Abstract
The cornea actively maintains its own avascular status to preserve its ultimate optical function. This corneal avascular state is also defined as “corneal angiogenic privilege”, which results from a critical and sensitive balance between anti-angiogenic and pro-angiogenic mechanisms. In our review, we aim to explore the complex equilibrium among multiple mediators which prevents neovascularization in the resting cornea, as well as to unveil the evolutive process which leads to corneal angiogenesis in response to different injuries.
1. Introduction
The cornea is the outer refractive element of the eye and exhibits a complex geometric shape and lamellar structure. The diameter and the organization of collagen fibrils composing the stromal lamellae guarantee corneal transparency, essential for vision. Hence to this end, the cornea is devoid of both lymphatic and blood vessels, deriving nutrients and oxygen supply from the tear film, peripheral nerves and posterior aqueous humor(Sabatino et al., 2017; Sridhar, 2018; Takatori et al., 2012). The corneal avascular status is also defined as “angiogenic privilege”.(Beebe, 2008)
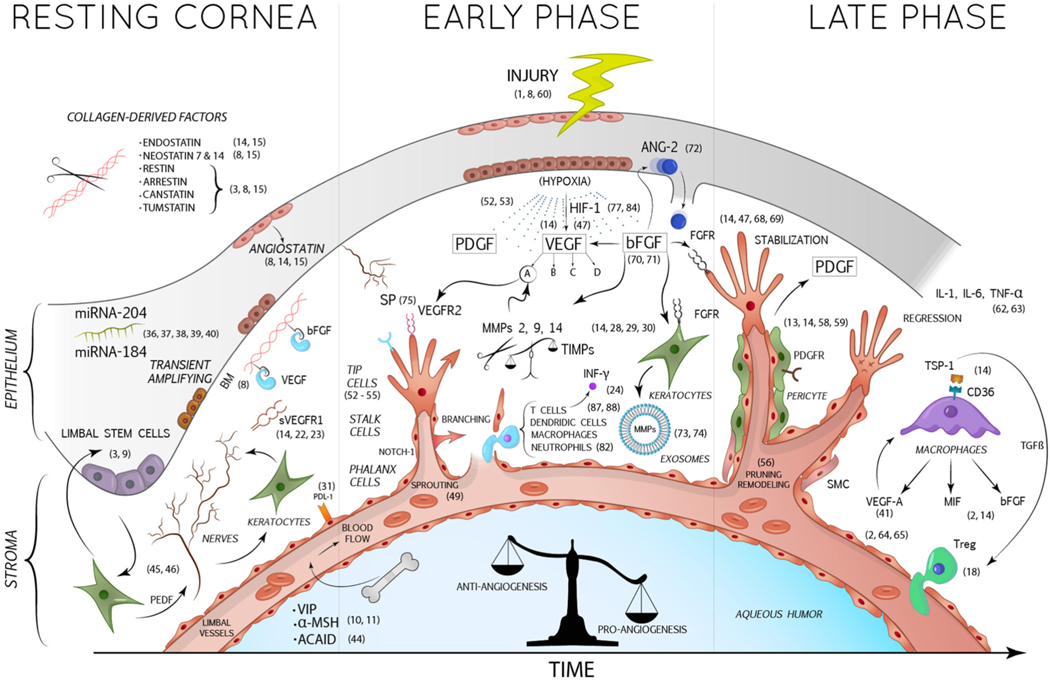
The corneal angiogenic privilege (CAP) results from a delicate balance between pro-angiogenic and anti-angiogenic factors. However, in some pathological conditions, upregulation of pro-angiogenic factors combined with downregulation of anti-angiogenic ones can occur, leading to invasion of capillaries from the limbal vascular plexus, consequently causing corneal neo-vascularization (CorNV) (Fig. 1). (Qazi et al., 2010).
Fig. 1.
In the resting cornea, essential anti-angiogenic factors are expressed by corneal keratocytes and epithelial cells, such as sVEGFR1, a soluble trap for VEGF-A, and angiostatin. The cleavage of stromal collagen by proteases (as MMPs) allows the production of numerous collagen-derived anti-angiogenic factors. Furthermore, BM acts by sequestering and inactivating angiogenic factors such as VEGF and bFGF. The rich corneal innervation prevents the growth of new blood vessels, as explained in the article. Nerves in turn are stimulated by the PEDF produced by the keratocytes, assuming indirectly an anti-angiogenic role. The corneal epithelium is also responsible for the secretion of some miRNAs (as miRNA 184 and 204) with angio-static properties. Within the aqueous humor also circulate anti-angiogenic factors such as α-MSH and VIP. After corneal injuries, damaged corneal cells produce 3 important mediators: VEGF, bFGF and PDGF. VEGF-A binds its receptor VEGFR2 expressed on the endothelial cells of new infiltrating vessels, promoting their growth. bFGF induces the production of pro-angiogenic MMPs and exosomes by keratocytes and the release of ANG-2 by corneal epithelium. PDGF is relevant for the recruitment of pericytes and SMC, which stabilize newly formed vessels. In the final stages, macrophages contribute to the pro-inflammatory and pro-angiogenic environment by releasing VEGF-A, MIF and bFGF.
AbbreviationsBM: Bowman’s membrane; VEGF: Vascular endothelial growth factor; VEGFR: Vascular endothelial growth factor receptor; PDGF: Platelet-derived growth factor; PDGFR: Platelet-derived growth factor receptor; bFGF: Basic fibroblast growth factor; FGFR: Fibroblast growth factor receptor; PEDF: Pigment epithelium-derived growth factor; TGF-β: Transforming growth factor-β; sVEGFR-1: Soluble vascular endothelial growth factor receptor-1; MMPs: Matrix metalloproteinases; TIMPs: Tissue inhibitors of metalloproteinases; INF-γ: Interferon γ; IL: Interleukin; TNF-α: Tumor necrosis factor-α; TSP: Trombospondin; MIF: Macrophage migration inhibitory factor; ANG-2: Angiopoietin-2; SP: Substance P; HIF-1: Hypoxia-inducible factor-1; miRNA: Micro RNA; VIP: Vasoactive intestinal peptide; α-MSH: α-Melanocyte-stimulating hormone; ACAID: Anterior chamber-associated immune deviation; SMC: Smooth muscle cells.
In this article we aim to analyze the available literature on CorNV, with a main focus on the molecular pathways involved in the preservation of CAP and on the mechanisms which may lead to its dysregulation in response to different corneal injuries.
2. Methods
Medline (PubMed), Web of Science, Google Scholar, Scopus and Embase online libraries were used for screening and selection of the available published studies on the issue. No language restrictions were applied. In addition, we used a snowballing method to include relevant studies not previously identified at the initial search. We initially found 3046 articles by using the search query presented in the search query box (Supplementary Fig. 1), from which 321 papers were selected. 2 reviewers (DG, MC), independently hand-searched and selected the relevant titles. A third reviewer (ADZ) then assessed the selected papers in order to resolve eventual discrepancies. After removal of duplicates, screening for relevance of the available titles and full-text assessment of the previously selected papers, a total of 120 published studies were included in our review (Supplementary Fig. 2).
3. Anti-angiogenic factors in the resting cornea
Clarity and avascularity are important corneal features, fundamental to guarantee optimal optical performance. A number of mechanisms act together physiologically in order to preserve this status(Azar, 2006).
3.1. Mechanical and biochemical factors
Corneal physiological anatomy is able to maintain its avascular state due to different mechanical properties. First of all, it has been demonstrated that the limbus plays a fundamental role in preserving CAP, though the mechanism by which the limbus prevents CorNV is not fully understood. In vivo studies have shown an increase in CorNV after experimental limbal damage (Espana et al., 2003; Grueterich et al., 2003), with a considerable regression in CorNV following limbal stem cell transplantation(Kenyon and Tseng, 1989; Tsai and Tseng, 1994). The limbus may act as a physical barrier towards invading vessels and conjunctival epithelial cells (Azar, 2006), but recent data suggests an inadequacy in this hypothesis(Ellenberg et al., 2010; Gao et al., 2019). Another important mechanical factor that preserves CAP is the compact corneal stromal anatomy comprised of tightly packed collagen lamellae with keratocyte networks among the lamellae and maintained as such due to a constant dehydration process. However studies have shown that stromal edema caused by corneal injuries, is inadequate to singularly cause CorNV(Azar, 2006; Bock et al., 2013). Some believe that even the Bowman’s membrane acts as an anti-angiogenic control mechanism by sequestering potent angiogenic factors, like vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF)(Ellenberg et al., 2010). Aqueous humor plays a role in preserving CAP by displaying significant anti-lymphangiogenic and mild hemangiostatic effects in vivo. This angiostatic effect on lymphatic endothelial cells and on blood endothelial cells is at least partially mediated by α− melanocyte stimulating hormone (α-MSH) and vasoactive intestinal peptide (VIP), which are two immunomodulatory factors present in aqueous humor(Bock et al, 2013, 2016). Moreover, even the lower corneal temperature could contribute to the anti-angiogenic state of the cornea, as it has been shown that hypothermia decreases both VEGF secretion and cellular metabolism, but this aspect requires further corroboration in literature (Azar, 2006; Bock et al., 2013; Coassin et al., 2010).
In addition, the interaction of different anti-angiogenic biochemical factors in the cornea plays a vital role in order to maintain CAP(Menzel-Severing, 2012). Some of them are directly produced in their active form, others are activated by proteolytic cleavage of larger precursors (Table 1). (Ellenberg et al., 2010)
Table 1.
