Abstract
Tropical almond (Terminalia catappa Linn.) is highly distributed within the tropics, but appears rather underutilized in developing countries like Nigeria. Specifically, relevant information regards the nutritional, health benefits, and pharmaceutical potential of roasted T. catappa nuts remains scanty. Comparing both raw and roasted T. catappa nuts should provide additional information especially from product development and potential commercial prospect standpoints. The changes in nutritional, health benefits, and pharmaceutical potentials of raw and roasted T. catappa nuts were, therefore, investigated. Whereas the raw T. catappa nuts obtained significantly (p < 0.05) higher protein, ash, moisture, crude fiber, as well as vitamins C, and B1-3 compared to the roasted ones, some contents like carbohydrates, energy, vitamin A, calcium, manganese, zinc, hydrogen cyanide, as well as oxalate would noticeably change (p < 0.05) after the roasting process. Twenty phytochemicals were identified in both raw and roasted samples with the concentrations of quinine, ribalinidine, sapogenin, flavan-3-ol and tannin significantly reduced, while catechin seemed enhanced upon roasting. Promising drug-likeness, pharmacokinetic properties, and safety profiles could be predicted among the phytochemicals. Overall, roasting T. catappa nuts should enhance the nutritional contents, which could aid both absorption and palatability.
Introduction
Identifying available and cheap nutrient-dense agro-foods could help solve the global burden of hunger/malnutrition, which have been triggered by the increasing population growth and unmatched food security plans. Developing healthy, quality, and safe food products from sustainable nutrient-dense agro-food sources is feasible via increased research, particularly those eco-friendly processes adaptable to consumers’ needs [1]. There is an increased global interest in nuts that remedies hunger and malnutrition given their enriched dietary fiber, high-quality plant protein, minerals, unsaturated fatty acids, vitamins, fat-soluble bioactives, and other phytonutrients [2–4]. Such phytoconstituents in nuts position them as promising agents that positively impact a myriad of health outcomes as well as natural pleiotropic nutraceuticals [3,5].
Nuts when incorporated as an essential constituent of human diet, either consumed whole (raw or roasted or salted) or as snack-type convenient food, contribute strongly to helping in sustaining healthy dietary patterns [4]. More so, the consumption of nuts would suppress hunger by modulating either the postprandial appetite or desire to eat, which creates a feeling of satiety [6]. More so, the processing methods applied to nuts generally enhances their organoleptic quality with long-term preservation [7]. From thermal (such as heating, blanching, roasting and frying) [8] to non-thermal (such as high hydrostatic pressure treatment and enzymatic treatment) types, the processing methods of nuts capably modify various characteristic qualities within the component matrix [7]. Given the aroma, flavor, texture, and color improvements that results, the roasting process continues to thrive as the increasingly preferred processing method for nuts [9].
Almond remains among the most consumed nut, especially in high-income countries. According to the International Nuts and Dried Fruits statistical yearbook 2018/2019, it accounted for about 39% of total consumption, with walnuts, cashews, and hazelnuts occupying the second, third, and fourth positions, respectively, in terms of consumption [10]. Tropical almond (Terminalia catappa Linn.) belongs to the Combretaceae family. Widely distributed within the tropics, including Nigeria, T. catappa plant thrives explicitly as shade at parks, along avenues as well as private gardens [11,12]. Besides its ellipsoid shape, the drupe of T. catappa plant is about 3–5.5 cm broad and 5–7 cm long, whereas its fruit, when ripe, turns from green to yellow or red [13]. Ripe mesocarp of T. catappa fruit is usually consumed by children. Further, the shell that is usually discarded covers the almond nut, the latter well established to comprise high protein, fat, carbohydrate, vitamin, and mineral contents [14–16].
However, relevant information regards the nutritional, health benefits, and pharmaceutical potential of roasted T. catappa nuts specific to Nigeria remains scanty. Essentially, the pharmacological and pharmacodynamic properties of nuts especially those possessing promising bioactive potentials need ascertaining through in silico integrative models of hits compounds. Achieving such feat (that is, the pharmacological and pharmacodynamic properties of nuts) especially within the biological system of drug candidates could help in predicting nut’s absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties with high precision [17]. More so, to actualize compounds of potential bioavailability with less toxicity that could be re-purposed for drug development might help to cumulatively save cost as well as time spent in drug discovery that would fail at clinical trials. To supplement existing information, this current work investigated the changes in nutritional, health benefits, and pharmaceutical potential of raw and roasted T. catappa nuts from Nigeria. Importantly, comparing both raw and roasted T. catappa nuts should provide additional information especially from product development and potential commercial prospect standpoints.
