Abstract
Patients with autosomal dominant polycystic kidney disease benefit from specialized care over their lifetimes, starting with diagnosis of the condition with ongoing discussion of both the renal course and extra-renal issues. Both renal and extra-renal issues may continue to cause major morbidity even after successful kidney transplant or initiation of RRT, and extra-renal disease aspects should always be considered as part of routine management. In this review, we will focus on updates in pain/depression screening, cardiac manifestations, liver and pancreatic cysts, kidney stone management, and genetic counseling. In some instances, we have shared our current clinical practice rather than an evidence-based guideline. We anticipate more standardization of care after the release of the Kidney Disease Improving Global Outcomes guidelines for management in autosomal dominant polycystic kidney disease later this year.
Keywords: ADPKD, liver cysts
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) patient care is often complex and multidisciplinary1 because ADPKD is a systemic disease affecting multiple organ systems.2 This review is not intended to be a comprehensive guide to management of all extra-renal ADPKD manifestations. Instead, we have chosen to highlight those areas in which there are advances affecting management of patients. We will cover pain and depression screening, cardiac manifestations of ADPKD, screening for intracranial aneurysms (ICAs) and vascular aneurysms, and management of liver and pancreatic cysts. Because genetic testing in cystic kidney disease is rapidly being adopted in practice, we offer some general suggestions on ordering and interpreting testing. We anticipate more standardization of care after the release of the Kidney Disease Improving Global Outcomes guidelines for management in ADPKD later this year and in the United States with harmonization of practice in the polycystic kidney disease (PKD) Foundation designated Centers of Excellence.
Pain and Depression
ADPKD significantly affects health-related quality of life.3,4 Pain is a primary driver of the mental and physical burden of the disease, contributing substantially to reductions in health-related quality of life.5–7 Pain related to increasing kidney size and cyst complications is reported in 50% of patients with ADPKD, with 20% reporting pain often, usually, or always.6 Pain remains an issue as disease progresses with 44% of transplant and 59% of dialysis patients reporting pain.3 Pain associated with ADPKD carries a physical and mental burden and is described by patients as inexplicable and unpredictable in qualitative studies,5 serving as a reminder of patients' diseased state.7 Patients desire support from their providers, open and honest conversations regarding pain management, and information and resources to learn more about their condition.8
Despite patients reporting pain management as a top priority in ADPKD outcomes,9 many feel that their pain is underestimated by their physician and wish for more discussion surrounding pain management and strategies.3,7 The ADPKD Pain and Discomfort Scale was developed and validated to specifically evaluate the severity and type of pain experienced by patients with ADPKD.10 Although this tool was designed for standardization of patient-reported outcomes research, routine questioning could normalize discussion of pain11 and could provide nephrologists with the ability to monitor changes in pain severity and interference over time.
We propose asking about each pain type (sharp, dull, or fullness or discomfort) at each clinical encounter (Table 1), with further exploration of the domains of severity or interference10 with a positive response as a way of engaging in a discussion of individual patient concerns.
Table 1.
Suggested screening at every encounter
| PKD-Specific Concerns | Screening | Additional Comments |
|---|---|---|
| Pain10 | Ask about change in chronic pain, acute pain, and fullness and discomfort | If positive, review the additional questions from ADPKD-PDS |
| Depression12 | Ask the following two questions: During the past month have you often been bothered by feeling down, depressed, or hopeless? During the past month have you often been bothered by little interest or pleasure in doing things? |
If positive, consider further screening, referral, and/or initiation of treatment |
| High BP13 | We encourage patients to check BP at home, with a goal BP of 110/75 for younger high-risk patients and 120/80 for older patients with kidney function decline | We avoid use of diuretics for those patients considering or taking tolvaptan |
| Diet and lifestyle14 | For patients with preserved kidney function, we encourage a low salt diet, high in potassium, with 3 L of fluid intake, and maintenance of an ideal body weight | For those patients interested in alternative strategies, we encourage intermittent fasting, with continued fluid intake but discuss concerns about high fat (ketogenic) diets |
ADPKD, autosomal dominant polycystic kidney disease; ADPKD-PDS, ADPKD Pain and Discomfort Scale.
Between 22% and 60% of patients with ADPKD have depression,15–17 and therefore, we also recommend screening. Although providers generally consider early ADPKD to be asymptomatic, the mental impact of an ADPKD diagnosis is immediate. On diagnosis, patients deal with feelings of uncertainty, fear, and a sense of loss.8 Furthermore, patients report lower satisfaction with life compared with healthy individuals even at the early stages of disease.18 Patients express guilt surrounding passing the condition to their children, and for those without children, ADPKD can complicate the decision to have a child.15 Tolvaptan, approved for treatment of ADPKD in 2018, has been shown to decrease symptoms of depression19; however, other studies have shown that patients maintain the same kidney-related quality-of-life concerns as untreated patients, despite improved treatment satisfaction.20,21
Depression is also associated with worse physical health of patients with ADPKD. Patients with depression are more likely to deal with pain and have lower scores on quality-of-life questionnaires and less sleep.15 Patients with more symptoms are less likely to comply with dietary guidelines, which may negatively affect disease progression, and depression is more common in patients with more severe disease.17 Patients in the Chronic Renal Insufficiency Cohort were more likely to have rapid nonlinear eGFR decline with moderate or high scores on the Beck Depression Index compared with patients with low scores.22
Given the negative effects of depression on mental and physical health of patients with ADPKD, it is important to screen for depression early on in ADPKD disease course and at regular intervals throughout management. The US Preventative Services Taskforce recommends that all adults should be screened for depression using evidence-based protocols, such as various iterations of the Patient Health Questionnaire.23 A short two-question survey should be sufficient in first-line screening (Table 1).12 If positive, this should be followed-up with more comprehensive depression measures.
Genetic Testing
Undoubtedly, genotype is an important predictor of clinical progression, but genetic testing is an evolving area in ADPKD. Opinion-based standards recommend genetic testing for patients with an unknown family history, with atypical imaging, for screening a young (younger than 40 years24) family member who wishes to be a kidney donor, and for use of in vitro fertilization with preimplantation genetic testing for mutation.24,25 Preimplantation genetic testing for mutation for ADPKD is possible if genetic studies in the affected parent are completed and conclusive before conception with cumulative success rates per couple of 58%–65%.26,27
The PKD spectrum of genes causing cystic diseases has expanded. Gene variants can be identified using specific targeted next-generation sequencing panels which are optimized for sequencing coding regions (exomes) of cystic kidney genes. The widespread availability of relatively inexpensive targeted next-generation sequencing panels has permitted rapid adoption into clinical practice. While this is welcome because many different genotypes may lead to a similar phenotype28 and knowing the genetic diagnosis may become a standard component of care, decisions regarding risk of progression (loss of kidney function) or screening for extra-renal features should not be made on the basis of genotype alone. Many nephrologists currently lack training in molecular genetic test interpretation. Geneticists, genetic counselors experienced in renal diseases, and nephrologists trained in clinical genomics will likely fill these gaps in the future.
