Abstract
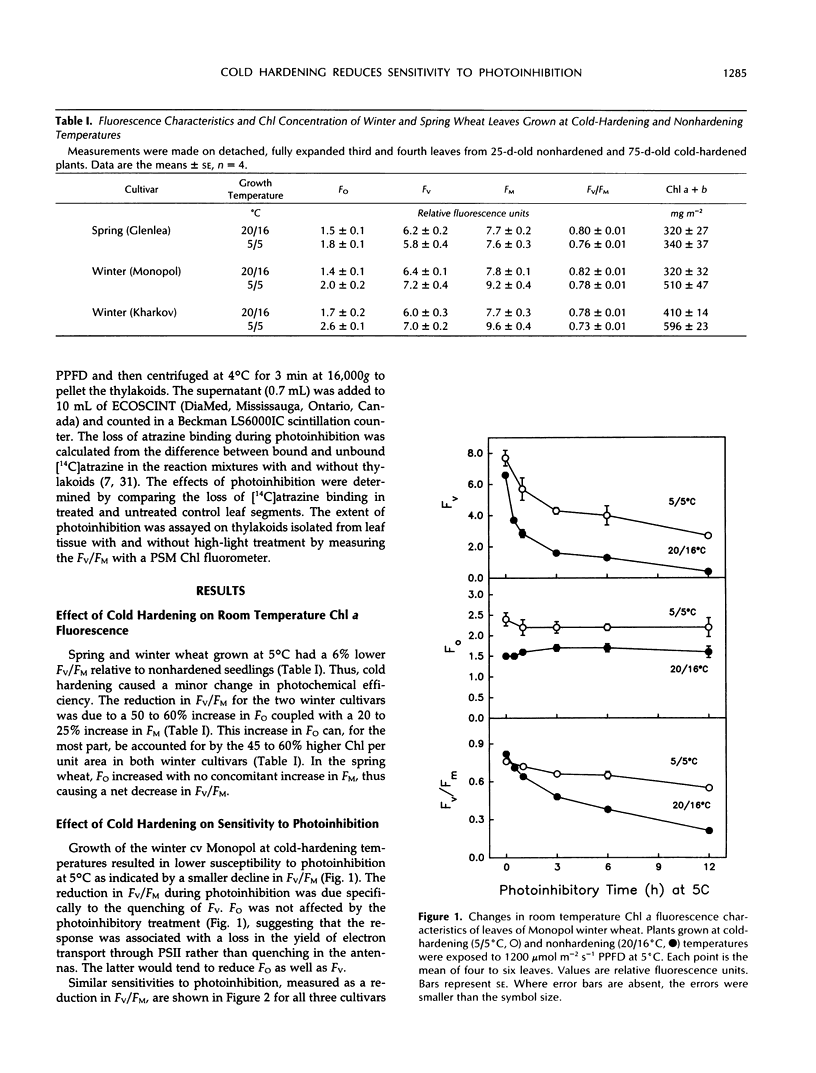
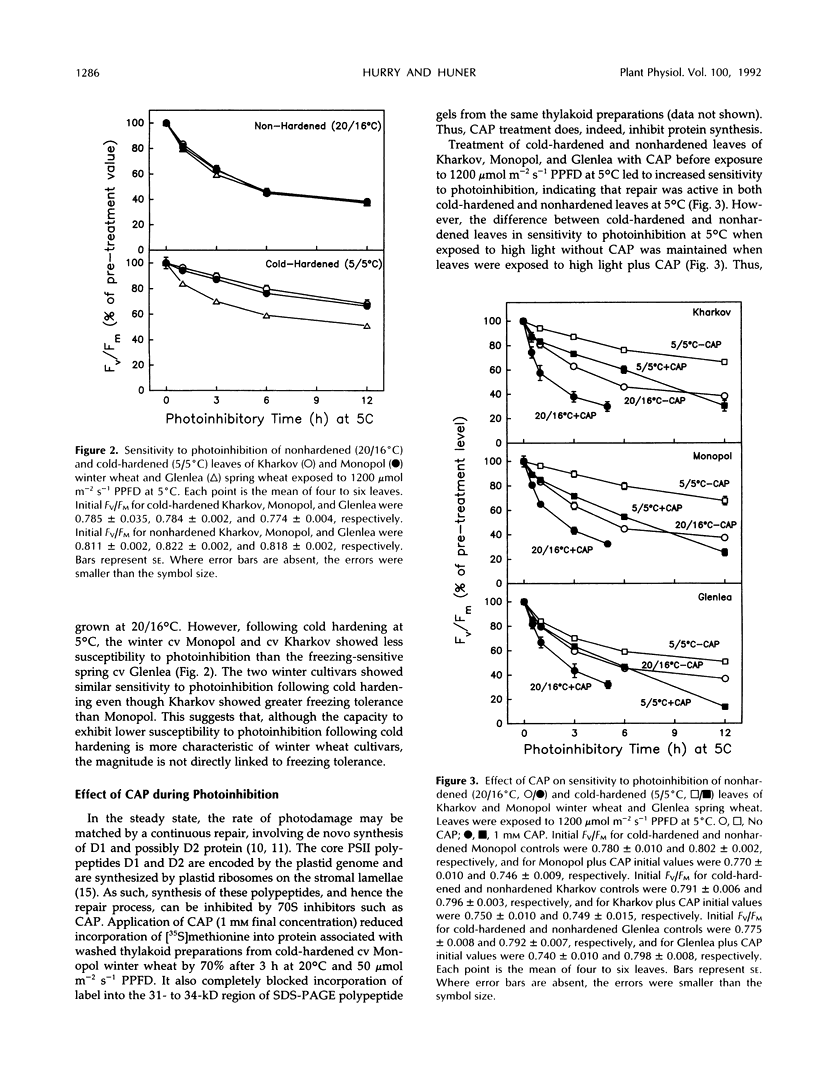
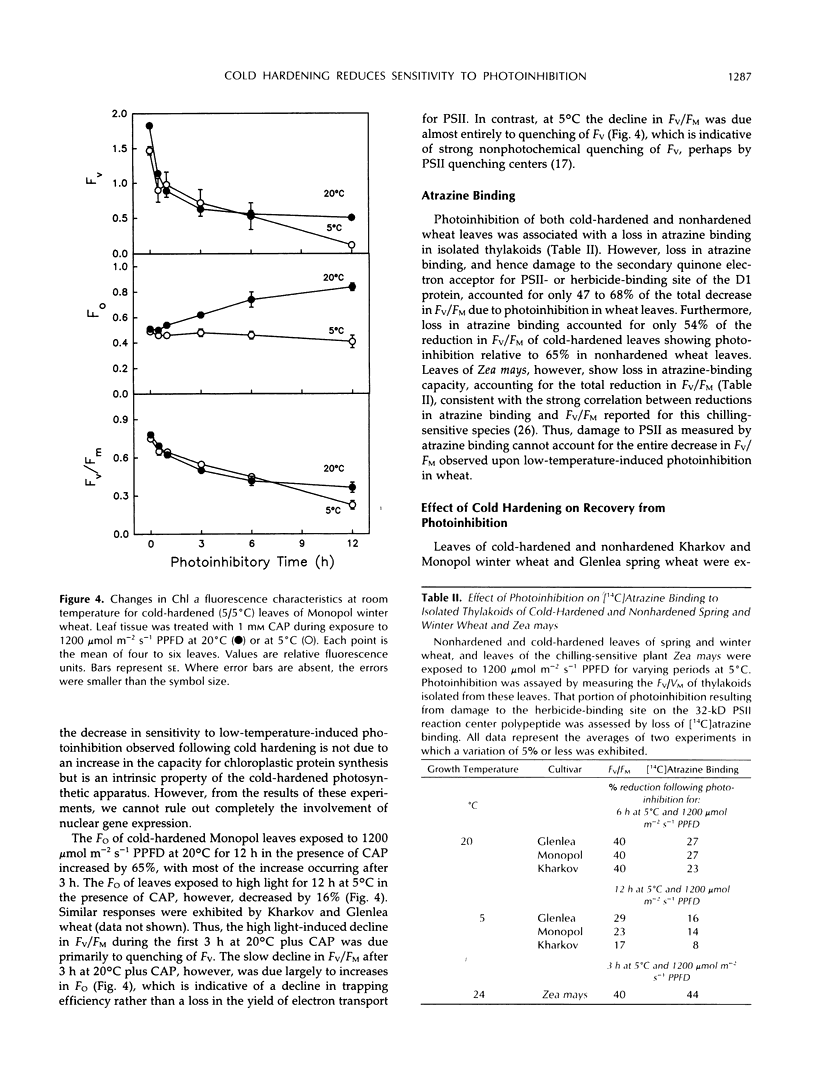
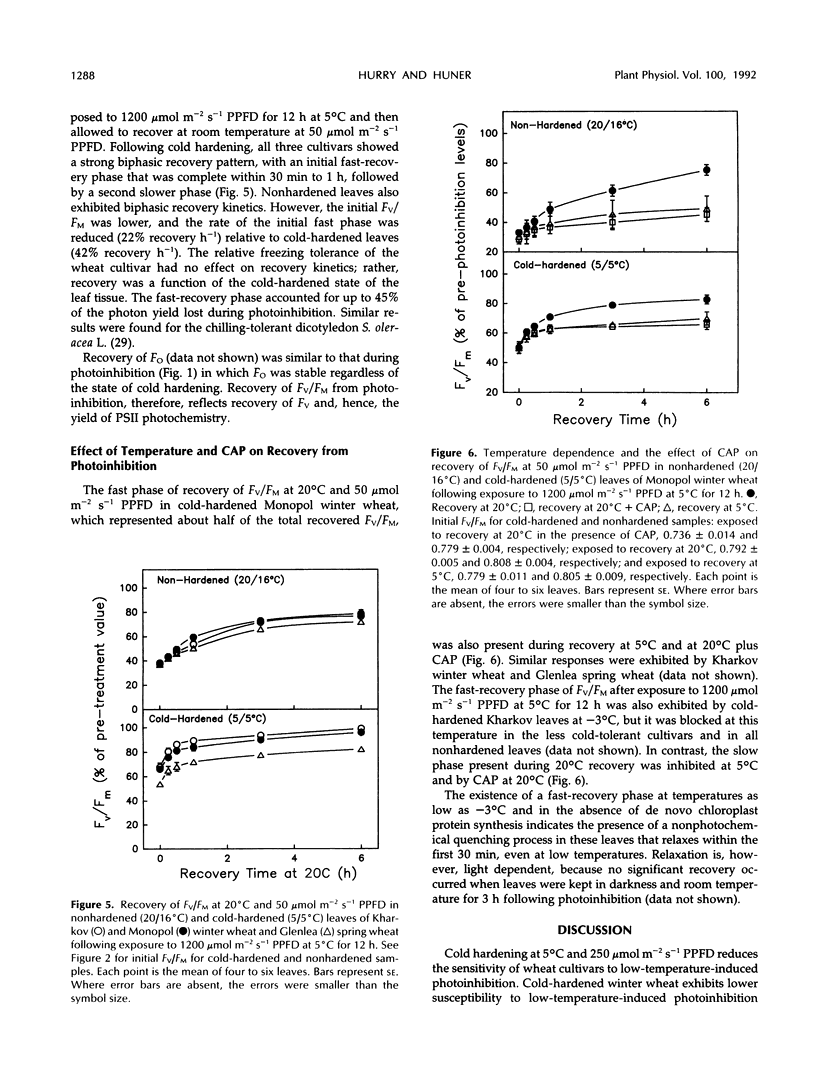
Photoinhibition of photosynthesis and its recovery were studied in wheat (Triticum aestivum L.) leaves grown at nonhardening (20°C) and cold-hardening (5°C) temperatures. Cold-hardened wheat leaves were less susceptible to photoinhibition at 5°C than nonhardened leaves, and the winter cultivars, Kharkov and Monopol, were less susceptible than the spring cultivar, Glenlea. The presence of chloramphenicol, a chloroplastic protein synthesis inhibitor, increased the susceptibility to photoinhibition, but cold-hardened leaves still remained less susceptible to photoinhibition than nonhardened leaves. Recovery at 50 μmol m−2 s−1 photosynthetic photon flux density and 20°C was at least biphasic, with a fast and a slow phase in all cultivars. Cold-hardened leaves recovered maximum fluorescence and maximum variable fluorescence in