Abstract
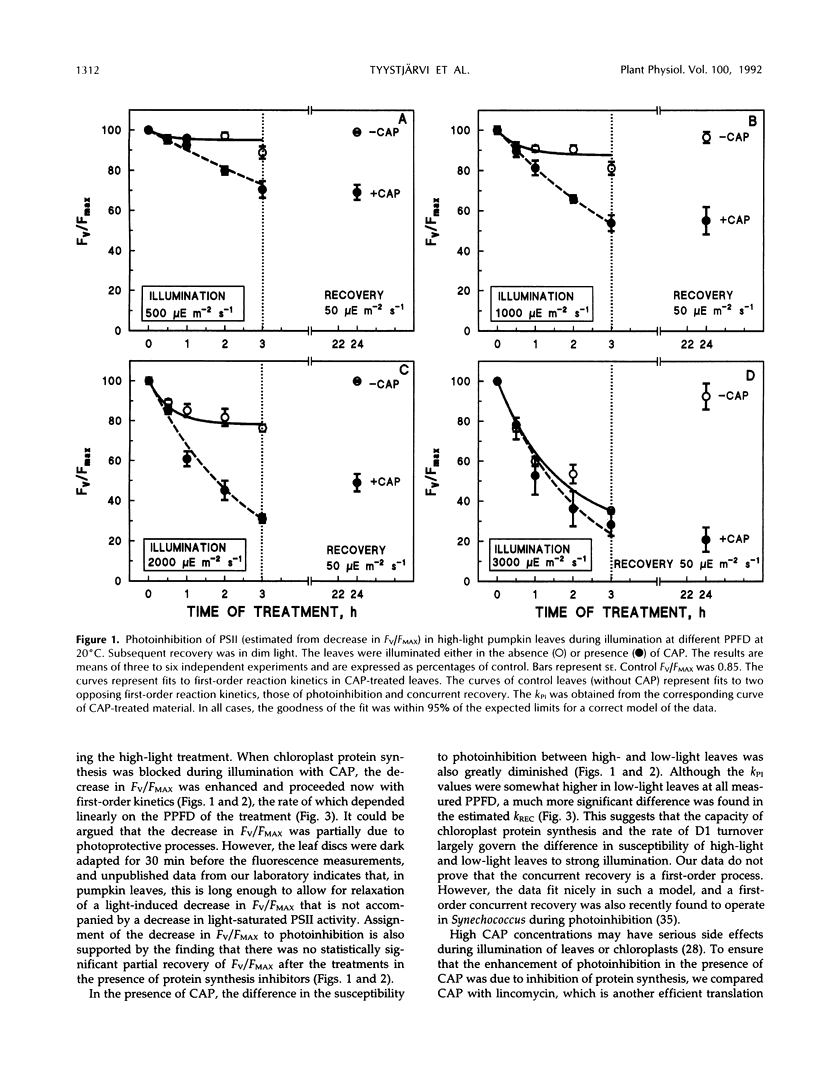
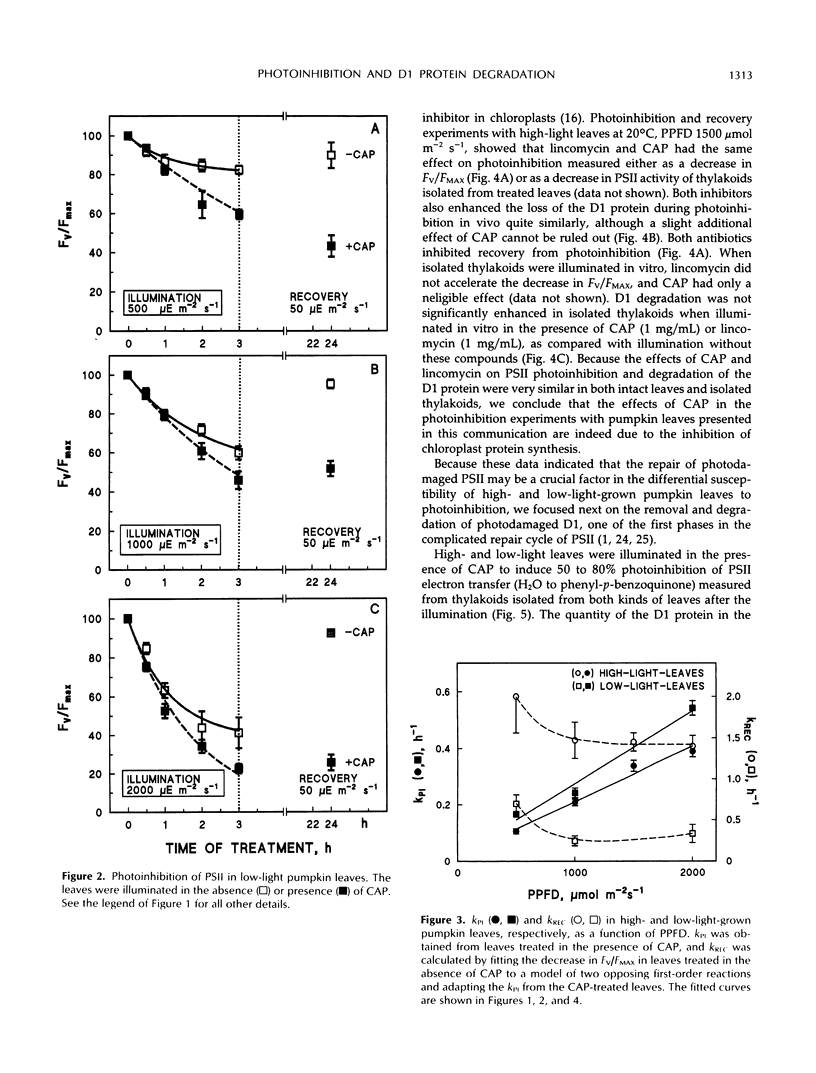
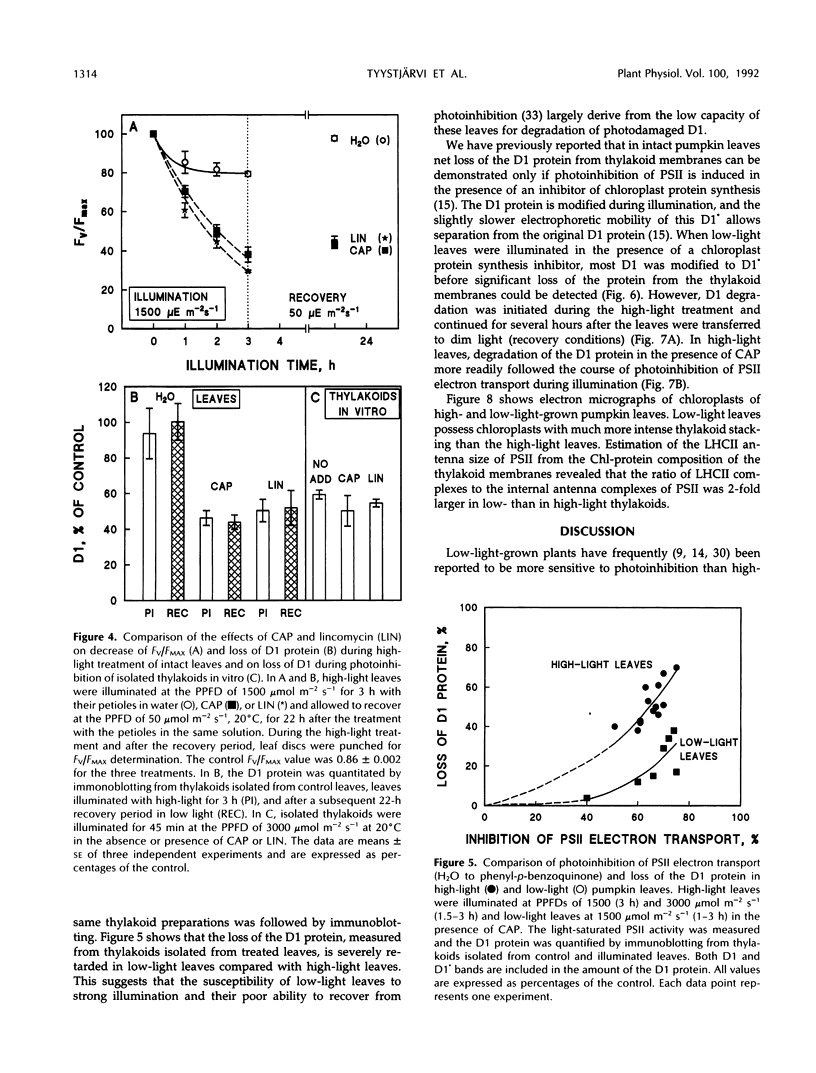
Photoinhibition of photosystem II (PSII) electron transport and subsequent degradation of the D1 protein were studied in pumpkin (Cucurbita pepo L.) leaves developed under high (1000 μmol m−2 s−1) and low (80 μmol m−2 s−1) photon flux densities. The low-light leaves were more susceptible to high light. This difference was greatly diminished when illumination was performed in the presence of chloramphenicol, indicating that a poor capacity to repair photodamaged PSII centers is decisive in the susceptibility of low-light leaves to photoinhibition. In fact, the first phases of the repair cycle, degradation and removal of photodamaged D1 protein from the reaction center complex, occurred slowly in low-light leaves, whereas in high-light leaves the degradation of the D1 protein more readily followed photoinhibition of PSII electron transport. A modified form of the D1 protein, with slightly slower electrophoretic mobility than the original D1, accumulated in the appressed thylakoid membranes of low-light leaves during illumination and was subsequently degraded only slowly.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adir N., Shochat S., Ohad I. Light-dependent D1 protein synthesis and translocation is regulated by reaction center II. Reaction center II serves as an acceptor for the D1 precursor. J Biol Chem. 1990 Jul 25;265(21):12563–12568. [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro E. M., Kettunen R., Tyystjärvi E. ATP and light regulate D1 protein modification and degradation. Role of D1* in photoinhibition. FEBS Lett. 1992 Feb 3;297(1-2):29–33. doi: 10.1016/0014-5793(92)80320-g. [DOI] [PubMed] [Google Scholar]