Liver Cysts
The liver is the most common extra-renal site of disease involvement in ADPKD. Most patients with polycystic liver disease (PLD) are asymptomatic and do not require treatment. Both liver cysts and parenchymal enlargement are responsible for most complications associated with PLD. The minority with symptomatic PLD complain of heartburn due to gastroesophageal reflux, early satiety, abdominal pain, abdominal distention, and so on29 (Table 2). Liver cyst infection can be life-threatening, and in suspected cases it is evaluated with blood cultures, possible cyst aspiration, and by positron emission tomography/computed tomography (CT) and treated with broad-spectrum, lipid-soluble antibiotics.30
Table 2.
Potential liver complications in autosomal dominant polycystic kidney disease
| Acute Complications from Single Cysts | Severe Hepatomegaly | Other Liver Complications |
|---|---|---|
| Infection or torsion Rupture or hemorrhage Obstructive jaundice Ascending cholangitis Biliary peritonitis (after cyst rupture) |
Abdominal fullness Abdominal distension GERD/anorexia/nausea/early satiety Dyspnea Abdominal hernia LFT abnormalities Ascites, hepatic outflow obstruction, portal hypertension, IVC compression, bile duct compression Pleural effusion Organ displacement Failure to thrive/malnutrition Budd-Chiari syndrome |
Isolated common bile duct dilation Hepatic fibrosis or biliary fibroadenomatosis Idiopathic biliary tract dilation (Caroli syndrome) |
GERD, gastroesophageal reflux disease, LFT; liver function test; IVC, inferior vena cava.
Known risk factors influence PLD severity, including female sex, parity, and exogenous estrogen exposure, and cysts are more prevalent, and cyst burden is generally higher in women than in men. Women who have multiple pregnancies or who have used oral contraceptives or estrogen replacement therapy have more severe disease, suggesting an estrogen effect on hepatic cyst growth.31–34 Growth in liver cysts declines after menopause.35,36 A history of exposure to estrogen-containing oral contraceptives correlated with PLD growth in premenopausal women. Every year of exposure correlated with a 1.45% higher height-adjusted total liver volume (hTLV), which corresponded to a 15.5% higher hTLV for every 10 years of use compared with unexposed women37 although there was no association between estrogen-containing oral contraceptive use and hTLV in the combined group of both premenopausal and postmenopausal women or in the postmenopausal subgroup.
Although there is an association with estrogen and liver cysts, two recent analyses of younger patients, either from Consortium of Radiologic Imaging Study of PKD or HALT Progression of Polycystic Kidney Disease Cohort A, did not find an independent effect of pregnancy or exogenous estrogen use on liver volume.36,38 This discrepancy may be because liver cyst complications still affect only a minority of patients, and average liver volume was only slightly increased in most patients, or because other factors drive liver cyst growth. Liver cyst volume adjusted for height and age and then stratified maybe a predictive biomarker to identify individuals at highest risk for progressive liver cyst disease burden.36 We discuss the use of hormonal therapy with our female patients, with a recommendation for avoidance for those at higher risk of PLD progression and individualized counseling for those at lower risk. Conservatively, imaging consistent with a total liver volume <1500 ml, or only a few cysts seen by CT or magnetic resonance imaging (MRI) imaging, maybe considered lower risk. Antiestrogen therapies are currently in clinical trials39
Somatostatin analogs administered by intramuscular injection (octreotide, lanreotide) are effective in decreasing liver growth and may be considered for symptomatic patients, for whom surgical intervention is not appropriate.40 A meta-analysis of pooled data from 592 patients shows that these treatments are effective in decreasing total liver and kidney volume; however, side effects include higher blood sugars, diarrhea, abdominal pain, cholelithiasis/cholecystitis, and alopecia.41 Younger patients42 with an elevated alkaline phosphatase43 are more likely to respond, with discontinuation of therapy if there is no symptomatic benefit after 6 months. We have occasionally tried short-acting subcutaneous twice daily dosing of octreotide for patients who are not able to obtain the long-acting (monthly) preparations because of insurance issues.
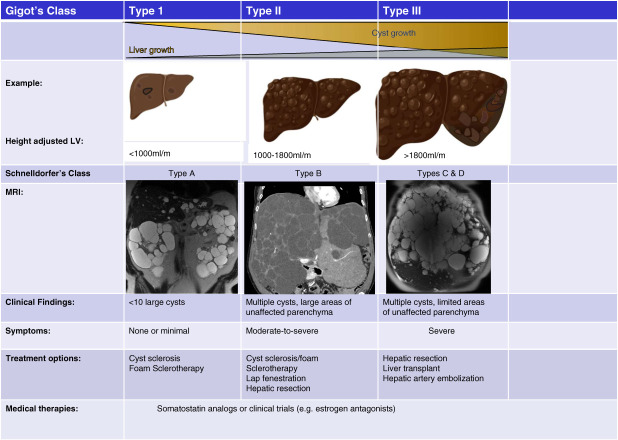
Several imaging classification systems are used in PLD (Figure 1); Gigot classification and Mayo Clinic classification stratify patients for operative procedure selection.44 Cyst aspiration is indicated for large symptomatic liver cysts (Figure 2). Percutaneous aspiration may be followed by injection of a sclerosing agent which causes destruction of the epithelial lining inhibiting fluid production. Several centers have adopted foaming sclerosants45,46 providing superior long-term efficacy and the procedure being less painful than other sclerosants. Without sclerosant, the cyst fluid may re-accumulate.
Figure 1.
Classification criteria for PLD severity, clinical presentation, symptoms, and treatment options. Gigot and Schnelldorfer classifications are based on the number and size of the cysts and the remaining liver parenchyma. Height adjusted liver volume criteria: <1000, 1000–1800, >1800 ml/m. Coronal T2-weighted MRI images are shown for each patient type. In type A, the patient has large kidney cysts, but well-preserved liver parenchyma; in the type B patient, there is a residual preserved area of liver parenchyma; and in the type C and D patient, there is very little nonaffected liver. LV, left ventricle; MRI, magnetic resonance imaging; PLD, polycystic liver disease.
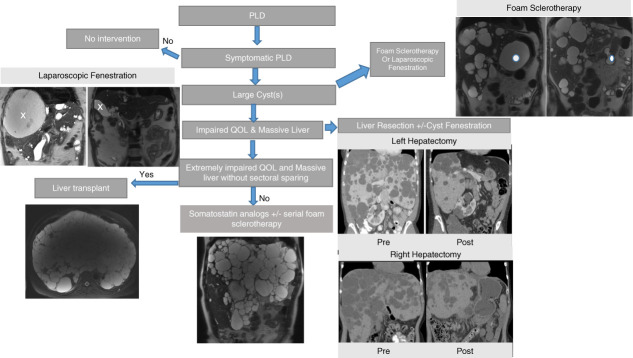
Figure 2.
