Abstract
Three independent Tn5-lac insertions in the S1 locus of Myxococcus xanthus inactivate the sglK gene, which is nonessential for growth but required for social motility and multicellular development. The sequence of sglK reveals that it encodes a homologue of the chaperone HSP70 (DnaK). The sglK gene is cotranscribed with the upstream grpS gene, which encodes a GrpE homologue. Unlike sglK, grpS is not required for social motility or development. Wild-type M. xanthus is encased in extracellular polysaccharide filaments associated with the multimeric fibrillin protein. Mutations in sglK inhibit cell cohesion, the binding of Congo red, and the synthesis or secretion of fibrillin, indicating that sglK mutants do not make fibrils. The fibR gene, located immediately upstream of the grpS-sglK operon, encodes a product which is predicted to have a sequence similar to those of the repressors of alginate biosynthesis in Pseudomonas aeruginosa and Pseudomonas putida. Inactivation of fibR leads to the overproduction of fibrillin, suggesting that M. xanthus fibril production and Pseudomonas alginate production are regulated in analogous ways. M. xanthus and Pseudomonas exopolysaccharides may play similar roles in a mechanism of social motility conserved in these gram-negative bacteria.
Many free-living microbes can move across surfaces without the use of flagella by mechanisms that have yet to be understood. Genetically, the phenomenon of gliding motility appears to be at least as complex as that of flagellar motility. The most-detailed genetic analysis of the nature of gliding has focused on the study of the complex soil bacterium Myxococcus xanthus. For M. xanthus, gliding motility plays critical roles both in its social feeding behaviors during vegetative growth and in its elaborate process of multicellular development triggered by starvation.
M. xanthus exercises two forms of gliding motility called A, or adventurous, and S, or social, motility, specified by separate subsets of genes (21). At least 1% of its 9.5-Mb genome appears to be dedicated to the production of essential components of these two motility systems. A combination of mutational and sequence analyses have revealed more than 50 different motility genes after several mutant hunts that have by no means saturated their desired targets (21, 22, 31, 55, 59).
Adventurous and social motility play different, independent roles during the vegetative growth and development of M. xanthus. Adventurous motility is required for the gliding of single cells in isolation, depends primarily on contacts between cells and a solid surface, and has an ancillary role in development, because only a minority of A mutants are defective in development. In contrast, social motility involves the movement of groups of cells, requires that cells remain in close proximity to move, and has a central role in development, because almost all S mutants are defective in development. M. xanthus strains with A mutations retain social motility, and strains with S mutations retain adventurous motility. However, double-mutant strains with one of the hundreds of possible pairs of a single A mutation and a single S mutation are not motile (21). We have used this double-mutant phenotype to isolate the mutants (31) described in this study.
S motility and the starvation-induced morphogenesis of fruiting structures depend on three different extracellular compartments, pili, fibrils, and O antigen. Pili are cell-length (ca. 10-μm-long) polymers of the pilin protein approximately 8 nm in diameter and located at the cell poles (41). Pilin is secreted by a type IV mechanism (59), resembling that required for the assembly of pili and the secretion of virulence factors in Pseudomonas aeruginosa, which displays a form of social gliding called twitching motility (35). Fibrils are peritrichous tubules 30 nm in diameter that can be as long as 10 cells, comprised of polysaccharide polymers associated with a multimeric protein, IFP-1 (4). We designate this protein fibrillin, because Behmlander and Dworkin (5) have shown that the majority of fibril-associated protein consists of multimers of a single small subunit. dsp mutants of M. xanthus do not make fibrils and are defective in S motility (49). The M. xanthus O-antigen composition is typical of gram-negative bacteria, including a lipid A anchor, a 2-keto-3-deoxyoctanate core, and a repeating polysaccharide unit comprised of mannose, galactose, glucosamine, and perhaps glucose and/or rhamnose (18, 40, 42, 51). Recently, Bowden and Kaplan (7) have shown that mutants of M. xanthus defective in the production of O antigen are also defective in S motility. All three components of social (S) motility are also required for the complex developmental cycle of M. xanthus.
In this study, we describe the detailed characterization of three adjacent genes, the cotranscribed grpS and sglK genes and the upstream fibR gene. The sglK gene, required for S motility, was identified by a cluster of Tn5-lac insertions. These insertions map to a region of the M. xanthus genome, the S1 locus (31, 32), which also includes the dsp genes required for fibril production. We find that the grpS and sglK genes encode homologues of the chaperones GrpE and DnaK, two of the three components of chaperone machines that play key roles in protein and polysaccharide secretion. Like the dsp mutants, mutants defective in sglK function do not make fibrils, suggesting that the GrpS, SglK, and their missing J-domain partner regulate the expression or secretion of extracellular fibrils. The fibR gene encodes a product which is predicted to have a sequence similar to those of the histone-like repressors of alginate exopolysaccharide biosynthesis in Pseudomonas spp., which acts as a negative regulator of exopolysaccharide production.
MATERIALS AND METHODS
Bacterial strains.
Escherichia coli JM107 (60) was used for the construction of plasmids and the preparation of plasmid DNA. Plasmids were introduced into JM107 by electroporation (52). Derivatives of JM107 with plasmids were grown in Luria-Bertani medium supplemented with ampicillin (100 μg/ml), kanamycin (40 μg/ml), or spectinomycin (50 μg/ml) and streptomycin sulfate (50 μg/ml). Plates used for screening β-galactosidase activity also contained 10 μg of isopropyl-β-d-thiogalactopyranoside per ml and 40 μg of 5-bromo-4-chloro-3-indolyl-β-d-thiogalactopyranoside per ml. M. xanthus strains are derivatives of the wild-type strain DK1622 (23) and are listed in Table 1. CTPM liquid medium (1% Casitone, 10 mM Tris [pH 7.6], 1 mM potassium phosphate [pH 7.5], 5 mM MgSO4) was used for growth of M. xanthus; TPM medium is CTPM without Casitone. Derivatives of M. xanthus MxH1379 (aglB1 sglK::Ω1252Tc) (31) with integrated plasmids were grown in CTPM medium with kanamycin (40 μg/ml). Strains with a Spr Smr substitution of plasmid pGB2 (11) for portions of grpS and sglK or an insertion of pGB2 in fibR were selected on medium with spectinomycin (0.8 mg/ml) and streptomycin sulfate (1.0 mg/ml). Plasmids were introduced into M. xanthus by electroporation (24). Antibiotics and chemicals were from Sigma or Aldrich. Oligonucleotides used for plasmid construction and mutagenesis were made by Biosource Inc. Restriction endonucleases and DNA-modifying enzymes were from New England Biolabs and were used under recommended conditions. Standard methods were used for the construction of plasmids.
TABLE 1.
M. xanthus strains and plasmids
| Bacterial strain or plasmid | Relevant genotype or phenotypea | Source or reference |
|---|---|---|
| Bacteria | ||
| DK1622 | Wild type | 23 |
| MxH1223 | sglK::Tn5-lacΩ1222 | 31 |
| MxH1253 | sglK::Tn5-lacΩ1252 | 31 |
| MxH1226 | sglK::Tn5-lacΩ1225 | 31 |
| MxH1256 | sglK::Tn5-lacΩ1255 | 31 |
| MxH1379 | aglB1 sglK::Tn5-lacΩ1252Tc | 31 |
| MxH1133 | aglB1 sglK::Tn5-lacΩ1252Tc (pPLH343) | This work |
| MxH1134 | aglB1 sglK::Tn5-lacΩ1252Tc (pAY844) | This work |
| MxH1135 | aglB1 sglK::Tn5-lacΩ1252Tc (pAY846) | This work |
| MxH1136 | aglB1 sglK::Tn5-lacΩ1252Tc (pAY847) | This work |
| MxH1137 | aglB1 sglK::Tn5-lacΩ1252Tc (pAY1081) | This work |
| MxH1138 | aglB1 sglK::Tn5-lacΩ1252Tc (pAY1082) | This work |
| MxH1139 | grpS-sglK::pGB2 | This work |
| MxH1140 | grpS-sglK::pGB2 (pPLH343) | This work |
| MxH1141 | grpS-sglK::pGB2 (pAY844) | This work |
| MxH1142 | grpS-sglK::pGB2 (pAY846) | This work |
| MxH1143 | grpS-sglK::pGB2 (pAY847) | This work |
| MxH1144 | grpS-sglK::pGB2 (pAY1081) | This work |
| MxH1145 | grpS-sglK::pGB2 (pAY1082) | This work |
| MxH1146 | fibR::pGB2 | This work |
| MxH1259 | S2::Ω1258 | 31 |
| Plasmids | ||
| pBLUESCRIPT KSII+ | AprlacZα | Stratagene |
| pLITMUS28 | AprlacZα | New England Biolabs |
| pUC18 | AprlacZα | 60 |
| pGB2 | Spr Smr | 11 |
| pAY647 | Apr Kmr (1–3390) | This work |
| pAY648 | Apr Kmr (1–3179) | This work |
| pAY649 | Apr (1–3390) | This work |
| pAY673 | Apr (1–3179) | This work |
| pAY685 | Apr Kmr (3390–6299) | This work |
| pAY670 | Apr (2840–3390) | This work |
| pAY692 | Apr (1–1457) | This work |
| pAY693 | Apr (1457–3505) | This work |
| pAY694 | Apr (1–3505) | This work |
| pAY696 | Apr (734–3505) | This work |
| pAY697 | Apr (1075–3505) | This work |
| pPLH343 | Kmrint+-attP+ | 33 |
| pAY894 | Kmr (1–3505) int+-attP+ | This work |
| pAY896 | Kmr (734–3505) int+-attP+ | This work |
| pAY897 | Kmr (1075–3505) int+-attP+ | This work |
| pAY1060 | Apr Spr Smr (1–1074 and 2457–3505) grpS-sglK::pGB2 | This work |
| pAY703 | KmrmglBA+ | 33 |
| pAY1081 | KmrmglBA+ int+-attP+ | 33 |
| pAY1072 | Kmr (1682–3505) | This work |
| pAY1079 | Apr (1682–3505) | This work |
| pAY1082 | KmrmglBA+-sglK+ int+-attP+ | This work |
| pAY1074 | Apr Spr Smr (1–240 and 240–3505) fibR::pGB2 | This work |
Numbers in parentheses are the coordinates of the S1 region cloned into plasmid vectors, according to GenBank accession no. U83800 and unpublished results. The substitution of the EcoRI-PstI backbone of pGB2 for a region internal to the grpS and sglK genes is designated grpS-sglK::pGB2, and the insertion of the entire pGB2 sequence in fibR is designated fibR::pGB2. Hosts carrying plasmids that have integrated due to site-specific recombination between the subcloned myxophage Mx8 int-attP genes (44) and the chromosomal attB locus, MxH1133-1138 and MxH1140-1144, are formally defective lysogens; their integrated plasmids (defective prophages) are shown in parentheses.