Synoptic overview of Anti-angiogenic Factors.
| ANTI ANGIOGENIC FACTORS | |
|---|---|
|
| |
| ENDOSTATIN | Inhibits the binding of VEGF to its cell surface receptor (VEGFR-1) and basic fibroblast growth factor (bFGF)- induced CNV. It enhances vascular endothelial cell apoptosis by increasing the activity of the intracellular protease caspase3. |
| NEOSTATIN 7–14 | Are both potent CNV inhibitors formed via MMP- mediated cleavage of collagen XVIII, by MMP-7 and membrane type 1-MMP respectively. |
| RESTIN | Derives from cleavage of collagen XV and possesses antiangiogenic activity. |
| ARRESTIN, CANSTATIN, TUMSTATIN | These are three type IV collagen–derived proteins that, acting via different integrin receptors, are able to suppress tumor growth, to inhibit endothelial cell proliferation and migration and to induce endothelial cell apoptosis. |
| TSP-1 | Interrupts VEGF signal transduction by inhibiting VEGFR2 phosphorylation and reduces the activation of the cell angiogenic Akt pathway. It is able to bind and sequester the angiogenic FGF-2, suppressing its bioavailability and activity. Promotes the activation of transforming growth factor β (TGFβ) 1. |
| TSP-2 | Contributes in CNV suppression by inhibiting endothelial cell migration. |
| sVEGFR1 | Acts as a competitive decoy by binding free VEGF-A, which is continuously produced in biologically functional amounts by corneal epithelial cells, preventing its activity on the endothelial VEGFR1. |
| ANGIOSTATIN | Reduces endothelial cells ATP production by binding ATP-synthase, consequently downregulating their migration and proliferation. |
| FAS-L | Binds its receptors on inflammatory and endothelial cells causing apoptosis, thence blocking angiogenesis. |
| AIF | It is a signaling molecule that preserves CAP, activating a caspase-independent pathway of apoptosis. |
| KLEIP | Modulates cell–cell adhesions through E-cadherins and regulates corneal epithelial integrity. The loss of KLEIP expression makes corneas more susceptible to mechanical injuries, increasing the risk of CNV after these events. |
| TIMPs | Are endogenous inhibitors of MMPs that act in synergy with MMPs in order to control ECM remodeling during corneal wound healing. |
| PDL-1 | Possesses negative regulatory effects on CNV by setting a higher threshold in cornea for angiogenic responses under robust angiogenic stimulation. |
| miRNA 184 | Prevents neovascularization by downregulating VEGF and Wnt/beta catenin expression and by attenuating Akt activity. |
| miRNA 204 | Is directly involved in CNV regulating both VEGF signaling pathway and corneal epithelial wound healing process. |
AbbreviationsAIF: apoptosis inducing factor; FAS-L: FAS ligand; KLEIP: Kelch- like ECT2-interacting protein; miRNA: micro- RNA; PDL-1: programmed death ligand 1; sVEGFR: soluble vascular endothelial growth factor receptor-1; TIMP: tissue inhibitor of metalloproteinases; TSP-1: thrombospondin 1; TSP-2: thrombospondin 2.
3.2. Epithelial basement membrane (EBM)-derived anti-angiogenic factors
Multiple potent anti-angiogenic factors are derived from extracellular matrix components of the EBM. Endostatin is a proteolytic fragment of collagen XVIII, cleaved into an active form by matrix metalloproteinases (MMPs), cathepsin-L and elastases (Rolfsen et al., 2013), and is one of the most potent endogenously produced inhibitors of angiogenesis. Endostatin inhibits the VEGF-induced endothelial cell proliferation and migration by impeding the binding of VEGF to its cell surface receptor, vascular endothelial growth factor receptor (VEGFR)-2 (Abdelfattah et al., 2016). Furthermore, endostatin inhibits bFGF-induced CorNV, enhances vascular endothelial cell apoptosis by increasing the activity of the intracellular protease caspase3, and even affects lymphogenesis by downregulating VEGF-C expression and by inducing apoptosis of VEGFR-3 expressing cells(Abdelfattah et al., 2016; Ellenberg et al., 2010). Like endostatin, neostatin 7 and 14 are both potent CorNV inhibitors formed via MMP-mediated cleavage of collagen XVIII, by MMP-7 and membrane type 1-MMP (MT1-MMP) respectively (Abdelfattah et al., 2016; Ellenberg et al., 2010). In particular, it has been proven that recombinant neostatin-7 blocks bFGF-induced corneal angiogenesis and lymphangiogenesis(Kojima et al., 2008). Collagen-derived angiogenesis inhibitor factors are restin, arresten, canstatin and tumstatin. Restin arises from cleavage of collagen XV and possesses anti-angiogenic activity. Arresten, canstatin and tumstatin are three type IV collagen–derived proteins that, acting via different integrin receptors, are able to suppress tumor growth, inhibit endothelial cell proliferation and migration and induce endothelial cell apoptosis (Abdelfattah et al., 2016; Azar, 2006; Ellenberg et al., 2010). Thrombospondin (TSP)-1 and TSP-2, deposited in the EBM, are signaling glycoproteins with strong anti-angiogenic effects constitutively expressed in the cornea(Adams and Lawler, 2011; Foulsham et al., 2019). TSP-1 expression is regulated by ocular surface inflammation. It reduces angiogenesis in response to injury by binding and activating CD36 receptors on both invading macrophages, inhibiting their VEGF secretion, and endothelial cells, starting the apoptosis process. For these reasons, CD36 agonists have been suggested as potential therapeutic agents in CorNV(Rolfsen et al., 2013). In addition, TSP1 interrupts VEGF signal transduction, by inhibiting VEGFR2 phosphorylation, and reduces the activation of the cell angiogenic Akt pathway. TSP1 is also known to bind and sequester the angiogenic FGF-2, suppressing its bioavailability and activity(Foulsham et al., 2019; Sun et al., 2009; Zhang et al., 2009). Lastly, TSP-1 also promotes the activation of transforming growth factor β (TGFβ) 1, which is required for development of Foxp3+Tregs, resulting in abolishment of the pro-inflammatory response(Foulsham et al., 2019). TGFβ1 also limits the activity of NK cells, B cells, Th1 and Th2 cells, thereby suppressing the immune response(Foulsham et al., 2019; Masli et al., 2014). On the other hand, TSP-2 contributes to CorNV suppression by inhibiting endothelial cell migration(Rolfsen et al., 2013).
3.3. Corneal soluble anti-angiogenic factors
Soluble vascular endothelial growth factor receptor 1 (sVEGFR1) is a soluble truncated form of VEGFR1 which lacks the transmembrane domain and intracellular region(Rolfsen et al., 2013). It acts as a competitive decoy by binding free VEGF-A, thus preventing its activity on the endothelial VEGFR1(Ambati et al, 2006, 2007). sVEGFR1 has been detected in healthy human cornea, but its expression has been shown to be significantly reduced during CorNV(Kommineni et al., 2008; Liu et al., 2017). The anti-angiogenic properties of sVEGFR1 have been elucidated in an in vivo rat model. Lacrimal glands transfection with sVEGFR1 gene was shown to be effective in preventing CorNV, with an apparently acceptable safety profile(Nominato et al., 2018). Angiostatin plays a remarkable role in maintaining CAP after injury. Produced as a fragment of plasminogen in corneal epithelial cells, it reduces endothelial cells ATP production by binding ATP-synthase, consequently down-regulating their migration and proliferation (Abdelfattah et al., 2016; Ellenberg et al., 2010; Rolfsen et al., 2013). In addition, other inhibitors of angiogenesis are also found in the cornea. Interferon-γ (INF-γ), primarily produced by T lymphocytes and NK cells, controls angiogenesis by down-regulating TGF-β-induced VEGF-A, and simultaneously enhancing sVEGFR-1 expression(Kommineni et al., 2008). Fas Ligand (FasL) binds its receptors on inflammatory and endothelial cells causing apoptosis, thereby blocking angiogenesis(Qazi et al., 2010; Rolfsen et al., 2013). Apoptosis-inducing factor (AIF), which has been found in mitochondria of macrophages and endothelial cells, is a signaling molecule that preserves CAP activating a caspase-independent pathway of apoptosis(Hisatomi et al., 2012; Rolfsen et al., 2013). Kelch-like ECT2 interacting protein (KLEIP), induced by hypoxic conditions, modulates cell–cell adhesions through E-cadherins and regulates corneal epithelial integrity. It has been shown that, in knockout mice corneas, the loss of KLEIP expression makes corneas more susceptible to mechanical injuries, increasing the risk of CorNV after these events(Hahn et al., 2012; Rolfsen et al., 2013). Tissue inhibitors of MMPs (TIMPs) 1–4 are endogenous inhibitors of MMPs that act in synergy with MMPs in order to control ECM remodeling during corneal wound healing(Chang et al., 2010; Rolfsen et al., 2013). It has been shown in in vitro and in vivo studies that TIMPs have the ability to inhibit angiogenesis(Anand-apte et al., 1997; Johnson et al., 1994). Programmed death ligand 1(PD-L1), expressed by vascular endothelial cells, possesses negative regulatory effects on CorNV by setting a higher threshold in cornea for angiogenic responses under robust angiogenic stimulation(Jin et al., 2011). It has been recently shown that local microRNAs (miRNAs) play a fundamental role in modulating CorNV (Ambros, 2004; Bartel, 2009; Friedman et al., 2009; Shen et al., 2008; Zong et al., 2016). In particular, miRNA-184, the most abundant corneal epithelial miRNA, prevents neovascularization by down-regulating VEGF and Wnt/beta catenin expression and by attenuating Akt activity (Park et al., 2017; Zong et al., 2016), whereas miRNA-204 is directly involved in CorNV regulating both VEGF signaling pathway (Zhang et al., 2018) and corneal epithelial wound healing process(An et al., 2015; Gao et al., 2015). Along with these, multiple miRNAs involved in CorNV are under investigation in order to individuate new potential therapeutical approaches(Mukwaya et al., 2019).
