Abstract
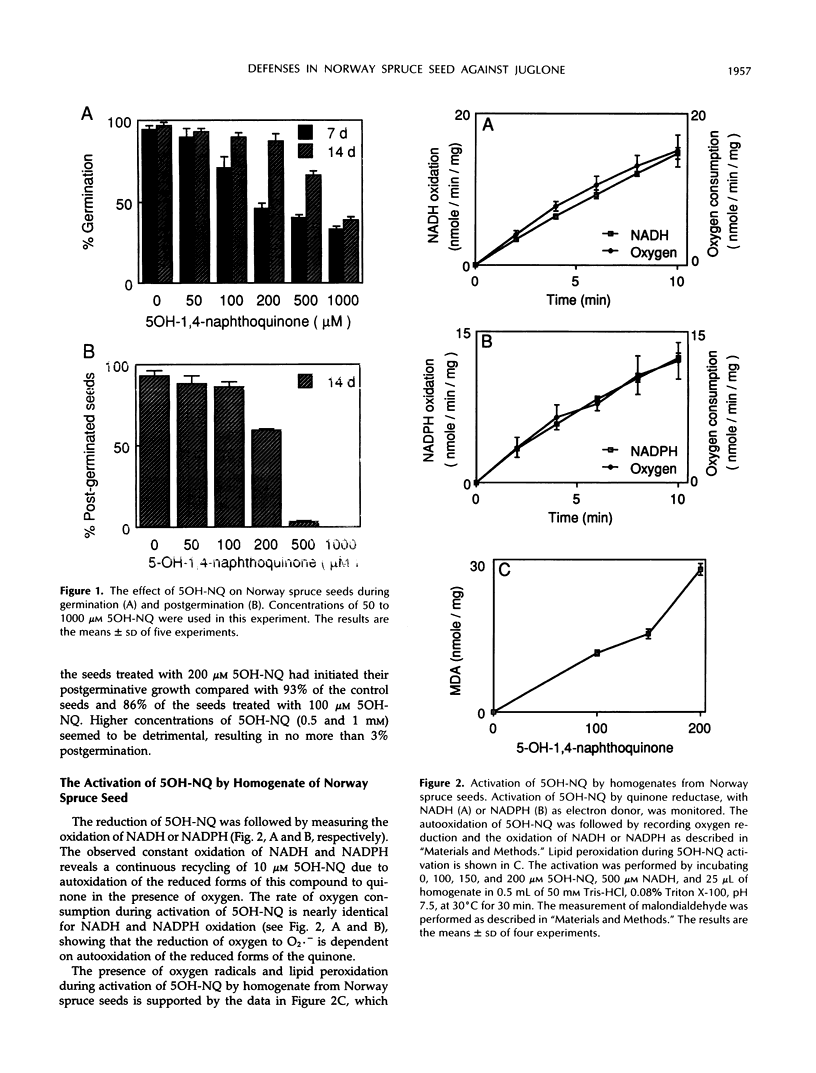
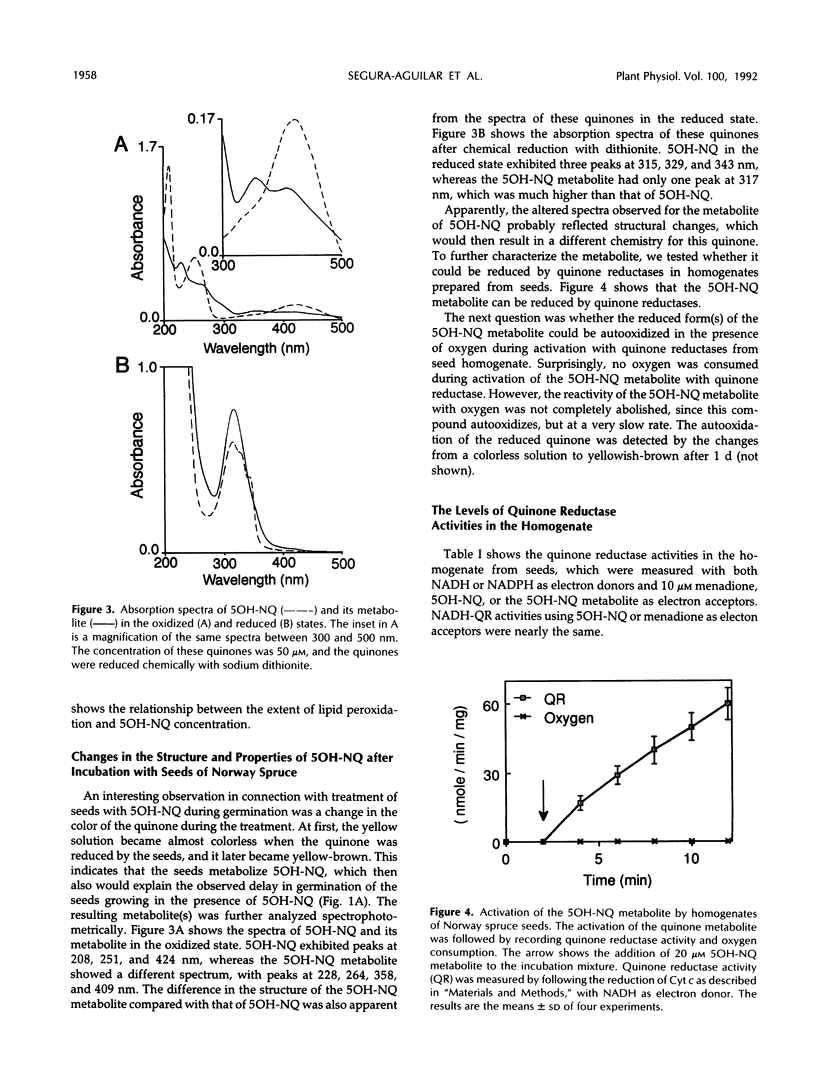
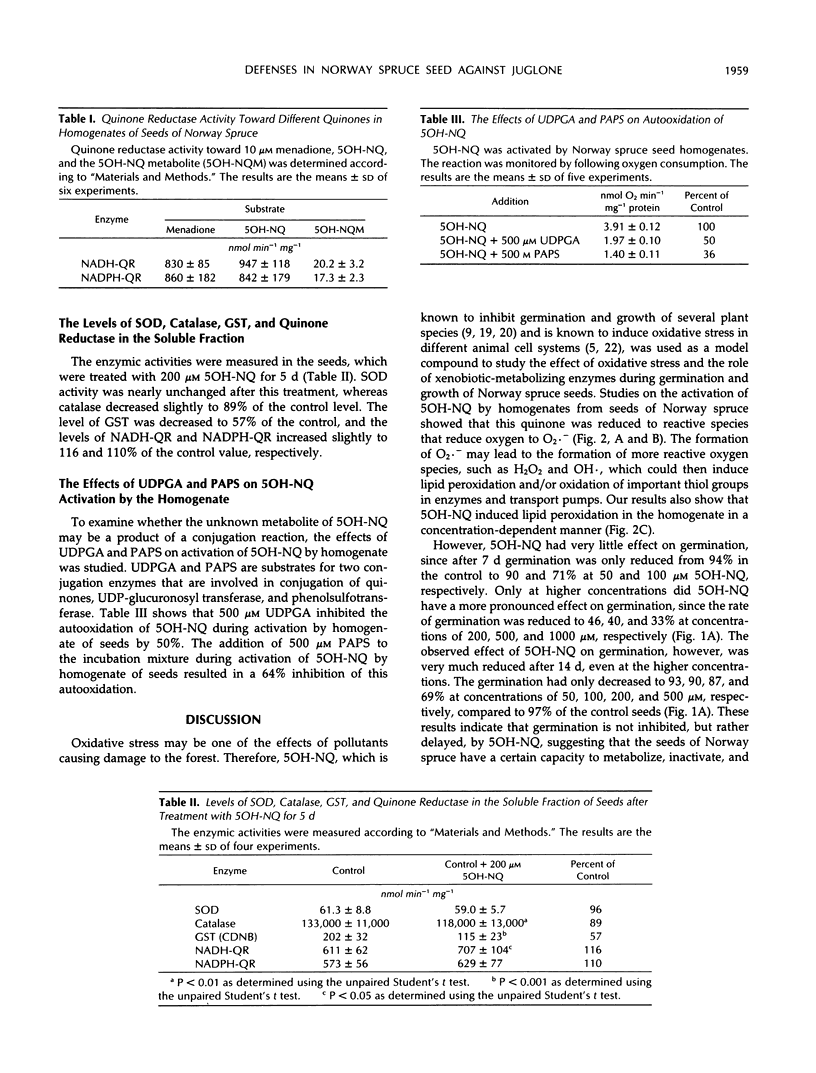
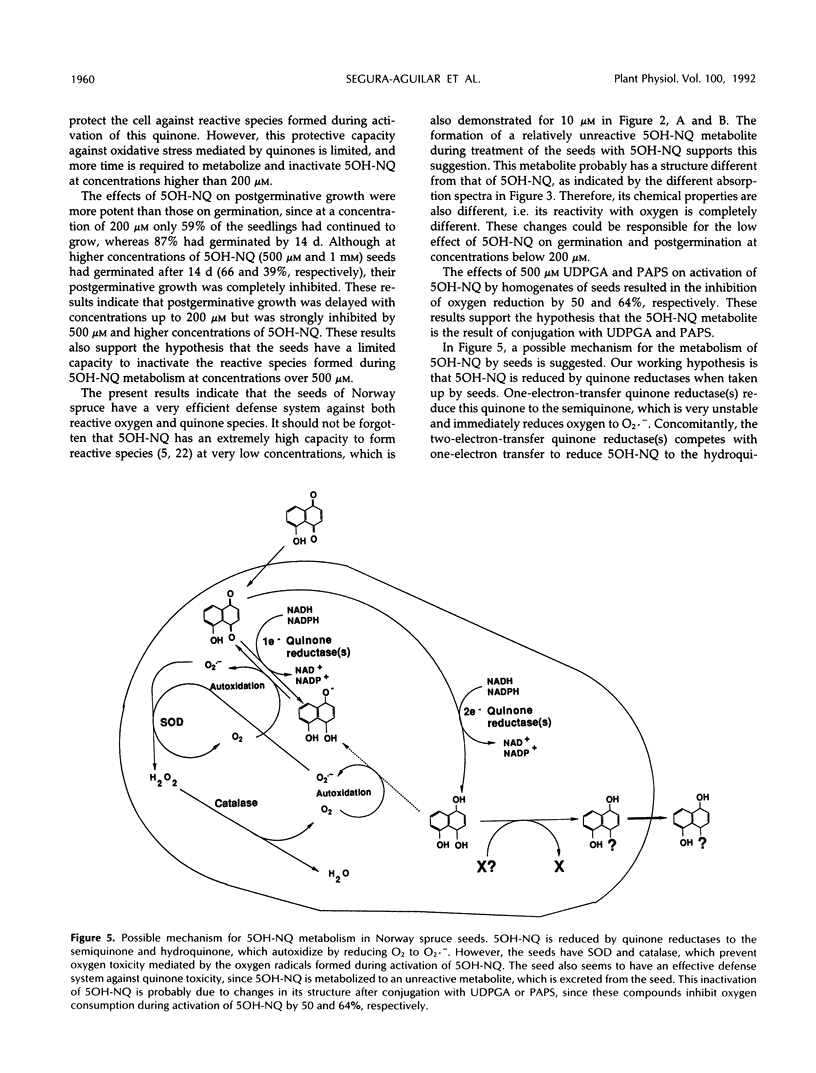
The effect of 5-OH-1,4-naphthoquinone (5OH-NQ), a known inhibitor of germination and growth and an inducer of oxidative stress, on seeds from Norway spruce (Picea abies) during germination was studied. 5OH-NQ was activated by homogenate from seeds to reactive species that reduce oxygen to superoxide radicals in vitro. Increasing concentrations of 5OH-NQ increased lipid peroxidation during this activation. Small effects of 5OH-NQ on germination of seeds were observed at concentrations up to 200 μm. However, higher concentrations, e.g. 500 and 1000 μm, exerted more pronounced effects on seeds. These results suggest that the effect of 5OH-NQ was a delay rather than an inhibition of germination. However, the effect of 5OH-NQ on postgerminative growth was more potent than that on germination, and higher concentrations inhibited growth >97%. These results suggest that the seeds have a very effective defense system against quinone and reactive oxygen species, since the small effects of 5OH-NQ on germination and postgermination at concentrations up to 200 μm can be explained by the formation of a metabolite of 5OH-NQ that is not as reactive with oxygen as the original quinone. The 5OH-NQ metabolite collected during germination experiments showed differences in its absorption spectrum in comparison