Materials and methods
Schematic overview of the experimental design
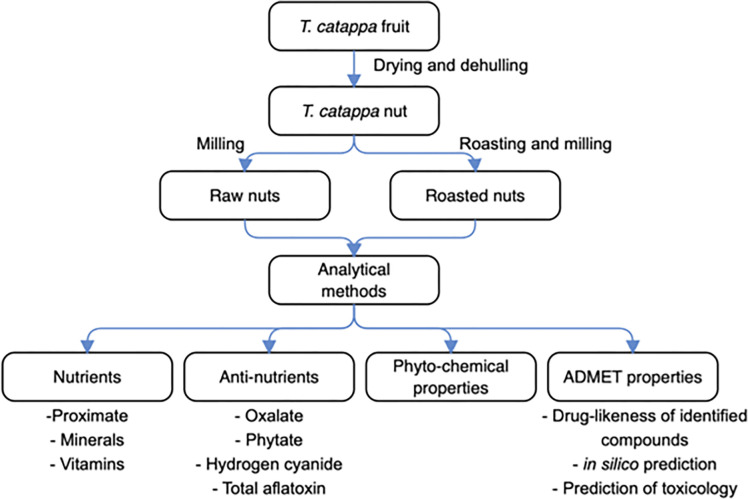
The schematic overview of the experimental design is depicted in Fig 1, which demonstrated the major stages, from the drying, and dehulling of T. catappa fruits, through the roasting/milling of nuts, to the analytical methods that involved the determinations of nutrients, anti-nutrients, phytochemical, and ADMET properties. For emphasis, this current work compared raw and roasted T. catappa nuts via nutritional (proximate, minerals, vitamins), anti-nutritional (oxalate, phytate, hydrogen cyanide, total aflatoxin), phytochemical, and ADMET (drug-likeness of the identified compounds, in silico prediction, and prediction of toxicity and toxicological effects) attributes. Importantly, all analytical measurements performed were in adherence to the relevant laboratory guidelines set out by the Department of Biochemistry, University of Nigeria, Nsukka, Enugu State, Nigeria.
Fig 1. The schematic overview of the experimental design showing the key stages, from the drying and dehulling of Terminalia catappa fruits, roasting/milling of the nuts, analytical methods involving determinations of nutrients, anti-nutrients, phytochemical, and ADMET properties.
Chemicals and reagents
The chemicals and reagents used in this study were of an analytical grade standard. Products purchased from British Drug House, England include sulphuric acid, ammonium sulphate, aqua regia (a mixture of HNO3 and HCl in the ratio of 1:3), anhydrous sodium sulfate, concentrated tetraoxosulphate (VI) acid, nitric acid, silver trioxonitrate, trioxoborate (III) acid, diethyl ether; from May and Baker, England include iron (II) chloride, potassium ferric cyanide, potassium iodide, potassium tetraoxomanganate; from Merck, Germany include ammonium iron (III) sulphate, calcium (III) chloride, sodium hydroxide, ferric chloride, potassium chiocyanate; from Sigma-Aldrich, USA. include acetone, butylalcohol, heptane, hexane, ethanol, methanol, thioglycolic acid, ammonium thiocyanote, bipyridine, diphenol indo 2, 6 –dichlorophenol, potassium iodide, anhydrous sodium sulphate, isopropanol, 4-amino phenol, ammonium hydroxide, glacial acetic acid, hydrogen peroxide, oxalic acid, sodium acetate anhydrous, potassium hydroxide, a-a 1–dipyridyl, hydrocyanic acid, potassium sulfocyanate; from Abcam, USA, include the total aflatoxin ELISA kit (ab285282, type).
