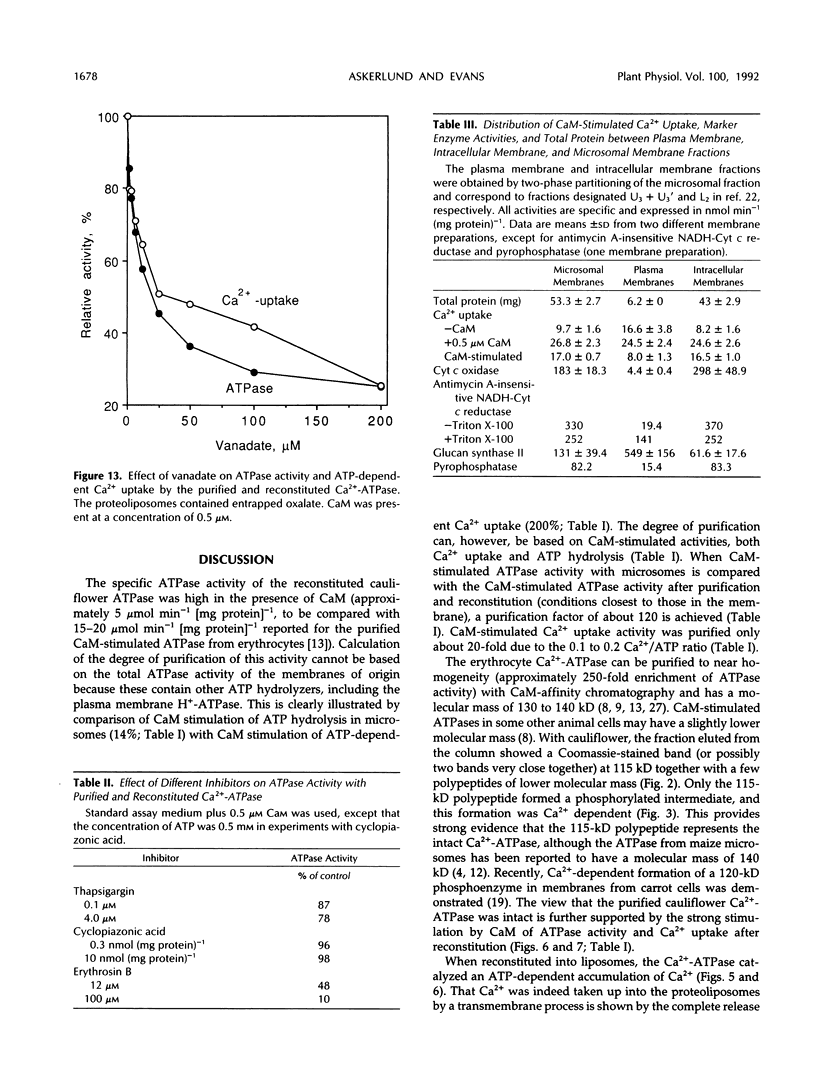
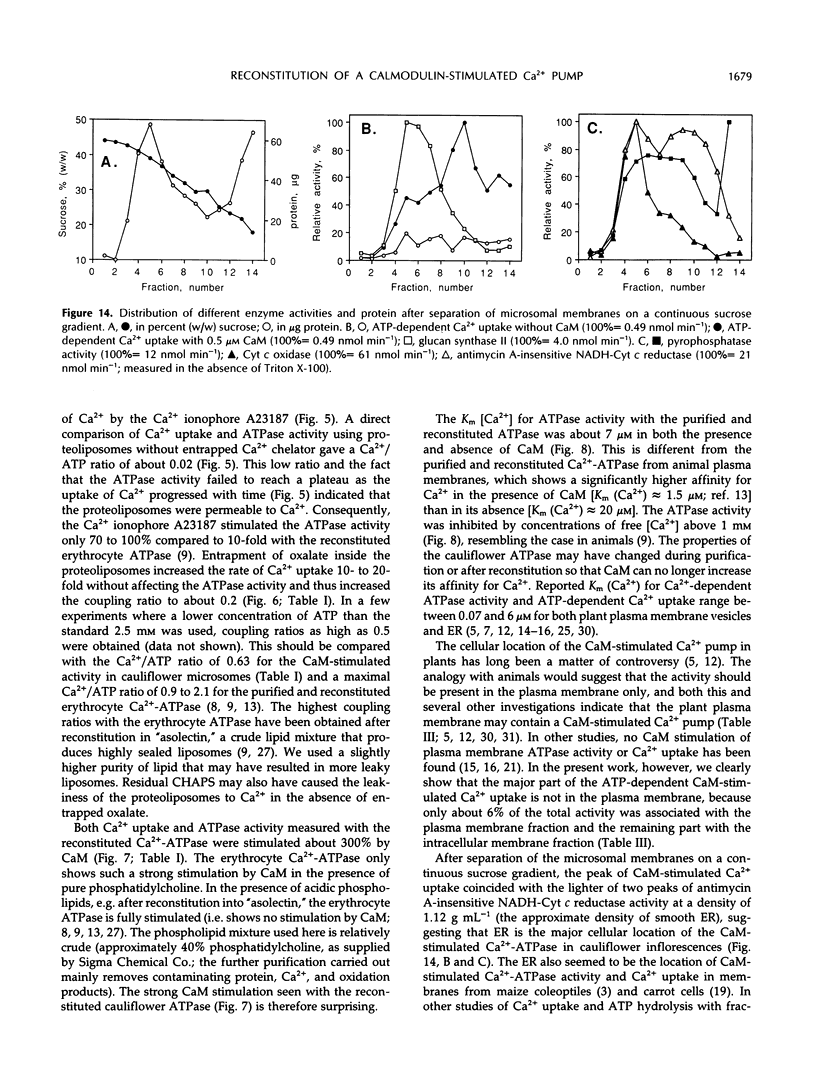
Abstract
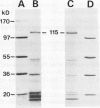
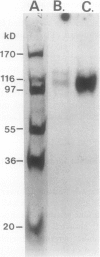
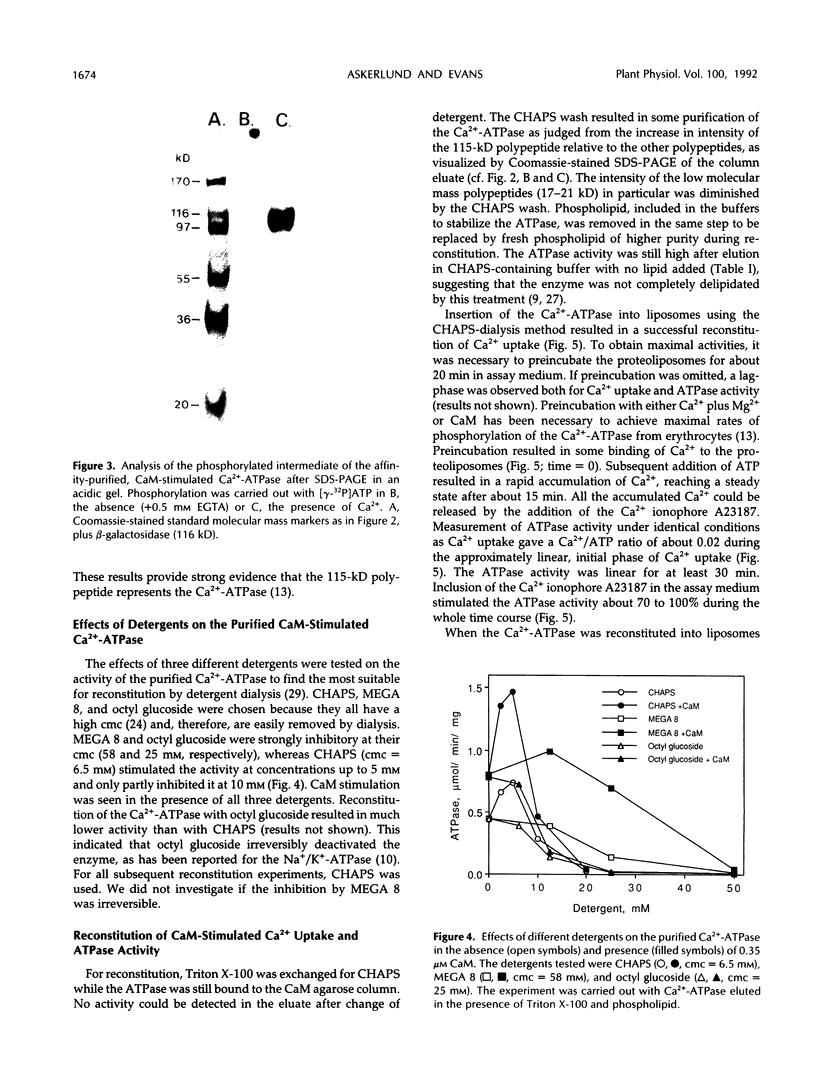
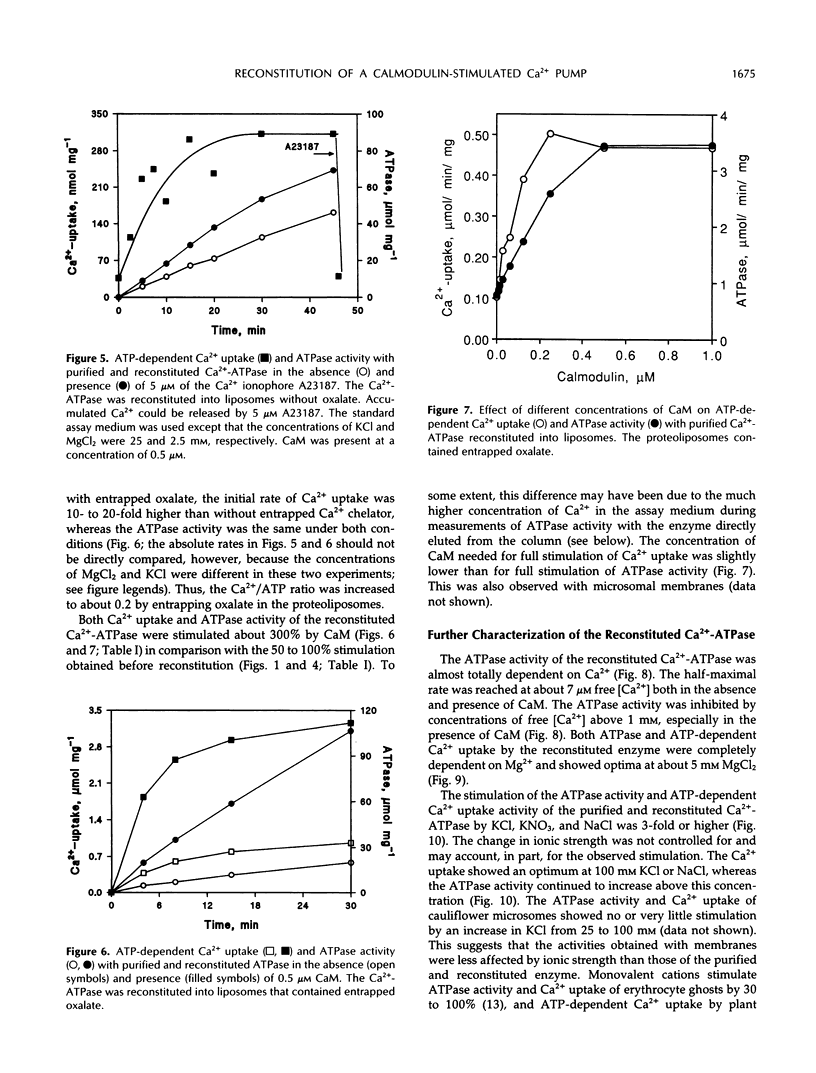
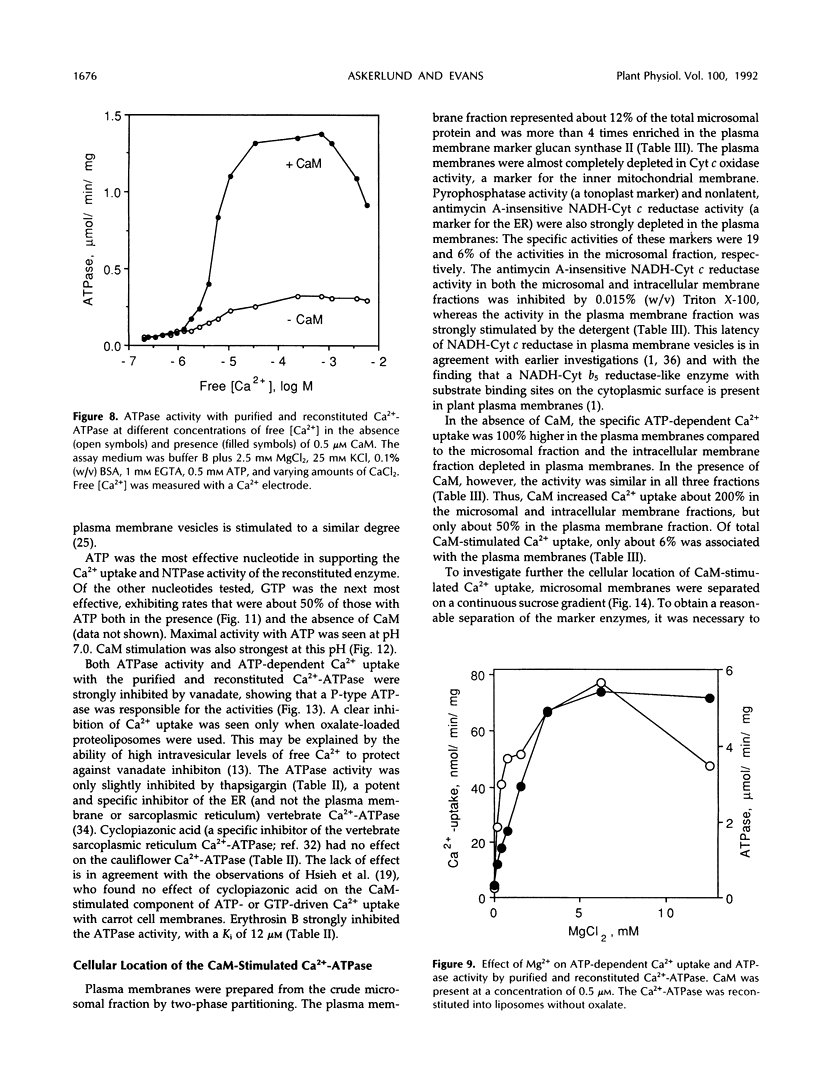
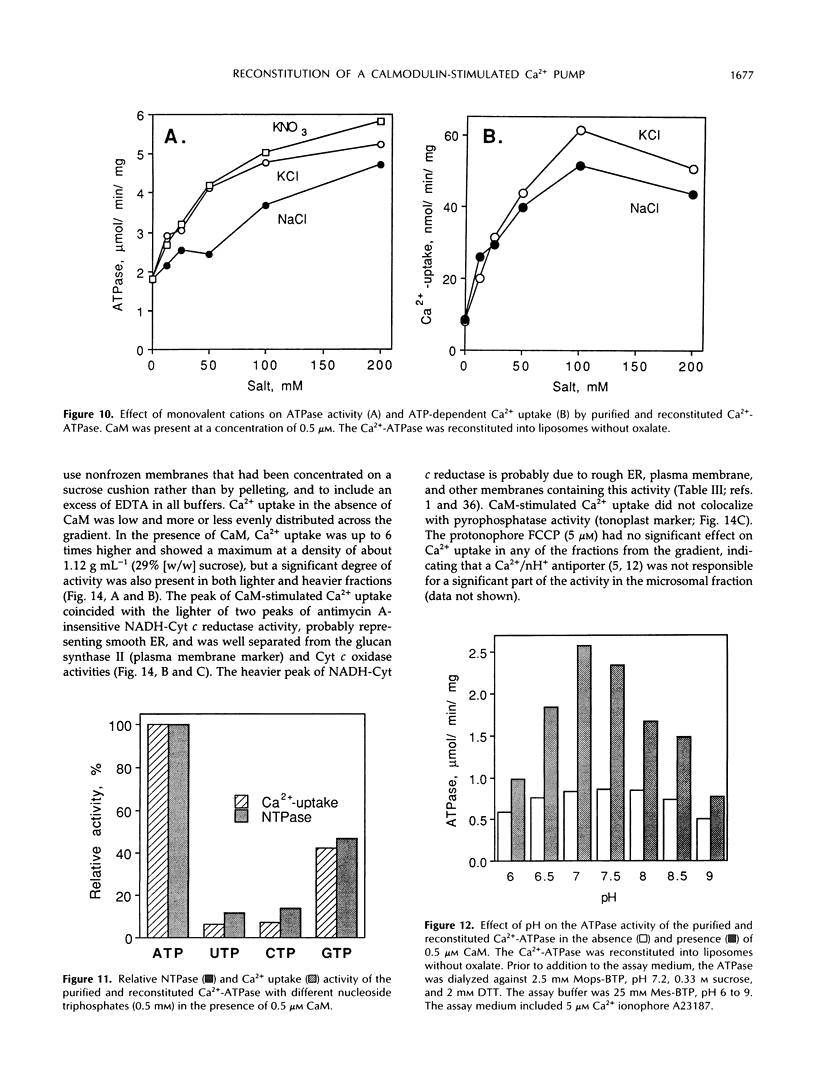
Purification and functional reconstitution of a calmodulin-stimulated Ca2+-ATPase from cauliflower (Brassica oleracea L.) is described. Activity was purified about 120-fold from a microsomal fraction using calmodulin-affinity chromatography. The purified fraction showed a polypeptide at 115 kD, which formed a phosphorylated intermediate in the presence of Ca2+, together with a few polypeptides with lower molecular masses that were not phosphorylated. The ATPase was reconstituted into liposomes by 3-([cholamidopropyl]-dimethylammonio-)1-propanesulfonate (CHAPS) dialysis. The proteoliposomes showed ATP-dependent Ca2+ uptake and ATPase activity, both of which were stimulated about 4-fold by calmodulin. Specific ATPase activity was about 5 μmol min−1 (mg protein)−1, and the Ca2+/ATP ratio was 0.1 to 0.5 when the ATPase was reconstituted with entrapped