Algorithm for PLD management in ADPKD. Asymptomatic or mildly symptomatic patients may require no long-term intervention. Patients who develop impaired quality of life may benefit from foam sclerotherapy, laparoscopic fenestration, liver resection (partial hepatectomy), or liver transplant. Patients who are not candidates for surgical treatment may benefit from a trial of somatostatin analogs with or without serial foam sclerotherapy. Coronal T2-weighted MRI images are shown. The x and the dot show the changes in a cyst before and after therapy. ADPKD, autosomal dominant polycystic kidney disease.
Fenestration surgery permits surgical deroofing of multiple cysts in a single laparoscopic procedure but is associated with significant side effects (ascites, pleural effusion, bleeding, biliary leakage).47 Segmental liver resection (with or without cyst fenestration) could be considered in individuals with symptomatic disease and/or massive hepatomegaly, who harbor cyst rich segments and have at least one segment with unaffected liver parenchyma.40,47 Careful preoperative selection and imaging is needed to select appropriate patients (Figure 2).
Liver transplantation is warranted in patients with extremely debilitating symptoms, seriously compromised functional status and reduced quality of life, untreatable complications, such as portal hypertension, and severe malnutrition with bilobar extensive cystic liver disease lacking adequate areas of parenchymal sparing. Since patients with PLD have essentially normal liver function, calculated model for end-stage liver disease (MELD) scores are low or normal, and hence United Network for Organ Sharing has developed a MELD exemption review process for PLD cases. An important update in 2021 allowed for liver transplant listing for patients with a GFR <20 ml/min or with a prior kidney transplant or with moderate-to-severe malnutrition, in addition to those on dialysis.48 This change expanded eligibility, as previously only patients on dialysis were eligible for MELD exception points for liver transplant listing. In individuals with GFR <30 or ESKD, simultaneous deceased donor combined liver-kidney transplant is the most optimal approach. A recent single-center study shows good survival outcomes for orthotopic liver transplant alone, living donor liver transplant, and simultaneous liver-kidney transplant.49
Pancreatic Lesions
Pancreatic cyst lesions are a recognized component of the ADPKD phenotype (seen in 15%–23% of patients)50 Most are incidental. As our practice has shifted to ordering more volumetric measurements of kidney and liver size, we have also frequently encountered incidental intraductal papillary mucinous neoplasms (IPMNs) in our patients with ADPKD (about 1% of patients). In general, these should be followed using standard protocols as developed for the general population. In a single-center review, IPMN patients were older, and seven of 12 had received kidney transplants.50 Thus, we suggest continued follow-up of patients with known IPMNs after kidney transplant, with appropriate surgical referral as indicated.
Diverticulosis and Hernias
The prevalence of colonic diverticulosis increases with increasing age in both ADPKD and non-ADPKD populations. A recent large retrospective study found that prevalence of diverticulosis was higher in patients with an ADPKD diagnosis, compared with those without, with a median age at onset of 64 years, compared with 72 years in those without an ADPKD diagnosis.51 Diverticulitis was also more prevalent in those patients with an ADPKD diagnosis, with a higher prevalence of diverticulitis in patients with an ADPKD diagnosis and a kidney transplant, compared with patients with a kidney transplant and no ADPKD diagnosis.51 It is reasonable, therefore, to have a high degree of suspicion for diverticular complications particularly in older patients or patients with ESKD from ADPKD.
Abdominal wall hernias are more common in ADPKD compared with other kidney disease or general surgical patients.52 Patients with ADPKD with ESKD on CAPD are at increased risk for indirect inguinal hernia53; however, in our experience many patients with ADPKD do well on peritoneal dialysis.
Cardiac Manifestations
Cardiac dysfunction is common in ADPKD because of both the activation of renin-angiotensin-aldosterone system and the presence of polycystins in vascular and cardiac tissue. A recent editorial reviewed many aspects of cardiac issues in ADPKD,54 and therefore, we have only highlighted a few areas for discussion below.
Hypertension and Left Ventricular Hypertrophy
Hypertension in ADPKD is associated with an increased risk of renal loss55 with onset well before declining kidney function. The onset of hypertension before age 35 years is a clinical risk factor for progression in the Predicting Renal Outcome in PKD risk score.56 ADPKD adults are more likely to be nondippers without physiologic drop in BP during 24-hour ambulatory monitoring than age-matched normotensive healthy controls, and 52% of children with ADPKD were characterized as nondippers.57,58 In patients with preserved renal function, a morning BP surge, or a swift rise in BP in the early hours of the morning, was independently associated with left ventricular hypertrophy (LVH).59
In the era before the widespread use of angiotensin converting enzyme inhibitors or angiotensin receptor blockers, LVH in ADPKD was common60; however, the prevalence of LVH was low at baseline in the HALT Progression of Polycystic Kidney Disease cohort, perhaps reflecting the widespread use of these medications.61 Two recent studies62,63 have found higher rates of LVH, perhaps reflecting differences in LVH measurement. Obesity, insulin resistance, or polycystin-dependent pathways may affect cardiac outcomes.64 We do not routinely screen with echocardiograms for hypertension management in ADPKD.
We advocate BP measurement and counseling at every clinical encounter with a BP target of 110/75 for high-risk patients with preserved kidney function13 and 120/8065 for patients with renal decline (Table 1). We also encourage home BP monitoring (2–3 times a week) particularly during medication titration. Because salt intake is also associated with hypertension and kidney function decline in ADPKD,66 we review dietary guidelines as well. For patients age older than 40 years, without diagnosed atherosclerotic cardiovascular disease, we use the American College of Cardiology risk estimator tool to determine the need for additional preventative therapy, such as a statin or aspirin.67
Valvular Abnormalities
Heart valve abnormalities are common in ADPKD affecting up to 39.5% of patients, with tricuspid valve regurgitation (16%), mitral valve regurgitation (15.3%), aortic valve regurgitation (4.8%), and mitral valve prolapse in 3.4%.68 Other studies have reported higher rates of mitral valve abnormalities ranging from 26% to 63% of patients.63,69 Aortic root aneurysms/dissection are more common in ADPKD,70 and patients with more rapidly progressive kidney disease (higher Mayo imaging classification) have larger aortic root diameters.63 Our practice is to offer a screening echocardiogram as part of initial screening only if there is a murmur on physical examination. If aortic root dilation is found, cardiac MRI or multidetector CT may be needed for further evaluation.
All our patients undergo echocardiograms as part of pretransplant evaluation regardless of physical examination findings as they approach kidney failure (Table 3).