Collection, preparation, and processing of T. catappa fruit samples
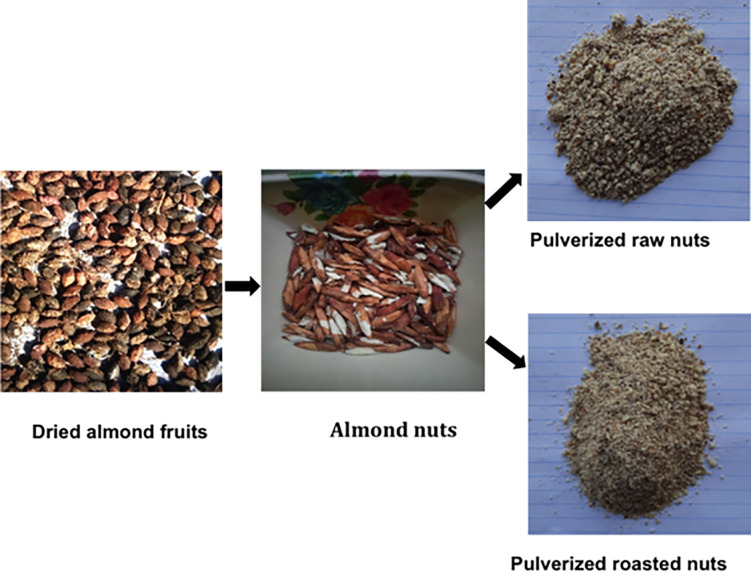
For this research, the almond (T. catappa) fruits (red morphotypes) were harvested from the trees abundantly situated at Zuba, Gwagwalada Local Government Area, Federal Capital Territory (FCT)- Abuja, Nigeria (9.1023° N, 7.1952° E). Specifically, the T. catappa fruit harvest process adhered to institutionally prescribed plant material collection guidelines. Taxonomic identification of T. catappa fruit was conducted and a voucher specimen for reference purposes (voucher reference number PCG/UNN/0126) deposited in the herbarium of the Department of Pharmacognosy, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Nigeria. Collected randomly from a batch, the almond fruits were first submitted to sun-drying for 14 days at temperature range of 34–40°C (93–104°F). The mesocarps were cracked open to remove the nuts and subsequently divided into two portions (500 g each) in line with the objective of this study. Specifically, one portion was designated for the raw sample and the other portion for the roasted sample. Following the method described by Tu et al. [9] with slight modifications, the nuts wrapped in aluminum foil were roasted using an electric rotary oven (Gallenkamp, England UK) at 125°C for 15 min based on the nut industry’s best practices. To prevent any quality deterioration, the roasting conditions applied were consistent with the good hygiene/manufacturing practices (GHP/GMP) prescribed by National Agency for Food and Drug Administration and Control (NAFDAC) for the Nigeria’s nut industry. The T. catappa nut samples were finely pulverized using a mechanical grinder (Vikas Ltd, England), and subsequently kept in a glass screw capped container at ambient temperature (25 ± 1°C) until required for further analyses. Fig 2 shows the pictures of almond fruits, almond nuts, and pulverized raw and roasted almond nuts.
Fig 2. Pictorial display of almond fruits, almond nuts, pulverized raw and roasted almond nuts (Terminalia catappa).
Analytical measurements of raw and roasted T. catappa nuts
Determination of nutritional contents. Proximate components
The proximate components (crude protein, crude fats, ash, moisture, crude fiber, and carbohydrate contents) of samples were determined by the Association of Official Analytical Chemists (AOAC) method [18]. Crude protein content was determined using the Kjeldahl method that employed firstly, the determination of total nitrogen, and secondly, the conversion factor of 6.25. Crude fat content was determined using the Soxhlet extraction method by non-polar organic solvent, wherein the residue left after evaporation were calculated. Ash content was determined by direct analysis in a muffle furnace of 600°C to burn off all organic materials, leaving the inorganic materials that did not volatilize at this temperature (as designated ash). Moisture content was determined using the vacuum oven method and calculated as a weight change after oven drying (100°C). The acid and alkaline digestive methods determined fiber content; weight loss on the ignition after digestion of a moisture-free ether extract of the samples was taken as the crude fiber content. Finally, total carbohydrate content was obtained by subtraction the percentages of all the other food components (moisture + ash + fat + fiber + protein contents) from 100. All the proximate components were expressed as percentages (%). In contrast, the energy was calculated using individual caloric factors of carbohydrate (caloric factor = 4), protein (caloric factor = 4), and fats (caloric factor = 9) as shown in Eq (1) below.
| (1) |
Mineral contents
The contents of selenium, calcium, copper, iron, manganese, and zinc in samples was determined according to AOAC [18] method with modifications using the atomic absorption spectrophotometer (AAS) model AA—7000 (Shimadzu, Japan). Pulverized samples (1 g each) were placed in a muffle furnace and heated for 3 h at 500°C. Subsequently, 5 mL of 1 N HNO3 was added to the cooled samples before evaporating them to dryness using a steam bath (Vikas Ltd, England). The dried samples were further heated for 10–15 min at 400°C until a greyish color was obtained. Then 20 mL of 1 N HCl was added to the cooled samples, and the resulting solution was filtrated into a 50 mL round volumetric flask. The obtained filtrate was used to perform analysis, followed concentration extrapolation from the standard curve automatically plotted by the instrument.
