Abstract
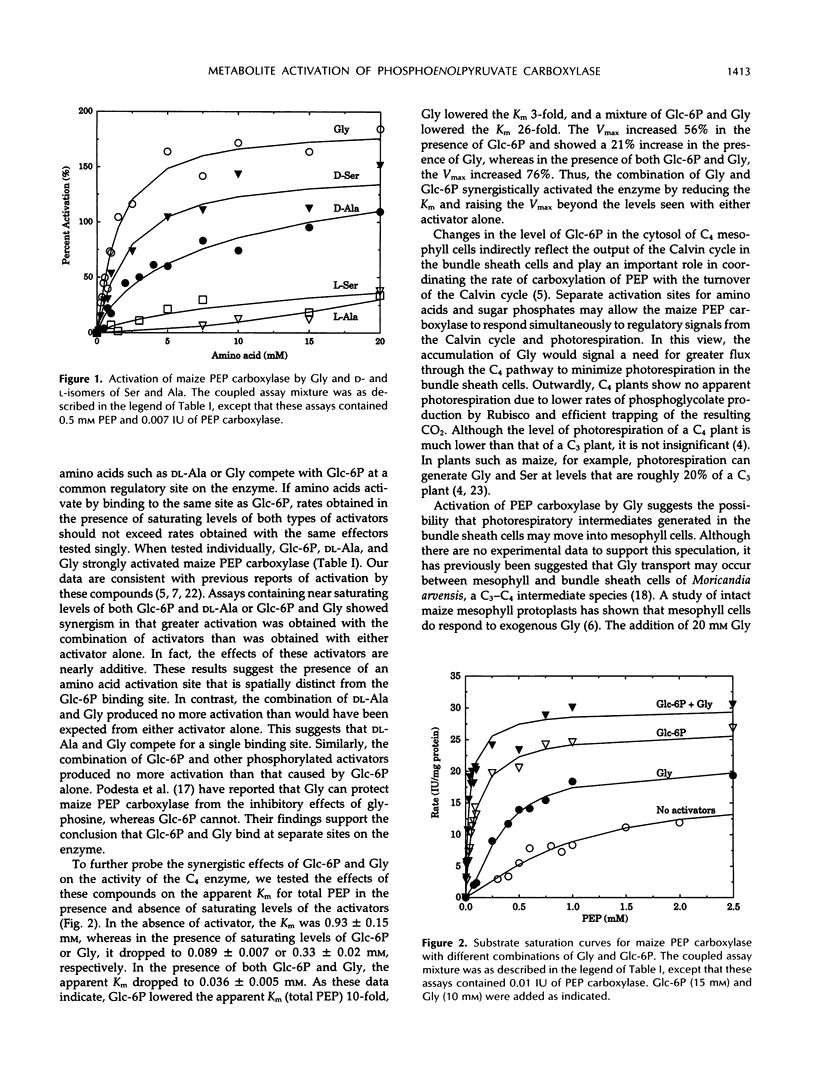
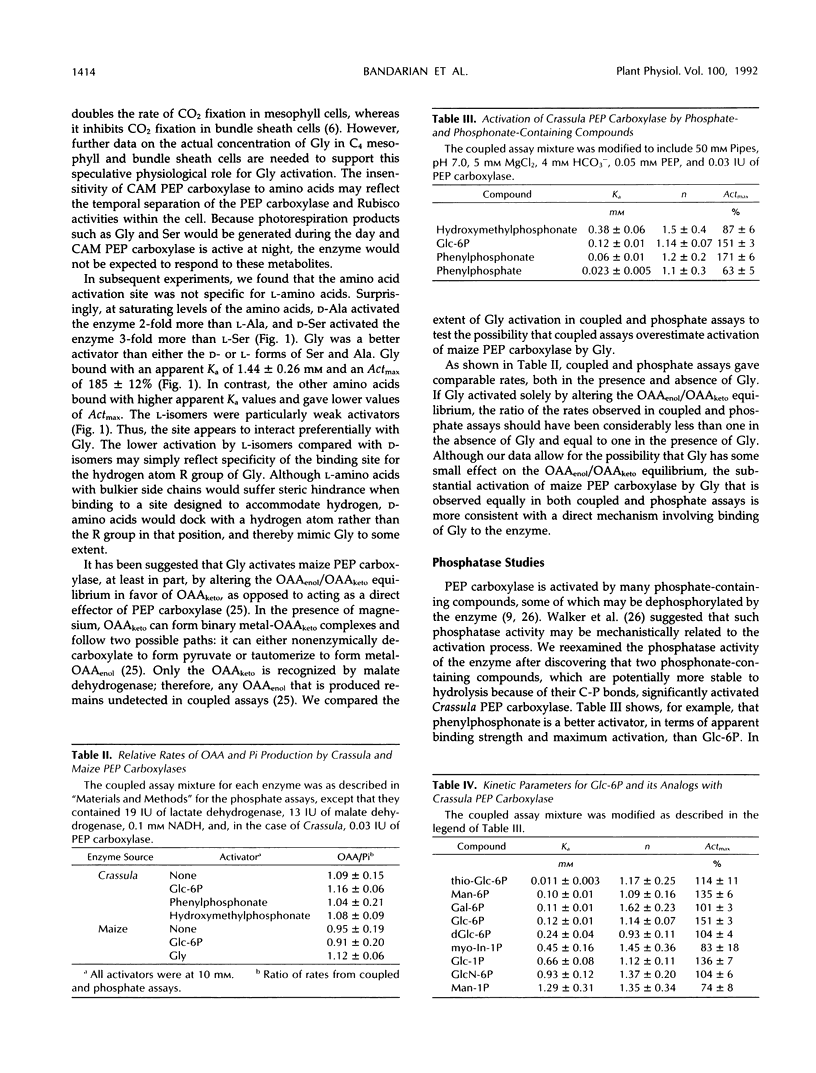
The effects of glycine, alanine, serine, and various phosphorylated metabolites on the activity of phosphoenolpyruvate (PEP) carboxylase from Zea mays and Crassula argentea were studied. The maize enzyme was found to be activated by amino acids at a site that is separate from the glucose 6-phosphate binding site. The combination of glycine and glucose 6-phosphate synergistically reduced the apparent Km of the enzyme for PEP and increased the apparent Vmax. Of the amino acids tested, glycine showed the lowest apparent Ka and caused the greatest activation. d-Isomers of alanine and serine were more effective activators than the l-isomers. Unlike the maize enzyme, the Crassula enzyme was not activated by amino acids. Activation of either the Crassula or maize enzyme by glucose 6-phosphate occurred without dephosphorylation of the activator molecule. Furthermore, the Crassula enzyme was activated by two compounds containing phosphonate groups whose carbon-phosphorus bonds were not cleaved by the enzyme. A study of analogs of glucose 6-phosphate with Crassula PEP carboxylase revealed that the identity of the ring heteroatom was a significant structural feature affecting activation. Activation was not highly sensitive to the orientation of the hydroxyl group at the second or fourth carbon positions or to the presence of a hydroxyl group at the second position. However, the position of the phosphate group was found to be a significant factor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Doncaster H. D., Leegood R. C. Regulation of phosphoenolpyruvate carboxylase activity in maize leaves. Plant Physiol. 1987 May;84(1):82–87. doi: 10.1104/pp.84.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garson L., Gray V. Regulation of phosphoenolpyruvate carboxylase from maize leaves by nitrate and alanine. Biochem Int. 1991 Jan;23(2):299–305. [PubMed] [Google Scholar]
- Gonzalez D. H., Iglesias A. A., Andreo C. S. Interaction of acetyl phosphate and carbamyl phosphate with plant phosphoenolpyruvate carboxylase. Biochem J. 1987 Jan 15;241(2):543–548. doi: 10.1042/bj2410543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J. A., Chollet R. Posttranslational regulation of phosphoenolpyruvate carboxylase in c(4) and crassulacean Acid metabolism plants. Plant Physiol. 