Cloning of M. xanthus genomic DNA flanking Tn5-lac insertions in sglK.
To subclone the regions upstream of sglK::Tn5-lac insertions Ω1222 and Ω1252, genomic DNA was isolated from vegetative cultures of strains MxH1223 and MxH1253, respectively, by the method for the rapid isolation of myxophage Mx8 DNA (33). Each strain was grown to a density of 5 × 108/ml in CTPM medium, and cells (2 ml) were pelleted by low-speed centrifugation and resuspended in one-fifth volume of distilled water immediately prior to DNA isolation. Approximately 1 μg of genomic DNA was mixed with 0.5 μg of pBluescript KSII+ DNA, and mixtures were cleaved with 10 U of XhoI for 12 h in 20 μl at 37°C. XhoI was heat inactivated, and the mixtures were placed on a 0.025-μm-pore-size filter (Millipore) and dialyzed against 1,000 volumes of distilled water for 30 min. Mixtures were then treated with T4 DNA ligase at 25°C for 4 to 12 h, incubated at 65°C for 20 min, and dialyzed prior to electroporation into E. coli host JM107. Approximately 2 × 10−6 Apr electroporants were also Kmr; these were found to carry plasmids pAY647 (from MxH1223) and pAY648 (from MxH1253) with the lacZ gene of Tn5-lac in an orientation inverted with respect to that of the 5′ portion of the lacZ gene carried by the pBluescript vector. Plasmids pAY647 and pAY648 were cleaved with BamHI to liberate smaller (ca. 3-kb) fragments carrying 50 bp of the outer end of IS50 from Tn5-lac joined to flanking M. xanthus DNA, and these fragments were ligated to the BamHI site of pLITMUS38 (New England Biolabs) to make plasmids pAY649 and pAY673, respectively.
The region of sglK downstream of the Ω1222 insertion was recovered in a similar way after cleavage of a mixture of MxH1223 and pLITMUS28 DNA with EcoRI. pAY685 is the Kmr plasmid with the EcoRI fragment containing this Tn5-lac chromosome junction. The region of M. xanthus DNA upstream of the Ω1225 insertion in strain MxH1226 was amplified by PCR. DNA derived from MxH1226 was used as template with primers 5′ AAAGAATTCGACGTCCTCCTGCTGGA and CCCAAGCTTGGATTCGCTGGAAAACGGGAAA to amplify a product of about 550 bp, and the product was cleaved with EcoRI and HindIII and ligated to pLITMUS28 to make pAY670. Amplification of template MxH1223 with the same primer pair yielded a product of identical size in a parallel reaction.
Reconstruction of the grpS-sglK operon.
A plasmid with the wild-type grpS-sglK operon was reconstructed in three steps. The smaller XhoI-PstI fragment of pAY673 was ligated to the same sites of pLITMUS28 to make pAY692; this insert (bp 1 to 1,457) includes the entire grpS gene and the 5′ half of sglK. The 3′ half of sglK was amplified from strain DK6204 DNA with primers GGACCGCATGCGCTGCA and AAAAAAGCTTCAGCTCGCCTGGCCCGTGTA, and the product was cleaved with PstI and HindIII and ligated to the same sites of pLITMUS28 to make pAY693. The PstI-HindIII fragment of pAY693 was ligated to the same sites of pAY692 to make pAY694, which carries the entire grpS-sglK operon (bp 1 to 3505).
Plasmids used to map the sglK promoter by complementation tests.
Two deletion derivatives of pAY694 were constructed to map the sglK promoter. Plasmid pAY694 was cleaved with SmaI and BglII, the BglII ends were filled in with DNA polymerase I large fragment, and the larger fragment was ligated to make pAY696, which has an insert with bp 734 to 3505 of the grpS-sglK sequence. pAY697 (bp 1075 to 3505) was made in a similar way after cleavage of plasmid pAY694 with BglII and EcoRI. Plasmids pAY844, pAY846, and pAY847 are Kmr derivatives of pAY694, pAY696, and pAY697, respectively, made by ligating SpeI-HindIII inserts from the latter plasmids to the XbaI and HindIII sites of plasmid pPLH343 (33), which carries the myxophage Mx8 int-attP site-specific recombination functions. Plasmid pAY1060 is a derivative of pAY694 that carries a substitution of the majority of Spr Smr plasmid pGB2 (11) for a region spanning the distal two-thirds of grpS and the proximal two-thirds of sglK. It was made by cleavage of both pAY694 and pGB2 with EcoRI and PstI and ligation of the larger products of cleavage.
To determine whether the grpS gene is essential for development, we constructed plasmid pAY1082, which expresses sglK from the mgl promoter. The sglK coding sequence was amplified with primers MSG3 (AAAGAATTCTCAGCTCGCCTGGCCCGTGTAGAT) and MSG5 (CCCGGTACCATGAGGAGGTTTAGTATGGGCAAGGTGATTGGAATCGACCTT), using pAY694 as the template. The amplified product was cleaved with Acc65I and EcoRI and ligated to the same sites of pAY703 (33) to make pAY1072. The EcoRI-HindIII fragment of pAY1072 was subcloned first into pUC18 (60) to make pAY1079 and then from pAY1079 into pPLH343 (33) to make pAY1082. pAY1082 expresses sglK as the third gene of the constitutive mglBA operon and carries the phage Mx8 int-attP genes. We also constructed the otherwise isogenic derivative of pAY1082, pAY1081, which lacks the sglK gene, by subcloning the EcoRI-HindIII fragment from pAY703 into pPLH343.
Disruption of the fibR gene.
To construct a disruption of fibR, plasmid pAY694, which carries a 3.5-kb insert of M. xanthus DNA with the full-length fibR, grpS, and sglK genes was cleaved at the unique AgeI site within fibR and ligated with XmaI-cleaved plasmid pGB2 to make plasmid pAY1074. pAY1074 was cleaved with SmaI, and the linear plasmid DNA was used to electroporate wild-type strain DK1622. About one-half of the Spr Smr electroporants were found to be Kms. DNA isolated from two Spr Smr Kms electroporants was amplified with primers TM5 (CCCCAAGCTTGGTACCACTAGTTATTTGCCGACTACCTTGGTGA) and TM6 (AAAAAAGCTTCCATGGTTTCATGGCTTGTTATGACTG) to confirm the presence of the aadA gene.
Assays for β-galactosidase activity.
β-Galactosidase assays were performed on vegetative cells, and cells were harvested from developmental assays as described previously (31, 32). To assay β-galactosidase activities in heat-shocked cells, cells were grown to a density of 5 × 108/ml in CTPM medium at 32°C, shifted to 42°C, and sampled at various times after temperature shift. Activities are expressed in nanomoles per minute per milligram of total protein, assayed by the method of Bradford (8).
Labeling M. xanthus proteins with [35S]methionine.
To label proteins made by vegetative M. xanthus cells with [35S]methionine, cells were grown in CTPM medium to a density of 5 × 108/ml at 32°C, washed twice in an equal volume of TPM buffer prewarmed to 32°C, and resuspended in an equal volume of TPM buffer at 32 or 42°C. After incubation of cells for 10 min, 50 μCi of [35S]methionine (5 μl) was added to 108 cells (200 μl) and incubation was continued for 5 min, at which time 100 μl of 2× sodium dodecyl sulfate (SDS) sample buffer was added to stop the incorporation of label. Samples were immediately heated to 100°C for 3 min, and 20-μl aliquots were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) on 10% acrylamide slab gels (29). Gels were dried on Whatman 3MM paper, and exposures were made by direct contact with Kodak XAR5 film.
Congo red binding assays.