Vitamin contents
To determine the vitamin A content, the AOAC [18] method with some modifications was used. A quantity of 1 g of each sample was weighed and added into a 100 mL flask fitted with a reflux condenser. Then, 20 mL of alcoholic sulphuric acid and absolute alcohol (10 mL) were added to the flask and wrapped with aluminum foil. The mixture was refluxed for 45 min and cooled. Following that, 5 mL of water was added into each flask and transferred to a separating funnel. Diethyl ether was used to extract non-saponified materials. The mixed ether extract was then washed to remove the acid and dried over anhydrous sodium sulphate. The extract was evaporated at low temperatures while protected from sunlight, with final traces of solvent removed in a stream of nitrogen and residues dissolved immediately in 10 mL isopropanol. The extinction of freshly prepared extract in isopropanol was measured at 325 nm against a solvent blank (T1) using a UV-spectrophotometer (Jenway 6305, Bibby Scientific Ltd, U.K.). Thereafter, samples were exposed to U.V. radiation until the extinction no longer decreased with time, and absorbance spectrophotometrically measured (T2). In the same way, the corresponding standard vitamin A solution was determined (for solvent blank = ST1; after exposure to radiation until extinction = ST2) using Eq 2.
| (2) |
To determine the vitamin B1 content, the AOAC [18] method with some modifications was used. Accurately, 2 g of each sample was weighed, then 0.5 mL of 4-amino phenol together with 5 mL of NH4OH (0.1 M), thereafter thoroughly mixed, followed by room temperature incubation for 5 min. Next, 10 mL of chloroform was added, which allowed for the observation of two layers. The absorbance of chloroform layer was measured at 430 nm against blank using a UV-spectrophotometer (Jenway 6305, Bibby Scientific Ltd, U.K.). Finally, the Vitamin B1 content was calculated using Eq 3 below.
| (3) |
To establish the vitamin B2 content, the AOAC [18] method was used with some modifications. The samples were weighed (2 g) and added to a calibrated test tube. In each test tube, 2 mL glacial acetic acid, 2 mL hydrochloric acid (1 M), 2 mL hydrogen peroxide, 2 mL potassium permanganate (15% w/v), and 2 mL phosphate buffer (pH 6.8) were added. The resulting mixture was mixed thoroughly before measuring its absorbance at 444 nm against blank using a UV-spectrophotometer (Jenway 6305, Bibby Scientific Ltd, U.K.). The content of vitamin B2 was calculated with the formula in Eq 4.
| (4) |
To determine the niacin content, the AOAC [18] method with some modifications was used. Samples (5 g) were autoclave digested with 1 N sulfuric acid with pH adjusted to 4.5 with 10 N sodium hydroxide, subsequently followed by filtration. Cyanogen bromide was complexed with the supernatants containing extracted niacin to form a purple color. Absorbance of solution was read at 470 nm against blank using a UV-spectrophotometer (Jenway 6305, Bibby Scientific Ltd, U.K.). The concentration of niacin was extrapolated from a standard curve.
To determine the vitamin C content, the AOAC [18] method with some modifications was employed. This involved the titration using diphenol indo 2, 6 –dichlorophenol (DPIP), which required sample (0.2 g) added to 4 mL of buffer solution (containing 1 g/L oxalic acid and 4 g/L sodium acetate anhydrous). The resultant solution was titrated against DPIP (295 mg/L) and sodium bicarbonate (100 mg/L). Vitamin C was calculated from Eq (5) below:
| (5) |
where: M = mass of ascorbic acid titrimetric equivalent to 0.001 M DPIP solution (mg)
100 is the dilution ratio of the sample taken. The second 100 is the scaling factor for conversion per 100 g of raw material, 10 is the titrate volume; V = titrant volume (0.00 1 M DPIP solution) ml; and B = weight of the sample extract used.