Table 3.
Suggested screening at initial encounter or at preparation for RRT
| Screening | Initial Encounter | At Preparation for RRT |
|---|---|---|
| Cardiac | ECHO only if physical examination is abnormal | ECHO routinely |
| ICAs | MRA for positive family history, symptoms, or patient preference | Discuss MRA if not already performed |
| AAA | Men age 65–75 years with a smoking history or men older than 60 years with a family history71 | |
| Liver cyst burden | Consider measuring liver volume at the time that total kidney volume measurements are obtained. Risk assessment may help tailor discussion of hormonal therapy | Re-evaluate liver cyst burden to assess need for possible liver transplant in the future |
| Kidney stones | 24-h urine with discussion of citrate therapy if hypocitraturia present |
ECHO, echocardiogram; ICA, intracranial aneurysm; MRA, magnetic resonance angiogram; AAA, abdominal aortic aneurysm.
Pericardial Effusion/Cardiomyopathy
The incidence of pericardial effusion is increased in ADPKD.72,73 Up to 35% of patients with clinically diagnosed ADPKD and serum creatinine ≥1.1 mg/dl were found to have pericardial effusion, frequently moderate to large but without clinical consequence.73 Importantly, these effusions were not associated with degree of loss of renal function. Clinically, if no other cause (uremia or infection for instance) is found for a pericardial effusion, we monitor as clinically indicated.
Either PKD1 or PKD2 variants may be associated with cardiomyopathy,74 but idiopathic dilated cardiomyopathy is more commonly associated with PKD2 disease-causing variants.74,75 Although most medications for management of heart failure are safe in ADPKD, sodium-glucose cotransporter-2 inhibitor (SGLT2i) could be used after a careful consideration of the individual risks and benefits for a patient.76Treatment with an SGLT2i may stimulate vasopressin and thus be detrimental patients with for ADPKD. In patients with ADPKD with a low ejection fraction, or at high risk of death, SGLT2i may be beneficial. A pilot feasibility study (NCT05510115) will evaluate the safety of SGLT2i in ADPKD.
Kidney Stone Management
Nephrolithiasis is common in ADPKD; the prevalence of stones is estimated to be 5–10 times greater in patients with ADPKD than in the general population.77 Patients with ADPKD are at a greater likelihood of having a hospital encounter for kidney stones compared with non-ADPKD patients.78 Kidney volume was significantly greater with nephrolithiasis than without; a kidney volume ≥500 ml was a significant predictor of nephrolithiasis in patients with ADPKD with normal renal function.79 The number of kidney cysts and the predominant cyst size are also significantly associated with nephrolithiasis in ADPKD.80
Because crystal deposition may accelerate cyst growth81 and hypocitraturia is associated with faster eGFR decline82 we have been evaluating for kidney stone risk factors by obtaining a 24-hour urine on patients with evidence of stones or mural calcification within a cyst (Table 3). Patients with ADPKD with stones were significantly more likely to be hypocitraturic with reduced 24-hour urine volume and urinary magnesium excretion, which are prolithogenic urinary risk factors.80 We treat with alkalinizing therapy as appropriate.
CT is the preferred imaging technique for stone evaluation.79,83 The presence of cysts within the sonographic field may degrade the diagnostic performance of ultrasound.77 CT is superior to ultrasound for exploration of kidney stones and detection of small stones, as well as detection of stones trapped in the ureters.84
Kidney stone management in patients with ADPKD generally follows the same guidance as management in patients without ADPKD. Ureteroscopy is safe and effective, with the advantage that it can be used in the presence of cyst-induced anatomic abnormalities and does not induce risk of traumatic nephron loss.85 Percutaneous surgical approaches are used in some cases with larger stones.86 Extracorporeal shock wave lithotripsy (ESWL) is the preferred and relatively least invasive intervention to directly treat stones, and it is best practice to use ESWL to treat stones smaller than 2 mm in adults with ADPKD. In addition, ESWL has also been shown to be an effective intervention in children with polycystic kidneys as one study reported a 94% stone-free status at a 12-month follow-up in 17 children with polycystic kidneys.11
Tolvaptan may confer protective effects against stone formation. A 2020 prospective, observational cohort study by Bargagli et al.87 found that urinary supersaturation ratios for calcium oxalate, brushite, and uric acid were significantly reduced with tolvaptan use. Higher rates of urinary citrate and calcium excretion, in conjunction with reduced net acid excretion, were observed in tolvaptan-treated patients with ADPKD.
ICAs
ICAs may rupture leading to subarachnoid, intraventricular, or intracerebral hemorrhage.88 Ruptured ICAs account for 4%–7% of deaths in patients with ADPKD.89 In addition, there are case reports of unilateral and bilateral subdural hematomas in ADPKD.90–93 Some have been associated with arachnoid cysts.
The prevalence of ICAs is four times higher in patients with ADPKD than in the general population (8%–12% versus 2%–3%, respectively).94 The incidence of ICA in patients with ADPKD with a positive family history of hemorrhagic stroke or ICA is higher compared with patients with ADPKD lacking such a family history (11.6% versus 23.5%).95 Although there is consensus to screen patients with ADPKD with a family history, there is no consensus on whether to screen every patient with ADPKD.94
We most commonly use a noncontrast magnetic resonance angiogram for screening (Table 1), but other modalities may be preferred because of patient issues (metallic surgical clips, etc).
Screening for ICA with mitral regurgitation angiography in patients with ADPKD every 5 years with annual follow-up in detected cases is cost-effective regardless of the family history of ICA.96 The existing literature recommends initial screening by the age of 30 years—earlier, if there is a family history of ICA.94 After a negative study, repeat screening every 5 years was also cost effective,96 but there is no consensus in the literature about rescreening after a negative study, particularly in patients without a family history.
Presymptomatic screening is generally not recommended before age 18 years. Because the average age of progression to ESKD is 55 years, and some studies have postulated that little benefit might be obtained from screening individuals older than 50 years.94 However, because the risk of intracranial hemorrhage increases with kidney failure,97 we discuss screening for ICA as part of preparation for RRT.
Annual surveillance mitral regurgitation angiography is optimal in patients with incidentally detected ICAs.96 Because most detected aneurysms are small and at low risk of rupture, many patients with incidentally found aneurysms will need long-term follow-up imaging.98 We collaborate with neurosurgery and neurointerventional radiology for monitoring of these patients.
In conclusion, ADPKD is a systemic disorder affecting multiple organ systems. Because most patients will be followed by a nephrology team, for these complex patients, the nephrologist is often the primary gatekeeper, referring to other specialties as appropriate. Our practices are based in academic tertiary medical care systems with access to interventional radiology, pain management, hepatology, neurosurgery, genetics, urology, cardiology, liver surgery, and high-risk obstetric care. In addition, we are supported by dieticians, social workers, and genetic counselors comfortable with ADPKD care and work closely with our pediatric nephrology and transplant specialists. For patients with complex ADPKD, close integration of care is our goal. Extra-renal manifestations of ADPKD continue post–kidney transplant, and therefore, monitoring and management should be lifelong.