Wild-type and mutant M. xanthus cells were grown to a density of 5 × 108 cells/ml in CTPM medium and harvested by centrifugation at 10,000 × g. Cells were suspended in 2 ml of agglutination buffer (10 mM morpholinepropanesulfonic acid [MOPS], 1 mM MgCl2, 1 mM CaCl2 [pH 6.8]) (3) to a density of 5 × 108 cells/ml. Congo red (2 mg/ml in agglutination buffer) was added to a 1-ml aliquot of cells to give a final concentration of 20 μg/ml; an equivalent amount of agglutination buffer was added to the remaining 1-ml aliquot of cells. Samples were incubated at room temperature for 30 min. Spectra were taken from 400 to 700 nm with a Perkin-Elmer λ-12 dual-beam UV-visible light spectrophotometer. The spectrum obtained from cells alone was subtracted from the spectrum obtained from cells with Congo red to determine the extent of binding. The absorption maxima for difference spectra were compared with the absorption maximum for Congo red alone in agglutination buffer.
Immunoblot assays for IFP-1.
Wild-type M. xanthus cells produce fibrils that are carbohydrate polymers associated with multimers of integral fibrillin protein (IFP-1). Mouse monoclonal antibody MAb2105 (5) was used to show that mutations in the S1 cluster prevent the production of mature IFP-1. Wild-type M. xanthus and mutants were grown in CTPM medium to a density of 5 × 108 cells/ml), harvested by centrifugation, and suspended in 0.2 volume of TPM buffer. An aliquot was removed and used for the determination of total protein concentration by the method of Bradford (8). An equal volume of 2× SDS sample buffer was added to the remaining cell suspension and heated at 90°C for 10 min. A volume corresponding to 7 μg of protein of each sample was separated by electrophoresis on an 8% polyacrylamide–SDS–Tricine gel (46). The gel was soaked in Towbin’s transfer buffer (53) for 20 min, and proteins were transferred to nitrocellulose by using a Bio-Rad semidry transfer cell. The nitrocellulose was probed with a 1:1,000 dilution of MAb2105 followed by a secondary horseradish peroxidase anti-mouse antibody at a 1:1,200 dilution; MAb2105 was a gift from Marty Dworkin. Binding was visualized with the Amersham ECL reagent by blocking and wash procedures recommended by the manufacturer. The apparent molecular mass of cross-reacting material was estimated by comparison of its mobility with the mobilities of proteins in the Kaleidoscope prestained high-molecular-mass protein standards (Bio-Rad) and Benchmark protein standards (Gibco-BRL).
Developmental assays.
To initiate development, derivatives of DK1622 were grown to a density of 5 × 108/ml in CTPM medium at 32°C, concentrated by low-speed centrifugation, and resuspended in 0.1 volume of TPM buffer. Multiple spots (20 μl) were made on TPM plates (1.5% agar), and plates were incubated at 32°C for 120 h. To measure viable spores, five 20-μl spots were harvested after incubation at 50°C for 2 h, scraped into 1 ml of TPM, and sonicated for 10 s at 20 W on a Microson cell dismembranator to disperse spores. Serial dilutions of spore suspensions were plated on CTPM plates and scored for growth after 72 h.
DNA sequence analysis.
Templates used for sequence analysis included plasmids pAY649, pAY673, pAY685, pAY670, and some smaller derivatives. Inserts were sequenced by the method of Sanger et al. (45), with M13 forward and reverse primers and additional primers, by Commonwealth Biotechnologies, Inc., Richland, Va. Sequencing runs were resolved on an ABI Prism model 377 automated sequencing apparatus. The sequence of each strand of each template was determined completely.
Nucleotide sequence accession number.
The sequence of the 4,849-bp region containing grpS and sglK has been assigned GenBank accession no. U83800.
RESULTS
Tn5-lac insertions in the S1 locus of M. xanthus disrupt the sglK gene, which encodes a homologue of HSP70.
In previous reports, we have described the isolation and initial genetic characterization of nine independent insertions of transposon Tn5-lac (designated by the symbol Ω) that disrupt functions required for social motility in M. xanthus. These nine insertions define three separate gene clusters, S1, S2, and S3. Insertions within each cluster are linked in generalized transduction crosses mediated by myxophage Mx4, suggesting that insertions within each cluster lie within the same 20-kb segment of the M. xanthus genome (31). Five of these nine insertions define the S1 cluster, within which four insertions, Ω1222, Ω1225, Ω1252, and Ω1601, are cotransduced with efficiencies of >95%, suggesting that they map within one gene or adjacent genes. The fifth insertion in the S1 cluster, Ω1255, is 15% linked with Ω1252, suggesting that it lies about 10 kb from the other four insertions.
To characterize the S1 locus in molecular detail, we cloned segments of DNA flanking three of the four tightly linked insertions within this cluster. Fragments of DNA with both the selectable kanamycin resistance (Kmr) determinant of Tn5-lac and M. xanthus genomic DNA adjacent to these insertions were cloned into ampicillin-resistant (Apr) plasmid vector pBluescript SKII+ or pLITMUS28. Apr Kmr plasmid subclones with M. xanthus DNA upstream of Ω1222, Ω1225, and Ω1252 were recovered, as was a subclone of DNA downstream of Ω1222. The sequences of the entire stretches of subcloned M. xanthus DNA upstream and downstream of Ω1222 were determined, as were the sequences of the junctions of insertions Ω1225 and Ω1252 with upstream DNA. The DNA sequence of this portion of the S1 locus reveals that all three insertions disrupt a single open reading frame, designated sglK (Fig. 1). The insertions Ω1222 and Ω1225 map at the same site, whereas the third, Ω1252, maps to a site 211 bp upstream of the other two. This result is consistent with the finding that the first two insertions are 97 to 99% linked with the third by generalized transduction with myxophage Mx4 (31). Surprisingly, an 8-bp repeat (bp 3390 to 3397) is found at the junctions of Ω1222, unlike the 9-bp repeats typically found at the junctions of Tn5 insertions in enteric host E. coli (6).
FIG. 1.
Physical map of the S1 region of M. xanthus. The locations of the fibR, grpS, sglK, and kinS genes are shown below the physical map. The black flag indicates the position of the insertion of Spr Smr plasmid pGB2 into the fibR gene on plasmid pAY1074 used to make MxH1146, and the white flags show the positions of Tn5-lac insertions Ω1252, Ω1222, and Ω1225. The substitution of the majority of pGB2 DNA for portions of the grpS and sglK genes, designated ΔgrpS-sglK in the text, is shown as a hatched bar below the genes. Restriction sites on the physical map are for XhoI (X; bp 1 and 4944), AgeI (A; bp 240), SmaI (S; bp 733), EcoRI (E; bp 1074), and PstI (P; bp 2457); a PstI site at bp 4824 and SmaI sites at bp 3911 and 4156 are not shown. The arrows at the top of the figure show the direction of transcription.
SglK and GrpS are members of the heat shock family of chaperones.
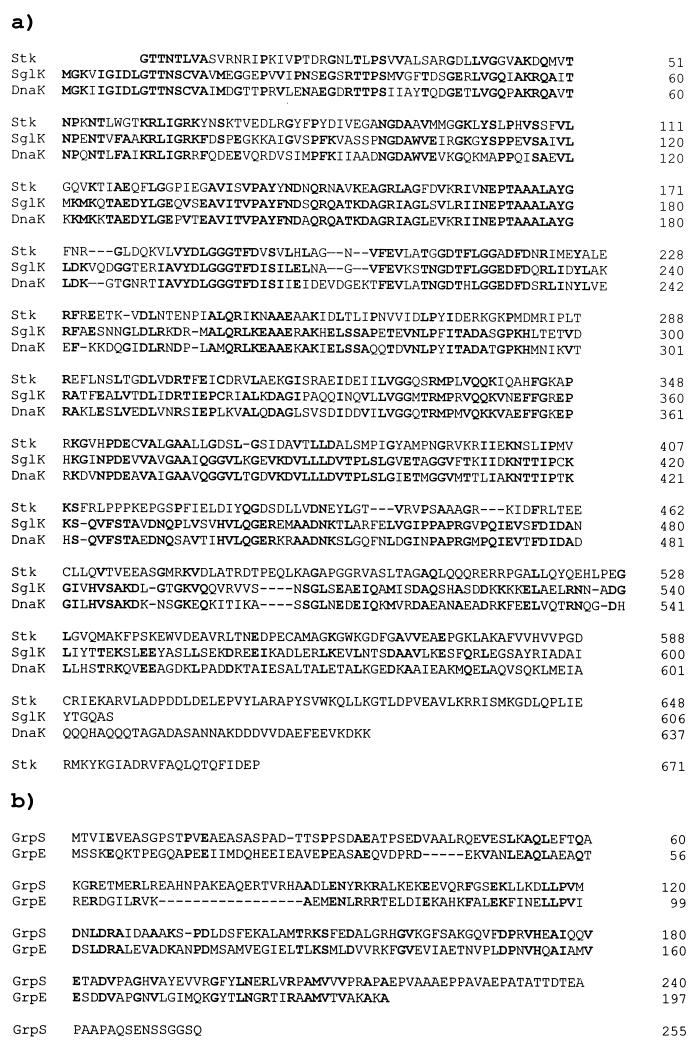
The 4,849-bp segment of the M. xanthus genome including sglK has a base composition of 68.2% G+C, and all four open reading frames exhibit codon usage typical for M. xanthus genes (47). No long open reading frames with typical codon usage are found on the bottom strand of this sequence. SglK shares striking identity with members of the HSP70 family of proteins and is closely related to E. coli DnaK as well as M. xanthus Stk, yet another nonessential member of this protein family (Fig. 2) (28).
