Abstract
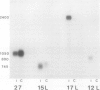
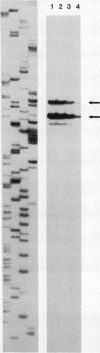
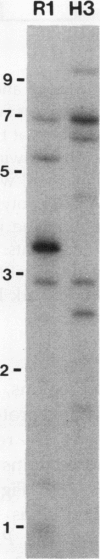
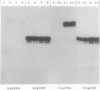
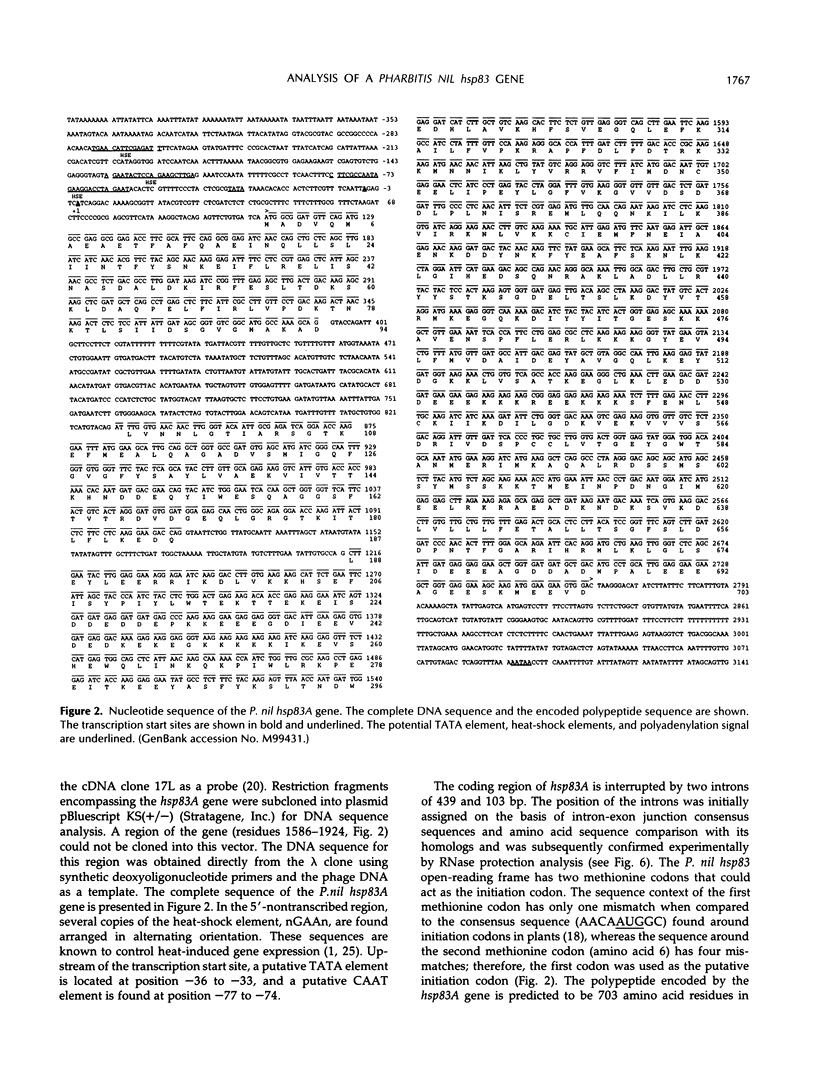
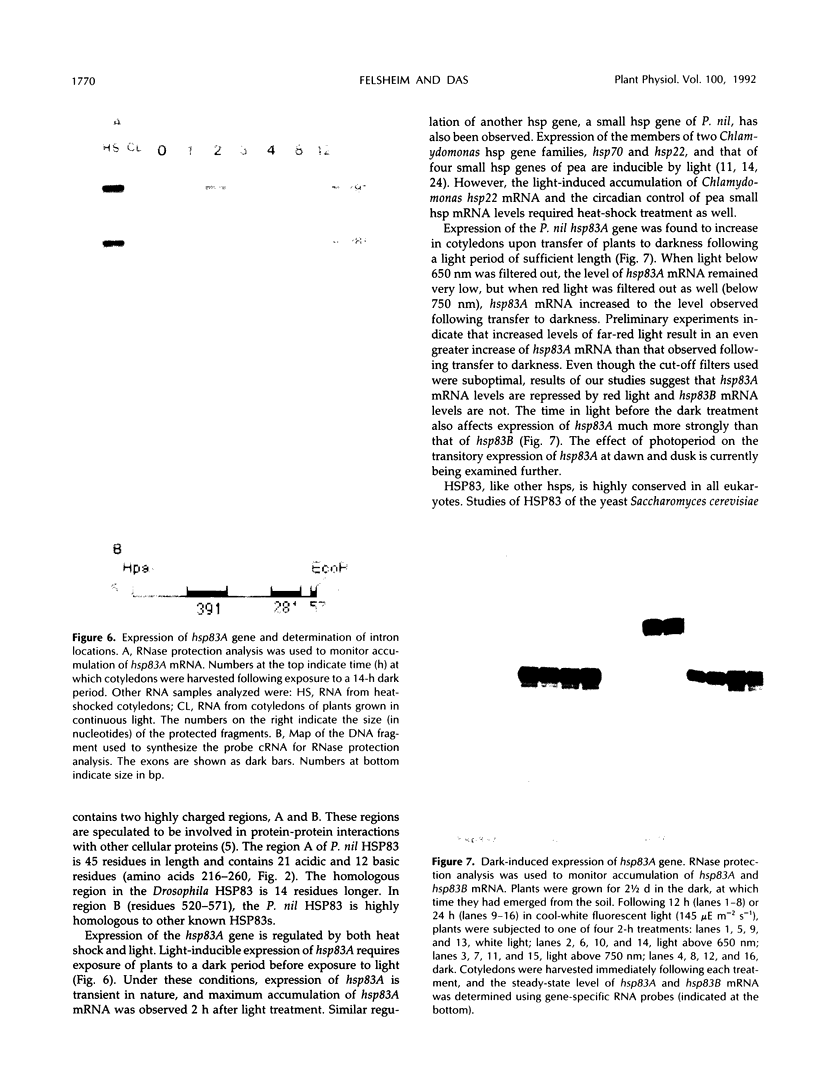
Four cDNA clones representing mRNAs whose levels were affected by a photoperiod that induces flowering in Pharbitis nil were isolated by a differential hybridization screening procedure. The level of mRNAs represented by three clones (12L, 15L, and 17L) increased following a photoperiod that induces flowering and that represented by the fourth clone (clone 27) increased under conditions in which flowering was inhibited. DNA sequence analysis showed that one cDNA, clone 17L, is homologous to members of the 83- to 90-kD heat-shock protein (hsp) gene family. The corresponding gene, hsp83A, was isolated and its DNA sequence was determined. hsp83A encodes a protein that exhibits 70% amino acid identity with Drosophila melanogaster HSP83. The P. nil hsp83A gene contains two introns within the coding region. hsp83A mRNA was not detectable in cotyledons of plants grown in continuous light, but its level increased transiently following a 14-h dark period and reached a maximum 2 h after the lights were turned on. A dramatic increase in the level of hsp83A mRNA was also found 2 h after an end-of-day dark treatment. Genomic Southern blot analysis demonstrated that the P. nil hsp83-90 gene family consists of at least six members, one of which appears to be constitutively expressed in the light.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amin J., Ananthan J., Voellmy R. Key features of heat shock regulatory elements. Mol Cell Biol. 1988 Sep;8(9):3761–3769. doi: 10.1128/mcb.8.9.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman R. K., Meselson M. Interspecific nucleotide sequence comparisons used to identify regulatory and structural features of the Drosophila hsp82 gene. J Mol Biol. 1986 Apr 20;188(4):499–515. doi: 10.1016/s0022-2836(86)80001-8. [DOI] [PubMed] [Google Scholar]
- Borkovich K. A., Farrelly F. W., Finkelstein D. B., Taulien J., Lindquist S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol. 1989 Sep;9(9):3919–3930. doi: 10.1128/mcb.9.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner T. W., Lafayette P. R., Nagao R. T., Key J. L. Sequence and Expression of a HSP83 from Arabidopsis thaliana. Plant Physiol. 1990 Dec;94(4):1689–1695. doi: 10.1104/pp.94.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Gardner S. M., Roeder W. D. Quantitative molecular hybridization with unfractionated, solubilized cells using RNA probes and polyacrylamide gel electrophoresis. Anal Biochem. 1987 Dec;167(2):381–386. doi: 10.1016/0003-2697(87)90180-1. [DOI] [PubMed] [Google Scholar]