Disclosures
N.K. Dahl reports the following: Consultancy: Otsuka Pharmaceuticals; Research Funding: I am a PI for clinical trials sponsored by Janssen, Reata, and Vertex; Honoraria: Natera and Otsuka Pharmaceutical; Advisory or Leadership Role: Natera Scientific Advisory Board and PKD Foundation; Speakers Bureau: I was on the unbranded speakers bureau for Otsuka until 12/2022; and Other Interests or Relationships: Medical Advisory Board, NKF NE Chapter. M.C. Hogan reports the following: Consultancy: Otsuka pharmaceuticals; Research Funding: Camurus, Reata, and Regulus Pharmaceuticals; Advisory or Leadership Role: Camurus Pharmaceuticals—no payment, Glaxo-Smith-Kline—no payment, Mayo Clinic Proceedings Quality & Outcomes Journal—no payment, and Sail Bio—no payment; and Other Interests or Relationships: American Society of Nephrology, PKD Disease Outcomes Consortium, and PKD Foundation. All remaining authors have nothing to disclose.
Funding
None.
Author Contributions
Conceptualization: Neera K. Dahl
Visualization: Neera K. Dahl, Marie C. Hogan.
Writing – original draft: Neera K. Dahl, Maryam Gondal, Marie C. Hogan, Kathryn Simmons, Lawrence Ullman.
Writing – review & editing: Neera K. Dahl, Marie C. Hogan.
References
- 1.Harris T, Sandford R., EAF co-chairs, EAF members, Roundtable participants. European ADPKD Forum multidisciplinary position statement on autosomal dominant polycystic kidney disease care: European ADPKD forum and multispecialist roundtable participants. Nephrol Dial Transplant. 2018;33(4):563–573. doi: 10.1093/ndt/gfx327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luciano RL, Dahl NK. Extra-renal manifestations of autosomal dominant polycystic kidney disease (ADPKD): considerations for routine screening and management. Nephrol Dial Transplant. 2014;29(2):247–254. doi: 10.1093/ndt/gft437 [DOI] [PubMed] [Google Scholar]
- 3.Eriksson D Karlsson L Eklund O, et al. Health-related quality of life across all stages of autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2017;32(12):2106–2111. doi: 10.1093/ndt/gfw335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suwabe T Ubara Y Mise K, et al. Quality of life of patients with ADPKD-Toranomon PKD QOL study: cross-sectional study. BMC Nephrol. 2013;14:179. doi: 10.1186/1471-2369-14-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heiwe S, Bjuke M. “An evil heritage”: interview study of pain and autosomal dominant polycystic kidney disease. Pain Manag Nurs. 2009;10(3):134–141. doi: 10.1016/j.pmn.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 6.Miskulin DC Abebe KZ Chapman AB, et al. Health-related quality of life in patients with autosomal dominant polycystic kidney disease and CKD stages 1-4: a cross-sectional study. Am J Kidney Dis. 2014;63(2):214–226. doi: 10.1053/j.ajkd.2013.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong A Rangan GK Ruospo M, et al. A painful inheritance-patient perspectives on living with polycystic kidney disease: thematic synthesis of qualitative research. Nephrol Dial Transplant. 2015;30(5):790–800. doi: 10.1093/ndt/gfv010 [DOI] [PubMed] [Google Scholar]
- 8.Baker A King D Marsh J, et al. Understanding the physical and emotional impact of early-stage ADPKD: experiences and perspectives of patients and physicians. Clin Kidney J. 2015;8(5):531–537. doi: 10.1093/ckj/sfv060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Natale P Perrone RD Tong A, et al. Establishing a core outcome measure for pain in patients with autosomal dominant polycystic kidney disease: a consensus workshop report. Clin Kidney J. 2022;15(3):407–416. doi: 10.1093/ckj/sfab110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oberdhan D, Cole JC, Atkinson MJ, Krasa HB, Davison SN, Perrone RD. Development of a patient-reported outcomes tool to assess pain and discomfort in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2023;18(2):213–222. doi: 10.2215/CJN.0000000000000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoggan J. An objective pain assessment for autosomal dominant polycystic kidney disease (ADPKD): a patient's perspective. Clin J Am Soc Nephrol. 2023;18(2):147–148. doi: 10.2215/CJN.0000000000000043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arroll B, Khin N, Kerse N. Screening for depression in primary care with two verbally asked questions: cross sectional study. BMJ. 2003;327(7424):1144–1146. doi: 10.1136/bmj.327.7424.1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chebib FT Perrone RD Chapman AB, et al. A practical guide for treatment of rapidly progressive ADPKD with tolvaptan. J Am Soc Nephrol. 2018;29(10):2458–2470. doi: 10.1681/ASN.2018060590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chebib FT, Torres VE. Recent advances in the management of autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2018;13(11):1765–1776. doi: 10.2215/CJN.03960318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simms RJ, Thong KM, Dworschak GC, Ong AC. Increased psychosocial risk, depression and reduced quality of life living with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2016;31(7):1130–1140. doi: 10.1093/ndt/gfv299 [DOI] [PubMed] [Google Scholar]
- 16.Barros BPd, Nishiura JL, Heilberg IP, Kirsztajn GM. Ansiedade, depressão e qualidade de vida em pacientes com glomerulonefrite familiar ou doença renal policística autossômica dominante. Jornal brasileiro de nefrologia. 2011;33(2):120–128. doi: 10.1590/s0101-2800201100020000221789424 [DOI] [Google Scholar]
- 17.Yarlioglu AM, Oguz EG, Gundogmus AG, Atilgan KG, Sahin H, Ayli MD. The relationship between depression, anxiety, quality of life levels, and the chronic kidney disease stage in the autosomal dominant polycystic kidney disease. Int Urol Nephrol. 2023;55(4):983–992. doi: 10.1007/s11255-022-03375-2 [DOI] [PubMed] [Google Scholar]
- 18.Jankowska M, Walerzak A, Harciarek M, Rutkowski B, Dębska-Ślizień A. Acceptance of illness, satisfaction with life, and emotional control in the early stage of autosomal dominant polycystic kidney disease [published online ahead of print October 12, 2022]. Nephron. 2022:1–6. doi: 10.1159/000526840 [DOI] [PubMed] [Google Scholar]
- 19.Lai S Mangiulli M Perrotta AM, et al. Cardiovascular risk and quality of life in autosomal dominant polycystic kidney disease patients on therapy with tolvaptan: a pilot study. Curr Vasc Pharmacol. 2021;19(5):556–564. doi: 10.2174/1570161118999200918094809 [DOI] [PubMed] [Google Scholar]