To determine the vitamin E content, the method described by Achikanu et al. [19] with some modifications was used. The sample (1 g) was macerated in 20 mL of n-hexane. Thereafter, the mixture was centrifuged for 10 min at 1500 rpm (4000 rpm, Abman, Canada), filtered, and treated with ethanol and alcoholic potassium hydroxide (0.5 N). Subsequently, 2 mL of the filtrate was evaporated to dryness in a boiling water bath (Gallenkamp, England). The residue was mixed with ethanol, ferric chloride (0.2%), and a-a 1-dipyridyl (0.5%). The absorbance of resultant solution was read at 520 nm against the blank using a UV-spectrophotometer (Jenway 6305, Bibby Scientific Ltd, U.K.), after which the concentration of vitamin E was calculated from Eq (6) below:
| (6) |
Determination of anti-nutritional properties
Oxalate content. To determine the oxalate content, the titration method as described by Day and Underwood [20] with some modifications was used. A quantity of 1 g of the sample was added into a 100 mL conical flask. Then, 75 mL of 3 M H2SO4 was added to the flask, and stirring was done for 1 h with a hot plate with a magnetic stirrer, after which it was filtered with Whatman No 1 filter paper (pore size of 11 m). Immediately and still heated, 25 mL of the filtrate was titrated against 0.05 M KMnO4 resulting in a faint pink color chromophore that persists for 1 min. The oxalate content was then calculated by taking 1 mL of 0.05 M KMnO4 as equivalent to 2.2 mg oxalate using Eq 7.
| (7) |
Phytate content. To determine the phytate content, the method of Lucas and Markaka [21] with some modifications was used. To weighed 1 g sample, 100 mL of 2% HCl was added and allowed to stand at room temperature for 3 h before filtering through a double-layer filter paper. Thereafter, 25 mL of the filtrate was added. The solution’s acidity was improved with 53 mL of distilled water. A volume of 10 mL of 0.3% ammonium thiocyanate solution was added to each sample solution as an indicator before titrating with standard iron chloride solution (0.00195 g iron/mL), until endpoint (brownish-yellow coloration) persisted for 5 min. Phytate concentration expressed in mg/100 g was calculated with Eq 8.
| (8) |
Where An = absorbance of the test sample; As = Absorbance of the standard solution; C = concentration of standard solution; W = weight of the sample used; Vf = total volume of the extract; and Va = volume of the extract analyzed.
Hydrogen cyanide content. To determine the hydrogen cyanide content, the AOAC [18] method with some modifications was used. This involved about 20 g of ground sample subject to distillation (2 h) to free all bound hydrocyanic acid, after which the collected distillate in 20 mL 0.01 N AgNO3 was acidified with 1 mL HNO3 (40 min). With 150 mL of distillate secured, filtration was performed with little water, followed by excess AgNO3 titrated with 0.02 N potassium sulfocyanate (KSCN) using a ferric alum indicator. The endpoint was observed (faint reddish color) upon the addition of 0.02 N KSCN. The volume of AgNO3 consumed by the complex was determined by the formula in Eq 9:
| (9) |
where V = volume of titer; 1 mL of AgNO3 = 0.27 mg HCN per 100.
Determination of total aflatoxin content
To determine the total aflatoxin content, the procedure described by Reza et al. [22] with some modifications, which involved enzyme-linked immunosorbent assay (ELISA) analysis and total aflatoxin ELISA kit (ab285282, Abcam, USA) The aflatoxin was extracted from 5 g of the samples using 33% methanol. The extract was filtered through Whatman no 1 filter paper. Then, 50 μL of the filtrate and 50 μL of 33% methanol, transferred to microtubes, were stored at -20°C until the analysis started. About 50 μL of distilled was added to the mixing well. Subsequently, 50 μL of varying standard concentrations namely, 0.1 μg/kg, 0.2 μg/kg, 0.5 μg/kg, and 1.4 μg/kg, were added to samples wells. By pipetting 50 μL of enzyme conjugate and 100 μL antibody (anti-aflatoxin) into all the wells, the reaction was initiated, gently mixed, and incubated at room temperature for 20 min. The content of the antibody–coated well was emptied, and washed intermittently, Afterwards, 200 μL of chromogen solution was added to each well and incubated at room temperature (20 min), then adding 50 μL of stop solution, followed again by gently mixing. The absorbance of aflatoxin in the samples and standard were read with an ELISA microplate reader (State Fax® 2100, Awareness, USA) at 450 nm. Thereafter, the total aflatoxin contents of each sample were calculated from a standard curve.
