Abstract
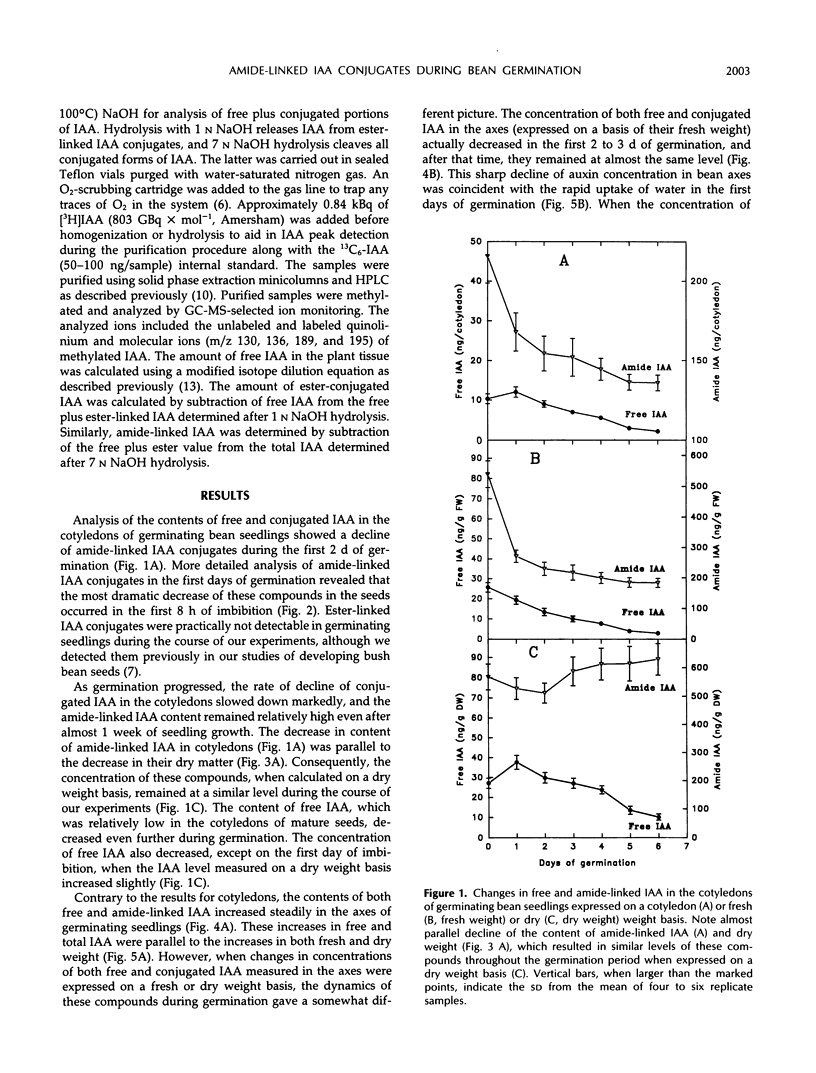
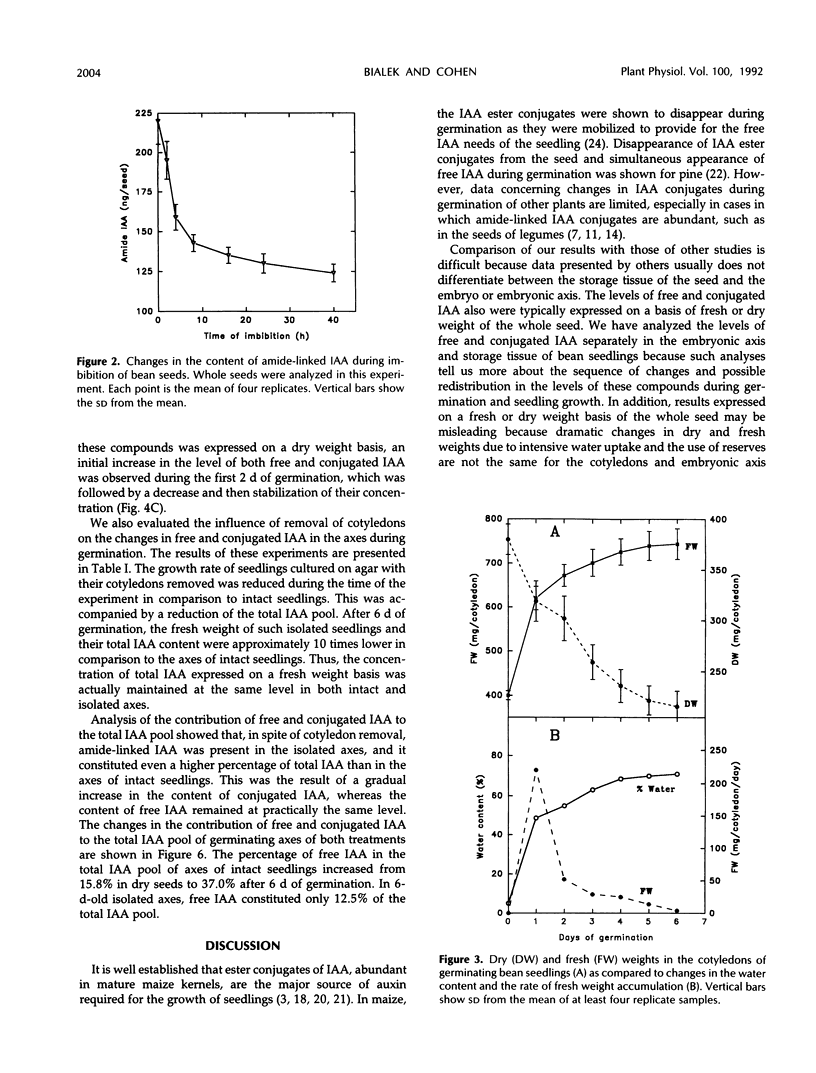
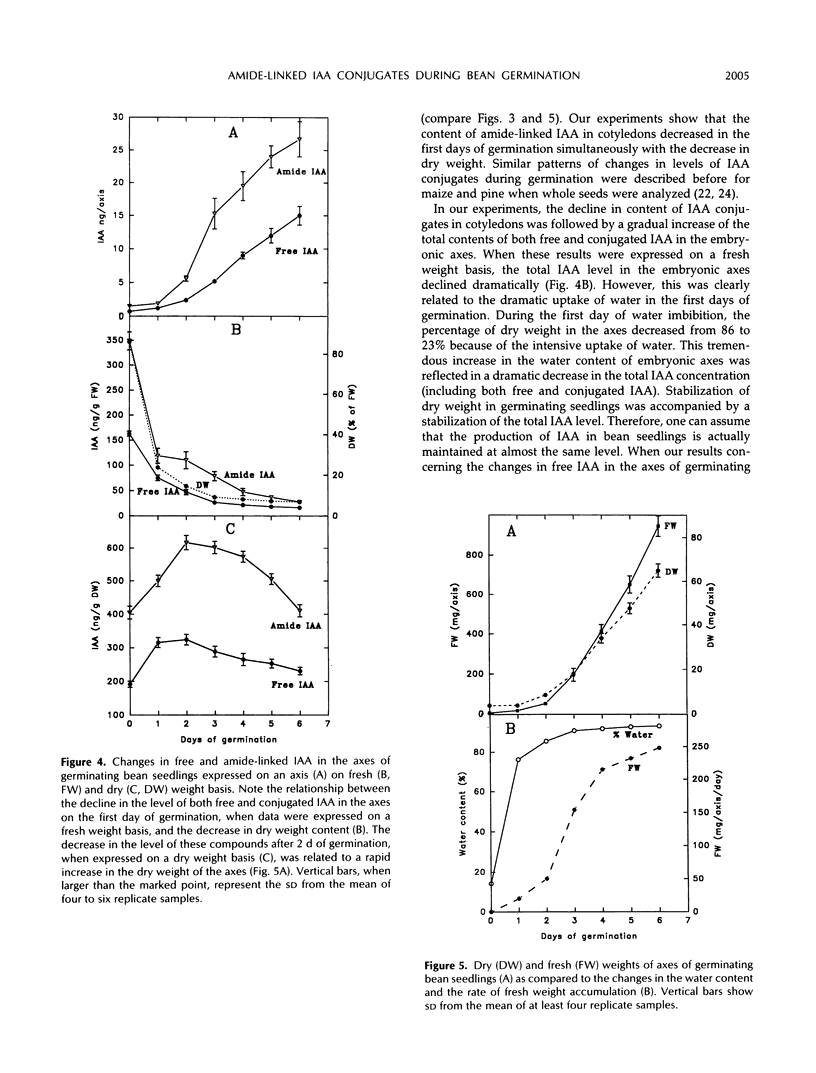
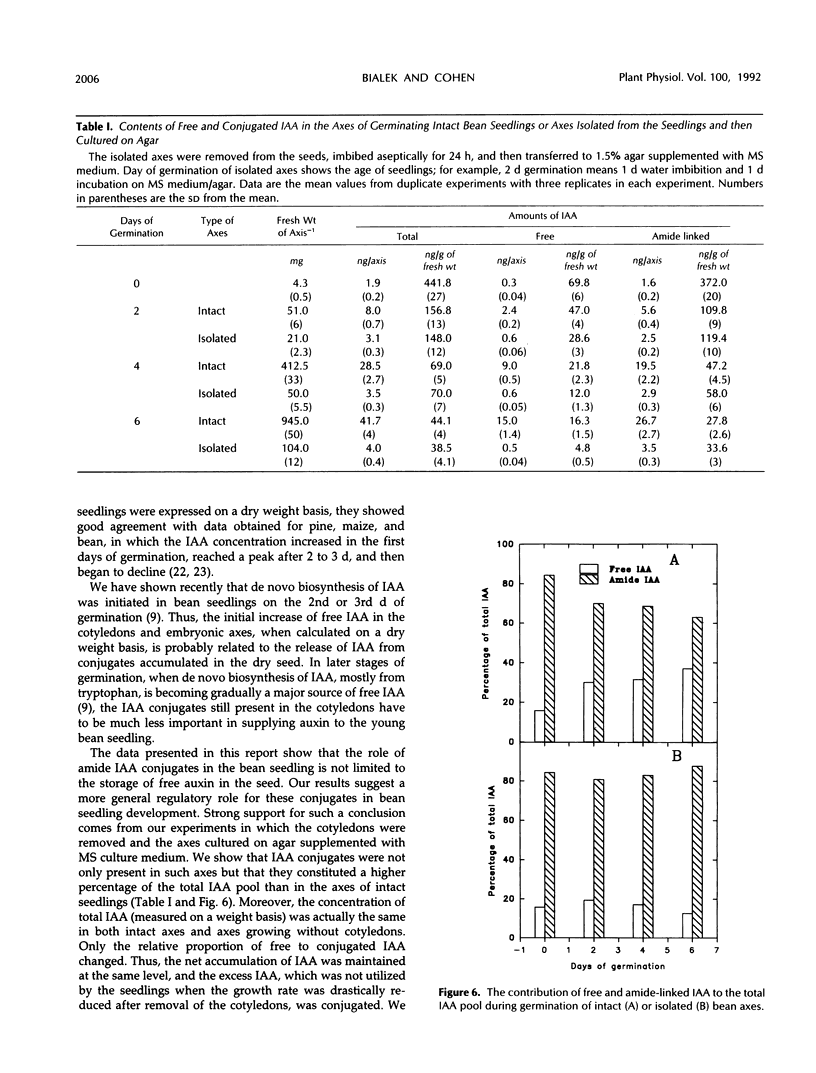
We have shown that amide-linked IAA (indole-3-acetic acid) conjugates accumulated to high levels during maturation of bean seeds (K. Bialek and J.D. Cohen [1989] Plant Physiol 91: 775-779). In the present study, we were interested in the fate of these and other IAA conjugates during seed germination. The content of amide-linked conjugates of IAA in cotyledons declined dramatically during the first hours of imbibition. The rate of decline slowed markedly during the period of the resumption of axis growth. The level of amide-linked IAA conjugates in cotyledons remained relatively high after almost 1 week of germination. The decline of IAA conjugates in cotyledons was followed by a steady increase in the content of both free and amide-linked IAA in the embryonic axes. Amide-linked IAA conjugates were also present in the axes cultured on agar after the cotyledons were removed, which suggests that de novo production of these IAA conjugates occurs in the axis of germinating bean seedlings. A comparison of relative amounts of free and conjugated IAA in the axes of intact seedlings and axes cultured on agar showed lower levels of free IAA and higher