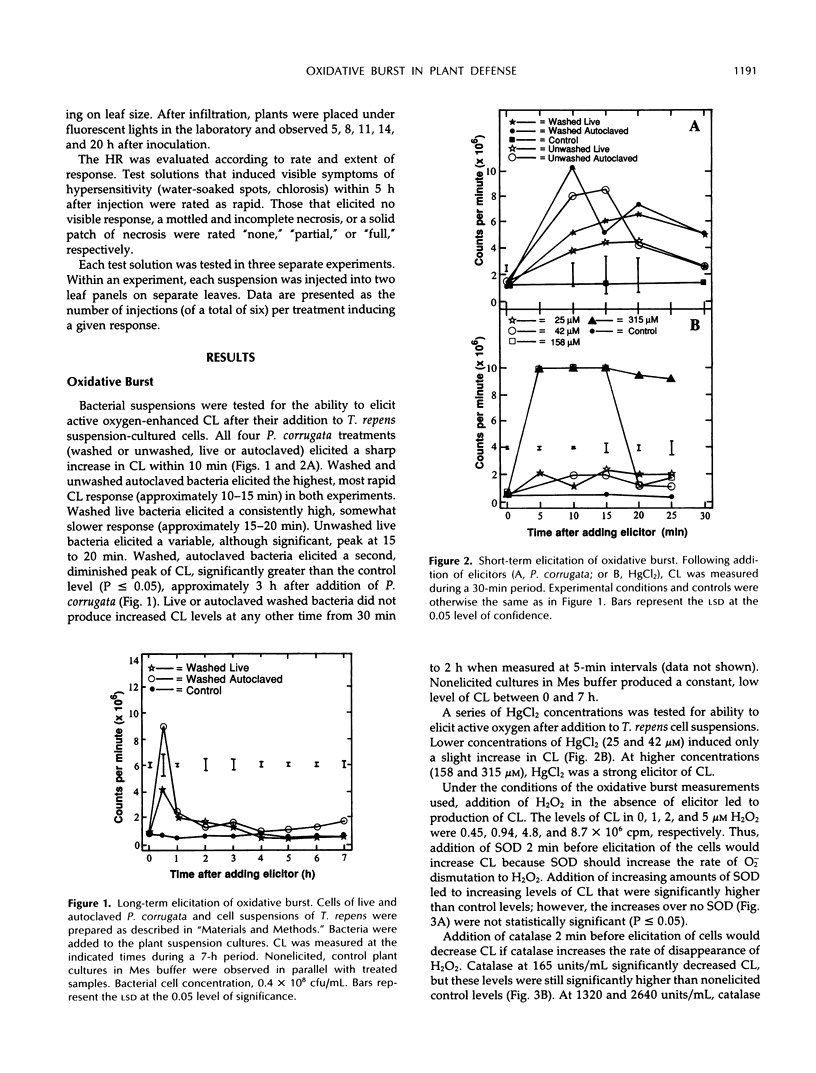
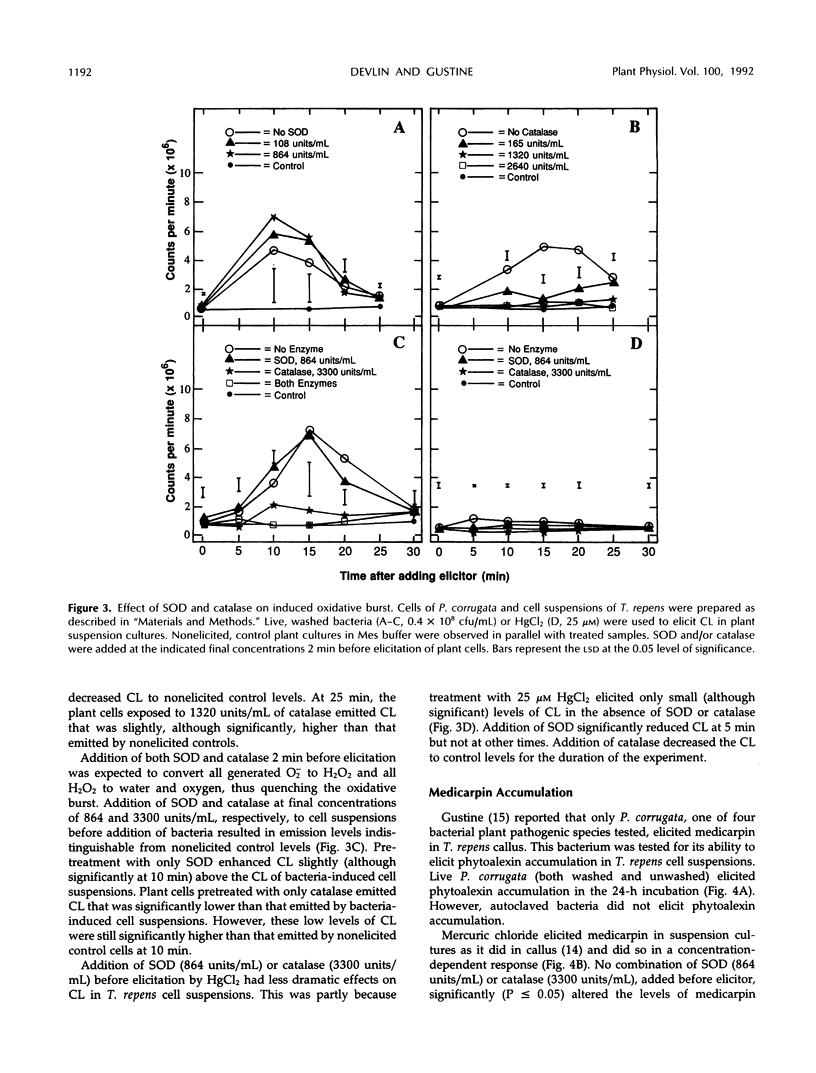
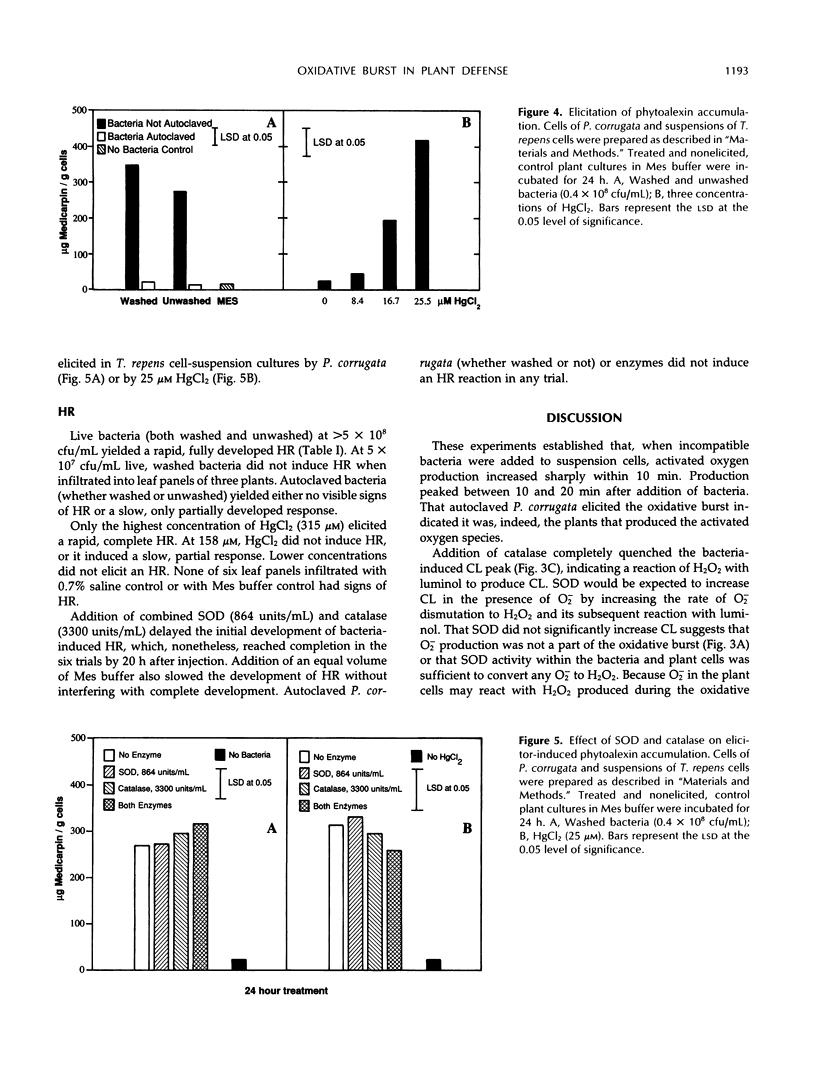
The role of the oxidative burst, transient production of activated oxygen species such as H2O2 and superoxide (O2−) in elicitation of phytoalexins and the hypersensitive reaction (HR) was investigated in white clover (Trifolium repens L.) and tobacco (Nicotiana tabacum L.). H2O2 and O2− production was measured as chemiluminescence (CL) mediated by luminol, which was added to suspension-cultured white clover just before measurement in an out-of-coincidence mode scintillation counter. Maximum CL occurred between 10 and 20 min after addition of 0.4 × 108 colony-forming units/mL of incompatible