Abstract
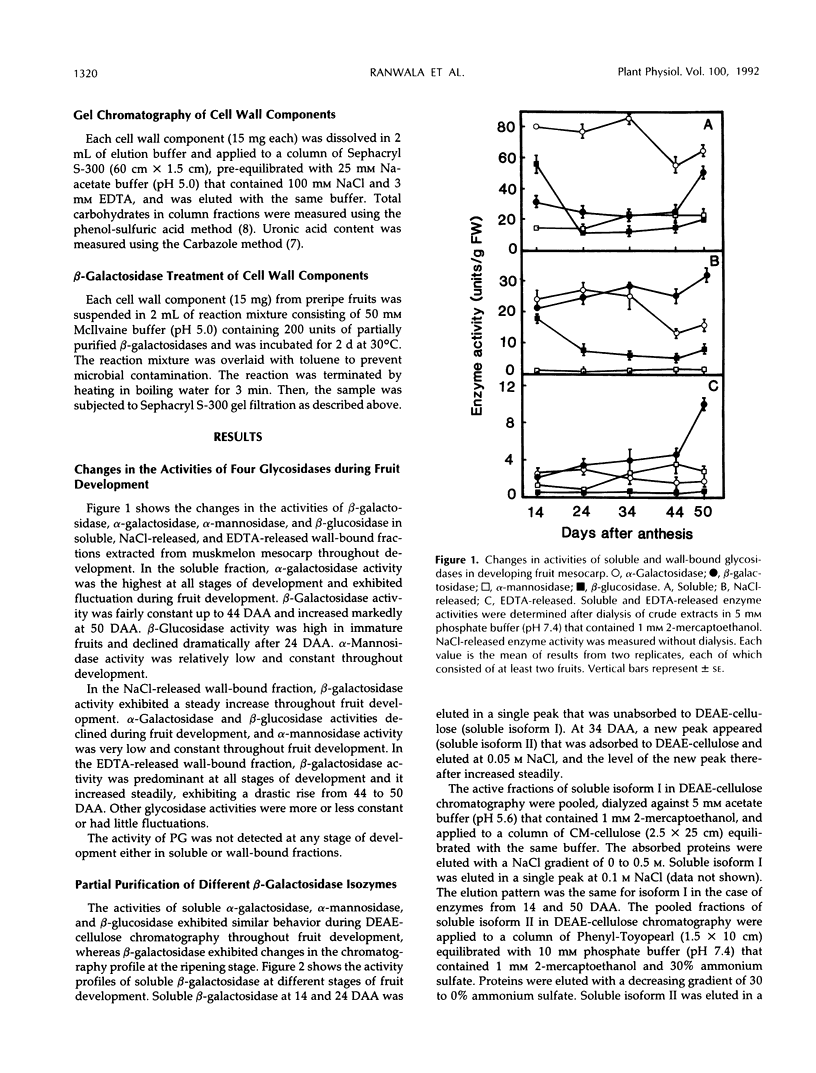
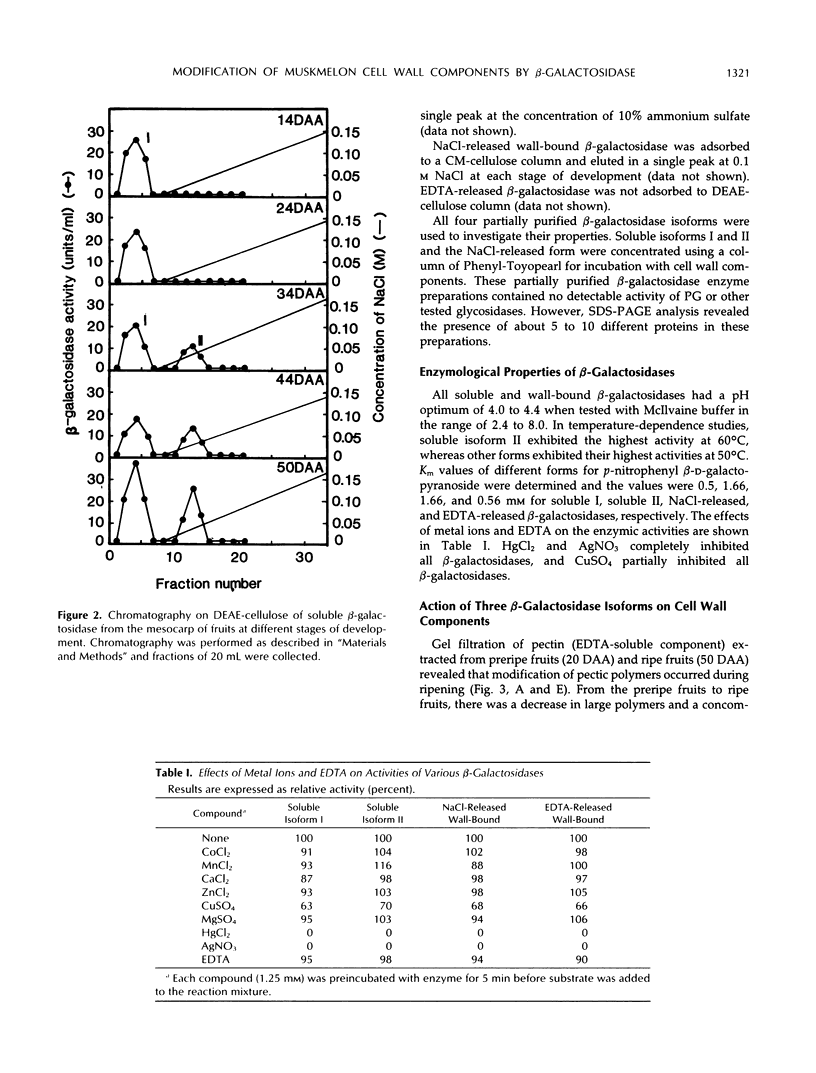
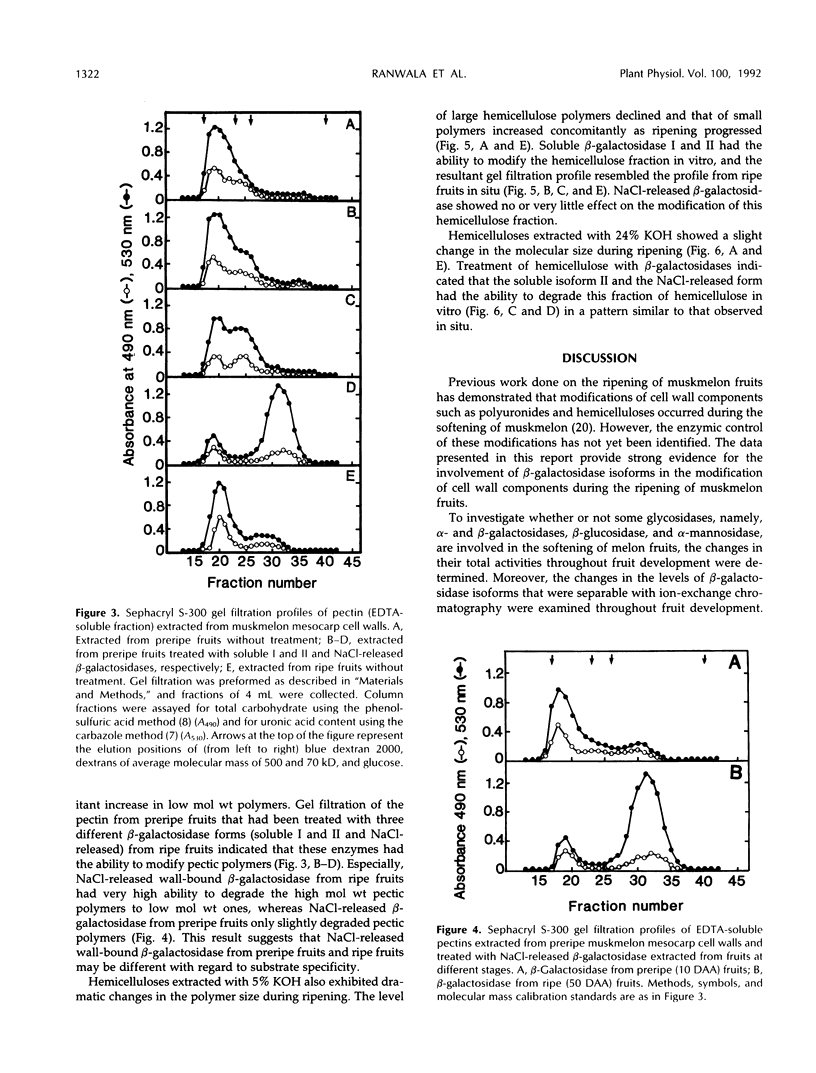
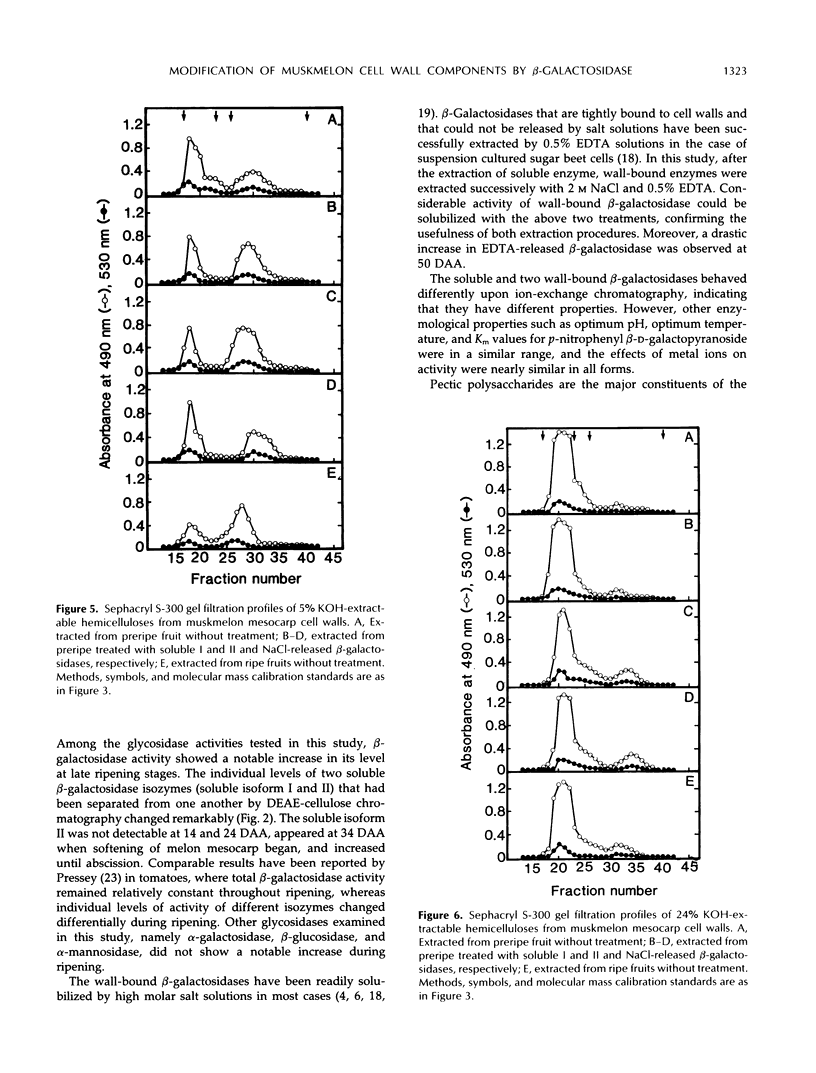
The changes in activities of soluble β-galactosidase and two forms of wall-bound β-galactosidases extracted with NaCl and EDTA were investigated throughout the development of muskmelon (Cucumis melo L. cv Prince) fruits. DEAE-cellulose ion-exchange chromatography of soluble β-galactosidase revealed the presence of two isoforms. Soluble isoform I was detected in all stages throughout the fruit development, whereas soluble isoform II appeared around 34 d after anthesis when fruit ripening initiated. Both NaCl- and EDTA-released β-galactosidase activities also increased as ripening proceeded. The soluble and wall-bound forms behaved differently upon ion-exchange chromatography. Enzymological properties such as optimum pH, optimum temperature, Km values for p-nitrophenyl β-d-galactopyranoside, and inhibition by metal ions were nearly similar in all forms. Molecular sizes of pectic polymers and hemicelluloses extracted from fruit mesocarp cell walls were shifted from larger to smaller polymers during ripening, as determined by gel filtration profiles. NaCl-released β-galactosidase from cell walls of ripe fruits