Abstract
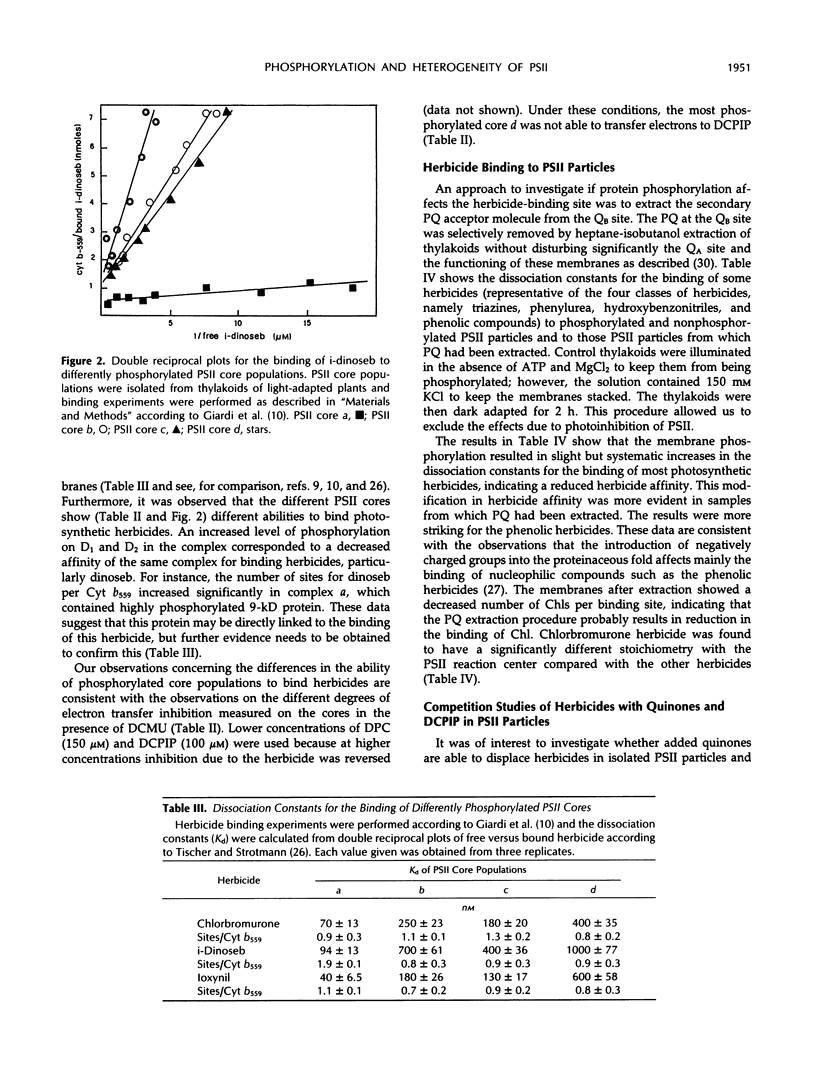
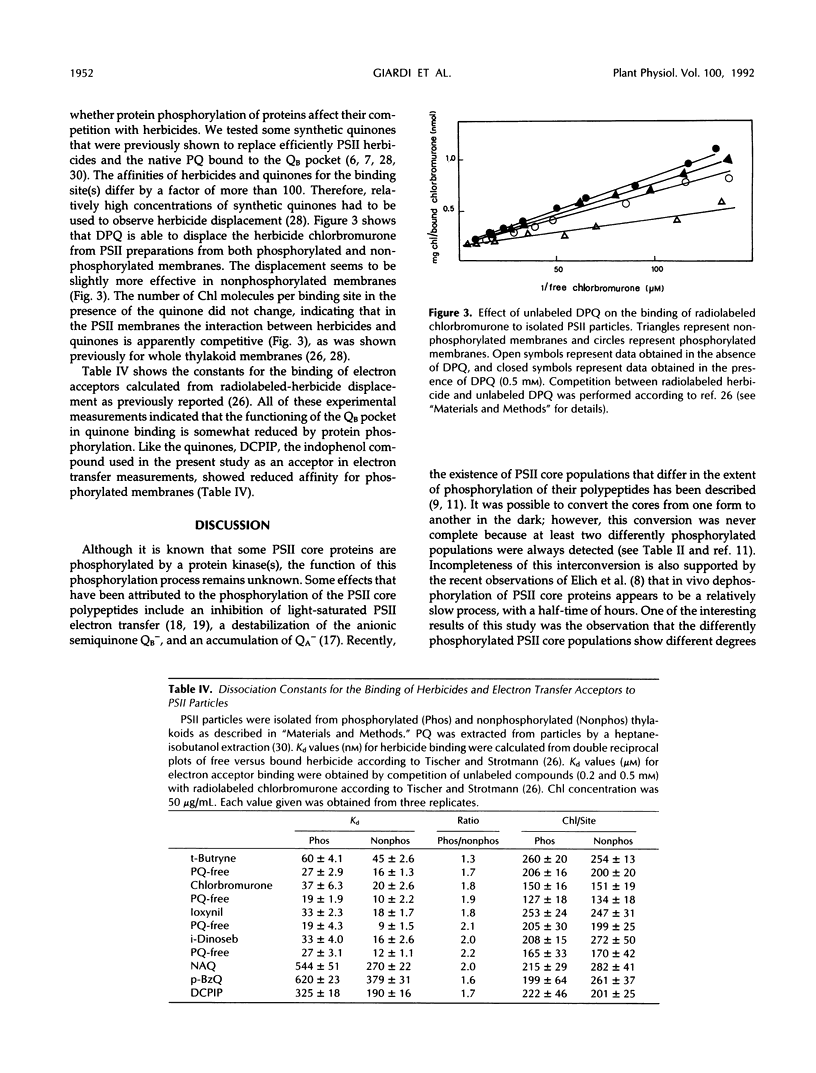
The effect of photosystem II core phosphorylation on the secondary quinone acceptor of photosystem II (QB) domain environment was analyzed by comparative herbicide-binding studies with photosystem II preparations from spinach (Spinacia oleracea L.). It was found that phosphorylation reduces the binding affinity for most photosynthetic herbicides. The binding of synthetic quinones and of the electron acceptor 2,6-dichlorophenolindophenol is also reduced by photosystem II phosphorylation. Four photosystem II core populations isolated from membranes showed different extents of phosphorylation as well as different degrees of affinity for photosynthetic herbicides. These findings support the idea that heterogeneity of photosystem II observed in vivo could be, in part, due to phosphorylation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbato R., Friso G., Giardi M. T., Rigoni F., Giacometti G. M. Breakdown of the photosystem II reaction center D1 protein under photoinhibitory conditions: identification and localization of the C-terminal degradation products. Biochemistry. 1991 Oct 22;30(42):10220–10226. doi: 10.1021/bi00106a021. [DOI] [PubMed] [Google Scholar]
- Elich T. D., Edelman M., Mattoo A. K. Identification, characterization, and resolution of the in vivo phosphorylated form of the D1 photosystem II reaction center protein. J Biol Chem. 1992 Feb 15;267(5):3523–3529. [PubMed] [Google Scholar]
