Abstract
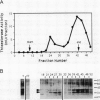
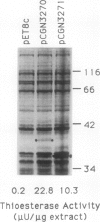
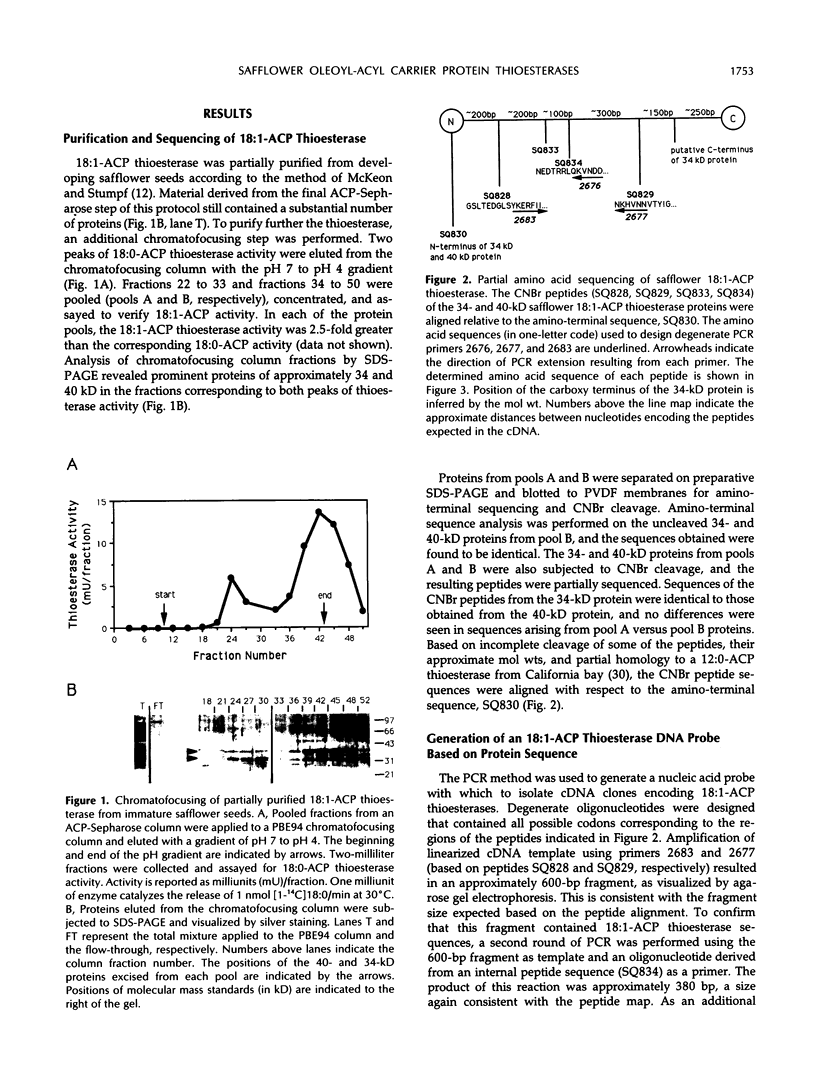
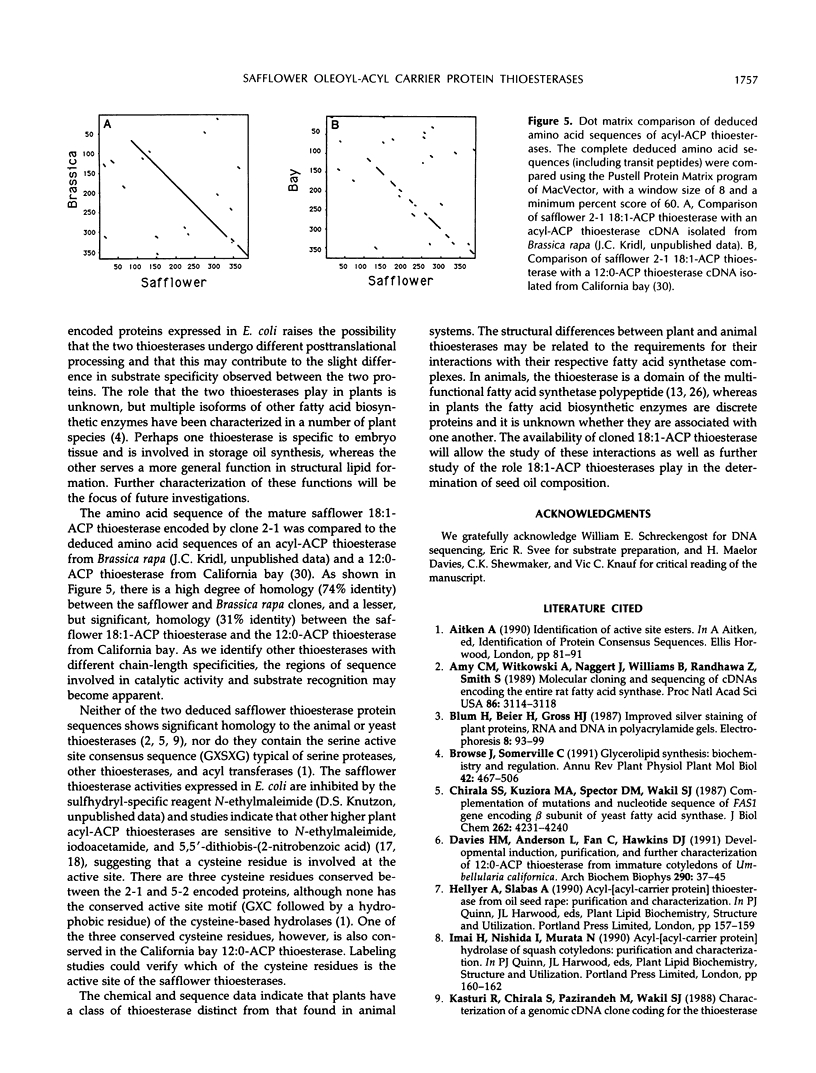
Oleoyl-acyl carrier protein (18:1-ACP) thioesterase has been partially purified from developing safflower (Carthamus tinctorius) seeds. Protein species with molecular masses of 34 and 40 kD associated with thioesterase activity were identified and partially sequenced. Analysis of amino-terminal and internal cyanogen bromide peptide sequences revealed no differences in the primary structure of the two species. Amino acid sequence was used to design degenerate oligonucleotides for primers in a polymerase chain reaction (PCR) using safflower embryo cDNA as a template. A 380-base pair PCR product was used to isolate two classes of cDNA clones, designated 2-1 and 5-2, from the embryo cDNA library. Clone 2-1 encodes a 389-amino acid protein including a 60-amino acid transit peptide, and contains all of the protein sequence determined from the 34- and 40-kD proteins. Clone 5-2 encodes a 385-amino acid protein with 80% identity to that encoded by 2-1. Expression of the two safflower cDNA clones in Escherichia coli resulted in a 50- to 100-fold increase in the level of 18:1-ACP thioesterase activity. Both thioesterases are most active on 18:1-ACP; however, the enzyme encoded by 5-2 shows less discrimination against saturated 16- and 18-carbon acyl-ACP substrates.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy C. M., Witkowski A., Naggert J., Williams B., Randhawa Z., Smith S. Molecular cloning and sequencing of cDNAs encoding the entire rat fatty acid synthase. Proc Natl Acad Sci U S A. 1989 May;86(9):3114–3118. doi: 10.1073/pnas.86.9.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirala S. S., Kuziora M. A., Spector D. M., Wakil S. J. Complementation of mutations and nucleotide sequence of FAS1 gene encoding beta subunit of yeast fatty acid synthase. J Biol Chem. 1987 Mar 25;262(9):4231–4240. [PubMed] [Google Scholar]