3.4. Immunological factors
In addition to the angiogenic privilege, the cornea also possesses immune privilege, which is an essential feature in order to maintain corneal transparency in response to inflammatory stimuli(Cursiefen, 2007). The cornea is both an immune-privileged site, with low rejection rates after allografts, and an immune-privileged tissue, due to its long-term survival once grafted into inflamed and vascularized sites (Hori et al., 2000). CAP and corneal immune privilege are strictly connected. The corneal immune privilege is preserved by two important mechanisms. The first one is the CAP itself, because the absence of blood inflow impedes immune effector cells from accessing the cornea and the absence of lymphatic vessels prevents cellular traffic to the regional lymph nodes(Patel and Dana, 2009). The second mechanism is the corneal capacity to actively suppress induction of delayed-type hypersensitivity (DTH) to intracameral specific antigens, a process known as anterior chamber-associated immune deviation (ACAID)(Dana, 2006). Corneal immune privilege plays a vital role in preserving the avascular state of the cornea. Macrophage recruitment, a potent source for all major angiogenic and lymphangiogenic growth factors (VEGF-A, VEGF-C and VEGF-D), is a critical event leading to CorNV. Moreover, VEGF-A and VEGF-C in turn stimulate the recruitment of macrophages and dendritic cells respectively, amplifying CorNV process(Cursiefen, 2007).
Different pathways are consequently activated in response to corneal inflammation in the attempt to suppress it and restore avascularity, in a sort of feedback mechanism. Therefore, TSP-1 (Cursiefen et al., 2011; Foulsham et al., 2019) IFN-γ (Di Zazzo et al., 2017b), mesenchymal stem cells (MSC) (Eslani et al., 2017) and regulatory T-cell immune mediators (Lužnik et al., 2020) are pivotal in the interplay between angiogenesis and inflammation. Neuromediators such as Substance P, also, have been involved in regulating CorNV in response to inflammation(Li et al., 2019).
Based on such insights several studies have been focusing on restoration of immune privilege by using anti-inflammatory drugs(Mirabelli et al., 2017; Nakao et al., 2007).
3.5. Neurogenic factors
In a clear, avascular cornea, the corneal nerve plexus plays a fundamental role in maintenance of local homeostasis, being responsible for both epithelial and stromal cell trophism. However, it has been shown that the corneal avascular state strictly depends on the constitutive expression of pigment epithelium-derived factor (PEDF) by corneal epithelial keratinocytes, with neurotrophic and angiostatic properties. Suppression of PEDF in a denervated cornea was responsible for the disinhibition of angiogenesis(Dawson et al., 1999; Ferrari et al., 2013).
4. Corneal angiogenesis
CorNV results from angiogenesis, that is the formation of new blood vessels from pre-existing vasculature. Under such conditions, blood and lymphatic vessel growth contribute naturally to wound healing and the resolution of edema, respectively, and can therefore be beneficial in cases of tissue injury. However, this healing response can be accompanied by loss of tissue transparency through vessel ingrowth, fluid leakage and fibrosis(Inomata et al., 2017). This differs from vasculogenesis, which is the formation of new blood vessels from bone marrow-derived angioblasts, that usually occurs during embryogenesis (Qazi et al., 2009; Rolfsen et al., 2013; Roshandel et al., 2018).
Within the cornea, several mechanisms act together in order to maintain corneal avascularity, showing that CAP is abundant. The genetic removal of one or more of the endogenous inhibitors of angiogenesis does not cause spontaneous CorNV, but the CAP can be overcome by pathological pro-angiogenic stimuli, leading to corneal neo-angiogenesis(Cursiefen, 2007).
5. Types and steps of angiogenesis
The angiogenesis process is characterized by two different branching mechanisms that act sequentially and in combination, depending on metabolic and hemodynamic factors: sprouting and intussusception (Djonov et al., 2003). Sprouting angiogenesis is characterized by sprouts of endothelial cells that grow toward an angiogenic stimulus by migrating, proliferating and remodeling the ECM. Therefore, it can add blood vessels to avascular portion of tissues. On the contrary, intussusceptive angiogenesis causes enlargement of existing capillary plexus and endothelial exchange surface by splitting one vessel into two parallel vessels without cell proliferation or basement membrane degradation, which in turn conserves more energy. Sprouting angiogenesis is normally driven by angiogenic factors such as VEGF, bFGF (also known as FGF-2) and platelet-derived growth factor (PDGF), whereas intussusception has been proved to be stimulated by various angiogenic molecules and hemodynamic parameters, even if the precise molecular and morphological mechanisms involved remain unclear(Baum et al., 2010; Djonov et al., 2003; Gerhardt, 2008).
In both physiological and pathological conditions, the neoangiogenesis process begins with the release of angiogenic growth factors induced by inflammatory or hypoxic conditions, which activate endothelial cells by binding their surface receptors(Blanco and Gerhardt, 2013; Lobov and Mikhailova, 2018). These activated endothelial cells can express two different specialized phenotypes, “tip” or “stalk”, which are both fundamental for realizing the new vascular network. Endothelial migratory “tip” cells, characterized by long and dynamic filopodia, lead the vascular sprouts growth following a VEGF gradient. On the contrary, high proliferative endothelial “stalk” cells stabilize the growing sprout, establishing adherent and tight junctions, forming the nascent vascular lumen. VEGF-A and Notch signaling pathways are key players governing tip and stalk cell specialization, ensuring an adequate ratio of both cells types in the sprout(Blanco and Gerhardt, 2013; Lobov and Mikhailova, 2018; Lobov et al., 2007; Pitulescu et al., 2017).
Subsequent vascular maturation requires the pruning of superfluous connections that can occur in two different ways: blood flow absent and blood flow present. In the first scenario with absent blood flow, the lumen of the pruning branches collapses at the beginning of the process and subsequently endothelial cells move away bidirectionally towards the neighboring major branches. In the second instance, if blood flow is present in the pruning branches, there is endothelial cell retraction, transforming the vascular structure into a unicellular tube and preserving the vascular lumen almost until the end, when it collapses(Lenard et al., 2015).
Experimentally, these phases have been observed in cornea models irritated by implantation of surgical silk sutures, chemical burning, micro-pellet implantation or experimental fungal keratitis(Ferrari et al., 2013; Inomata et al., 2015; Kilarski et al., 2009; Saika et al., 2005; Yuan and Wilhelmus, 2009). In a recent wounded mouse cornea model analyzed by high-resolution time-lapse in vivo microscopy, endothelial cell behavior in vascular morphogenesis, including sprouting, fusion and pruning, has been assessed. A third novel mode of regression termed “reverse” intussusception, due to its resemblance to longitudinal splitting of vessels in reverse has been described(Wang et al., 2018).
The final step in neoangiogenesis is the maturation and stabilization of the new vessels, accomplished by pericytes and smooth muscle cells coverage, which marks the end of the transient pruning phase. The recruitment of pericytes by new vessels is promoted by endothelial cells release of PDGF and is a clinically relevant phase. Any angioregressive therapy, acting via inhibition of angiogenic factors, including VEGF and PDGF, is effective only on newly sprouted, immature vessels not yet or only partially covered by pericytes. In human CorNV, it has been shown that 80% of new vessels are already coated by pericytes within two weeks of the onset of neoangiogenesis process(Cursiefen et al., 2003; Menzel-Severing, 2012; Pérez-Santonja et al., 2010; Rolfsen et al., 2013). As a consequence, an angioregressive strategy during CorNV should be started within the first few weeks(Mirabelli et al., 2014).