levels of conjugated IAA in much slower growing isolated axes. These results suggest a more general role for IAA conjugates in the control of seedling growth than simply to serve as a seed storage form of auxin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandurski R. S., Schulze A., Cohen J. D. Photo-regulation of the ratio of ester to free indole-3-acetic acid. Biochem Biophys Res Commun. 1977 Dec 21;79(4):1219–1223. doi: 10.1016/0006-291x(77)91136-6. [DOI] [PubMed] [Google Scholar]
- Bandurski R. S., Schulze A. Concentration of Indole-3-acetic Acid and Its Derivatives in Plants. Plant Physiol. 1977 Aug;60(2):211–213. doi: 10.1104/pp.60.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialek K., Cohen J. D. Free and conjugated indole-3-acetic Acid in developing bean seeds. Plant Physiol. 1989 Oct;91(2):775–779. doi: 10.1104/pp.91.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialek K., Cohen J. D. Isolation and Partial Characterization of the Major Amide-Linked Conjugate of Indole-3-Acetic Acid from Phaseolus vulgaris L. Plant Physiol. 1986 Jan;80(1):99–104. doi: 10.1104/pp.80.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialek K., Cohen J. D. Quantitation of indoleacetic Acid conjugates in bean seeds by direct tissue hydrolysis. Plant Physiol. 1989 Jun;90(2):398–400. doi: 10.1104/pp.90.