FIG. 2.
Deduced amino acid sequence homologies of the SglK (a) and GrpS (b) chaperones with M. xanthus Stk protein and E. coli DnaK and GrpE proteins. Alignments were performed by using the gapped BLAST 2.0 algorithm (1). Residues identical to those in SglK and GrpS are shown in bold type. The sequence of the M. xanthus Stk protein is from reference 28. Gaps introduced to optimize alignment are indicated by dashes.
Located immediately upstream of sglK is a second open reading frame, designated grpS. The sequence of the predicted product of the grpS gene shows that it is a member of the family of GrpE chaperones (Fig. 2). As shown in Fig. 1, the sequence of this region includes several additional open reading frames, all of which are transcribed from the same (top) strand of DNA. The translational stop codon for grpS precedes the sglK start codon by only 5 bp, a distance less than that separating most known pairs of cotranscribed M. xanthus genes. About 200 bp upstream of grpS is an open reading frame, fibR, predicted to encode a product of 169 amino acids, with a sequence similar to those of repressors of alginate biosynthesis in pseudomonads (Fig. 3). Initiating about 600 bp downstream of sglK is a large open reading frame, kinS, predicted to encode a product with a C-terminal sequence most similar to Pkn6, a member of a large family of serine-threonine protein kinases made by M. xanthus (61).
FIG. 3.
FibR is similar to PprB and AlgP, repressors of alginate production in Pseudomonas spp. Identical residues in the N termini of the three proteins, FibR, PprB (54), and AlgP (16, 26), are shown in bold type. Tetrapeptide repeats similar to the consensus sequence KPAA found repeated in the C termini of the pseudomonad repressors are underlined (16). Gaps introduced to optimize alignment are indicated by dashes.
The grpS and sglK genes are cotranscribed.
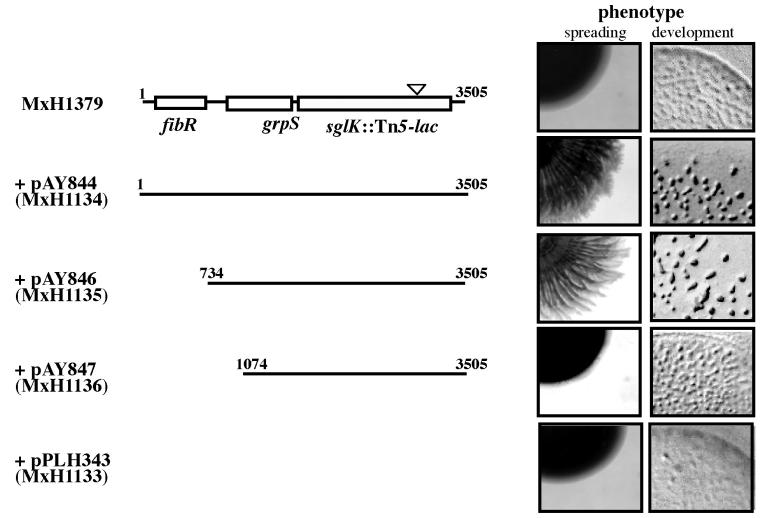
The close proximity of the grpS and sglK genes suggests that these two genes are part of the same transcription unit. This is not surprising, because the grpE and dnaK genes of several gram-positive bacteria, including Bacillus subtilis and Staphylococcus aureus are cotranscribed in the same order (39, 56). To determine whether grpS and sglK are cotranscribed, we asked what sequences upstream of sglK are required for its expression. We tested whether a nonmotile double A and S mutant strain with an insertion in sglK could be complemented for sglK function by a second, ectopic copy of sglK. The chromosomal locus we chose for the integration of a second copy of sglK is attB, the preferred bacterial attachment site for the temperate myxophage Mx8. The attB locus is located about 2.5 Mb from the sglK locus on the 9.5-Mb physical map of wild-type strain DK1622 (31, 47). Plasmids with the phage Mx8 int-attP genes integrate efficiently into the attB locus after electroporation into M. xanthus (30). Therefore, we cloned the sglK gene with various extents of flanking, upstream DNA into Kmr plasmid pPLH343 (33) and electroporated these plasmids into strain MxH1379.
Tetracycline-resistant (Tcr) strain MxH1379 carries the A-motility mutation, aglB1, as well as insertion Ω1252Tc in sglK. This insertion was constructed by replacement of an internal portion of the Kmr Tn5-lac element with a Tcr determinant in parental strain MxH1252 (aglB1 sglK::Ω1252) (31). Because host strain MxH1379 carries a combination of two mutations, one in an A-motility gene, and one in an S-motility gene, it is nonmotile. When plasmid pPLH343 integrates into the attB locus of MxH1379 after electroporation, the recombinant strain, MxH1379(pPLH343), like its parent, cannot glide. Both strains form uniformly dark tan colonies when spotted on CTPM plates with 0.3% agar and grown for 5 days at 32°C (Fig. 4). In contrast, strain MxH1379(pAY844), with bp 1 to 3505 of the S1 locus, including the full-length fibR, grpS, and sglK genes, is motile, and forms a starburst colony comprised of alternating tan and yellow annuli on its interior. Strain MxH1379(pAY846), which has the entire grpS and sglK genes, but not fibR, also is motile. In contrast, strain MxH1379(pAY847) is not motile. The integrated plasmid in this strain carries bp 1074 to 3505 of the S1 locus, including the 3′ two-thirds of grpS and the entire sglK gene. This complementation test shows that, unlike the inserts in pAY844 and pAY846, the insert in pAY847 is missing an element required for the expression of sglK. Therefore, the promoter for sglK must lie between bp 734 and 1074 of the S1 locus and most likely is immediately proximal to grpS.
FIG. 4.
The grpS and sglK genes are in the same transcription unit. The structure of the S1 locus in strain MxH1379 (aglB1 sglK::Ω1252Tc) is represented at the top left; the position of the Tn5-lac insertion is represented by the triangle. S1 inserts integrated at the attB bacterial attachment locus for prophage Mx8 in derivatives of MxH1379 are indicated below. Plasmid pPLH343 is the Kmr plasmid vector with the Mx8 int-attP genes and no S1 insert. The phenotypes of each strain are shown on the right as photographs (magnification of ×10) of 20-μl spots of cells made on CTPM medium with 0.3% agar (spreading) and TPM agar (development) after 5 days of incubation at 32°C.
Figure 4 also shows that the double-mutant aglB sglK strain, MxH1379, forms ill-defined, translucent mounds upon development. In contrast, derivatives of this strain with plasmids pAY844 and pAY846 that complement the sglK defect for motility form well-defined fruiting bodies, whereas derivatives with plasmids pPLH343 or pAY847 do not.
Although plasmid pAY847 carries the wild-type allele of the sglK::Ω1252Tc allele, we did not detect recombinants in which the wild-type allele of sglK at the ectopic attB locus was recovered by the S1 locus as the result of an allelic exchange or gene conversion event in strain MxH1379(pAY847). The selection for motility is a powerful one, enabling the detection of as few as 10−9 motile revertants from a population of otherwise nonmotile M. xanthus cells (20). Therefore, we took advantage of this strain as a way to estimate the frequency with which such a rare gene conversion event might occur. Strain MxH1379(pAY847) was grown to a density of 5 × 108 cells/ml in CTPM medium, and 10 spots (20 μl) of 107 cells each were made on a CTPM plate with 1.0% agar. After the cells grew for 2 days, we recovered >2 × 108 viable cells from each spot. After the cells grew for 14 days, we recovered only four recombinant strains arising as motile flares emerging from these spots. All four motile flares were formed by cells with a Kmr Tcs phenotype, indicating that they had arisen from gene conversion events in which the wild-type allele of sglK at attB had replaced the mutant allele at S1 and had been retained at attB.
The results of this simple experiment provide us with an estimate of the frequency of gene conversion events that occur between duplicated genes (which share >2 kb of perfect homology) at different, distant loci in the M. xanthus genome. This frequency is about 2 × 10−9, near the limit of detection of the sensitive genetic selection for motility. These results also reveal the power of using plasmids integrated at the ectopic attB locus for complementation analyses in M. xanthus.
Both the grpS and sglK genes are not essential for vegetative growth.