Pseudomonas corrugata or 158 μm HgCl2. Autoclaved P. corrugata produced a slightly higher response. Elicitation of cells with 25 μm HgCl2 did not produce CL. Preincubation of plant cells in superoxide dismutase, which converts O2− to H2O2, for 2 min before addition of bacteria did not significantly increase maximum CL levels (P ≥ 0.05). Preincubation of plant cells with catalase for 2 min before addition of bacteria prevented the increase in CL, confirming that H2O2 is the substrate for the luminol reaction. Addition of live bacteria or HgCl2 (25 and 158 μm) to white clover increased levels of the phytoalexin medicarpin during a 24-h period, but addition of autoclaved bacteria did not elicit formation of medicarpin. Preincubation of plant cells with catalase, which quenched the bacteria-induced oxidative burst, did not decrease phytoalexin accumulation. Live bacteria infiltrated into Havana 44 tobacco leaf panels induced development of the HR, but autoclaved bacteria did not. Incubation of live bacteria with superoxide dismutase and catalase before infiltration into tobacco leaves did not interfere with development of the HR. Tobacco leaf panels infiltrated with up to 158 μm HgCl2 did not develop an HR. These results suggest that an oxidative burst consisting of H2O2 and O2− does occur during these two plant defense responses, but it may not be a necessary element of the signaling system for HR and phytoalexin formation.