the dark-adapted state during the fast phase at a rate of 42% h−1 compared with 22% h−1 for nonhardened leaves. The slow phase occurred at similar rates (2% h−1) in cold-hardened and nonhardened leaves. Full recovery required up to 30 h. Fast-recovery phase was not reduced by either lowering the recovery temperature to 5°C or by the presence of chloramphenicol. Slow-recovery phase was inhibited by both treatments. Hence, the fast phase of recovery does not require de novo chloroplast protein synthesis. In addition, only approximately 60% of the photochemical efficiency lost through photoinhibition at 5°C was associated with lost [14C]atrazine binding and, hence, with damage to the secondary quinone electron acceptor for photosystem II-binding site. We conclude that the decrease in susceptibility to photoinhibition exhibited following cold hardening of winter and spring cultivars is not due to an increased capacity for repair of photoinhibitory damage at 5°C but reflects intrinsic properties of the cold-hardened photosynthetic apparatus. A model to account for the fast component of recovery is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boese S. R., Huner N. P. Effect of growth temperature and temperature shifts on spinach leaf morphology and photosynthesis. Plant Physiol. 1990 Dec;94(4):1830–1836. doi: 10.1104/pp.94.4.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurry V. M., Huner N. P. Low growth temperature effects a differential inhibition of photosynthesis in spring and winter wheat. Plant Physiol. 1991 Jun;96(2):491–497. doi: 10.1104/pp.96.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa Z., Oquist G., Gustafsson P. Photoinhibition and Recovery of Photosynthesis in psbA Gene-Inactivated Strains of Cyanobacterium Anacystis nidulans. Plant Physiol. 1990 May;93(1):1–6. doi: 10.1104/pp.93.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe L., Huner N. P., Carpentier R., Ottander C. Resistance to low temperature photoinhibition is not associated with isolated thylakoid membranes of winter rye. Plant Physiol. 1991 Oct;97(2):804–810. doi: 10.1104/pp.97.2.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad I., Adir N., Koike H., Kyle D. J., Inoue Y. Mechanism of photoinhibition in vivo. A reversible light-induced conformational change of reaction center II is related to an irreversible modification of the D1 protein. J Biol Chem. 1990 Feb 5;265(4):1972–1979. [PubMed] [Google Scholar]
- Tischer W., Strotmann H. Relationship between inhibitor binding by chloroplasts and inhibition of photosynthetic electron transport. Biochim Biophys Acta. 1977 Apr 11;460(1):113–125. doi: 10.1016/0005-2728(77)90157-8. [DOI] [PubMed] [Google Scholar]
- Yalovsky S., Schuster G., Nechushtai R. The apoprotein precursor of the major light-harvesting complex of photosystem II (LHCIIb) is inserted primarily into stromal lamellae and subsequently migrates to the grana. Plant Mol Biol. 1990 May;14(5):753–764. doi: 10.1007/BF00016508. [DOI] [PubMed] [Google Scholar]