Characterization of phytochemical composition by GC-FID
The characterization of phytochemical compounds followed the method described by Bezerra and Filho [23] with some modifications. Specifically, the Shimadzu G.C. 2010 gas chromatograph directly connected to a mass spectrometer (GCMS), has been equipped with a flame ionization detector (FID). In addition to the injection mode, the carrier gas used helium. The mass spectra were kept in electron impact mode between 40 and 600 amu using a quadruple analyzer. In the end, the phytochemical contents in raw and roasted samples were reported in μg/mg based on the peak area produced in the chromatogram.
Determination of ADMET properties
Drug-likeness of the identified compounds. The canonical smiles of the identified compounds retrieved from the PubChem database (www.pubchem.com) were used to predict the compound’s absorption, distribution, metabolism, and excretion (ADME) using the SwissADME web server (http://www.swissadme.ch/). The physicochemical properties of molecular weight (M.W.), no of rotatable bond (Nrot), Hydrogen Bond Donor and Acceptor (HBD and H.B.A.), Topological Polar Surface Area (TPSA), n-octanol/water partition coefficient (Log P) were subjected to drug-likeness filters proposed by Egan et al. [24], Veber et al. [25], and Lipinski et al. [26].
In silico prediction of the pharmacokinetics of the identified compounds. Oral bioavailability and brain barrier permeability were predicted with lipophilicity profiles (WLogP) and polarity (TPSA) using the method of Brain or Intestinal Estimation Permeation (BOILED-Egg) from the Swiss ADME platform. At the same time, P-glycoprotein inhibition/substrate, as well as cytochrome P450 inhibition and skin permeation, were also estimated from the SwissADME platform (http://www.swissadme.ch/) [27].
Prediction of toxicity and toxicological effects of the identified compounds. ProTox-II webserver (https://tox-new.charite.de/protox_II/) was employed to predict toxicity and toxicological effects of the identified compounds, which is considered an essential part of the drug design development process. The ProTox-II web server incorporates pharmacophores, molecular similarity, fragment propensities, and machine-learning models to predict oral toxicity, organ toxicity (hepatotoxicity), and toxicity endpoints (carcinogenicity, immunogenicity, mutagenicity, and cytogenecity).
Statistical analysis
All data of triplicate determination were subject to Student T-test. The emergent results were presented as mean±standard deviation (SD). The level of statistical significance was set at p < 0.05 (95% confidence level). Statistical Package for the Social Sciences (SPSS) for Windows version 23 (IBM® SPSS Inc., Chicago, IL- USA.) was used to run the data.
Results and discussion
Changes in nutritional value
Nutritional value of raw and roasted T. catappa nut samples, including proximate composition and energy, vitamins, and minerals were depicted in Tables 1–3. Significant differences (p < 0.05) across proximate components happened comparing raw and roasted nuts, with the exception of crude fat (p > 0.05). Specifically, raw T. catappa nuts’ crude protein (31.24 ± 0.75%), ash (7.25 ± 0.14%), moisture (10.01 ± 0.32%) and crude fiber contents (5.00 ± 0.24%) were significantly higher (p < 0.05) than those of roasted (crude protein content = 27.56 ± 1.96%; ash content = 6.76 ± 0.23%; moisture content = 5.38 ± 0.04%; and crude fiber content = 2.23 ± 0.06%). High ash content depicts the total amount of minerals present [28] in roasted T. catappa nuts, which appeared comparable with those of macadamia nuts [9]. Moreover, the progress of Maillard reaction may relate with decreasing crude protein herein, potentially rendering the amino acid inaccessible for digestion [15]. High protein content of T. catappa nut might help avert the global challenge of protein malnutrition [29]. By facilitating both cholesterol excretion and food digestion, crude fiber enhance the immune system [28]. Favorably, crude protein of raw and roasted T. catappa compared well with other conventional oilseeds like black-eyed beans (27.13%), brown beans (28.00%), cowpea (27.80%), groundnut (25.00%) Bambara nuts (23.41%), and pigeon pea (21.88%) [13]. Roasted T. catappa nuts obtained significantly higher (p < 0.05) carbohydrate (38.68%) and energy (439.2 kcal/100 g) compared to raw (carbohydrate content = 25.10%; energy content = 417.6 kcal/100 g) samples. These (carbohydrate and energy) results suggest thermal processing method of roasting able to modify the biochemical characteristics within the nut matrix [7,8]. Despite the somewhat high energy content of raw and roasted samples [14], the proximate data herein corroborated those found in sesame varieties [30], but contrary to those in sunflower seeds [31].