Full text
PDF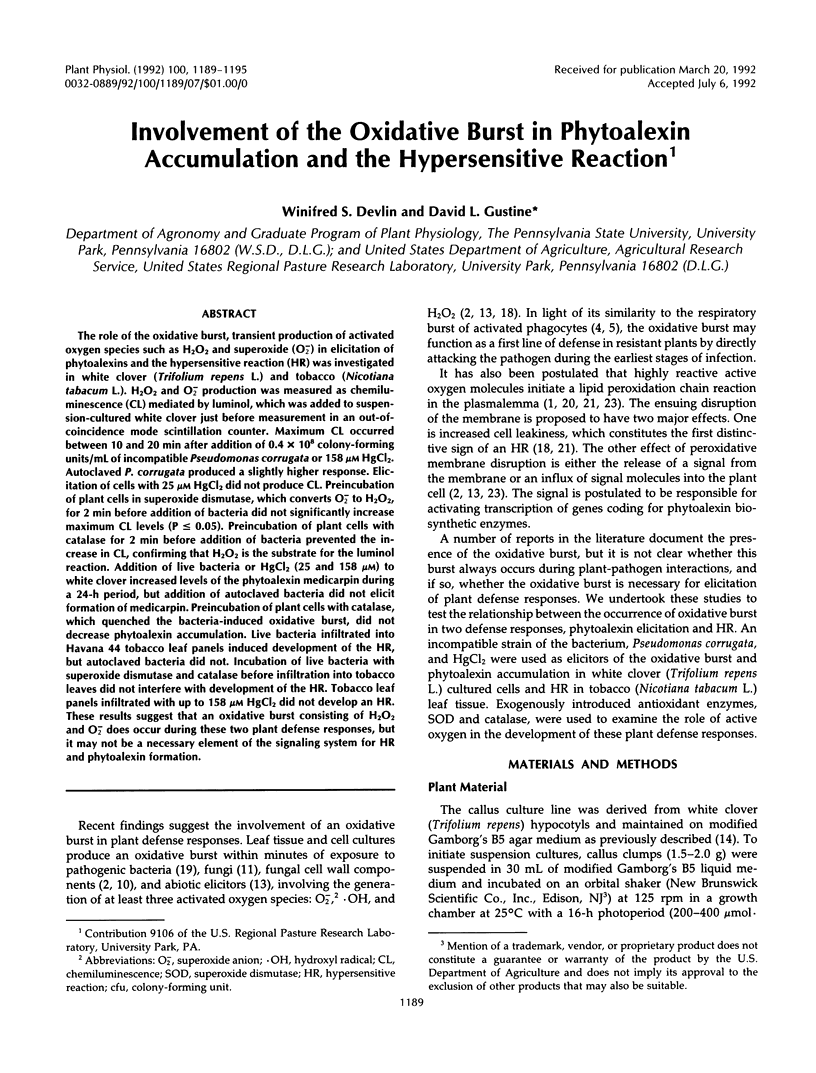



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apostol I., Heinstein P. F., Low P. S. Rapid Stimulation of an Oxidative Burst during Elicitation of Cultured Plant Cells : Role in Defense and Signal Transduction. Plant Physiol. 1989 May;90(1):109–116. doi: 10.1104/pp.90.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson M. M., Keppler L. D., Orlandi E. W., Baker C. J., Mischke C. F. Involvement of plasma membrane calcium influx in bacterial induction of the k/h and hypersensitive responses in tobacco. Plant Physiol. 1990 Jan;92(1):215–221. doi: 10.1104/pp.92.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Buege J. A., Aust S. D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- Gustine D. L. Evidence for Sulfhydryl Involvement in Regulation of Phytoalexin Accumulation in Trifolium repens Callus Tissue Cultures. Plant Physiol. 1981 Dec;68(6):1323–1326. doi: 10.1104/pp.68.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustine D. L. Induction of Medicarpin Biosynthesis in Ladino Clover Callus by p-Chloromercuribenzoic Acid Is Reversed by Dithiothreitol. Plant Physiol. 1987 May;84(1):3–6. doi: 10.1104/pp.84.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEMENT Z. RAPID DETECTION OF THE PATHOGENICITY OF PHYTOPATHOGENIC PSEUDOMONADS. Nature. 1963 Jul 20;199:299–300. doi: 10.1038/199299b0. [DOI] [PubMed] [Google Scholar]
- Rogers K. R., Albert F., Anderson A. J. Lipid peroxidation is a consequence of elicitor activity. Plant Physiol. 1988 Feb;86(2):547–553. doi: 10.1104/pp.86.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater T. F. Free-radical mechanisms in tissue injury. Biochem J. 1984 Aug 15;222(1):1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]