6. Injuries and angiogenesis
As mentioned, CorNV results from a disruption in the balance of pro- angiogenic (Table 2) and anti-angiogenic factors and it can be caused by several ocular insults, including infectious keratitis, inflammatory disorders, autoimmune diseases, ischemia, hypoxia, degeneration, trauma, alkali burns, and loss of the limbal stem cell barrier(Beebe, 2008; Ellenberg et al., 2010; Sharif and Sharif, 2019). However, regardless of the causative condition, the corneal angiogenesis process starts as a consequence of the upregulated expression of VEGF and its signaling cascade in response to hypoxic and/or inflammatory stimuli(Qazi et al., 2009). The VEGF family includes VEGFs A–E and placenta growth factor (PlGF) and it is a member of the PDGFs. Among all the VEGFs, VEGF-A is the most important in blood vessel formation. It acts by binding tyrosine-kinase VEGFR-1 (Flt-1) and VEGFR-2 (KDR or Flk-1) on the endothelial cell surface, promoting their proliferation and migration. VEGF-C and VEGF-D are the main promoters of lymphangiogenesis, by binding to their high-affinity receptor VEGFR-3 (Flt-4)(Cursiefen et al., 2004). VEGF can be directly secreted by corneal endothelial and epithelial cells, fibroblasts and limbal vessel vascular endothelial cells, as seen in hypoxic conditions(Rolfsen et al., 2013). However, in an inflamed cornea, invading macrophages are the major source of VEGF. Under inflammatory conditions, various chemokines, particularly interleukin-1β (IL-1β), CCL2 and tumor necrosis factor (TNF)-α (Dana et al., 1998; Ferrari et al., 2015; Nakao et al., 2005; Wolf et al., 2019), recruit macrophages to the cornea by binding specific receptors on their surface, particularly CCR-5, and, once there, they start producing several pro-inflammatory and pro-angiogenic factors, including VEGF via nuclear factor (NF)-kB. Furthermore, secreted VEGF provides positive feedback enabling recruitment of more macrophages, which in turn produce more VEGF(Ambati et al., 2003; Kiriakidis et al., 2003; Mwaikambo et al., 2006; Qazi et al., 2010). In an inflammatory setting, the transcription factor NF-kB has a notable relevance in promoting angiogenesis. When activated by inflammatory signals like TNF-α, NF-kB promotes the transcription of several pro-angiogenic genes besides VEGF, such as CCL2, an inflammatory chemokine mediating monocyte extravasation, and CXCL5, that recruits neutrophils, promotes angiogenesis and remodels connective tissue(Lennikov et al., 2018; Madalli et al., 2015; Rowland et al., 2014; Sierra-Filardi et al., 2014). In a corneal rat model it was shown that CCL2 and CXCL5 are among the most upregulated genes in inflammatory CorNV(Mukwaya et al., 2016). Invading macrophages also release other important pro-inflammatory and pro-angiogenic pleiotropic factors, especially macrophage migratory inhibitory factor (MIF) and bFGF(Qazi et al., 2010; Rolfsen et al., 2013). MIF stimulates CorNV either directly, promoting migration of endothelial cells and angiogenesis, or indirectly, inducing and promoting the expression of angiogenic factors such as VEGF and IL-8(Oh et al., 2010; Usui et al., 2007). Even bFGF is a potent pro-angiogenic factor that regulates expression of integrins and cadherins inducing endothelial cell proliferation and migration and neovessel maturation, by binding its FGF receptor (FGFR) on endothelial cells, (Hajrasouliha et al., 2012; Murakami and Simons, 2008; Qazi et al., 2009; Rolfsen et al., 2013),. In addition, bFGF mediates the expression of other pro-angiogenic factors, such as IL-6, angiopoietin-2 (ANG2) and, via MT1-MMP, VEGF(Chen et al., 2020; Onguchi et al., 2009). ANG2 is constitutively expressed in the epithelium of the human avascular cornea and it can diffuse into the stroma upon injury, eliciting a pro-angiogenic response(Ferrari et al., 2016). Recent data demonstrates the impact of MMP-14 delivered via exosomes, produced by corneal fibroblasts, in supporting neoangiogenesis process by increasing VEGF-A-induced endothelial cell proliferation and migration. MMP-14-containing exosomes degrade VEGFR-1, which possesses a weak kinase activity and high binding affinity for VEGF-A approximately 10 times stronger than that of VEGFR-2(Han et al., 2019). VEGFR-1 acts as a competitive decoy receptor for free VEGF-A, preventing its principal activity by VEGFR-2(Shibuya, 2011). MMP-14 activates others pro-angiogenic pro-MMPs, like MMP-2(Han et al., 2015). Finally, Substance P (SP), a neuropeptide released from sensory nerve endings, plays a remarkable role in inducing CorNV during inflammation. SP is a key mediator of neurogenic inflammation which induces increased vascular permeability and vasodilatation, causing corneal edema, and stimulates macrophages release of inflammatory mediators and pro-angiogenic growth factors(Bignami et al., 2016).
Table 2.
Synoptic overview of Pro-angiogenic Factors.
| PRO ANGIOGENIC FACTORS | |
|---|---|
|
| |
| VEGF-A | Acts by binding tyrosine-kinase VEGFR-1 (Flt-1) and VEGFR-2 (KDR or Flk-1) on the endothelial cells surface, promoting their proliferation and migration. |
| PDGF | Induces the recruitment of pericyte and smooth muscle cells by new vessels. |
| MIF | Promotes migration of endothelial cells and angiogenesis and induces the expression of angiogenic factors, such as VEGF and IL-8. |
| bFGF | Regulates expression of integrins and cadherins, inducing endothelial cell proliferation and migration and neovessels maturation, by binding its FGF receptor (FGFR) on endothelial cells. Mediates the expression of VEGF, IL-6 and angiopoietin-2 (Ang-2). In particular, bFGF upregulates VEGF via MT1-MMP |
| IL-1β | Stimulates endothelial cell migration and proliferation, adhesion molecule expression, inflammatory mediator production and leukocyte recruitment. |
| NF-kB | During inflammation promotes the transcription of several genes, among which VEGF, CCL2 and CXCL5 |
| CCL2 | Promotes macrophage recruitment to the corneal stromal following chemical injury and viral infection |
| CXCL5 | Recruits neutrophils, promotes angiogenesis and remodels connective tissue |
| Substance P | Mediator of neurogenic inflammation which induces vasodilatation and stimulates macrophages release of proangiogenic factors. |
| IL-17A | Induces IL-6 production by corneal fibroblasts, acting in concert to further up- regulate VEGF-A production. Directly enhances MMP-9 production. |
| HIF-1 | Activates the transcription of VEGF genes by binding the hypoxia response element (HRE) in the VEGF promoter region. |
|
Allospecific
<!–cellbgcolor color: #auto; background-color: #FFFFFF;– > T- cells |
Directly produce VEGF-A and mediate a decrease in antiangiogenic signals by reducing corneal endostatin. |
AbbreviationsbFGF: basic fibroblast growth factor; HIF-1: Hypoxia-inducible factor 1; IL17 A: interleukin 17 A; MIF: macrophage inhibitor factor; PDGF: platelet-derived growth factor; VEGF-A: vascular endothelial growth factor A; IL-1β: interleukin 1β; NF-kB: nuclear factor-kB.
CorNV patterns can generally be grouped into three clinical entities: deep NV, that overlies Descemet’s membrane as seen in herpetic and luetic interstitial keratitis; stromal NV, that is mainly associated with stromal keratitis; and vascular pannus, which is fibrovascular connective tissue proliferating in the superficial corneal periphery, mainly associated with ocular surface disorders(Abdelfattah et al., 2015).
6.1. Corneal infections and angiogenesis
The most common culprits in CorNV are infections. Infectious keratitis leading to neoangiogenesis may be caused by various bacterial, viral and parasitic pathogens. Chlamydia trachomatis infection and onchocerciasis are the two most common infectious causes of blindness worldwide, and can both result in CorNV. However, in developed countries the most common infectious cause is herpes simplex virus-1 (HSV-1)(Gonzalez et al., 2013; Stevenson et al., 2012). Recurrent herpetic stromal keratitis (HSK) promotes CorNV by disrupting the balance between pro- and anti-angiogenic factors, increasing corneal levels of VEGF-A and decreasing the sVEGFR-1(Suryawanshi et al., 2011). It has been shown that HSV-1 induces the transcription of VEGF-A in infected epithelial cells through the binding of infected cell protein 4 (ICP4), that is the HSV-1’s major transcriptional regulator, to the VEGF-A promoter. The human VEGF-A promoter is very similar to a promoter sequence (GC-box) of HSV-1 early genes, that ICP4 normally transactivates(Wuest et al., 2011). In addition, HSV-1 infection causes inflammation with a prominent neutrophilic response(Suryawanshi et al., 2011). These infiltrating inflammatory cells are another VEGF-A source, maintaining high levels even after the viral replication has ceased. Moreover, CorNV during HSK is promoted by the reduced amount of corneal sVEGFR-1. HSV-1 infection inhibits the expression of new sVEGFR-1 in corneal epithelial cells, whereas free stromal sVEGFR-1 is degraded by MMP-2, MMP-7 and MMP-9 enzymes, produced by infiltrating neutrophils and infected epithelial cells(Abdelfattah et al., 2015; Kaye and Choudhary, 2006; Suryawanshi et al., 2011; Wuest et al., 2011). These pro-angiogenic processes are promoted by various cytokines released by infected corneal epithelial cells. Among these cytokines, it has been shown that IL-17A, which is rapidly upregulated in the eye after HSV infection, plays a central role in regulating corneal angiogenesis. IL-17A induces IL-6 production by corneal fibroblasts, acting in concert to further up-regulate VEGF-A production. In addition, IL-17A directly enhances MMP-9 production and induces the neutrophil chemoattractant CXCL1/KC in the cornea, increasing neutrophils recruitment and further VEGF-A production(Suryawanshi et al., 2012).
6.2. Contact lens overwear and angiogenesis
Over the past several decades the popularity of contact lens wear has increased and, currently, a large number of people, from children to elderly, wear contact lenses. However, as a consequence, complications related to inappropriate contact lens use and care have increased, among which CorNV, primarily due to hypoxia, has been reported in up to 20% of users(Liesegang, 2002). Contact lenses reduce oxygen delivery to the cornea in the range of 8%–15%, depending on the gas permeability of the lens material, and the hypoxic status is exacerbated by prolonged and overnight wear. Prolonged hypoxia depletes corneal glycogen reserves and ATP, causing accumulation of lactic acid in the stroma and a decrease in the pumping action of endothelium, resulting in corneal edema(Chen et al., 2012; Liesegang, 2002). The major regulator of the hypoxic response is the hypoxia-inducible factor 1 (HIF-1), which is composed of two subunits, α and β. Low oxygen tension induces the translocation of HIF-1α into the nucleus, where, after binding with the constitutively expressed HIF-1β, НІF-1 activates the transcription of the VEGF genes by binding the hypoxia response element (HRE) in the VEGF promoter region(Chen et al., 2012; Gonzalez et al., 2013). In addition, even if hypoxia is the main stimulus, contact lenses induce CorNV by mechanically irritating the limbal sulcus, leading to limbal inflammation(Ellenberg et al., 2010). Contact lens-associated CorNV process arises in several steps. The first response to hypoxic stimulus is limbal hyperemia, which is common and reversible. Later, neovessels invade the superficial cornea causing superficial neovascularization. In the third step chronic hypoxia causes deep stromal neovascularization, that may progress to an inflammatory or fibrovascular deep pannus. Finally, in some cases intracorneal hemorrhages may occur(Abdelfattah et al., 2015; Chen et al., 2012).