- 20.Anderegg MA Dhayat NA Sommer G, et al. Quality of life in autosomal dominant polycystic kidney disease patients treated with tolvaptan. Kidney Med. 2020;2(2):162–171. doi: 10.1016/j.xkme.2019.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lecardeur L, Joly D. Quality of life in patients with autosomal dominant polycystic kidney disease. Nephrol Ther. 2017;13(7):505–510. doi: 10.1016/j.nephro.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 22.Missikpode C Ricardo AC Brown J, et al. Association between depressive symptom trajectory and CKD progression: findings from the chronic renal insufficiency cohort (CRIC) study. Kidney360. 2023;4(5):606–614. doi: 10.34067/KID.0000000000000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Screening for depression in adults: recommendation statement. Am Fam Physician. 2016;94(4):Online. [PubMed] [Google Scholar]
- 24.Cornec-Le Gall E, Alam A, Perrone RD. Autosomal dominant polycystic kidney disease. Lancet. 2019;393(10174):919–935. doi: 10.1016/S0140-6736(18)32782-X [DOI] [PubMed] [Google Scholar]
- 25.Pei Y, Watnick T. Diagnosis and screening of autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17(2):140–152. doi: 10.1053/j.ackd.2009.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gimpel C, Bergmann C, Mekahli D. The wind of change in the management of autosomal dominant polycystic kidney disease in childhood. Pediatr Nephrol. 2022;37(3):473–487. doi: 10.1007/s00467-021-04974-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berckmoes V Verdyck P De Becker P, et al. Factors influencing the clinical outcome of preimplantation genetic testing for polycystic kidney disease. Hum Reprod. 2019;34(5):949–958. doi: 10.1093/humrep/dez027 [DOI] [PubMed] [Google Scholar]
- 28.Cornec-Le Gall E, Torres VE, Harris PC. Genetic complexity of autosomal dominant polycystic kidney and liver diseases. J Am Soc Nephrol. 2018;29(1):13–23. doi: 10.1681/ASN.2017050483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neijenhuis MK Gevers TJ Hogan MC, et al. Development and validation of a disease-specific questionnaire to assess patient-reported symptoms in polycystic liver disease. Hepatology. 2016;64(1):151–160. doi: 10.1002/hep.28545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jouret F, Hogan MC, Chebib FT. A practical guide for the management of acute abdominal pain with fever in patients with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2022;37(8):1426–1428. doi: 10.1093/ndt/gfab040 [DOI] [PubMed] [Google Scholar]
- 31.Sherstha R McKinley C Russ P, et al. Postmenopausal estrogen therapy selectively stimulates hepatic enlargement in women with autosomal dominant polycystic kidney disease. Hepatology. 1997;26(5):1282–1286. doi: 10.1002/hep.510260528 [DOI] [PubMed] [Google Scholar]
- 32.Gabow PA, Johnson AM, Kaehny WD, Manco-Johnson ML, Duley IT, Everson GT. Risk factors for the development of hepatic cysts in autosomal dominant polycystic kidney disease. Hepatology. 1990;11(6):1033–1037. doi: 10.1002/hep.1840110619 [DOI] [PubMed] [Google Scholar]
- 33.Kanaan N, Devuyst O, Pirson Y. Renal transplantation in autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2014;10(8):455–465. doi: 10.1038/nrneph.2014.104 [DOI] [PubMed] [Google Scholar]
- 34.Kerlin BA, Stephens JA, Hogan MJ, Smoyer WE, O'Brien SH. Development of a pediatric-specific clinical probability tool for diagnosis of venous thromboembolism: a feasibility study. Pediatr Res. 2015;77(3):463–471. doi: 10.1038/pr.2014.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chebib FT Jung Y Heyer CM, et al. Effect of genotype on the severity and volume progression of polycystic liver disease in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2016;31(6):952–960. doi: 10.1093/ndt/gfw008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bae KT Tao C Feldman R, et al. Volume progression and imaging classification of polycystic liver in early autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2022;17(3):374–384. doi: 10.2215/CJN.08660621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Aerts RMM Bernts LHP Gevers TJG, et al. Estrogen-containing oral contraceptives are associated with polycystic liver disease severity in premenopausal patients. Clin Pharmacol Ther. 2019;106(6):1338–1345. doi: 10.1002/cpt.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hogan MC Abebe K Torres VE, et al. Liver involvement in early autosomal-dominant polycystic kidney disease. Clin Gastroenterol Hepatol. 2015;13(1):155–164.e6. doi: 10.1016/j.cgh.2014.07.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aapkes SE, Bernts LHP, Barten TRM, van den Berg M, Gansevoort RT, Drenth JPH. Estrogens in polycystic liver disease: a target for future therapies? Liver Int. 2021;41(9):2009–2019. doi: 10.1111/liv.14986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gevers TJG, Drenth JPH. Diagnosis and management of polycystic liver disease. Nat Rev Gastroenterol Hepatol. 2013;10(2):101–108. doi: 10.1038/nrgastro.2012.254 [DOI] [PubMed] [Google Scholar]
- 41.Griffiths J, Mills MT, Ong AC. Long-acting somatostatin analogue treatments in autosomal dominant polycystic kidney disease and polycystic liver disease: a systematic review and meta-analysis. BMJ Open. 2020;10(1):e032620. doi: 10.1136/bmjopen-2019-032620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gevers TJ Inthout J Caroli A, et al. Young women with polycystic liver disease respond best to somatostatin analogues: a pooled analysis of individual patient data. Gastroenterology. 2013;145(2):357–365.e1-2. doi: 10.1053/j.gastro.2013.04.055 [DOI] [PubMed] [Google Scholar]
- 43.Gevers TJ, Nevens F, Torres VE, Hogan MC, Drenth JP. Alkaline phosphatase predicts response in polycystic liver disease during somatostatin analogue therapy: a pooled analysis. Liver Int. 2016;36(4):595–602. doi: 10.1111/liv.12986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schnelldorfer T, Torres VE, Zakaria S, Rosen CB, Nagorney DM. Polycystic liver disease: a critical appraisal of hepatic resection, cyst fenestration, and liver transplantation. Ann Surg. 2009;250(1):112–118. doi: 10.1097/SLA.0b013e3181ad83dc [DOI] [PubMed] [Google Scholar]
- 45.Iliuta I-A Shi B Pourafkari M, et al. Foam sclerotherapy for cyst volume reduction in autosomal dominant polycystic kidney disease: a prospective cohort study. Kidney Med. 2019;1(6):366–375. doi: 10.1016/j.xkme.2019.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bugazia S Gregory A Torres VE, et al. Four Year Outcomes, Efficacy, and Safety of Foam Sclerotherapy in Cysts for ADPKD and Autosomal-Dominant Polycystic Liver Disease (ADPLD). ASN Kidney Week Abstract; 2022. TH-PO395. [Google Scholar]