Like many members of the HSP70 chaperone family in other organisms, the sglK gene does not appear to be essential for vegetative growth. Presumably, this is because the HSP70 family of chaperones, as well as those of its GrpE and DnaJ partners, has evolved by the multiplication and divergence of an ancestral dnaK gene. This ancestral gene conferred the enormous selective advantage of maintaining the function of other proteins under extreme conditions that promote their denaturation, a role reflected by the fact that the DnaK sequence is deeply rooted in protein evolution. Thus, many prokaryotes possess both dnaK homologues with functions essential for vegetative growth and dnaK homologues like sglK with more-specialized, nonessential functions. To determine whether the grpS gene is essential for vegetative growth, we constructed a plasmid carrying a Spr Smr substitution for the majority of the coding sequences of both genes and inserted these substituted genes in the M. xanthus genome by crosses. Plasmid pAY1060 DNA was made linear by cleavage at a site adjacent to its subcloned region of the S1 locus and electroporated into wild-type M. xanthus DK1622. One of four Spr Smr recombinants screened after electroporation was found to have lost S motility and to have acquired the substitution of plasmid pGB2 DNA for a region of genomic DNA spanning both grpS and sglK. These recombinants are formed by pairs of crossover events between the plasmid and chromosome in the regions of homology flanking this substitution. This result shows that grpS plays a nonessential role in the vegetative growth of M. xanthus.
The grpS gene is not required for motility or development.
The finding that grpS and sglK are in the same transcription unit and encode products that are homologues of interacting chaperones suggested that grpS also may be essential for S motility and development. However, this is not the case.
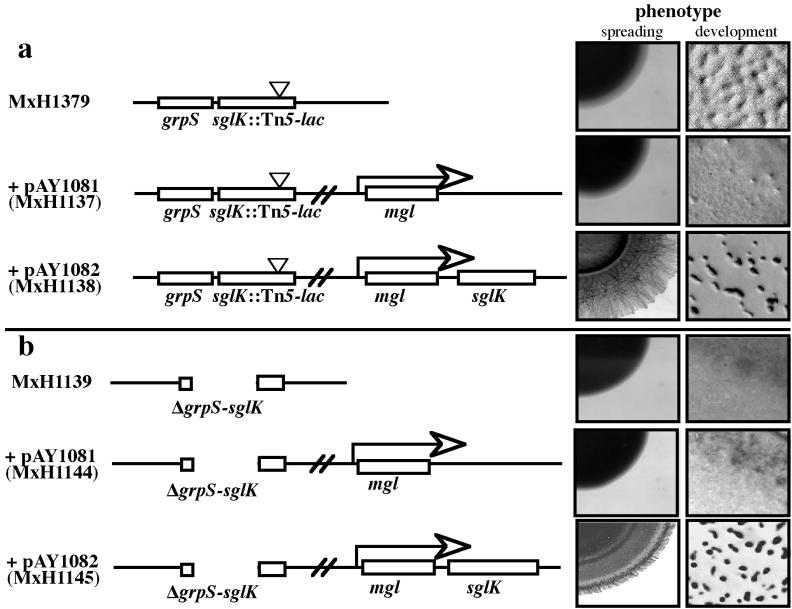
To determine whether grpS is required for S motility and development, we constructed a mutant of M. xanthus that expresses sglK but not grpS. In previous reports, we have shown that several different genes, including the temperate myxophage Mx8 mox (33) and int (44) genes, can be expressed from the constitutive mglBA promoter carried by a plasmid integrated at the attB locus. To construct a strain that expresses sglK but not grpS, we made plasmid pAY1082, in which sglK is positioned as the third gene in the mglBA operon, and integrated pAY1082 into the attB locus of the Spr Smr grpS-sglK double-mutant strain.
To show that plasmid pAY1082 can express the sglK gene from the attB locus, we also introduced pAY1082 into MxH1379 (aglB1 sglK::Ω1252) by electroporation. As shown in Fig. 5, MxH1379(pAY1082) displays social motility and can form erect, dark fruiting bodies in response to starvation. In contrast, MxH1379(pAY1081) which has only the mglBA genes integrated at attB (without sglK), like its parent, neither glides nor forms fruit. This result shows that expression of sglK from the mgl operon integrated at attB can complement the sglK mutation in MxH1379.
FIG. 5.
The grpS gene is not required for social motility or fruiting body morphogenesis. The structures of the S1 loci in strains MxH1379 (aglB1 sglK::Ω1252Tc) and MxH1139 (ΔgrpS-sglK) are shown on the left (a and b, respectively). Derivatives of these strains carry second, ectopic copies of the mgl operon integrated at the Mx8 attB locus, without (pAY1081) or with (pAY1082) a second, wild-type copy of the sglK gene. A description of the photographs of mutant phenotypes is provided in the legend to Fig. 4.
When plasmid pAY1082 is introduced into the grpS-sglK double mutant strain, it also complements this strain for both motility and development, whereas the pAY1081 control plasmid does not (Fig. 5). This surprising result shows that grpS function is not required for social motility or development.
Expression of the grpS-sglK operon is induced by conditions that trigger development but not by temperature shift.
The high degree of sequence similarity between the predicted product of the sglK gene and DnaK (HSP70) prompted us to ask whether sglK plays a role in the heat shock response in addition to its established role in S motility and multicellular development (31, 32). To identify the SglK protein and monitor its regulation in response to environmental stresses, we examined the profiles of proteins produced in otherwise wild-type and sglK mutant strains by two methods. As shown in Fig. 6, when the proteins produced by vegetative M. xanthus cells growing at 32°C are compared with those made after an increase in temperature to 42°C, several prominent proteins that incorporate label in the form of [35S]methionine are found to be expressed at higher levels after heat shock. These include four prominent bands representing proteins with apparent molecular masses of about 90, 70, 50, and 15 kDa, consistent with the results of Nelson and Killeen (38). We presume that the major, heat shock-inducible band with an apparent molecular mass of 70 kDa is HSP70 (DnaK), and this result shows that SglK and DnaK are different proteins, because sglK mutants with Tn5-lac insertion mutations produce this prominent band after heat shock. Furthermore, we observe no differences in the patterns of [35S]methionine-labeled proteins in wild-type and mutant cells in comparisons of samples prepared from cells that have been heat shocked and from cells that have not. Thus, either the SglK protein is produced at low levels, or it is obscured by a more-prominent band with a similar apparent molecular mass, such as DnaK. We also attempted to resolve the DnaK and SglK proteins by SDS-PAGE in extracts prepared from wild-type and sglK mutant cells by a more-sensitive method of detection. Resolved proteins were stained with monoclonal antibody specific for the ATP-binding site of DnaK (27), and both wild-type and mutant cells were found to produce a single, heat-inducible protein band with an apparent molecular mass of 70 kDa that cross-reacts with this antibody (data not shown).
FIG. 6.
Mutants with insertions in the sglK gene produce HSP70 after heat shock. An autoradiogram of an SDS-polyacrylamide gel in which radiolabeled M. xanthus proteins from three different strains were fractionated is shown. Wild-type (WT) strain DK1622 and sglK mutant strains MxH1223 (Ω1222) and MxH1253 (Ω1252) were grown to exponential phase at 32°C and pulse-labeled with [35S]methionine after incubation at 32°C (−) or 42°C (+), as described in Materials and Methods. Both wild-type and mutant cells produce an abundant heat shock protein with an apparent molecular mass of 70 kDa, presumably the M. xanthus homologue of HSP70 (38). MW, molecular mass.
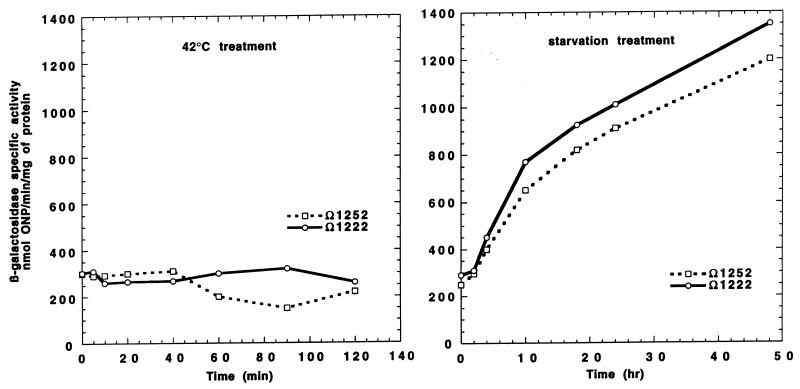
Because we could not detect the wild-type SglK protein directly by SDS-PAGE against the background of abundant, essential DnaK protein, we measured the expression of the lacZ gene from the sglK promoter to monitor its regulation in response to environmental stresses. As shown in Fig. 7, the expression of β-galactosidase activity from from the lacZ transcriptional fusions generated by the Ω1222 and Ω1252 insertions in the sglK gene increases more than fourfold in response to starvation, confirming our previous results (31). In contrast, LacZ activity does not increase in these fusion strains after heat shock, consistent with our finding that SglK is not the major, presumably essential, HSP70. LacZ activity in these strains also increases after treatment of vegetative cultures with 0.5 M glycerol (data not shown), which triggers an alternative developmental pathway leading to the rapid sporulation of M. xanthus in the absence of fruiting body formation (17).
FIG. 7.
Starvation, but not heat shock, induces sglK expression. Specific activities of β-galactosidase produced from the lacZ transcriptional fusions generated with the sglK operon by Tn5-lac insertions Ω1252 and Ω1222 in treated exponential-phase cultures of strains MxH1253 and MxH1223 after heat shock (42°C) and starvation are shown.
SglK function is required for the production of fibrils.
