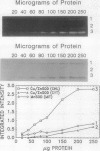
Abstract
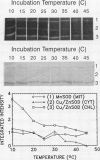
The activity of pea (Pisum sativum L.) Cu/Zn and Mn superoxide dismutase isoforms was evaluated across a range of temperatures from 10 to 45°C. Maximal activity of the Cu/Zn and Mn superoxide dismutase isoforms was observed at 10°C. Both cytoplasmic and chloroplast Cu/Zn superoxide dismutases exhibit a reduction in staining intensity with increasing temperatures. Mn superoxide dismutase, however, maintained a relatively constant staining intensity across the range of temperatures evaluated. An unrelated enzyme used as a control, malate dehydrogenase, exhibited the expected increase in staining activity with increasing temperatures. These results describe a unique response of a protection enzyme to temperature.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannister J. V., Bannister W. H., Rotilio G. Aspects of the structure, function, and applications of superoxide dismutase. CRC Crit Rev Biochem. 1987;22(2):111–180. doi: 10.3109/10409238709083738. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem. 1989 May 15;264(14):7761–7764. [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: inactivation of the enzyme. Biochemistry. 1975 Dec 2;14(24):5294–5299. doi: 10.1021/bi00695a010. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. Superoxide dismutase and peroxidase: a positive activity stain applicable to polyacrylamide gel electropherograms. Arch Biochem Biophys. 1977 Oct;183(2):511–515. doi: 10.1016/0003-9861(77)90386-1. [DOI] [PubMed] [Google Scholar]
- Tsang E. W., Bowler C., Hérouart D., Van Camp W., Villarroel R., Genetello C., Van Montagu M., Inzé D. Differential regulation of superoxide dismutases in plants exposed to environmental stress. Plant Cell. 1991 Aug;3(8):783–792. doi: 10.1105/tpc.3.8.783. [DOI] [PMC free article] [PubMed] [Google Scholar]