- 47.Drenth JP, Chrispijn M, Nagorney DM, Kamath PS, Torres VE. Medical and surgical treatment options for polycystic liver disease. Hepatology. 2010;52(6):2223–2230. doi: 10.1002/hep.24036 [DOI] [PubMed] [Google Scholar]
- 48.Transplantion LaIO. Further enhancements to the National liver review board: guidance to liver transplant programs and the National liver review board for adult MELD exception review. In: Transplantion LaIO; 2021. [Google Scholar]
- 49.Alsager M Neong SF Gandhi R, et al. Liver transplantation in adult polycystic liver disease: the Ontario experience. BMC Gastroenterol. 2021;21(1):115. doi: 10.1186/s12876-021-01703-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McNicholas BA Kotaro Y Martin W, et al. Pancreatic cysts and intraductal papillary mucinous neoplasm in autosomal dominant polycystic kidney disease. Pancreas. 2019;48(5):698–705. doi: 10.1097/MPA.0000000000001306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duarte-Chavez R Stoltzfus J Yellapu V, et al. Colonic diverticular disease in autosomal dominant polycystic kidney disease: is there really an association? A nationwide analysis. Int J Colorectal Dis. 2021;36(1):83–91. doi: 10.1007/s00384-020-03736-2 [DOI] [PubMed] [Google Scholar]
- 52.Morris-Stiff G, Coles G, Moore R, Jurewicz A, Lord R. Abdominal wall hernia in autosomal dominant polycystic kidney disease. Br J Surg. 1997;84(5):615–617. doi: 10.1046/j.1365-2168.1997.02616.x [DOI] [PubMed] [Google Scholar]
- 53.Modi KB, Grant AC, Garret A, Rodger RS. Indirect inguinal hernia in CAPD patients with polycystic kidney disease. Adv Perit Dial. 1989;5:84–86. PMID: 2577435. [PubMed] [Google Scholar]
- 54.Kuo IY. Defining cardiac dysfunction in ADPKD. Kidney360. 2023;4(2):126–127. doi: 10.34067/KID.0000000000000066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rahbari-Oskoui F, Williams O, Chapman A. Mechanisms and management of hypertension in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2014;29(12):2194–2201. doi: 10.1093/ndt/gft513 [DOI] [PubMed] [Google Scholar]
- 56.Cornec-Le Gall E Audrézet MP Rousseau A, et al. The PROPKD score: a new Algorithm to predict renal survival in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2016;27(3):942–951. doi: 10.1681/ASN.2015010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Massella L Mekahli D Paripović D, et al. Prevalence of hypertension in children with early-stage ADPKD. Clin J Am Soc Nephrol. 2018;13(6):874–883. doi: 10.2215/CJN.11401017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valero FA Martinez-Vea A Bardají A, et al. Ambulatory blood pressure and left ventricular mass in normotensive patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1999;10(5):1020–1026. doi: 10.1681/ASN.V1051020 [DOI] [PubMed] [Google Scholar]
- 59.Yildiz A Sag S Gul CB, et al. Morning blood pressure surge in early autosomal dominant polycystic kidney disease and its relation with left ventricular hypertrophy. Ren Fail. 2021;43(1):223–230. doi: 10.1080/0886022X.2020.1864403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chapman AB, Johnson AM, Rainguet S, Hossack K, Gabow P, Schrier RW. Left ventricular hypertrophy in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1997;8(8):1292–1297. doi: 10.1681/ASN.V881292 [DOI] [PubMed] [Google Scholar]
- 61.Perrone RD Abebe KZ Schrier RW, et al. Cardiac magnetic resonance assessment of left ventricular mass in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6(10):2508–2515. doi: 10.2215/CJN.04610511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen H, Watnick T, Hong SN, Daly B, Li Y, Seliger SL. Left ventricular hypertrophy in a contemporary cohort of autosomal dominant polycystic kidney disease patients. BMC Nephrol. 2019;20(1):386. doi: 10.1186/s12882-019-1555-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arjune S Grundmann F Todorova P, et al. Cardiac manifestations in patients with autosomal dominant polycystic kidney disease (ADPKD): a single-center study. Kidney360. 2023;4(2):150–161. doi: 10.34067/KID.0002942022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oto OA, Edelstein CL. The pathophysiology of left ventricular hypertrophy, beyond hypertension, in autosomal dominant polycystic kidney disease [published online ahead of print July 27, 2022]. Nephron. 2022:1–9. doi: 10.1159/000525944 [DOI] [PubMed] [Google Scholar]
- 65.Kidney Disease: Improving Global Outcomes KDIGO Blood Pressure Work Group. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99(3S):S1–S87. doi: 10.1016/j.kint.2020.11.003 [DOI] [PubMed] [Google Scholar]
- 66.Kramers BJ Koorevaar IW Drenth JPH, et al. Salt, but not protein intake, is associated with accelerated disease progression in autosomal dominant polycystic kidney disease. Kidney Int. 2020;98(4):989–998. doi: 10.1016/j.kint.2020.04.053 [DOI] [PubMed] [Google Scholar]
- 67.Goff DC Jr. Lloyd-Jones DM Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of cardiology/American heart association task force on practice guidelines. Circulation. 2014;129(25 suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 68.Pfeferman MB, Rocha DRD, Rodrigues FG, Pfeferman E, Heilberg IP. Echocardiographic abnormalities in autosomal dominant polycystic kidney disease (ADPKD) patients. J Clin Med. 2022;11(20):5982. doi: 10.3390/jcm11205982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hossack KF, Leddy CL, Johnson AM, Schrier RW, Gabow PA. Echocardiographic findings in autosomal dominant polycystic kidney disease. New Engl J Med. 1988;319(14):907–912. doi: 10.1056/NEJM198810063191404 [DOI] [PubMed] [Google Scholar]
- 70.Nunes R Gouveia E Melo R Almeida AG, et al. Does autosomal dominant polycystic kidney disease increase the risk of aortic aneurysm or dissection: a point of view based on a systematic review and meta-analysis. J Nephrol. 2022;35(6):1585–1593. doi: 10.1007/s40620-022-01309-w [DOI] [PubMed] [Google Scholar]
- 71.Guirguis-Blake JM, Beil TL, Senger CA, Coppola EL. Primary care screening for abdominal aortic aneurysm: updated evidence report and systematic review for the US preventive Services task force. JAMA. 2019;322(22):2219–2238. doi: 10.1001/jama.2019.17021 [DOI] [PubMed] [Google Scholar]
