Abstract
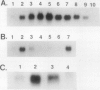
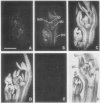
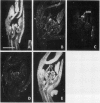
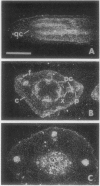
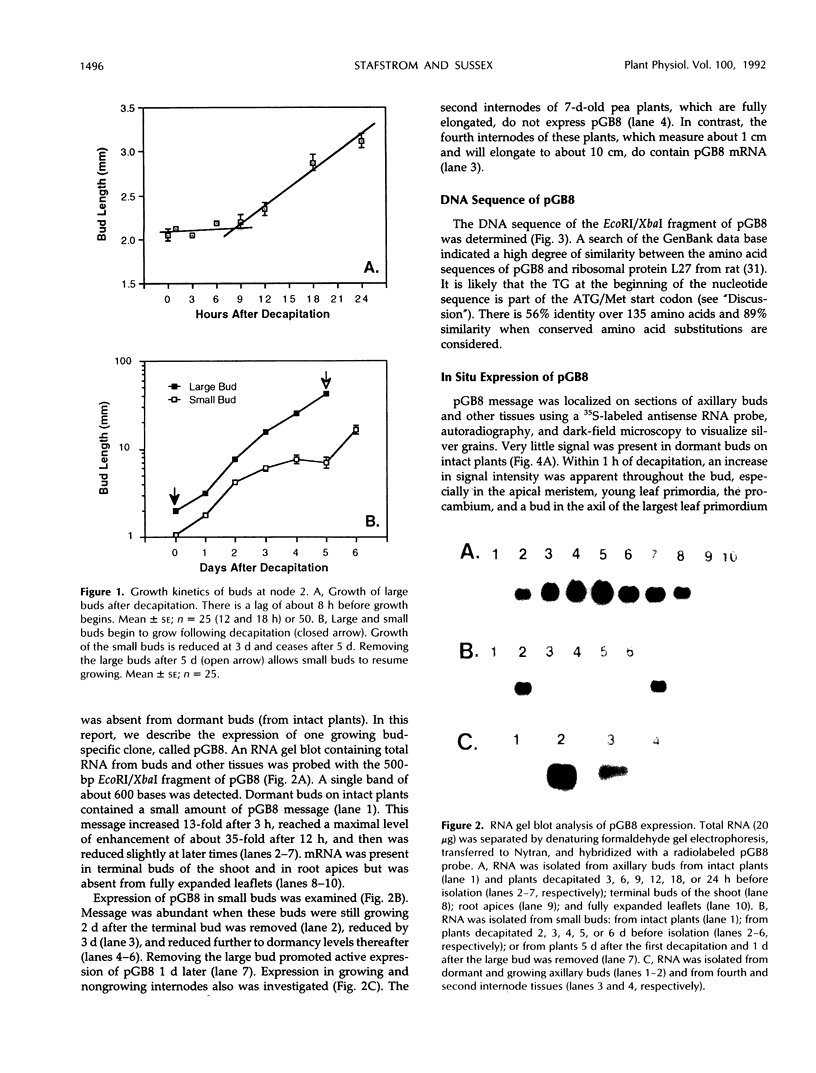
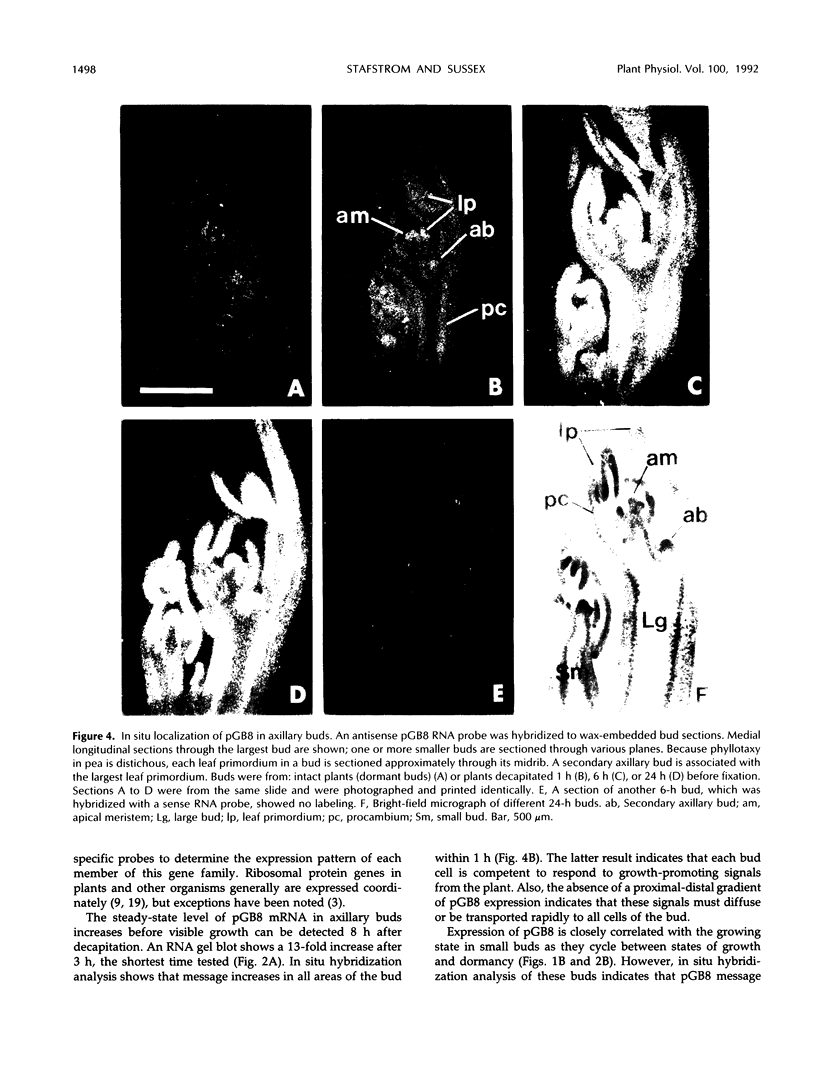
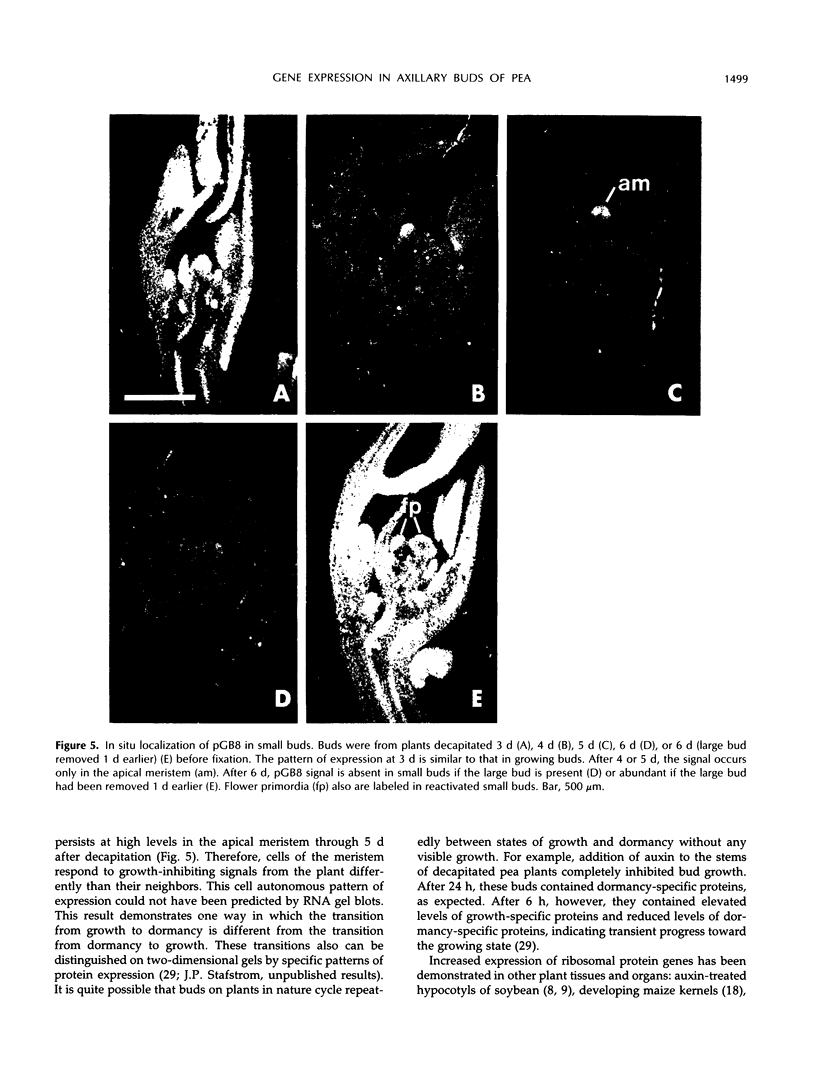
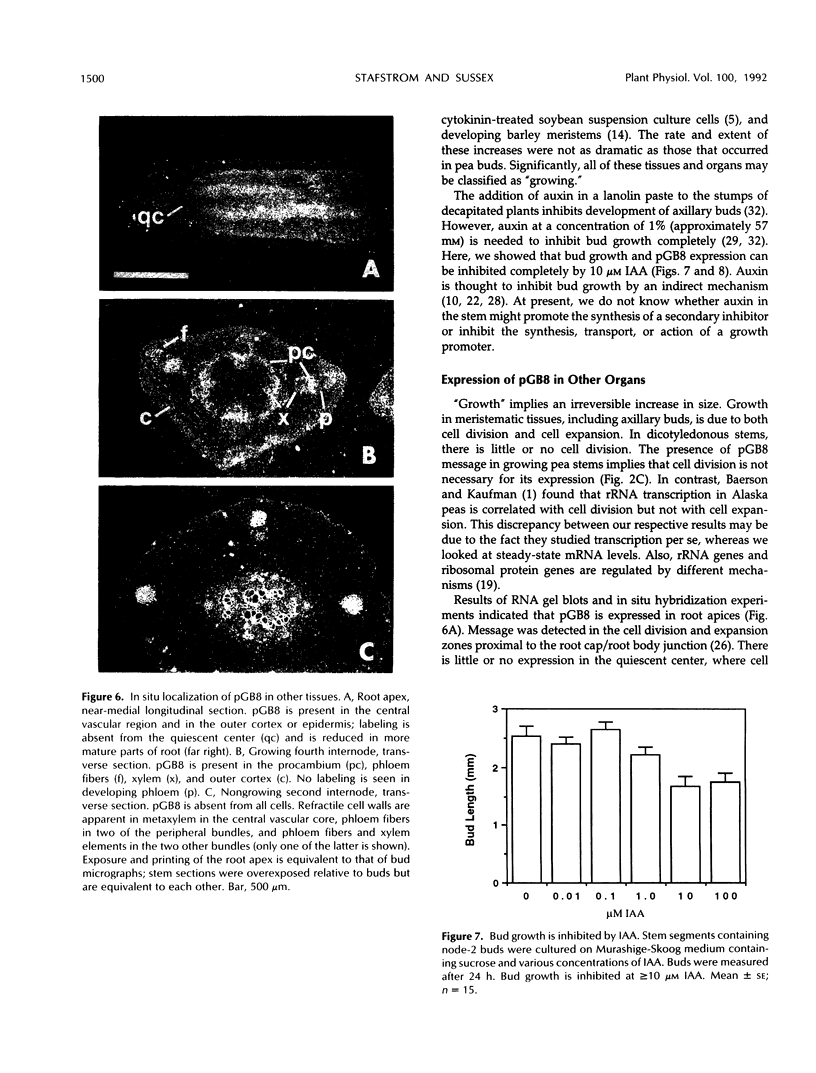
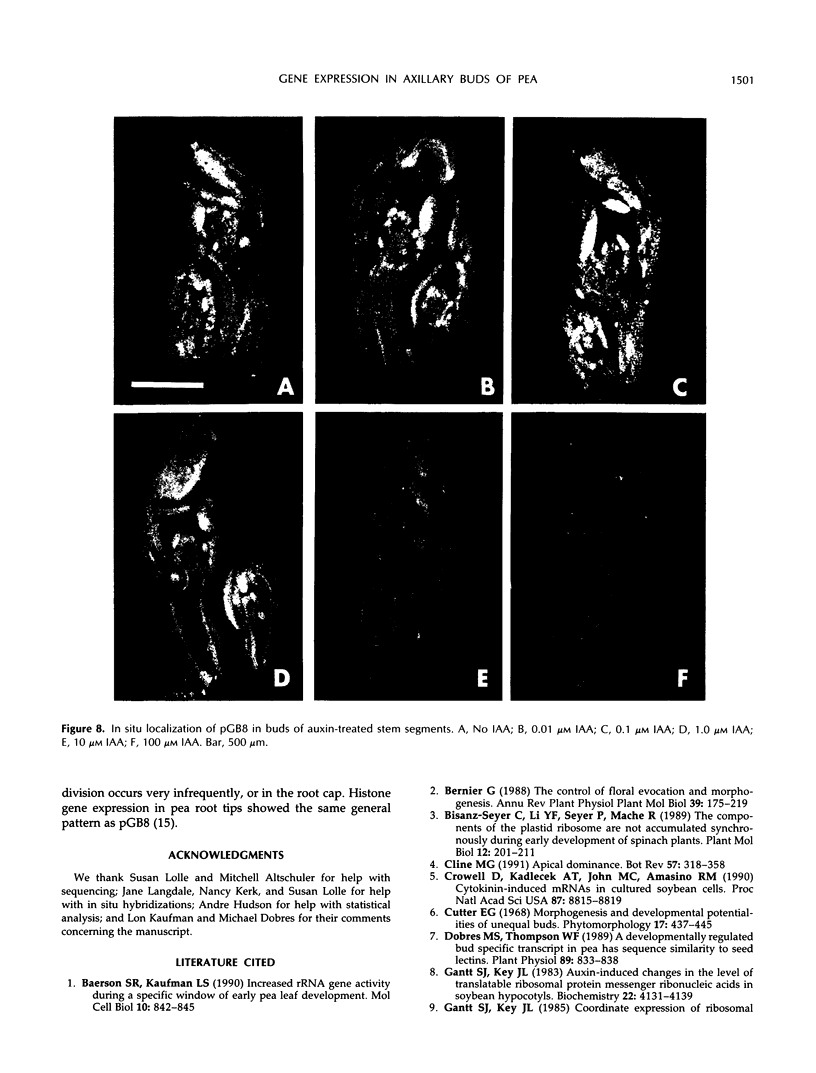
Axillary buds of intact pea seedlings (Pisum sativum L. cv Alaska) do not grow and are said to be dormant. Decapitation of the terminal bud promotes the growth of these axillary buds, which then develop in the same manner as terminal buds. We previously showed that unique sets of proteins are expressed in dormant and growing buds. Here we describe the cloning, sequencing, and expression of a cDNA clone (pGB8) that is homologous to ribosomal protein L27 from rat. RNA corresponding to this clone increases 13-fold 3 h after decapitation, reaches a maximum enhancement of about 35-fold after 12 h, and persists at slightly reduced levels at later times. Terminal buds, root apices, and