6.3. Surgical induced corneal angiogenesis
Corneal transplantation commonly causes neovascularization, especially when grafts are placed in inflamed host beds, both by mechanical injury and inflammatory response to foreign graft antigens(Inomata et al., 2017). The pathophysiology of CorNV in corneal transplants has been shown to be related to an increased expression of VEGF, supported by the fact that VEGF inhibition postoperatively leads to a decreased rate of CorNV(Bachmann et al., 2009; Rolfsen et al., 2013). The angiogenic process after corneal transplantation develops in two phases. The early phase is related to the postoperative healing process, mediated by innate immune cells recruited by inflammation. The late phase is mediated, at least in part, by alloreactive infiltrating T cells, which promote CorNV in vitro by directly producing VEGF-A and by inducing vascular endothelial cells expression of VEGF-A, VEGF-C and VEGFR-2 (Di Zazzo et al., 2017b). In addition, it has been reported that graft-infiltrating allospecific T cells mediate a decrease in anti-angiogenic signals by reducing corneal endostatin, a broad inhibitor of angiogenic factors(Tan et al., 2012). CorNV is a very important feature in keratoplasty because it is the major risk factor for corneal graft (CG) rejection. Both the extent and depth of corneal vessels directly correlate with the risk of allograft rejection(Di Zazzo et al., 2017a). For this reason, the Collaborative Corneal Transplantation Study (CCTS) defined a cornea with two or more quadrants of deep stromal vessels prior to surgery as “high risk”, and “low risk” if less than two quadrants were involved(‘The Collaborative Corneal Transplantation Studies (CCTS): Effectiveness of Histocompatibility Matching in High-Risk Corneal Transplantation’, 1992). Blood vessels are critical for delivering immune effector cells to the graft site, particularly T helper 1 (Th1) cells, which are the principal mediators of graft rejection in corneal transplantation(Di Zazzo et al, 2017b, 2020). In a low risk environment, the post-transplantation transient vascular engorgement or vascular sprouting in the limbus is quickly inhibited, and the CAP is restored. In contrast, in a high risk scenario there is a loss of CAP, and blood and lymphatic vessels invade the corneal graft, increasing the risk for allograft rejection(Di Zazzo et al, 2017a, 2017b). For these reasons, graft survival rates are over 90% when performed in non-vascularized and uninflamed low risk host beds. Graft rejection rates dramatically increase to nearly 50% when transplants are placed into inflamed and vascularized high risk host beds (Di Zazzo et al., 2017b; Inomata et al., 2015; Lam and Dana, 2009).
6.4. Chemical burns and angiogenesis
Chemical burns can lead to CorNV both by inducing a vigorous inflammatory response and by damaging the corneal limbus, causing limbal stem cell deficiency. In particular, the lack of limbal stem cells can lead to the conjunctivalization of the cornea, providing an avenue for the extension of blood vessels into the cornea with loss of the barrier function(Rolfsen et al., 2013; Stevenson et al., 2012).
7. Future directions
CorNV is a significant ocular complication associated with several pathologies affecting a large number of people globally. It may result in visual impairment and an increased risk of corneal graft rejection. The maintenance of the corneal avascular state is an active process based on continuous interactions between multiple pro- and anti-angiogenic factors. VEGF is the most influential molecule in inducing corneal neoangiogenesis. There is now emerging evidence that in addition to the myriad of cornea-derived pro- and anti-angiogenic factors, neighboring tissues (such as the aqueous humor and corneal nerves) and infiltrating immune cells play critical roles in corneal angiogenic privilege and CorNV. As the field of CorNV research has successfully identified and examined many individual cellular/molecular targets using a reductionist approach, there is a need to put these ‘building blocks’ of our knowledge on CorNV back together to compose an aerial view of how these individual targets and various ocular surface components interconnect and interact in homeostasis and diseases.
At the cornea, as well as in other tissues, angiogenic and immune privilege, are critical components of tissue homeostasis(Medzhitov, 2008). In particular, the interconnectivity of the vascular, neuronal, and immunological systems in the cornea and, by extension, the ocular surface is part of a functional system (Di Zazzo et al., 2019). Then, corneal angiogenic privilege, is part of dynamic homeostatic mechanisms, and works actively to maintain cornea function in response to environmental insults or stimuli. Therefore, in such view angiogenesis may be considered an adaptive reaction to restore specific tissue homeostasis and preserving its final function. In fact, as herein reported, several insults lead to a specific and transitory loss of corneal privilege to allow tissue wound healing and homeostasis(Inomata et al., 2017). In fact such responses are usually self-limiting and directly proportional to the insults. For instance, in the context of a low risk corneal transplantation, IFNγ produced by specific conventional T effector cells inhibits VEGF-A produced by other immune cells and indirectly increase endostatin production, as well as proangiogenic VEGF-C and -D stimulate regulatory T cells to suppress effector T cells, further downregulating VEGF-A production(Di Zazzo et al., 2017b; Lužnik et al., 2020; Tan et al., 2012). Unfortunately, sometimes these delicately balanced homeostatic mechanisms go awry and become excessive or persistent compared to the severity of insults, leading to prolonged inflammation (Neumann et al., 1991; Stephen Foster et al., 1982) and irreversible loss of angiogenic privilege, such as in the setting of a high risk corneal transplantation(Di Zazzo et al., 2017b; Inomata et al., 2017). In line with this, the aim of treatment would not be to treat the blood vessels alone, but to treat the underlying dysregulation of corneal angiogenic privilege, taking into account the right timing of intervention, as well as the types of insults. Principal limitations of the present review were due to the paucity of controlled or randomized studies available in literature and the higher numbers of studies conducted only in animal models.
8. Conclusions
Despite several decades of active research in CorNV, clinical management of CorNV remains stagnant with the exception of anti-VEGF therapy. Given the pivotal role of VEGF in angiogenesis and the advent of anti-VEGF therapy for vitreo-retinal vascular diseases, it is not surprising that anti-VEGF treatment is becoming mainstream for curbing CorNV clinically. The long-term efficacy, however, is unclear and there is concern that prolonged suppression of VEGF signaling may lead to neurotrophic keratopathy, which highlights the intimate relationship between corneal angiogenesis and innervation. Apart from the anti- VEGF therapy, there lacks a pipeline to translate existing anti-CorNV experimental therapeutics into clinical use. Therefore, raising awareness of this unmet medical need to a broad R&D audience and developing regulatorily and commercially viable products have the potential to benefit patients with CorNV or high-risk corneal transplantation.
Supplementary Material
Acknowledgement
Thanks to Maria Poddi and Simone Stefanini for helping in searching for the articles.
Footnotes
Declaration of competing interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.exer.2021.108457.
References
- Abdelfattah NS, Amgad M, Zayed AA, Hussein H, El-Baky NA, 2016. Molecular underpinnings of corneal angiogenesis: advances over the past decade. Int. J. Ophthalmol 9, 768–779. 10.18240/ijo.2016.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelfattah NS, Amgad M, Zayed AA, Salem H, Elkhanany AE, Hussein H, El- Baky NA, 2015. Clinical correlates of common corneal neovascular diseases: a literature review. Int. J. Ophthalmol 10.3980/j.issn.2222-3959.2015.01.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JC, Lawler J, 2011. The thrombospondins. Cold Spring Harb. Perspect. Biol 3, 1–29. 10.1101/cshperspect.a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambati BK, Anand A, Joussen AM, Kuziel WA, Adamis AP, Ambati J, 2003. Sustained inhibition of corneal neovascularization by genetic ablation of CCR5. Investig. Ophthalmol. Vis. Sci 44, 590–593. 10.1167/iovs.02-0685. [DOI] [PubMed] [Google Scholar]
- Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, Albuquerque RJC, Richter E, Sakurai E, Newcomb MT, Kleinman ME, Caldwell RB, Lin Q, Ogura Y, Orecchia A, Samuelson DA, Agnew DW, St Leger J, Green WR, Mahasreshti PJ, Curiel DT, Kwan D, Marsh H, Ikeda S, Leiper LJ, Collinson JM, Bogdanovich S, Khurana TS, Shibuya M, Baldwin ME, Ferrara N, Gerber HP, De Falco S, Witta J, Baffi JZ, Raisler BJ, Ambati J, 2006. Corneal avascularity is due to soluble VEGF receptor-1. Nature 443, 993–997. 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambati BK, Patterson E, Jani P, Jenkins C, Higgins E, Singh N, Suthar T, Vira N, Smith K, Caldwell R, 2007. Soluble vascular endothelial growth factor receptor-1 contributes to the corneal antiangiogenic barrier. Br. J. Ophthalmol 91, 505–508. 10.1136/bjo.2006.107417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros V, 2004. The functions of animal microRNAs. Nature. 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- An J, Chen X, Chen W, Liang R, Reinach PS, Yan D, Tu LL, 2015. MicroRNA expression profile and the role of miR-204 in corneal wound healing. Investig. Ophthalmol. Vis. Sci 56, 3673–3683. 10.1167/iovs.15-16467. [DOI] [PubMed] [Google Scholar]