- Fuzzi M., Di Febo G., Carnevale G., Giardi G., Angelini E. Chiusura marginale di corone parziali in oro. Valutazione in vivo prima e dopo cementazione. Dent Cadmos. 1988 Apr 30;56(7):62-4, 69-71. [PubMed] [Google Scholar]
- Giardi M. T., Rigoni F., Barbato R., Giacometti G. M. Relationships between heterogeneity of the PSII core complex from grana particles and phosphorylation. Biochem Biophys Res Commun. 1991 May 15;176(3):1298–1305. doi: 10.1016/0006-291x(91)90427-9. [DOI] [PubMed] [Google Scholar]
- Mattoo A. K., Marder J. B., Edelman M. Dynamics of the photosystem II reaction center. Cell. 1989 Jan 27;56(2):241–246. doi: 10.1016/0092-8674(89)90897-0. [DOI] [PubMed] [Google Scholar]
- Tischer W., Strotmann H. Relationship between inhibitor binding by chloroplasts and inhibition of photosynthetic electron transport. Biochim Biophys Acta. 1977 Apr 11;460(1):113–125. doi: 10.1016/0005-2728(77)90157-8. [DOI] [PubMed] [Google Scholar]
- Vermaas W. F., Steinback K. E., Arntzen C. J. Characterization of chloroplast thylakoid polypeptides in the 32-kDa region: polypeptide extraction and protein phosphorylation affect binding of photosystem II-directed herbicides. Arch Biochem Biophys. 1984 May 15;231(1):226–232. doi: 10.1016/0003-9861(84)90382-5. [DOI] [PubMed] [Google Scholar]