- 72.Liu J Fujikura K Dev H, et al. Pericardial effusion on MRI in autosomal dominant polycystic kidney disease. J Clin Med. 2022;11(4):1127. doi: 10.3390/jcm11041127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qian Q, Hartman RP, King BF, Torres VE. Increased occurrence of pericardial effusion in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2007;2(6):1223–1227. doi: 10.2215/CJN.01920507 [DOI] [PubMed] [Google Scholar]
- 74.Chebib FT Hogan MC El-Zoghby ZM, et al. Autosomal dominant polycystic kidney patients may Be predisposed to various cardiomyopathies. Kidney Int Rep. 2017;2(5):913–923. doi: 10.1016/j.ekir.2017.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paavola J Schliffke S Rossetti S, et al. Polycystin-2 mutations lead to impaired calcium cycling in the heart and predispose to dilated cardiomyopathy. J Mol Cell Cardiol. 2013;58:199–208. doi: 10.1016/j.yjmcc.2013.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patel DM, Dahl NK. Examining the role of novel CKD therapies for the ADPKD patient. Kidney360. 2021;2(6):1036–1041. doi: 10.34067/KID.0007422020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mallett A, Patel M, Tunnicliffe DJ, Rangan GK. KHA-CARI autosomal dominant polycystic kidney disease guideline: management of renal stone disease. Semin Nephrol. 2015;35(6):603–606.e3. doi: 10.1016/j.semnephrol.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 78.Kalatharan V Welk B Nash DM, et al. Risk of hospital encounters with kidney stones in autosomal dominant polycystic kidney disease: a cohort study. Can J Kidney Health Dis. 2021;8:20543581211000227. doi: 10.1177/20543581211000227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nishiura JL, Neves RF, Eloi SR, Cintra SM, Ajzen SA, Heilberg IP. Evaluation of nephrolithiasis in autosomal dominant polycystic kidney disease patients. Clin J Am Soc Nephrol. 2009;4(4):838–844. doi: 10.2215/CJN.03100608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grampsas SA Chandhoke PS Fan J, et al. Anatomic and metabolic risk factors for nephrolithiasis in patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2000;36(1):53–57. doi: 10.1053/ajkd.2000.8266 [DOI] [PubMed] [Google Scholar]
- 81.Torres JA Rezaei M Broderick C, et al. Crystal deposition triggers tubule dilation that accelerates cystogenesis in polycystic kidney disease. J Clin Invest. 2019;129(10):4506–4522. doi: 10.1172/JCI128503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rocha DR Xue L Gomes Sousa HM, et al. Urinary citrate is associated with kidney outcomes in early polycystic kidney disease. Kidney360. 2022;3(12):2110–2115. doi: 10.34067/KID.0004772022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chapman AB Devuyst O Eckardt KU, et al. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2015;88(1):17–27. doi: 10.1038/ki.2015.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rahbari-Oskoui F, Mittal A, Mittal P, Chapman A. Renal relevant radiology: radiologic imaging in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2014;9(2):406–415. doi: 10.2215/CJN.08940813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mufti UB, Nalagatla SK. Nephrolithiasis in autosomal dominant polycystic kidney disease. J Endourol. 2010;24(10):1557–1561. doi: 10.1089/end.2010.0093 [DOI] [PubMed] [Google Scholar]
- 86.Xiao B Zhang G Ji C, et al. Percutaneous nephrolithotomy under X-ray-free technique in upper urinary stone patients with autosomal dominant polycystic kidney disease: experience from a large-volume stone management center. J Endourol. 2021;35(7):967–972. doi: 10.1089/end.2020.0827 [DOI] [PubMed] [Google Scholar]
- 87.Bargagli M Dhayat NA Anderegg M, et al. Urinary lithogenic risk profile in ADPKD patients treated with tolvaptan. Clin J Am Soc Nephrol. 2020;15(7):1007–1014. doi: 10.2215/CJN.13861119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chauveau D, Pirson Y, Verellen-Dumoulin C, Macnicol A, Gonzalo A, Grünfeld JP. Intracranial aneurysms in autosomal dominant polycystic kidney disease. Kidney Int. 1994;45(4):1140–1146. doi: 10.1038/ki.1994.151 [DOI] [PubMed] [Google Scholar]
- 89.Fick GM, Johnson AM, Hammond WS, Gabow PA. Causes of death in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1995;5(12):2048–2056. doi: 10.1681/ASN.V5122048 [DOI] [PubMed] [Google Scholar]
- 90.Holthouse D, Wong G. Chronic subdural hematoma in a 50-year-old man with polycystic kidney disease. Am J Kidney Dis. 2001;38(2):E6. doi: 10.1053/ajkd.2001.26116 [DOI] [PubMed] [Google Scholar]
- 91.Wijdicks EF, Torres VE, Schievink WI. Chronic subdural hematoma in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2000;35(1):40–43. doi: 10.1016/S0272-6386(00)70299-X [DOI] [PubMed] [Google Scholar]
- 92.Abderrahim E Hedri H Lâabidi J, et al. Chronic subdural haematoma and autosomal polycystic kidney disease: report of two new cases. Nephrology (Carlton). 2004;9(5):331–333. doi: 10.1111/j.1440-1797.2004.00270.x [DOI] [PubMed] [Google Scholar]
- 93.Takata T Kokudo Y Kume K, et al. Dialysis-induced subdural hematoma in an arachnoid cyst associated with autosomal dominant polycystic kidney disease. Intern Med. 2016;55(15):2065–2067. doi: 10.2169/internalmedicine.55.6295 [DOI] [PubMed] [Google Scholar]
- 94.Perrone RD, Malek AM, Watnick T. Vascular complications in autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2015;11(10):589–598. doi: 10.1038/nrneph.2015.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu HW, Yu SQ, Mei CL, Li MH. Screening for intracranial aneurysm in 355 patients with autosomal-dominant polycystic kidney disease. Stroke. 2011;42(1):204–206. doi: 10.1161/STROKEAHA.110.578740 [DOI] [PubMed] [Google Scholar]
- 96.Malhotra A, Wu X, Matouk CC, Forman HP, Gandhi D, Sanelli P. MR angiography screening and surveillance for intracranial aneurysms in autosomal dominant polycystic kidney disease: a cost-effectiveness analysis. Radiology. 2019;291(2):400–408. doi: 10.1148/radiol.2019181399 [DOI] [PubMed] [Google Scholar]
- 97.Yoo DJ, Agodoa L, Yuan CM, Abbott KC, Nee R. Risk of intracranial hemorrhage associated with autosomal dominant polycystic kidney disease in patients with end stage renal disease. BMC Nephrol. 2014;15(1):39. doi: 10.1186/1471-2369-15-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Irazabal MV Huston JI Kubly V, et al. Extended follow-up of unruptured intracranial aneurysms detected by presymptomatic screening in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6(6):1274–1285. doi: 10.2215/CJN.09731110 [DOI] [PMC free article] [PubMed] [Google Scholar]