oxalate. The purified, reconstituted Ca2+-ATPase was inhibited by vanadate and erythrosin B, but not by cyclopiazonic acid and thapsigargin. Activity was supported by ATP (100%) and GTP (50%) and had a pH optimum of about 7.0. The effect of monovalent and divalent cations (including Ca2+) on activity is described. Assay of membranes purified by two-phase partitioning indicated that approximately 95% of the activity was associated with intracellular membranes, but only about 5% with plasma membranes. Sucrose gradient centrifugation suggests that the endoplasmic reticulum is the major cellular location of calmodulin-stimulated Ca2+-pumping ATPase in Brassica oleracea inflorescences.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askerlund P., Laurent P., Nakagawa H., Kader J. C. NADH-Ferricyanide Reductase of Leaf Plasma Membranes : Partial Purification and Immunological Relation to Potato Tuber Microsomal NADH-Ferricyanide Reductase and Spinach Leaf NADH-Nitrate Reductase. Plant Physiol. 1991 Jan;95(1):6–13. doi: 10.1104/pp.95.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borke J. L., Caride A. J., Yaksh T. L., Penniston J. T., Kumar R. Cerebrospinal fluid calcium homeostasis: evidence for a plasma membrane Ca2+-pump in mammalian choroid plexus. Brain Res. 1989 Jun 12;489(2):355–360. doi: 10.1016/0006-8993(89)90870-6. [DOI] [PubMed] [Google Scholar]