- Callahan F. E., Ghirardi M. L., Sopory S. K., Mehta A. M., Edelman M., Mattoo A. K. A novel metabolic form of the 32 kDa-D1 protein in the grana-localized reaction center of photosystem II. J Biol Chem. 1990 Sep 15;265(26):15357–15360. [PubMed] [Google Scholar]
- Elich T. D., Edelman M., Mattoo A. K. Identification, characterization, and resolution of the in vivo phosphorylated form of the D1 photosystem II reaction center protein. J Biol Chem. 1992 Feb 15;267(5):3523–3529. [PubMed] [Google Scholar]
- Greenberg B. M., Gaba V., Canaani O., Malkin S., Mattoo A. K., Edelman M. Separate photosensitizers mediate degradation of the 32-kDa photosystem II reaction center protein in the visible and UV spectral regions. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6617–6620. doi: 10.1073/pnas.86.17.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettunen R., Tyystjärvi E., Aro E. M. D1 protein degradation during photoinhibition of intact leaves. A modification of the D1 protein precedes degradation. FEBS Lett. 1991 Sep 23;290(1-2):153–156. doi: 10.1016/0014-5793(91)81247-6. [DOI] [PubMed] [Google Scholar]
- Klaff P., Gruissem W. Changes in Chloroplast mRNA Stability during Leaf Development. Plant Cell. 1991 May;3(5):517–529. doi: 10.1105/tpc.3.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle D. J., Ohad I., Arntzen C. J. Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4070–4074. doi: 10.1073/pnas.81.13.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marder J. B., Goloubinoff P., Edelman M. Molecular architecture of the rapidly metabolized 32-kilodalton protein of photosystem II. Indications for COOH-terminal processing of a chloroplast membrane polypeptide. J Biol Chem. 1984 Mar 25;259(6):3900–3908. [PubMed] [Google Scholar]