- Davies H. M., Anderson L., Fan C., Hawkins D. J. Developmental induction, purification, and further characterization of 12:0-ACP thioesterase from immature cotyledons of Umbellularia californica. Arch Biochem Biophys. 1991 Oct;290(1):37–45. doi: 10.1016/0003-9861(91)90588-a. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lütcke H. A., Chow K. C., Mickel F. S., Moss K. A., Kern H. F., Scheele G. A. Selection of AUG initiation codons differs in plants and animals. EMBO J. 1987 Jan;6(1):43–48. doi: 10.1002/j.1460-2075.1987.tb04716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon T. A., Stumpf P. K. Purification and characterization of the stearoyl-acyl carrier protein desaturase and the acyl-acyl carrier protein thioesterase from maturing seeds of safflower. J Biol Chem. 1982 Oct 25;257(20):12141–12147. [PubMed] [Google Scholar]
- Naggert J., Witkowski A., Mikkelsen J., Smith S. Molecular cloning and sequencing of a cDNA encoding the thioesterase domain of the rat fatty acid synthetase. J Biol Chem. 1988 Jan 25;263(3):1146–1150. [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J. B., Shine W. E., Stumpf P. K. Fat metabolism in higher plants. Characterization of plant acyl-ACP and acyl-CoA hydrolases. Arch Biochem Biophys. 1978 Aug;189(2):382–391. doi: 10.1016/0003-9861(78)90225-4. [DOI] [PubMed] [Google Scholar]
- Pollard M. R., Anderson L., Fan C., Hawkins D. J., Davies H. M. A specific acyl-ACP thioesterase implicated in medium-chain fatty acid production in immature cotyledons of Umbellularia californica. Arch Biochem Biophys. 1991 Feb 1;284(2):306–312. doi: 10.1016/0003-9861(91)90300-8. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rock C. O., Cronan J. E., Jr Acyl carrier protein from Escherichia coli. Methods Enzymol. 1981;71(Pt 100):341–351. doi: 10.1016/0076-6879(81)71043-7. [DOI] [PubMed] [Google Scholar]