- Briars S. A., Evans D. E. The calmodulin-stimulated ATPase of maize coleoptiles forms a phosphorylated intermediate. Biochem Biophys Res Commun. 1989 Feb 28;159(1):185–191. doi: 10.1016/0006-291x(89)92421-2. [DOI] [PubMed] [Google Scholar]
- Briskin D. P. Ca-translocating ATPase of the plant plasma membrane. Plant Physiol. 1990 Oct;94(2):397–400. doi: 10.1104/pp.94.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckhout T. J. Characterization of Ca Transport in Purified Endoplasmic Reticulum Membrane Vesicles from Lepidium sativum L. Roots. Plant Physiol. 1984 Dec;76(4):962–967. doi: 10.1104/pp.76.4.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. The calcium pumping ATPase of the plasma membrane. Annu Rev Physiol. 1991;53:531–547. doi: 10.1146/annurev.ph.53.030191.002531. [DOI] [PubMed] [Google Scholar]
- Carafoli E., Zurini M. The Ca2+-pumping ATPase of plasma membranes. Purification, reconstitution and properties. Biochim Biophys Acta. 1982 Dec 31;683(3-4):279–301. doi: 10.1016/0304-4173(82)90004-0. [DOI] [PubMed] [Google Scholar]
- Cornelius F. Functional reconstitution of the sodium pump. Kinetics of exchange reactions performed by reconstituted Na/K-ATPase. Biochim Biophys Acta. 1991 Mar 7;1071(1):19–66. doi: 10.1016/0304-4157(91)90011-k. [DOI] [PubMed] [Google Scholar]
- Giannini J. L., Gildensoph L. H., Reynolds-Niesman I., Briskin D. P. Calcium Transport in Sealed Vesicles from Red Beet (Beta vulgaris L.) Storage Tissue : I. Characterization of a Ca-Pumping ATPase Associated with the Endoplasmic Reticulum. Plant Physiol. 1987 Dec;85(4):1129–1136. doi: 10.1104/pp.85.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräf P., Weiler E. W. Functional Reconstitution of an ATP-Driven Ca-Transport System from the Plasma Membrane of Commelina communis L. Plant Physiol. 1990 Oct;94(2):634–640. doi: 10.1104/pp.94.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmke S. M., Howard B. D. Fractionation and reconstitution of the sarcoplasmic reticulum Ca2+ pump solubilized and stabilized by CHAPS/lipid micelles. Membr Biochem. 1987;7(1):1–22. doi: 10.3109/09687688709029426. [DOI] [PubMed] [Google Scholar]