- Mattoo A. K., Edelman M. Intramembrane translocation and posttranslational palmitoylation of the chloroplast 32-kDa herbicide-binding protein. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1497–1501. doi: 10.1073/pnas.84.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo A. K., Hoffman-Falk H., Marder J. B., Edelman M. Regulation of protein metabolism: Coupling of photosynthetic electron transport to in vivo degradation of the rapidly metabolized 32-kilodalton protein of the chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1380–1384. doi: 10.1073/pnas.81.5.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale P. J., Melis A. Activation of a Reserve Pool of Photosystem II in Chlamydomonas reinhardtii Counteracts Photoinhibition. Plant Physiol. 1990 Apr;92(4):1196–1204. doi: 10.1104/pp.92.4.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K., Satoh K., Katoh S. Chloramphenicol is an inhibitor of photosynthesis. FEBS Lett. 1991 Dec 16;295(1-3):155–158. doi: 10.1016/0014-5793(91)81407-y. [DOI] [PubMed] [Google Scholar]
- Powles S. B., Critchley C. Effect of Light Intensity during Growth on Photoinhibition of Intact Attached Bean Leaflets. Plant Physiol. 1980 Jun;65(6):1181–1187. doi: 10.1104/pp.65.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster G., Timberg R., Ohad I. Turnover of thylakoid photosystem II proteins during photoinhibition of Chlamydomonas reinhardtii. Eur J Biochem. 1988 Nov 1;177(2):403–410. doi: 10.1111/j.1432-1033.1988.tb14389.x. [DOI] [PubMed] [Google Scholar]
- Tyystjärvi E., Koivuniemi A., Kettunen R., Aro E. M. Small light-harvesting antenna does not protect from photoinhibition. Plant Physiol. 1991 Oct;97(2):477–483. doi: 10.1104/pp.97.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin I., Ghanotakis D. F., Andersson B. Light-induced D1-protein degradation in isolated photosystem II core complexes. FEBS Lett. 1990 Aug 20;269(1):45–48. doi: 10.1016/0014-5793(90)81115-5. [DOI] [PubMed] [Google Scholar]