- Anand-apte B, Pepper MS, Voest E, Montesano R, Olsen B, Murphy G, Apte SS, Zetter B, 1997. Inhibition of Angiogenesis by Tissue Inhibitor of 38. [PubMed] [Google Scholar]
- Azar D, 2006. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing. Trans. Am. Ophthalmol. Soc 104, 264–302. [PMC free article] [PubMed] [Google Scholar]
- Bachmann BO, Luetjen-Drecoll E, Bock F, Wiegand SJ, Hos D, Dana R, Kruse FE, Cursiefen C, 2009. Transient postoperative vascular endothelial growth factor (VEGF)-neutralisation improves graft survival in corneas with partly regressed inflammatory neovascularisation. Br. J. Ophthalmol 93, 1075–1080. 10.1136/bjo.2008.145128. [DOI] [PubMed] [Google Scholar]
- Bartel DP, 2009. MicroRNAs: target recognition and regulatory functions. Cell. 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum O, Suter F, Gerber B, Tschanz SA, Buergy R, Blank F, Hlushchuk R, Djonov V, 2010. VEGF-A promotes intussusceptive angiogenesis in the developing chicken chorioallantoic membrane. Microcirculation 17, 447–457. 10.1111/j.1549-8719.2010.00043.x. [DOI] [PubMed] [Google Scholar]
- Beebe DC, 2008. Maintaining transparency: a review of the developmental physiology and pathophysiology of two avascular tissues. Semin. Cell Dev. Biol 10.1016/j.semcdb.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami F, Rama P, Ferrari G, 2016. Substance P and its inhibition in ocular inflammation. Curr. Drug Targets 17, 1265–1274. 10.2174/1389450116666151019100216. [DOI] [PubMed] [Google Scholar]
- Blanco R, Gerhardt H, 2013. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb. Perspect. Med 3 10.1101/cshperspect.a006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock F, Maruyama K, Regenfuss B, Hos D, Steven P, Heindl LM, Cursiefen C, 2013. Novel anti(lymph)angiogenic treatment strategies for corneal and ocular surface diseases. Prog. Retin. Eye Res. 34, 89–124. 10.1016/j.preteyeres.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Bock F, Onderka J, Braun G, Schneider AC, Hos D, Bi Y, Bachmann BO, Cursiefen C, 2016. Identification of novel endogenous anti(Lymph)angiogenic factors in the aqueous humor. Investig. Ophthalmol. Vis. Sci 57, 6554–6560. 10.1167/iovs.15-18526. [DOI] [PubMed] [Google Scholar]
- Chang JH, Han KY, Azar DT, 2010. Wound healing fibroblasts modulate corneal angiogenic privilege: interplay of basic fibroblast growth factor and matrix metalloproteinases in corneal angiogenesis. Jpn. J. Ophthalmol 54, 199–205. 10.1007/s10384-010-0801-5. [DOI] [PubMed] [Google Scholar]
- Chen M, Bao L, Zhao M, Cao J, Zheng H, 2020. Progress in research on the role of FGF in the formation and treatment of corneal neovascularization. Front. Pharmacol 10.3389/fphar.2020.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Yin H, Wang Ye, Wang Yao, Xie L, 2012. Inhibition of VEGF expression and corneal neovascularization by shRNA targeting HIF-1α in a mouse model of closed eye contact lens wear. Mol. Vis 18, 864–873. [PMC free article] [PubMed] [Google Scholar]
- Coassin M, Duncan KG, Bailey KR, Singh A, Schwartz DM, 2010. Hypothermia reduces secretion of vascular endothelial growth factor by cultured retinal pigment epithelial cells. Br. J. Ophthalmol 94, 1678–1683. 10.1136/bjo.2009.168864. [DOI] [PubMed] [Google Scholar]
- Cursiefen C, 2007. Immune privilege and angiogenic privilege of the cornea. Chem. Immunol. Allergy 92, 50–57. 10.1159/000099253. [DOI] [PubMed] [Google Scholar]
- Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D’Amore PA, Dana MR, Wiegand SJ, Streilein JW, 2004. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Invest 113, 1040–1050. 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cursiefen C, Hofmann-Rummelt C, Küchle M, Schlotzer-Schrehardt U, 2003. ¨ Pericyte recruitment in human corneal angiogenesis: an ultrastructural study with clinicopathological correlation. Br. J. Ophthalmol 87, 101–106. 10.1136/bjo.87.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cursiefen C, Maruyama K, Bock F, Saban D, Sadrai Z, Lawler J, Dana R, Masli S, 2011. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J. Exp. Med 208, 1083–1092. 10.1084/jem.20092277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana M, 2006. Angiogenesis and lymphangiogenesis - implications for corneal immunity. Semin. Ophthalmol 21, 19–22. 10.1080/08820530500509358. [DOI] [PubMed] [Google Scholar]
- Dana MR, Zhu SN, Yamada J, 1998. Topical modulation of interleukin-1 activity in corneal neovascularization. Cornea 17, 403–409. 10.1097/00003226-199807000-00011. [DOI] [PubMed] [Google Scholar]
- Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu HJ, Benedict W, Bouck NP, 1999. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science 285, 245–248. 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- Di Zazzo A, Kheirkhah A, Abud TB, Goyal S, Dana R, 2017a. Management of high- risk corneal transplantation. Surv. Ophthalmol 10.1016/j.survophthal.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Zazzo A, Lee S-M, Sung J, Niutta M, Coassin M, Mashaghi A, Inomata T, 2020. Variable responses to corneal grafts: insights from immunology and systems biology. J. Clin. Med 9, 586. 10.3390/jcm9020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Zazzo A, Micera A, Coassin M, Varacalli G, Foulsham W, De Piano M, Bonini S, 2019. Inflammaging at ocular surface: clinical and biomolecular analyses in healthy volunteers. Investig. Ophthalmol. Vis. Sci 60, 1769–1775. 10.1167/iovs.18-25822. [DOI] [PubMed] [Google Scholar]
- Di Zazzo A, Tahvildari M, Subbarayal B, Yin J, Dohlman TH, Inomata T, Mashaghi A, Chauhan SK, Dana R, 2017b. Proangiogenic function of T cells in corneal transplantation. Transplantation 101, 778–785. 10.1097/TP.0000000000001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djonov V, Baum O, Burri PH, 2003. Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res. 10.1007/s00441-003-0784-3. [DOI] [PubMed] [Google Scholar]
- Ellenberg D, Azar DT, Hallak JA, Tobaigy F, Han KY, Jain S, Zhou Z, Chang JH, 2010. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog. Retin. Eye Res. 29, 208–248. 10.1016/j.preteyeres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslani M, Putra I, Shen X, Hamouie J, Afsharkhamseh N, Besharat S, Rosenblatt MI, Dana R, Hematti P, Djalilian AR, 2017. Corneal mesenchymal stromal cells are directly antiangiogenic via PEDF and sFLT-1. Investig. Ophthalmol. Vis. Sci 58, 5507–5517. 10.1167/iovs.17-22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana EM, Ti SE, Grueterich M, Touhami A, Tseng SCG, 2003. Corneal stromal changes following reconstruction by ex vivo expanded limbal epithelial cells in rabbits with total limbal stem cell deficiency. Br. J. Ophthalmol 87, 1509–1514. 10.1136/bjo.87.12.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari G, Bignami F, Rama P, 2015. Tumor necrosis factor-α inhibitors as a treatment of corneal hemangiogenesis and lymphangiogenesis. Eye Contact Lens. 10.1097/ICL.0000000000000071. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Giacomini C, Bignami F, Moi D, Ranghetti A, Doglioni C, Naldini L, Rama P, Mazzieri R, 2016. Angiopoietin 2 expression in the cornea and its control of corneal neovascularisation. Br. J. Ophthalmol 100, 1005–1010. 10.1136/bjophthalmol-2015-307901. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Hajrasouliha AR, Sadrai Z, Ueno H, Chauhan SK, Dana R, 2013. Nerves and neovessels inhibit each other in the cornea. Investig. Ophthalmol. Vis. Sci 54, 813–820. 10.1167/iovs.11-8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulsham W, Dohlman TH, Mittal SK, Taketani Y, Singh RB, Masli S, Dana R, 2019. Thrombospondin-1 in ocular surface health and disease. Ocul. Surf 17, 374–383. 10.1016/j.jtos.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman RC, Farh KKH, Burge CB, Bartel DP, 2009. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19, 92–105. 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Wang Y, Zhao X, Chen P, Xie L, 2015. MicroRNA-204–5p–mediated regulation of SIRT1 contributes to the delay of epithelial cell cycle traversal in diabetic corneas. Investig. Ophthalmol. Vis. Sci 56, 1493–1504. 10.1167/iovs.14-15913. [DOI] [PubMed] [Google Scholar]
- Gao X, Guo K, Santosa SM, Montana M, Yamakawa M, Hallak JA, Han KY, Doh SJ, Rosenblatt MI, Chang JH, Azar DT, 2019. Application of corneal injury models in dual fluorescent reporter transgenic mice to understand the roles of the cornea and limbus in angiogenic and lymphangiogenic privilege. Sci. Rep 9, 1–13. 10.1038/s41598-019-48811-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H, 2008. VEGF and endothelial guidance in angiogenic sprouting. Organogenesis 4, 241–246. 10.4161/org.4.4.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez L, Loza RJ, Han KY, Sunoqrot S, Cunningham C, Purta P, Drake J, Jain S, Hong S, Chang JH, 2013. Nanotechnology in corneal neovascularization therapy - a review. J. Ocul. Pharmacol. Therapeut 29, 124–134. 10.1089/jop.2012.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueterich M, Espana EM, Tseng SCG, 2003. Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Surv. Ophthalmol 48, 631–646. 10.1016/j.survophthal.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Hahn N, Dietz CT, Kühl S, Vossmerbaeumer U, Kroll J, 2012. KLEIP deficiency in mice causes progressive corneal neovascular dystrophy. Invest. Ophthalmol. Vis. Sci 53, 3260–3268. 10.1167/iovs.12-9676. [DOI] [PubMed] [Google Scholar]