- Hsieh W. L., Pierce W. S., Sze H. Calcium-pumping ATPases in vesicles from carrot cells : stimulation by calmodulin or phosphatidylserine, and formation of a 120 kilodalton phosphoenzyme. Plant Physiol. 1991 Dec;97(4):1535–1544. doi: 10.1104/pp.97.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R. S., Pedersen P. L. Sensitive protein assay in presence of high levels of lipid. Methods Enzymol. 1989;172:393–399. doi: 10.1016/s0076-6879(89)72025-5. [DOI] [PubMed] [Google Scholar]
- Kasai M., Muto S. Solubilization and reconstitution of ca pump from corn leaf plasma membrane. Plant Physiol. 1991 Jun;96(2):565–570. doi: 10.1104/pp.96.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Neugebauer J. M. Detergents: an overview. Methods Enzymol. 1990;182:239–253. doi: 10.1016/0076-6879(90)82020-3. [DOI] [PubMed] [Google Scholar]
- Penniston J. T., Filoteo A. G., McDonough C. S., Carafoli E. Purification, reconstitution, and regulation of plasma membrane Ca2+-pumps. Methods Enzymol. 1988;157:340–351. doi: 10.1016/0076-6879(88)57089-1. [DOI] [PubMed] [Google Scholar]
- Pohl T. Concentration of proteins and removal of solutes. Methods Enzymol. 1990;182:68–83. doi: 10.1016/0076-6879(90)82009-q. [DOI] [PubMed] [Google Scholar]