- Rock C. O., Garwin J. L., Cronan J. E., Jr Preparative enzymatic synthesis of acyl-acyl carrier protein. Methods Enzymol. 1981;72:397–403. doi: 10.1016/s0076-6879(81)72029-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimakata T., Stumpf P. K. The procaryotic nature of the fatty acid synthetase of developing Carthamus tinctorius L. (Safflower) seeds. Arch Biochem Biophys. 1982 Aug;217(1):144–154. doi: 10.1016/0003-9861(82)90488-x. [DOI] [PubMed] [Google Scholar]
- Shine W. E., Mancha M., Stumpf P. K. Fat metabolism in higher plants. The function of acyl thioesterases in the metabolism of acyl-coenzymes A and acyl-acyl carrier proteins. Arch Biochem Biophys. 1976 Jan;172(1):110–116. doi: 10.1016/0003-9861(76)90054-0. [DOI] [PubMed] [Google Scholar]
- Simcox P. D., Reid E. E., Canvin D. T., Dennis D. T. Enzymes of the Glycolytic and Pentose Phosphate Pathways in Proplastids from the Developing Endosperm of Ricinus communis L. Plant Physiol. 1977 Jun;59(6):1128–1132. doi: 10.1104/pp.59.6.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S., Agradi E., Libertini L., Dileepan K. N. Specific release of the thioesterase component of the fatty acid synthetase multienzyme complex by limited trypsinization. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1184–1188. doi: 10.1073/pnas.73.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Thompson G. A., Scherer D. E., Foxall-Van Aken S., Kenny J. W., Young H. L., Shintani D. K., Kridl J. C., Knauf V. C. Primary structures of the precursor and mature forms of stearoyl-acyl carrier protein desaturase from safflower embryos and requirement of ferredoxin for enzyme activity. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2578–2582. doi: 10.1073/pnas.88.6.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker T. A., Worrell A. C., Anderson L., Bleibaum J., Fan C., Hawkins D. J., Radke S. E., Davies H. M. Fatty acid biosynthesis redirected to medium chains in transgenic oilseed plants. Science. 1992 Jul 3;257(5066):72–74. doi: 10.1126/science.1621095. [DOI] [PubMed] [Google Scholar]
- Weaire P. J., Kekwick R. G. The synthesis of fatty acids in avocado mesocarp and cauliflower bud tissue. Biochem J. 1975 Feb;146(2):425–437. doi: 10.1042/bj1460425. [DOI] [PMC free article] [PubMed] [Google Scholar]