Electron microscopic examination of sglK mutant cells shows that they have pili (58). These mutants also retain sensitivity to myxophages Mx4 and Mx8, indicating that they produce O antigen. The behavior of sglK mutant cells in liquid culture suggested to us that they are defective in fibril production. Unlike wild-type M. xanthus cells, which tend to form aggregates or clumps even when grown in rapidly shaking liquid cultures, sglK mutant cells do not aggregate during growth. This phenotype is shared by the cohesion-defective dsp (dispersed) mutants, a large cluster of S mutations linked with one of the Tn5-lac insertions in the S1 cluster (31). The dsp mutants, like the sglK mutants, are defective in the early steps of development and fail to aggregate and form fruit or spores (32, 49). To quantify the ability of wild-type cells to aggregate, Shimkets has developed an agglutination assay in which exponentially growing cells are suspended in buffer, and the optical density of the suspended cells is monitored over time. As cells agglutinate, the optical density of the cell suspension decreases (48). As shown in Fig. 8, when wild-type cells are assayed for their ability to agglutinate, the A600 of the resuspended cells drops sharply after incubation at 32°C for 60 min. In contrast, sglK mutant cells do not agglutinate.
FIG. 8.
The sglK gene is required for the agglutination of M. xanthus cells. Exponentially growing cells of wild-type strain DK1622 and sglK mutant strain MxH1253 were concentrated and suspended in agglutination buffer, and the absorbance (A) at 600 nm of each suspension was monitored over time at 32°C. Unlike an equal mixture of wild-type and dsp mutant cells (2), which agglutinates with the same kinetics as wild-type cells, an equal mixture of wild-type and sglK mutant cells (wild type + MxH1223) agglutinates with intermediate kinetics.
When equal titers of wild-type and mutant dsp cells are mixed, the mixed population of cells agglutinates with kinetics similar to those for wild-type cells (49). This result shows that the ability of dsp mutant cells to agglutinate can be rescued by fibrils released from or associated with the surfaces of wild-type cells. Mutant dsp strains are unable to produce fibrils, long polysaccharide filaments about 50 nm in diameter containing multimers of the fibrillin protein IFP-1 (5, 9). The addition of purified fibrils to dsp mutant cells rescues their ability to agglutinate and develop (9). The dsp mutants require additional second-step mutations called fbd (for fibril binding defective) to prevent their complementation by purified fibrils for agglutination (10). As shown in Fig. 8, when equal titers of sglK mutant cells and wild-type cells are mixed, the mixtures do not agglutinate with wild-type kinetics. This result shows that, unlike dsp mutants and like dsp fbp double mutants, sglK mutants are defective both as recipients and donors in the agglutination assay.
The histological dye Congo red, which binds to bacterial extracellular polysaccharides, inhibits the agglutination, S motility, and fruiting of wild-type M. xanthus cells. Arnold and Shimkets (3) have shown that dsp mutants defective in fibril production have a substantially lower affinity for Congo red than do wild-type cells, suggesting that the major receptor for Congo red binding is associated with fibrils. To test whether sglK mutants have the major receptor for Congo red binding, wild-type and mutant M. xanthus cells were incubated with Congo red, and continuous spectra of the samples were taken. The spectra obtained from the absorbance of Congo red alone was compared with the difference spectra obtained from a comparison of the absorbance of a mixture of wild-type cells and Congo red with wild-type cells alone and from a comparison of the absorbance of a mixture of sglK mutant cells and Congo red with mutant cells alone (Fig. 9). Wild-type cells bind Congo red with a higher affinity than do sglK mutant cells (data not shown). Furthermore, whereas Congo red has an absorbance maximum of 482 nm in aqueous solution, this maximum is redshifted by 22 nm upon binding to wild-type cells. The absorbance maximum of Congo red bound to sglK mutant cells is redshifted by only 10 nm, indicating that the dye binds to a different receptor on mutant cells than on wild-type cells, because changes in the absorption maximum of a chromophore result from changes in its immediate electronic environment. This result, taken together with the results of our agglutination assays, suggests that the sglK mutants do not produce fibrils.
FIG. 9.
Continuous difference spectra of the polysaccharide-binding dye Congo red in association with wild-type and sglK mutant (MxH1223) cells. A, absorbance.
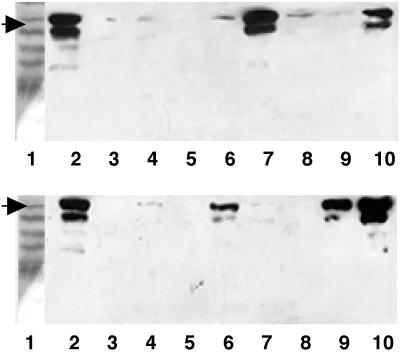
To test this hypothesis directly, we grew wild-type strain DK1622, sglK mutant strains, and sglK mutant strains with integrated plasmids to exponential phase, concentrated the cells by centrifugation, and lysed the cells in SDS sample buffer. Proteins in cell lysates were resolved by electrophoresis and transferred to nitrocellulose. Transferred proteins were incubated with monoclonal anti-IFP-1 antibody (MAb2105) (5), and cross-reacting material was identified by staining in an enzyme-linked assay. As shown in Fig. 10, MxH1379 and its derivatives with integrated plasmids that do not express sglK do not produce the protein that cross-reacts with anti-IFP-1 antibody; this is also true for sglK mutant strains MxH1223 and MxH1253. In contrast, derivatives of MxH1379 that carry plasmid pAY696 or pAY1082, both of which express the sglK gene, produce fibrillin. Similar results are observed with derivatives of the double-mutant grpS-sglK strain, MxH1139. These results show that sglK function, but not grpS function, is required for the expression of fibrillin. Taken together, the results of agglutination assays, Congo red binding experiments, and antibody assays for fibrillin, show that sglK mutants are defective in fibril production.
FIG. 10.
Mutations in the sglK and fibR genes affect the level of fibrillin production by M. xanthus. Proteins present in wild-type and mutant M. xanthus cells were fractionated and assayed by Western immunoblot analysis, as described in Materials and Methods. (Top and bottom) Lanes 1, molecular mass markers; lanes 2, material cross-reacting with MAb2105 in extracts prepared from wild-type cells; lanes 3, MxH1223 (sglK::Ω1222). (Top) Lane 4, MxH1256 (sglK::Ω1255); lane 5, MxH1139 (grpS-sglK::pGB2); lane 6, MxH1140 [grpS-sglK::pGB2 (pPLH343)]; lane 7, MxH1142 [grpS-sglK::pGB2 (pAY846)]; lane 8, MxH1143 [grpS-sglK::pGB2 (pAY847)]; lane 9, MxH1144 [grpS-sglK::pGB2 (pAY1081)]; lane 10, MxH1145 [grpS-sglK::pGB2 (pAY1082)]. (Bottom) Lane 4, MxH1259 (S2::Ω1258); lane 5, MxH1133 [aglB1 sglK::Ω1252Tc (pPLH343)]; lane 6, MxH1134 [aglB1 sglK::Ω1252Tc (pAY846)]; lane 7, MxH1135 [aglB1 sglK::Ω1252Tc (pAY847)]; lane 8 MxH1136 [aglB1 sglK::Ω1252Tc (pAY1081)]; lane 9, MxH1135 [aglB1 sglK::Ω1252Tc (pAY1081)]; lane 10, MxH1146 (fibR::pGB2). The fibR mutant (bottom, lane 10) produces an increased level of fibrillin, the phenotype expected for a mutant with a repressor mutation. Also note that the Tn5-lac insertion, Ω1258, which maps in the unlinked S2 cluster of mutant S insertions (31), is defective in fibrillin production.
The fibR gene encodes a negative regulator of fibril production.
To investigate the function of the fibR gene, which is located immediately upstream of the grpS-sglK operon, we constructed an insertion mutation in fibR and placed this insertion in the M. xanthus chromosome by crosses. The mutant fibR strain is almost indistinguishable in phenotype from its wild-type parent, showing that fibR, like grpS and sglK, is not essential for growth. Like its DK1622 parent, the mutant fibR::pGB2 strain has S motility and produces fruit containing a full complement of heat-resistant spores (data not shown). The fibR gene is predicted to encode a product with a sequence similar to those of the histone-like repressors of alginate biosynthesis in the pseudomonads (Fig. 3) (16, 26, 50). Although the polysaccharide fibrils made by M. xanthus do not appear to contain the charged uronic acid residues typically found in alginate (4), their composition is similar to that of the neutral exopolysaccharides made by P. aeruginosa (34). Furthermore, like M. xanthus fibrils, the combination of charged and neutral exopolysaccharides secreted by P. aeruginosa is associated with a substantial protein fraction (36). Therefore, we tried to ascertain whether the fibR gene encodes a negative regulator of fibril production by examining the relative level of fibrillin produced by the fibR mutant strain. As shown in Fig. 10, the fibR mutant strain produces about three- to fourfold-higher levels of fibrillin than does its wild-type parent, DK1622.
DISCUSSION
We have characterized a subset of Tn5-lac insertions that define the S1 locus of M. xanthus required for social motility and multicellular development. Sequence analysis of the sites of these insertions shows that they inactivate a gene, sglK, which encodes a nonessential homologue of DnaK (HSP70). The SglK protein is not the only M. xanthus HSP70 homologue that may be involved in S motility. An insertion of Tn5 that inactivates the M. xanthus stk gene, predicted to encode another member of the DnaK family of chaperones, suppresses a subset of S mutations. Insertions in stk are simply recessive alleles that result in increased cohesion and fibril production (15). In contrast, we have shown that insertions in sglK are simply recessive alleles with the opposite phenotype and abolish fibril synthesis. Thus, there may be two M. xanthus HSP70 homologues that play opposing roles in fibril production.