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialek K., Meudt W. J., Cohen J. D. Indole-3-acetic Acid (IAA) and IAA Conjugates Applied to Bean Stem Sections: IAA Content and the Growth Response. Plant Physiol. 1983 Sep;73(1):130–134. doi: 10.1104/pp.73.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialek K., Michalczuk L., Cohen J. D. Auxin Biosynthesis during Seed Germination in Phaseolus vulgaris. Plant Physiol. 1992 Sep;100(1):509–517. doi: 10.1104/pp.100.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. H., Miller A. N., Patterson G. W., Cohen J. D. A Rapid and Simple Procedure for Purification of Indole-3-Acetic Acid Prior to GC-SIM-MS Analysis. Plant Physiol. 1988 Mar;86(3):822–825. doi: 10.1104/pp.86.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. D., Baldi B. G., Slovin J. P. C(6)-[benzene ring]-indole-3-acetic Acid: a new internal standard for quantitative mass spectral analysis of indole-3-acetic Acid in plants. Plant Physiol. 1986 Jan;80(1):14–19. doi: 10.1104/pp.80.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. D. Identification and Quantitative Analysis of Indole-3-Acetyl-l-Aspartate from Seeds of Glycine max L. Plant Physiol. 1982 Sep;70(3):749–753. doi: 10.1104/pp.70.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E., Baldi B. G., Cohen J. D. Identification of Indole-3-Acetylglutamate from Seeds of Glycine max L. Plant Physiol. 