with 5OH-NQ, which suggest changes in structure. This metabolite was reduced by quinone reductase, but reduction of oxygen to superoxide radicals was not detected during its activation with homogenate from seeds. This metabolite may arise via a conjugation reaction, since the addition of 500 μm uridine 5′-diphosphoglucuronic acid or 3′-phosphoadenosine-5′-phosphosulfate to the incubation mixture during activation of this metabolite by homogenate from seeds in vitro inhibited reduction of oxygen to superoxide radicals by 50 and 64%, respectively. The constitutive levels of superoxide dismutase and catalase were sufficient to prevent oxygen toxicity during activation of 5OH-NQ, since these enzymes were not induced when the seeds were treated with 200 μm 5OH-NQ.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Bachur N. R., Gordon S. L., Gee M. V., Kon H. NADPH cytochrome P-450 reductase activation of quinone anticancer agents to free radicals. Proc Natl Acad Sci U S A. 1979 Feb;76(2):954–957. doi: 10.1073/pnas.76.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlack W. R., Okita R. T., Hochstein P. The role of NADPH-cytochrome b 5 reductase in microsomal lipid peroxidation. Biochem Biophys Res Commun. 1973 Jul 17;53(2):459–465. doi: 10.1016/0006-291x(73)90684-0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buffinton G. D., Ollinger K., Brunmark A., Cadenas E. DT-diaphorase-catalysed reduction of 1,4-naphthoquinone derivatives and glutathionyl-quinone