The sglK gene is the second gene in an operon that also includes grpS, which encodes a homologue of a chaperone partner of HSP70, GrpE. Surprisingly, unlike sglK, grpS is not required for cell cohesion, S motility, or development. Presumably, M. xanthus encodes yet another homologue of GrpE that functions together with SglK in a role that is redundant to that of GrpS. Alternatively, GrpS may function together with SglK to control some physiological process other than social motility, and SglK may interact with a different GrpE-like partner to control motility. We consider these possibilities because DnaK and the nonessential homologues of HSP70 are known to function as components of tripartite chaperone machines, involving combinations of members of each of the DnaK, GrpE, and DnaJ (or J-domain) families of proteins. Alternatively, SglK may not require a GrpE homologue for its role in motility and development.
In other gram-negative bacteria, DnaK and GrpE are known to interact with membrane proteins containing J domains that control the synthesis and secretion of exopolysaccharides. Both Coxiella burnetti (62) and E. coli (12, 13, 25) encode transmembrane proteins with J domains required for the secretion of the exopolysaccharide colanic acid, necessary for mucoidy. The Shigella flexneri rol gene encodes a J-domain transmembrane protein that regulates O-antigen chain length (37). Therefore, it is not surprising that M. xanthus makes a specialized homologue of HSP70 that controls fibril production, and we now are looking for the transmembrane J-domain partner of SglK.
The precise natures of the roles that the exopolysaccharide fibrils play in the social motility and development of M. xanthus remain a mystery. Fibrils play critical roles in the motility and development of wild-type cells. Mutations that abolish fibril production, such as dsp and sglK, are required for both motility and development (48), and purified fibrils can rescue the defects in motility and development caused by dsp lesions (10). Mutants with defective sglK, however, have a more severe phenotype than dsp mutants, because they are defective as both donors and recipients in agglutination assays with wild-type cells.
Chang and Dworkin have isolated second-step mutations called fbp in a mutant dsp genetic background that restore cohesion, social motility, and development without restoring fibril production (10). Their surprising results show that the physical presence of fibrils per se is not required for motility and development and suggest that fibrils are an intermediate in a signal transduction pathway required for these phenotypes. They have proposed that the perception of fibrils is required for motility and development, perhaps by mediating the exchange of intercellular signal molecules. Consistent with this model, M. xanthus produces a development-specific protein related in sequence to IFP-1 found associated with fibrils (14).
We have shown that the gene upstream of the grpS-sglK operon, fibR, encodes a negative regulator of fibril production. Taken together with the observation that its predicted product is similar to the histone-like repressors of alginate production in pseudomonads, this result suggests that the control of slime exopolysaccharide manufacture in these distantly related bacteria may be similar. Indeed, the pseudomonads not only make the charged alginate exopolysaccharides, containing the signature, oxidized uronic acids, but also secrete neutral exopolysaccharides. The ratio of charged to neutral pseudomonad exopolysaccharides, the latter of which are similar in composition to M. xanthus fibrils, varies as a function of carbon source. It is intriguing that the best inducer of alginate production by P. aeruginosa, glycerol (34), acts as a morphogen for M. xanthus as well. Glycerol triggers an alternative pathway for M. xanthus development, in which vegetative cells undergo sporulation without the concomitant morphogenesis of fruit (17, 43), and is thought to play a key regulatory role during the late steps of starvation-induced development (19).
Finally, we note that for two of the three cases in which a DnaK–GrpE–J-domain protein chaperone machine is involved in the secretion of bacterial exopolysaccharides, this machine controls the function of a two-component regulatory system that activates secretion (25). The algR-algS response regulator and histidine kinase genes control the production of pseudomonad exopolysaccharide (57) and are in turn controlled by a repressor homologue of fibR. Therefore, we will not be surprised if M. xanthus is found to have homologues of this two-component regulatory system and if pseudomonads are found to have homologues of the grpS-sglK operon encoding components of a chaperone machine that regulates exopolysaccharide production.
ACKNOWLEDGMENTS
We thank Samuel Wu and Dale Kaiser for their electron microscopic examination of pili on the surfaces of sglK mutant cells, Marty Dworkin for his gift of MAb2105 antibody, Paul Blum for his gift of anti-DnaK antibody, and John Downard for allowing us to use the sequence of stk to complete Fig. 2.
This work was supported in part by grants GM50962 and GM53392 to P.L.H. and P.Y., respectively, from the National Institutes of Health and by EPSCoR grant OSR-9350539 to P.L.H.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold J W, Shimkets L J. Cell surface properties correlated with cohesion in Myxococcus xanthus. J Bacteriol. 1988;170:5771–5777. doi: 10.1128/jb.170.12.5771-5777.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold J W, Shimkets L J. Inhibition of cell-cell interactions in Myxococcus xanthus by Congo red. J Bacteriol. 1988;170:5765–5770. doi: 10.1128/jb.170.12.5765-5770.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behmlander R M, Dworkin M. Biochemical and structural analyses of the extracellular matrix fibrils of Myxococcus xanthus. J Bacteriol. 1994;176:6295–6303. doi: 10.1128/jb.176.20.6295-6303.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behmlander R M, Dworkin M. Integral proteins of the extracellular matrix fibrils of Myxococcus xanthus. J Bacteriol. 1994;176:6304–6311. doi: 10.1128/jb.176.20.6304-6311.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg D E, Schmandt M A, Lowe J B. Specificity of transposon Tn5 insertion. Genetics. 1983;105:813–828. doi: 10.1093/genetics/105.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowden, M. G., and H. B. Kaplan. Myxococcus xanthus O antigen mutants are defective in gliding motility and multicellular development. Mol. Microbiol., in press. [DOI] [PubMed]
- 8.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 9.Chang B Y, Dworkin M. Isolated fibrils rescue cohesion and development in the Dsp mutant of Myxococcus xanthus. J Bacteriol. 1994;176:7190–7196. doi: 10.1128/jb.176.23.7190-7196.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang B Y, Dworkin M. Mutants of Myxococcus xanthus dsp defective in fibril binding. J Bacteriol. 1996;178:697–700. doi: 10.1128/jb.178.3.697-700.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Churchward G, Belin D, Nagamine Y. A pSC101-derived plasmid which shows no sequence homology to other commonly used cloning vectors. Gene. 1984;31:165–171. doi: 10.1016/0378-1119(84)90207-5. [DOI] [PubMed] [Google Scholar]
- 12.Clarke D J, Holland I B, Jacq A. Point mutations in the transmembrane domain of DjlA, a membrane-linked DnaJ-like protein, abolish its function in promoting colanic acid production via the Rcs signal transduction pathway. Mol Microbiol. 1997;25:933–944. doi: 10.1111/j.1365-2958.1997.mmi528.x. [DOI] [PubMed] [Google Scholar]
- 13.Clarke D J, Jacq A, Holland I B. A novel DnaJ-like protein in Escherichia coli inserts into the cytoplasmic membrane with a type III topology. Mol Microbiol. 1996;20:1273–1286. doi: 10.1111/j.1365-2958.1996.tb02646.x. [DOI] [PubMed] [Google Scholar]
- 14.Clemans D L, Chance C M, Dworkin M. A development-specific protein in Myxococcus xanthus is associated with the extracellular fibrils. J Bacteriol. 1991;173:6749–6759. doi: 10.1128/jb.173.21.6749-6759.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dana J R, Shimkets L J. Regulation of cohesion-dependent cell interactions in Myxococcus xanthus. J Bacteriol. 1993;175:3636–3647. doi: 10.1128/jb.175.11.3636-3647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deretic V, Konyecsni W M. A procaryotic regulatory factor with a histone H1-like carboxy-terminal domain: clonal variation of repeats within algP, a gene involved in regulation of mucoidy in Pseudomonas aeruginosa. J Bacteriol. 1990;172:5544–5554. doi: 10.1128/jb.172.10.5544-5554.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dworkin M, Sadler W. Induction of cellular morphogenesis in Myxococcus xanthus. I. General description. J Bacteriol. 1966;91:1516–1519. doi: 10.1128/jb.91.4.1516-1519.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fink J M, Zissler J F. Characterization of lipopolysaccharide from Myxococcus xanthus by use of monoclonal antibodies. J Bacteriol. 1989;171:2028–2032. doi: 10.1128/jb.171.4.2028-2032.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frasch S C, Dworkin M. Increases in the intracellular concentration of glycerol during development in Myxococcus xanthus. FEMS Microbiol Lett. 1994;122:321–325. [PubMed] [Google Scholar]