had the ability to degrade in vitro the pectin extracted from preripe fruit cell walls to smaller sizes of pectin similar to those that were observed in ripe cell walls in situ. Both soluble isoform I and II were able to degrade in vitro the 5% KOH-extractable hemicellulose from preripe fruit cell walls to sizes of molecules similar to those that were observed in ripe cell walls in situ. Soluble isoform I and the NaCl-released form from ripe fruits were able to modify in vitro 24% KOH-extractable hemicellulose from preripe cell walls to sizes of molecules similar to those that were observed in ripe fruits in situ.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Dellapenna D., Alexander D. C., Bennett A. B. Molecular cloning of tomato fruit polygalacturonase: Analysis of polygalacturonase mRNA levels during ripening. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6420–6424. doi: 10.1073/pnas.83.17.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey P. M., Del Campillo E. Biochemistry of the multiple forms of glycosidases in plants. Adv Enzymol Relat Areas Mol Biol. 1984;56:141–249. doi: 10.1002/9780470123027.ch3. [DOI] [PubMed] [Google Scholar]
- Giovannoni J. J., DellaPenna D., Bennett A. B., Fischer R. L. Expression of a chimeric polygalacturonase gene in transgenic rin (ripening inhibitor) tomato fruit results in polyuronide degradation but not fruit softening. Plant Cell. 1989 Jan;1(1):53–63. doi: 10.1105/tpc.1.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOBSON G. E. Determination of polygalacturonase in fruits. Nature. 1962 Aug 25;195:804–805. doi: 10.1038/195804a0. [DOI] [PubMed] [Google Scholar]
- Keegstra K., Talmadge K. W., Bauer W. D., Albersheim P. The Structure of Plant Cell Walls: III. A Model of the Walls of Suspension-cultured Sycamore Cells Based on the Interconnections of the Macromolecular Components. Plant Physiol. 1973 Jan;51(1):188–197. doi: 10.1104/pp.51.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey R. beta-Galactosidases in Ripening Tomatoes. Plant Physiol. 1983 Jan;71(1):132–135. doi: 10.1104/pp.71.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner S. J., Walker J. E. Glycosidases in Cell Wall-degrading Extracts of Ripening Tomato Fruits. Plant Physiol. 1975 Jan;55(1):94–98. doi: 10.1104/pp.55.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauberman G., Schiffmann-Nadel M. Pectin methylesterase and polygalacturonase in avocado fruit at various stages of development. Plant Physiol. 1972 May;49(5):864–865. doi: 10.1104/pp.49.5.864. [DOI] [PMC free article] [PubMed] [Google Scholar]