elongating internodes also contain pGB8 mRNA but fully expanded leaflets and fully elongated internodes do not. In situ hybridization analysis demonstrates that pGB8 mRNA increases in all parts of the bud within 1 h of decapitation. Under appropriate conditions, growing buds can be made to stop growing and become dormant; these buds subsequently can grow again. Therefore, buds have the capacity to undergo multiple cycles of growth and dormancy. RNA gel blots show that pGB8 expression is reduced to dormancy levels as soon as buds stop growing. However, in situ hybridization experiments show that pGB8 expression continues at growing-bud levels in the apical meristem for 2 d after it is reduced in the rest of the bud. When cultured stems containing buds are treated with indoleacetic acid at concentrations ≥10 μm, bud growth and expression of pGB8 in the buds are inhibited.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baerson S. R., Kaufman L. S. Increased rRNA gene activity during a specific window of early pea leaf development. Mol Cell Biol. 1990 Feb;10(2):842–845. doi: 10.1128/mcb.10.2.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell D. N., Kadlecek A. T., John M. C., Amasino R. M. Cytokinin-induced mRNAs in cultured soybean cells. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8815–8819. doi: 10.1073/pnas.87.22.8815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobres M. S., Thompson W. F. A developmentally regulated bud specific transcript in pea has sequence similarity to seed lectins. Plant Physiol. 1989 Mar;89(3):833–838. doi: 10.1104/pp.89.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt J. S., Key J. L. Coordinate expression of ribosomal protein mRNAs following auxin treatment of soybean hypocotyls. J Biol Chem. 1985 May 25;260(10):6175–6181. [PubMed] [Google Scholar]
- Glazer R. I., Morris H. P. Multiple nuclear protein kinase activities in rat liver and hepatoma 3924A. Cancer Biochem Biophys. 1977;2(2):91–96. [PubMed] [Google Scholar]
- Keller B., Lamb C. J. Specific expression of a novel cell wall hydroxyproline-rich glycoprotein gene in lateral root initiation. Genes Dev. 1989 Oct;3(10):1639–1646. doi: 10.1101/gad.3.10.1639. [DOI] [PubMed] [Google Scholar]