- 20.Hartzell P L. Complementation of sporulation and motility defects in a prokaryote by a eukaryotic GTPase. Proc Natl Acad Sci USA. 1997;94:9881–9886. doi: 10.1073/pnas.94.18.9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodgkin J, Kaiser D. Genetics of gliding motility in Myxococcus xanthus (Myxobacteriales): two gene systems control movement. Mol Gen Genet. 1979;171:177–191. [Google Scholar]
- 22.Hodgkin J, Kaiser D. Genetics of gliding motility in Myxococcus xanthus (Myxobacteriales): genes controlling movement of single cells. Mol Gen Genet. 1979;171:167–176. [Google Scholar]
- 23.Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci USA. 1979;76:5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kashefi K, Hartzell P L. Genetic suppression and phenotypic masking of a Myxococcus xanthus frzF-defect. Mol Microbiol. 1995;15:483–494. doi: 10.1111/j.1365-2958.1995.tb02262.x. [DOI] [PubMed] [Google Scholar]
- 25.Kelley W L, Georgopoulos C. Positive control of the two-component RcsC/B signal transduction network by DjlA: a member of the DnaJ family of molecular chaperones in Escherichia coli. Mol Microbiol. 1997;25:913–931. doi: 10.1111/j.1365-2958.1997.mmi527.x. [DOI] [PubMed] [Google Scholar]
- 26.Konyecsni W M, Deretic V. DNA sequence and expression analysis of algP and algQ, components of the multigene system transcriptionally regulating mucoidy in Pseudomonas aeruginosa: algP contains multiple direct repeats. J Bacteriol. 1990;172:2511–2520. doi: 10.1128/jb.172.5.2511-2520.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krska J, Elthon T, Blum P. Monoclonal antibody recognition and function of a DnaK (HSP70) epitope found in gram-negative bacteria. J Bacteriol. 1993;175:6433–6440. doi: 10.1128/jb.175.20.6433-6440.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kupfer D M, Downard J, Roe B A. The STK locus of Myxococcus xanthus. 1996. GenBank accession no. AC000101. [Google Scholar]
- 29.Laemmli U K. Cleavage of structural proteins during the assembly of the bacteriophage T4 head. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 30.Li S F, Shimkets L J. Site-specific integration and expression of a developmental promoter in Myxococcus xanthus. J Bacteriol. 1988;170:5552–5556. doi: 10.1128/jb.170.12.5552-5556.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacNeil S D, Calara F, Hartzell P L. New clusters of genes required for gliding motility in Myxococcus xanthus. Mol Microbiol. 1994;14:61–71. doi: 10.1111/j.1365-2958.1994.tb01267.x. [DOI] [PubMed] [Google Scholar]
- 32.MacNeil S D, Mouzeyan A, Hartzell P L. Genes required for both gliding motility and development in Myxococcus xanthus. Mol Microbiol. 1994;14:785–795. doi: 10.1111/j.1365-2958.1994.tb01315.x. [DOI] [PubMed] [Google Scholar]
- 33.Magrini V, Salmi D, Thomas D, Herbert S K, Hartzell P L, Youderian P. Temperate Myxococcus xanthus phage Mx8 encodes a DNA adenine methylase, Mox. J Bacteriol. 1997;179:4254–4263. doi: 10.1128/jb.179.13.4254-4263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marty N, Dournes J L, Chabanon G, Montrozier H. Influence of nutrient media on the chemical composition of the exopolysaccharide from mucoid and non-mucoid Pseudomonas aeruginosa. FEMS Microbiol Lett. 1992;77:35–44. doi: 10.1016/0378-1097(92)90128-b. [DOI] [PubMed] [Google Scholar]
- 35.Mattick J S, Whitchurch C B, Alm R A. The molecular genetics of type-4 fimbriae in Pseudomonas aeruginosa—a review. Gene. 1996;179:147–155. doi: 10.1016/s0378-1119(96)00441-6. [DOI] [PubMed] [Google Scholar]
- 36.McAvoy M J, Newton V, Paull A, Morgan J, Gacesa P, Russell N J. Isolation of mucoid strains of Pseudomonas aeruginosa from non-cystic-fibrosis patients and characterisation of the structure of their secreted alginate. J Med Microbiol. 1989;28:183–189. doi: 10.1099/00222615-28-3-183. [DOI] [PubMed] [Google Scholar]
- 37.Morona R, van den Bosch L, Manning P A. Molecular, genetic, and topological characterization of O-antigen chain length regulation in Shigella flexneri. J Bacteriol. 1995;177:1059–1068. doi: 10.1128/jb.177.4.1059-1068.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson D R, Killeen K P. Heat shock proteins of vegetative and fruiting Myxococcus xanthus cells. J Bacteriol. 1986;168:1100–1106. doi: 10.1128/jb.168.3.1100-1106.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohta T, Saito K, Kuroda M, Honda K, Hirata H, Hayashi H. Molecular cloning of two new heat shock genes related to the hsp70 genes in Staphylococcus aureus. J Bacteriol. 1994;176:4779–4783. doi: 10.1128/jb.176.15.4779-4783.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panasenko S M, Jann B, Jann K. Novel change in the carbohydrate portion of Myxococcus xanthus lipopolysaccharide during development. J Bacteriol. 1989;171:1835–1840. doi: 10.1128/jb.171.4.1835-1840.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenbluh A, Eisenbach M. Effect of mechanical removal of pili on gliding motility of Myxococcus xanthus. J Bacteriol. 1992;174:5406–5413. doi: 10.1128/jb.174.16.5406-5413.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenfelder G, Luderitz O, Westphal O. Composition of lipopolysaccharides from Myxococcus fulvus and other fruiting and non-fruiting myxobacteria. Eur J Biochem. 1974;44:411–420. doi: 10.1111/j.1432-1033.1974.tb03499.x. [DOI] [PubMed] [Google Scholar]
- 43.Sadler W, Dworkin M. Induction of cellular morphogenesis in Myxococcus xanthus. II. Macromolecular synthesis and mechanism of inducer action. J Bacteriol. 1966;91:1520–1525. doi: 10.1128/jb.91.4.1520-1525.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salmi D, Magrini V, Hartzell P L, Youderian P. Genetic determinants of immunity and integration of temperate Myxococcus xanthus phage Mx8. J Bacteriol. 1998;180:614–621. doi: 10.1128/jb.180.3.614-621.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanger F S, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1998;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schagger H, VonJagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 47.Shimkets L. The myxobacterial genome. In: Dworkin M, Kaiser D, editors. Myxobacteria II. Washington, D.C: American Society for Microbiology; 1993. pp. 85–107. [Google Scholar]
- 48.Shimkets L J. Correlation of energy-dependent cell cohesion with social motility in Myxococcus xanthus. J Bacteriol. 1986;166:837–841. doi: 10.1128/jb.166.3.837-841.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimkets L J. Role of cell cohesion in Myxococcus xanthus fruiting body formation. J Bacteriol. 1986;166:842–848. doi: 10.1128/jb.166.3.842-848.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strom M S, Bergman P, Lory S. Identification of active-site cysteines in the conserved domain of PilD, the bifunctional type IV pilin leader peptidase/N-methyltransferase of Pseudomonas aeruginosa. J Biol Chem. 1993;268:15788–15794. [PubMed] [Google Scholar]
- 51.Sutherland I W, Thomson S. Comparison of polysaccharides produced by Myxococcus strains. J Gen Microbiol. 1975;89:124–132. doi: 10.1099/00221287-89-1-124. [DOI] [PubMed] [Google Scholar]
- 52.Taketo A. DNA transfection of Escherichia coli by electroporation. Biochim Biophys Acta. 1988;949:318–324. doi: 10.1016/0167-4781(88)90158-3. [DOI] [PubMed] [Google Scholar]
- 53.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Venturi V, Otten M, Korse V, Brouwer B, Leong J, Weisbeek P. Alginate regulatory and biosynthetic gene homologs in Pseudomonas putida WCS358: correlation with the siderophore regulatory gene pfrA. Gene. 1995;155:83–88. doi: 10.1016/0378-1119(94)00868-s. [DOI] [PubMed] [Google Scholar]
- 55.Wall D, Kaiser D. Alignment enhances the cell-to-cell transfer of pilus phenotype. Proc Natl Acad Sci USA. 1998;95:3054–3058. doi: 10.1073/pnas.95.6.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wetzstein M, Schumann W. Nucleotide sequence of a Bacillus subtilis gene homologous to the grpE gene of E. coli located immediately upstream of the dnaK gene. Nucleic Acids Res. 1990;18:1289. doi: 10.1093/nar/18.5.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whitchurch C B, Alm R A, Mattick J S. The alginate regulator AlgR and an associated sensor FimS are required for twitching motility in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1996;93:9839–9843. doi: 10.1073/pnas.93.18.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu, S. S., and D. Kaiser. Personal communication.
- 59.Wu S S, Kaiser D. Genetic and functional evidence that Type IV pili are required for social gliding motility in Myxococcus xanthus. Mol Microbiol. 1995;18:547–558. doi: 10.1111/j.1365-2958.1995.mmi_18030547.x. [DOI] [PubMed] [Google Scholar]
- 60.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 61.Zhang W, Munoz-Dorado J, Inouye M, Inouye S. Identification of a putative eukaryotic-like protein kinase family in the developmental bacterium Myxococcus xanthus. J Bacteriol. 1992;174:5450–5453. doi: 10.1128/jb.174.16.5450-5453.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zuber M, Hoover T A, Court D L. Analysis of a Coxiella burnetti gene product that activates capsule synthesis in Escherichia coli: requirement for the heat shock chaperone DnaK and the two-component regulator RcsC. J Bacteriol. 1995;177:4238–4244. doi: 10.1128/jb.177.15.4238-4244.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]