conjugates. Effect of substituents on autoxidation rates. Biochem J. 1989 Jan 15;257(2):561–571. doi: 10.1042/bj2570561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas E., Mira D., Brunmark A., Lind C., Segura-Aguilar J., Ernster L. Effect of superoxide dismutase on the autoxidation of various hydroquinones--a possible role of superoxide dismutase as a superoxide:semiquinone oxidoreductase. Free Radic Biol Med. 1988;5(2):71–79. doi: 10.1016/0891-5849(88)90032-9. [DOI] [PubMed] [Google Scholar]
- ERNSTER L., DANIELSON L., LJUNGGREN M. DT diaphorase. I. Purification from the soluble fraction of rat-liver cytoplasm, and properties. Biochim Biophys Acta. 1962 Apr 9;58:171–188. doi: 10.1016/0006-3002(62)90997-6. [DOI] [PubMed] [Google Scholar]
- Goodman J., Hochstein P. Generation of free radicals and lipid peroxidation by redox cycling of adriamycin and daunomycin. Biochem Biophys Res Commun. 1977 Jul 25;77(2):797–803. doi: 10.1016/s0006-291x(77)80048-x. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Lind C., Hochstein P., Ernster L. DT-diaphorase as a quinone reductase: a cellular control device against semiquinone and superoxide radical formation. Arch Biochem Biophys. 1982 Jun;216(1):178–185. doi: 10.1016/0003-9861(82)90202-8. [DOI] [PubMed] [Google Scholar]
- Lown J. W., Sim S. K., Majumdar K. C., Chang R. Y. Strand scission of DNA by bound adriamycin and daunorubicin in the presence of reducing agents. Biochem Biophys Res Commun. 1977 Jun 6;76(3):705–710. doi: 10.1016/0006-291x(77)91557-1. [DOI] [PubMed] [Google Scholar]
- Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974 Sep 16;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Sandalio L. M., Fernández V. M., Rupérez F. L., Del Río L. A. Superoxide free radicals are produced in glyoxysomes. Plant Physiol. 1988 May;87(1):1–4. doi: 10.1104/pp.87.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura-Aguilar J., Jönsson K., Tidefelt U., Paul C. The cytotoxic effects of 5-OH-1,4-naphthoquinone and 5,8-diOH-1,4-naphthoquinone on doxorubicin-resistant human leukemia cells (HL-60). Leuk Res. 1992 Jun-Jul;16(6-7):631–637. doi: 10.1016/0145-2126(92)90013-w. [DOI] [PubMed] [Google Scholar]
- Segura-Aguilar J., Lind C. On the mechanism of the Mn3(+)-induced neurotoxicity of dopamine:prevention of quinone-derived oxygen toxicity by DT diaphorase and superoxide dismutase. Chem Biol Interact. 1989;72(3):309–324. doi: 10.1016/0009-2797(89)90006-9. [DOI] [PubMed] [Google Scholar]
- Sinha B. K., Sik R. H. Binding of [14C]-adriamycin to cellular macromolecules in vivo. Biochem Pharmacol. 1980 Jun 15;29(12):1867–1868. doi: 10.1016/0006-2952(80)90156-2. [DOI] [PubMed] [Google Scholar]
- Svingen B. A., Powis G. Pulse radiolysis studies of antitumor quinones: radical lifetimes, reactivity with oxygen, and one-electron reduction potentials. Arch Biochem Biophys. 1981 Jun;209(1):119–126. doi: 10.1016/0003-9861(81)90263-0. [DOI] [PubMed] [Google Scholar]