1986 Jan;80(1):256–258. doi: 10.1104/pp.80.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E., Cohen J. D., Bandurski R. S. Concentration and Metabolic Turnover of Indoles in Germinating Kernels of Zea mays L. Plant Physiol. 1980 Mar;65(3):415–421. doi: 10.1104/pp.65.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momonoki Y. S., Schulze A., Bandurski R. S. Effect of Deseeding on the Indole-3-acetic Acid Content of Shoots and Roots of Zea mays Seedlings. Plant Physiol. 1983 Jun;72(2):526–529. doi: 10.1104/pp.72.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowacki J., Bandurski R. S. Myo-Inositol Esters of Indole-3-acetic Acid as Seed Auxin Precursors of Zea mays L. Plant Physiol. 1980 Mar;65(3):422–427. doi: 10.1104/pp.65.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillberg E. Indoleacetic Acid Levels in Phaseolus, Zea, and Pincus during Seed Germination. Plant Physiol. 1977 Aug;60(2):317–319. doi: 10.1104/pp.60.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M., Bandurski R. S. A Quantitative Estimation of Alkali-labile Indole-3-Acetic Acid Compounds in Dormant and Germinating Maize Kernels. Plant Physiol. 1969 Aug;44(8):1175–1181. doi: 10.1104/pp.44.8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]