- Hajrasouliha AR, Sadrai Z, Chauhan SK, Dana R, 2012. B-FGF induces corneal blood and lymphatic vessel growth in a spatially distinct pattern. Cornea 31, 804–809. 10.1097/ICO.0b013e31823f8b5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KY, Chang JH, Azar DT, 2019. MMP14-containing exosomes cleave VEGFR1 and promote VEGFA-induced migration and proliferation of vascular endothelial cells. Investig. Ophthalmol. Vis. Sci 60, 2321–2329. 10.1167/iovs.18-26277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KY, Dugas-Ford J, Seiki M, Chang JH, Azar DT, 2015. Evidence for the involvement of MMP14 in MMP2 processing and recruitment in exosomes of corneal fibroblasts. Investig. Ophthalmol. Vis. Sci 56, 5323–5329. 10.1167/iovs.14-14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisatomi T, Nakao S, Murakami Y, Noda K, Nakazawa T, Notomi S, Connolly E, She H, Almulki L, Ito Y, Vavvas DG, Ishibashi T, Miller JW, 2012. The regulatory roles of apoptosis-inducing factor in the formation and regression processes of ocular neovascularization. Am. J. Pathol 181, 53–61. 10.1016/j.ajpath.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori J, Joyce N, Streilein JW, 2000. Epithelium-deficient corneal allografts display immune privilege beneath the kidney capsule. Invest. Ophthalmol. Vis. Sci 41, 443–452. [PubMed] [Google Scholar]
- Inomata T, Mashaghi A, Di Zazzo A, Dana R, 2015. Ocular surgical models for immune and angiogenic responses. J. Biol. Methods 2, 27. 10.14440/jbm.2015.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata T, Mashaghi A, Di Zazzo A, Lee SM, Chiang H, Dana R, 2017. Kinetics of angiogenic responses in corneal transplantation. Cornea 36, 491–496. 10.1097/ICO.0000000000001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Chauhan SK, Annan JEI, Sage PT, Sharpe AH, Dana R, 2011. A novel function for programmed death ligand-1 regulation of angiogenesis. Am. J. Pathol 178, 1922–1929. 10.1016/j.ajpath.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, Kim HR, Chesler L, Tsao-Wu G, Bouck N, Polverini PJ, 1994. Inhibition of angiogenesis by tissue inhibitor of metalloproteinase. J. Cell. Physiol 160, 194–202. 10.1002/jcp.1041600122. [DOI] [PubMed] [Google Scholar]
- Kaye S, Choudhary A, 2006. Herpes simplex keratitis. Prog. Retin. Eye Res. 10.1016/j.preteyeres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Kenyon KR, Tseng SCG, 1989. Limbal autograft transplantation for ocular surface disorders. Ophthalmology 96, 709–723. 10.1016/S0161-6420(89)32833-8. [DOI] [PubMed] [Google Scholar]
- Kilarski WW, Samolov B, Petersson L, Kvanta A, Gerwins P, 2009. Biomechanical regulation of blood vessel growth during tissue vascularization. Nat. Med 15, 657–664. 10.1038/nm.1985. [DOI] [PubMed] [Google Scholar]
- Kiriakidis S, Andreakos E, Monaco C, Foxwell B, Feldmann M, Paleolog E, 2003. VEGF expression in human macrophages is NF-κB-dependent: studies using adenoviruses expressing the endogenous NF-κB inhibitor IκBα and a kinase-defective form of the IκB kinase 2. J. Cell Sci. 10.1242/jcs.00286. [DOI] [PubMed] [Google Scholar]
- Kojima T, Azar DT, Chang J-H, 2008. Neostatin-7 regulates bFGF-induced corneal lymphangiogenesis. FEBS Lett. 582, 2515–2520. 10.1016/j.febslet.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommineni VK, Nagineni CN, William A, Detrick B, Hooks JJ, 2008. IFN-gamma acts as anti-angiogenic cytokine in the human cornea by regulating the expression of VEGF-A and sVEGF-R1. Biochem. Biophys. Res. Commun 374, 479–484. 10.1016/j.bbrc.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam H, Dana MR, 2009. Corneal graft rejection. Int. Ophthalmol. Clin 10.1097/IIO.0b013e3181924e23. [DOI] [PubMed] [Google Scholar]
- Lenard A, Daetwyler S, Betz C, Ellertsdottir E, Belting H-G, Huisken J, Affolter M, 2015. Endothelial cell self-fusion during vascular pruning. PLoS Biol. 13, e1002126 10.1371/journal.pbio.1002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennikov A, Mirabelli P, Mukwaya A, Schaupper M, Thangavelu M, Lachota M, Ali Z, Jensen L, Lagali N, 2018. Selective IKK2 inhibitor IMD0354 disrupts NF-κB signaling to suppress corneal inflammation and angiogenesis. Angiogenesis 21, 267–285. 10.1007/s10456-018-9594-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Yang W, Jiang H, Guo C, Huang AJW, Hu H, Liu Q, 2019. TRPV1 activity and substance P release are required for corneal cold nociception. Nat. Commun 10 10.1038/s41467-019-13536-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesegang TJ, 2002. Physiologic changes of the cornea with contact lens wear. CLAO J. 28, 12–27. [PubMed] [Google Scholar]
- Liu CH, Wang Z, Sun Y, Chen J, 2017. Animal models of ocular angiogenesis: from development to pathologies. Faseb. J 31, 4665–4681. 10.1096/fj.201700336R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobov I, Mikhailova N, 2018. The role of dll4/notch signaling in normal and pathological ocular angiogenesis: dll4 controls blood vessel sprouting and vessel remodeling in normal and pathological conditions. J. Ophthalmol. 2018 10.1155/2018/3565292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ, 2007. Delta-like ligand 4 (DII4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc. Natl. Acad. Sci. U.S.A. 104, 3219–3224. 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lužnik Z, Anchouche S, Dana R, Yin J, 2020. Regulatory T cells in angiogenesis. J. Immunol 205, 2557–2565. 10.4049/jimmunol.2000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madalli S, Beyrau M, Whiteford J, Duchene J, Nandhra IS, Patel NSA, Motwani MP, Gilroy DW, Thiemermann C, Nourshargh S, Scotland RS, 2015. Sex-specific regulation of chemokine Cxcl5/6 controls neutrophil recruitment and tissue injury in acute inflammatory states. Biol. Sex Differ. 6 10.1186/s13293-015-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masli S, Sheibani N, Cursiefen C, Zieske J, 2014. Matricellular protein thrombospondins: influence on ocular angiogenesis, wound healing and immuneregulation. Curr. Eye Res. 39, 759–774. 10.3109/02713683.2013.877936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, 2008. Origin and physiological roles of inflammation. Nature. 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Menzel-Severing J, 2012. Emerging techniques to treat corneal neovascularisation. Eye. 10.1038/eye.2011.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabelli P, Mukwaya A, Lennikov A, Xeroudaki M, Peebo B, Schaupper M, Lagali N, 2017. Genome-wide expression differences in anti-Vegf and dexamethasone treatment of inflammatory angiogenesis in the rat cornea. Sci. Rep 7 10.1038/s41598-017-07129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabelli P, Peebo BB, Xeroudaki M, Koulikovska M, Lagali N, 2014. Early effects of dexamethasone and anti-VEGF therapy in an inflammatory corneal neovascularization model. Exp. Eye Res. 125, 118–127. 10.1016/j.exer.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Mukwaya A, Jensen L, Peebo B, Lagali N, 2019. MicroRNAs in the cornea: role and implications for treatment of corneal neovascularization. Ocul. Surf 10.1016/j.jtos.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Mukwaya A, Peebo B, Xeroudaki M, Ali Z, Lennikov A, Jensen L, Lagali N, 2016. Factors regulating capillary remodeling in a reversible model of inflammatory corneal angiogenesis. Sci. Rep 6 10.1038/srep32137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Simons M, 2008. Fibroblast growth factor regulation of neovascularization. Curr. Opin. Hematol 10.1097/MOH.0b013e3282f97d98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwaikambo BR, Sennlaub F, Ong H, Chemtob S, Hardy P, 2006. Activation of CD36 inhibits and induces regression of inflammatory corneal neovascularization. Investig. Ophthalmol. Vis. Sci 47, 4356–4364. 10.1167/iovs.05-1656. [DOI] [PubMed] [Google Scholar]
- Nakao S, Hata Y, Miura M, Noda K, Kimura YN, Kawahara S, Kita T, Hisatomi T, Nakazawa T, Jin Y, Dana MR, Kuwano M, Ono M, Ishibashi T, Hafezi-Moghadam A, 2007. Dexamethasone inhibits interleukin-1β-induced corneal neovascularization: role of nuclear factor-κB-activated stromal cells in inflammatory angiogenesis. Am. J. Pathol 171, 1058–1065. 10.2353/ajpath.2007.070172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao S, Kuwano T, Tsutsumi-Miyahara C, Ueda SI, Kimura YN, Hamano S, Sonoda KH, Saijo Y, Nukiwa T, Strieter RM, Ishibashi T, Kuwano M, Ono M, 2005. Infiltration of COX-2-expressing macrophages is a prerequisite for IL- 1β-induced neovascularization and tumor growth. J. Clin. Invest 115, 2979–2991. 10.1172/JCI23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann R, Tauber J, Foster CS, 1991. Remission and recurrence after withdrawal of therapy for ocular cicatricial pemphigoid. Ophthalmology 98, 858–862. 10.1016/S0161-6420(91)32209-7. [DOI] [PubMed] [Google Scholar]