- Kelly A. J., Zagotta M. T., White R. A., Chang C., Meeks-Wagner D. R. Identification of genes expressed in the tobacco shoot apex during the floral transition. Plant Cell. 1990 Oct;2(10):963–972. doi: 10.1105/tpc.2.10.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning A. J., Tanimoto E. Y., Kiehne K., Rost T., Comai L. Cell-specific expression of plant histone H2A genes. Plant Cell. 1991 Jul;3(7):657–665. doi: 10.1105/tpc.3.7.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdale J. A., Rothermel B. A., Nelson T. Cellular pattern of photosynthetic gene expression in developing maize leaves. Genes Dev. 1988 Jan;2(1):106–115. doi: 10.1101/gad.2.1.106. [DOI] [PubMed] [Google Scholar]
- Larkin J. C., Hunsperger J. P., Culley D., Rubenstein I., Silflow C. D. The organization and expression of a maize ribosomal protein gene family. Genes Dev. 1989 Apr;3(4):500–509. doi: 10.1101/gad.3.4.500. [DOI] [PubMed] [Google Scholar]
- Mager W. H. Control of ribosomal protein gene expression. Biochim Biophys Acta. 1988 Jan 25;949(1):1–15. doi: 10.1016/0167-4781(88)90048-6. [DOI] [PubMed] [Google Scholar]
- Medford J. I., Elmer J. S., Klee H. J. Molecular cloning and characterization of genes expressed in shoot apical meristems. Plant Cell. 1991 Apr;3(4):359–370. doi: 10.1105/tpc.3.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer S., Majewski D. M., Apel K. Early Changes in Gene Expression during the Transition from Vegetative to Generative Growth in the Long-Day Plant Sinapis alba. Plant Cell. 1990 Oct;2(10):953–961. doi: 10.1105/tpc.2.10.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussex I. M. Developmental programming of the shoot meristem. Cell. 1989 Jan 27;56(2):225–229. doi: 10.1016/0092-8674(89)90895-7. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Kuwano Y., Ishikawa K., Ogata K. Nucleotide sequence of cloned cDNA specific for rat ribosomal protein L27. Eur J Biochem. 1988 Apr 5;173(1):53–56. doi: 10.1111/j.1432-1033.1988.tb13965.x. [DOI] [PubMed] [Google Scholar]