- Hightower L. E. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991 Jul 26;66(2):191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Ish-Shalom D., Kloppstech K., Ohad I. Light regulation of the 22 kd heat shock gene transcription and its translation product accumulation in Chlamydomonas reinhardtii. EMBO J. 1990 Sep;9(9):2657–2661. doi: 10.1002/j.1460-2075.1990.tb07450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J. C., Felsheim R., Das A. Floral determination in the terminal bud of the short-day plant Pharbitis nil. Dev Biol. 1990 Feb;137(2):434–443. doi: 10.1016/0012-1606(90)90268-n. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lütcke H. A., Chow K. C., Mickel F. S., Moss K. A., Kern H. F., Scheele G. A. Selection of AUG initiation codons differs in plants and animals. EMBO J. 1987 Jan;6(1):43–48. doi: 10.1002/j.1460-2075.1987.tb04716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Kavanagh T. Speeding-up the sequencing of double-stranded DNA. Nucleic Acids Res. 1988 Jun 10;16(11):5198–5198. doi: 10.1093/nar/16.11.5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Otto B., Grimm B., Ottersbach P., Kloppstech K. Circadian Control of the Accumulation of mRNAs for Light- and Heat-Inducible Chloroplast Proteins in Pea (Pisum sativum L.). Plant Physiol. 1988 Sep;88(1):21–25. doi: 10.1104/pp.88.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perisic O., Xiao H., Lis J. T. Stable binding of Drosophila heat shock factor to head-to-head and tail-to-tail repeats of a conserved 5 bp recognition unit. Cell. 1989 Dec 1;59(5):797–806. doi: 10.1016/0092-8674(89)90603-x. [DOI] [PubMed] [Google Scholar]
- Picard D., Khursheed B., Garabedian M. J., Fortin M. G., Lindquist S., Yamamoto K. R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990 Nov 8;348(6297):166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost H. J., Lindquist S. RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell. 1986 Apr 25;45(2):185–193. doi: 10.1016/0092-8674(86)90382-x. [DOI] [PubMed] [Google Scholar]
- von Gromoff E. D., Treier U., Beck C. F. Three light-inducible heat shock genes of Chlamydomonas reinhardtii. Mol Cell Biol. 1989 Sep;9(9):3911–3918. doi: 10.1128/mcb.9.9.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]