- Nominato LF, Dias AC, Dias LC, Fantucci MZ, da Silva LECM, de Andrade Murashima A, Rocha EM, 2018. Prevention of corneal neovascularization by adenovirus encoding human vascular endothelial growth factor soluble receptor (s-VEGFR1) in lacrimal gland. Investig. Ophthalmol. Vis. Sci 59, 6036–6044. 10.1167/iovs.17-22322. [DOI] [PubMed] [Google Scholar]
- Oh SY, Choi J-S, Kim E-J, Chuck RS, Park CY, 2010. The role of macrophage migration inhibitory factor in ocular surface disease pathogenesis after chemical burn in the murine eye. Mol. Vis 16, 2402. [PMC free article] [PubMed] [Google Scholar]
- Onguchi T, Han KY, Chang JH, Azar DT, 2009. Membrane type-1 matrix metalloproteinase potentiates basic fibroblast growth factor-induced corneal neovascularization. Am. J. Pathol 174, 1564–1571. 10.2353/ajpath.2009.080452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JK, Peng H, Yang W, Katsnelson J, Volpert O, Lavker RM, 2017. MiR-184 exhibits angiostatic properties via regulation of Akt and VEGF signaling pathways. Faseb. J 31, 256–265. 10.1096/fj.201600746R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Dana R, 2009. Corneal lymphangiogenesis: implications in immunity. Semin. Ophthalmol 24, 135–138. 10.1080/08820530902801320. [DOI] [PubMed] [Google Scholar]
- Pérez-Santonja JJ, Campos-Mollo E, Lledó-Riquelme M, Javaloy J, Alió JL, 2010. Inhibition of corneal neovascularization by topical bevacizumab (Anti-VEGF) and sunitinib (Anti-VEGF and Anti-PDGF) in an animal model. Am. J. Ophthalmol 150 10.1016/j.ajo.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Pitulescu ME, Schmidt I, Giaimo BD, Antoine T, Berkenfeld F, Ferrante F, Park H, Ehling M, Biljes D, Rocha SF, Langen UH, Stehling M, Nagasawa T, Ferrara N, Borggrefe T, Adams RH, 2017. Dll4 and Notch signalling couples sprouting angiogenesis and artery formation. Nat. Cell Biol. 19, 915–927. 10.1038/ncb3555. [DOI] [PubMed] [Google Scholar]
- Qazi Y, Maddula S, Ambati BK, 2009. Mediators of ocular angiogenesis. J. Genet 88, 495–515. 10.1007/s12041-009-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qazi Y, Wong G, Monson B, Stringham J, Ambati BK, 2010. Corneal transparency: genesis, maintenance and dysfunction. Brain Res. Bull 81, 198–210. 10.1016/j.brainresbull.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfsen ML, Frisard NE, Stern EM, Foster TP, Bhattacharjee PS, McFerrin HE, Clement C, Rodriguez PC, Lukiw WJ, Bergsma DR, Ochoa AC, Hill JM, 2013. Corneal neovascularization: a review of the molecular biology and current therapies. Expet Rev. Ophthalmol 8, 167–189. 10.1586/eop.13.8. [DOI] [Google Scholar]
- Roshandel D, Eslani M, Baradaran-Rafii A, Cheung AY, Kurji K, Jabbehdari S, Maiz A, Jalali S, Djalilian AR, Holland EJ, 2018. Current and emerging therapies for corneal neovascularization. Ocul. Surf 16, 398–414. 10.1016/j.jtos.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland KJ, Diaz-Miron J, Guo J, Erwin CR, Mei J, Worthen GS, Warner BW, 2014. CXCL5 is required for angiogenesis, but not structural adaptation after small bowel resection. J. Pediatr. Surg 49, 976–980. 10.1016/j.jpedsurg.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino F, Di Zazzo A, De Simone L, Bonini S, 2017. The intriguing role of neuropeptides at the ocular surface. Ocul. Surf 10.1016/j.jtos.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Saika S, Miyamoto T, Yamanaka O, Kato T, Ohnishi Y, Flanders KC, Ikeda K, Nakajima Y, Kao WWY, Sato M, Muragaki Y, Ooshima A, 2005. Therapeutic effect of topical administration of SN50, an inhibitor of nuclear factor-κB, in treatment of corneal alkali burns in mice. Am. J. Pathol 166, 1393–1403. 10.1016/S0002-9440(10)62357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif Z, Sharif W, 2019. Corneal neovascularization: updates on pathophysiology, investigations & management. Rom. J. Ophthalmol 63, 15–22. 10.22336/rjo.2019.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen JK, Yang X, Xie B, Chen Y, Swaim M, Hackett SF, Campochiaro PA, 2008. MicroRNAs regulate ocular neovascularization. Mol. Ther 16, 1208–1216. 10.1038/mt.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M, 2011. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Gene Canc. 2, 1097–1105. 10.1177/1947601911423031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Filardi E, Nieto C, Domínguez-Soto Á, Barroso R, Sánchez-Mateos P, Puig-Kroger A, López-Bravo M, Joven J, Ardavín C, Rodríguez-Fernández JL, Sánchez-Torres C, Mellado M, Corbí ÁL, 2014. CCL2 shapes macrophage polarization by GM-CSF and M-CSF: identification of CCL2/CCR2-dependent gene expression profile. J. Immunol 192, 3858–3867. 10.4049/jimmunol.1302821. [DOI] [PubMed] [Google Scholar]
- Sridhar MS, 2018. Anatomy of cornea and ocular surface. Indian J. Ophthalmol 10.4103/ijo.IJO_646_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen Foster C, Wilson LA, Ekins MB, 1982. Immunosuppressive therapy for progressive ocular cicatncial pemphigoid. Ophthalmology 89, 340–353. 10.1016/S0161-6420(82)34791-0. [DOI] [PubMed] [Google Scholar]
- Stevenson W, Cheng SF, Dastjerdi MH, Ferrari G, Dana R, 2012. Corneal neovascularization and the utility of topical VEGF inhibition: ranibizumab (Lucentis) vs bevacizumab (Avastin). Ocul. Surf 10, 67–83. 10.1016/j.jtos.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Hopkins BD, Tsujikawa K, Perruzzi C, Adini I, Swerlick R, Bornstein P, Lawler J, Benjamin LE, 2009. Thrombospondin-1 modulates VEGF-A-mediated Akt signaling and capillary survival in the developing retina. Am. J. Physiol. Heart Circ. Physiol 296, H1344–H1351. 10.1152/ajpheart.01246.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawanshi A, Mulik S, Sharma S, Reddy PBJ, Sehrawat S, Rouse BT, 2011. Ocular neovascularization caused by herpes simplex virus type 1 infection results from breakdown of binding between vascular endothelial growth factor A and its soluble receptor. J. Immunol 186, 3653–3665. 10.4049/jimmunol.1003239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawanshi A, Veiga-Parga T, Reddy PBJ, Rajasagi NK, Rouse BT, 2012. IL-17A differentially regulates corneal vascular endothelial growth factor (VEGF)-A and soluble VEGF receptor 1 expression and promotes corneal angiogenesis after herpes simplex virus infection. J. Immunol 188, 3434–3446. 10.4049/jimmunol.1102602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatori SC, de la Jara PL, Holden B, Ehrmann K, Ho A, Radke CJ, 2012. In vivo oxygen uptake into the human cornea. Investig. Ophthalmol. Vis. Sci 53, 6331–6337. 10.1167/iovs.12-10059. [DOI] [PubMed] [Google Scholar]
- Tan Y, Cruz-Guilloty F, Medina-Mendez CA, Cutrufello NJ, Martinez RE, Urbieta M, Wilson D, Li Y, Perez VL, 2012. Immunological disruption of antiangiogenic signals by recruited allospecific T cells leads to corneal allograft rejection. J. Immunol 188, 5962–5969. 10.4049/jimmunol.1103216. [DOI] [PubMed] [Google Scholar]
- The Collaborative Corneal Transplantation Studies (CCTS), 1992. Effectiveness of histocompatibility matching in high-risk corneal transplantation. Arch. Ophthalmol 110, 1392–1403. 10.1001/archopht.1992.01080220054021. [DOI] [PubMed] [Google Scholar]
- Tsai RJF, Tseng SCG, 1994. Human allograft limbal transplantation for corneal surface reconstruction. Cornea 13, 389–400. 10.1097/00003226-199409000-00003. [DOI] [PubMed] [Google Scholar]
- Usui T, Yamagami S, Kishimoto S, Seiich Y, Nakayama T, Amano S, 2007. Role of macrophage migration inhibitory factor in corneal neovascularization. Investig. Ophthalmol. Vis. Sci 48, 3545–3550. 10.1167/iovs.06-0695. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jin Y, Laviña B, Jakobsson L, 2018. Characterization of multi-cellular dynamics of angiogenesis and vascular remodelling by intravital imaging of the wounded mouse cornea. Sci. Rep 8, 1–12. 10.1038/s41598-018-28770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M, Clay SM, Zheng S, Pan P, Chan MF, 2019. MMP12 inhibits corneal neovascularization and inflammation through regulation of CCL2. Sci. Rep 9 10.1038/s41598-019-47831-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuest T, Zheng M, Efstathiou S, Halford WP, Carr DJJ, 2011. The herpes simplex virus-1 transactivator infected cell protein-4 drives VEGF-A dependent Neovascularization. PLoS Pathog. 7 10.1371/journal.ppat.1002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Wilhelmus KR, 2009. Corneal neovascularization during experimental fungal keratitis. Mol. Vis 15, 1988–1996. [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Di G, Dong M, Qu M, Zhao X, Duan H, Hu X, Liu T, Zhou Q, Shi W, 2018. Epithelium-derived miR-204 inhibits corneal neovascularization. Exp. Eye Res. 167, 122–127. 10.1016/j.exer.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kazerounian S, Duquette M, Perruzzi C, Nagy JA, Dvorak HF, Parangi S, Lawler J, 2009. Thrombospondin-1 modulates vascular endothelial growth factor activity at the receptor level. Faseb. J 23, 3368–3376. 10.1096/fj.09-131649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong R, Zhou T, Lin Z, Bao X, Xiu Y, Chen Y, Chen L, Ma JX, Liu Z, Zhou Y, 2016. Down-regulation of microRNA-184 is associated with corneal neovascularization. Investig. Ophthalmol. Vis. Sci 57, 1398–1407. 10.1167/iovs.15-17417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.