- Preiss J., Danner S., Summers P. S., Morell M., Barton C. R., Yang L., Nieder M. Molecular Characterization of the Brittle-2 Gene Effect on Maize Endosperm ADPglucose Pyrophosphorylase Subunits. Plant Physiol. 1990 Apr;92(4):881–885. doi: 10.1104/pp.92.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racker E. Reconstitution of membrane processes. Methods Enzymol. 1979;55:699–711. doi: 10.1016/0076-6879(79)55078-2. [DOI] [PubMed] [Google Scholar]
- Rasi-Caldogno F., Carnelli A., De Michelis M. I. Plasma Membrane Ca-ATPase of Radish Seedlings : II. Regulation by Calmodulin. Plant Physiol. 1992 Mar;98(3):1202–1206. doi: 10.1104/pp.98.3.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler N. W., Jona I., Vegh M., Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989 Oct 25;264(30):17816–17823. [PubMed] [Google Scholar]
- Stoscheck C. M. Quantitation of protein. Methods Enzymol. 1990;182:50–68. doi: 10.1016/0076-6879(90)82008-p. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Ca2+-selective electrodes: a novel PVC-gelled neutral carrier mixture compared with other currently available sensors. J Neurosci Methods. 1981 Jun;4(1):73–86. doi: 10.1016/0165-0270(81)90020-0. [DOI] [PubMed] [Google Scholar]