1991 Apr;95(4):981–985. doi: 10.1104/pp.95.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R. S., Pedersen P. L. Characterization of phosphate efflux pathways in rat liver mitochondria. Biochem J. 1983 May 15;212(2):279–288. doi: 10.1042/bj2120279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton G. A., Fewson C. A., Wilkins M. B., Nimmo H. G. Purification, oligomerization state and malate sensitivity of maize leaf phosphoenolpyruvate carboxylase. Biochem J. 1989 Jul 15;261(2):349–355. doi: 10.1042/bj2610349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C. R., Rustin P., Wedding R. T. A simple and accurate spectrophotometric assay for phosphoenolpyruvate carboxylase activity. Plant Physiol. 1988 Feb;86(2):325–328. doi: 10.1104/pp.86.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikido T., Takanashi H. Glycine activation of PEP carboxylase from monocotyledoneous C4 plants. Biochem Biophys Res Commun. 1973 Jul 2;53(1):126–133. doi: 10.1016/0006-291x(73)91410-1. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Sotres R., Muñoz-Clares R. A. Kinetic evidence of the existence of a regulatory phosphoenolpyruvate binding site in maize leaf phosphoenolpyruvate carboxylase. Arch Biochem Biophys. 1990 Jan;276(1):180–190. doi: 10.1016/0003-9861(90)90025-t. [DOI] [PubMed] [Google Scholar]
- Rustin P., Meyer C., Wedding R. The Effect of Adenine Nucleotides on Purified Phosphoenolpyruvate Carboxylase from the CAM Plant Crassula argentea. Plant Physiol. 1988 Sep;88(1):153–157. doi: 10.1104/pp.88.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. H., Ku M. S., Edwards G. E. Activity of maize leaf phosphoenolpyruvate carboxylase in relation to tautomerization and nonenzymatic decarboxylation of oxaloacetate. Arch Biochem Biophys. 1986 Aug 1;248(2):489–501. doi: 10.1016/0003-9861(86)90502-3. [DOI] [PubMed] [Google Scholar]
- Wedding R. T., Black M. K., Meyer C. R. Activation of higher plant phosphoenolpyruvate carboxylases by glucose-6-phosphate. Plant Physiol. 1989 Jun;90(2):648–652. doi: 10.1104/pp.90.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Veau E. J., Burris J. E. Photorespiratory rates in wheat and maize as determined by o-labeling. Plant Physiol. 1989 Jun;90(2):500–511. doi: 10.1104/pp.90.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]