Table 1. Changes in proximate composition and energy of raw and roasted Terminalia catappa nut samples.
| Proximate composition | Raw nut | Roasted nut | p -values |
|---|---|---|---|
| Protein (%) | 31.24 ± 0.75b | 27.56 ± 1.96a | 0.039 |
| Crude fat (%) | 21.38 ± 0.90a | 19.52 ± 1.60a | 0.155 |
| Ash (%) | 7.25 ± 0.14b | 6.76 ± 0.23a | 0.036 |
| Moisture (%) | 10.01 ± 0.32b | 5.38 ± 0.04a | 0.000 |
| Crude fiber (%) | 5.00 ± 0.24b | 2.23 ± 0.06a | 0.001 |
| Carbohydrate (%) | 25.10 ± 1.10a | 38.68 ± 0.61b | 0.000 |
| Energy (kcal/100 g) | 417.6 ± 2.50a | 439.2 ± 8.10b | 0.012 |
Data represent mean ± standard deviation (SD) of triple measurements. Values with different superscripts in a row are statistically different at p < 0.05.
Table 3. Changes in mineral composition of raw and roasted Terminalia catappa nut samples.
| Minerals (ppm) | *Raw nuts | *Roasted nuts | p -values |
#RNI (Adult, 19+) References Male Female |
|---|---|---|---|---|
| Selenium | 0.45 ± 0.03a | 0.42 ± 0.03a | 0.288 | 34 26 μg/day (RDA, 1989) |
| Calcium | 56.67 ± 1.00a | 77.32 ± 1.80b | 0.000 | 1000 1000 mg/day (RDA, 1989) |
| Copper | 0.03 ± 0.01a | ND | 0.000 | 900 900 μg/day (RDA, 1989) |
| Iron | 1.22 ± 0.04a | 1.22 ± 0.60a | 1.000 | 10 15 mg/day (RDA, 1989) |
| Manganese | 0.03 ± 0.01a | 0.16 ± 0.01b | 0.000 | 5.0 2.0 mg/day (RDA, 1989) |
| Zinc | 0.26 ± 0.03a | 0.32 ± 0.01b | 0.027 | 15 12 mg/day (RDA, 1989) |
Key
*Data of the current study
# Published references; ND = Not detected; RNI = Recommended nutrient intake; Data represent mean ± SD of triple determination; Values with different superscripts in a row are statistically different at p < 0.05.
As shown in Table 2, roasted T. catappa nuts obtained significant increases (p < 0.05) in vitamins A (0.28 ± 0.01 mg/100 g) and E (26.00 ± 1.00 mg/100 g) and at the same time, reductions (p < 0.05) in B1-3 (vitamin B1 = 0.15 ± 0.04 mg/100 g; vitamin B2 = 0.67 ± 0.10 mg/100 g; vitamin B3 = 2.52 ± 0.20 mg/100 g) compared to the corresponding values in raw samples (vitamin A = 0.23 ± 0.20 mg/100 g; vitamin E = 24.77 ± 2.25 mg/100 g; vitamin B1 = 0.32 ± 0.03 mg/100 g; vitamin B2 = 1.14 ± 0.02 mg/100 g; vitamin B3 = 3.52 ± 0.10 mg/100 g). High temperatures emanating from roasting probably decreased the water-soluble vitamin concentrations [15]. Typically, vitamin A would enhance good vision, cell growth, and provide a healthy immune system. Herein, vitamin E of roasted T. catappa nuts compared favorably with previously published data of walnuts (0.70 mg/100 g), cashew (0.90 mg/100 g), pistachio (2.86 mg/100 g), pecans (1.40 mg/100 g), pine nuts (9.33 mg/100 g) and hazelnuts (15.03 mg/100 g) [32]. Moreover, thiamin, riboflavin, and niacin of roasted T. catappa nuts were significantly (p < 0.05) lower than the raw samples, probably owed to their vulnerability to high temperatures. Additionally, to extend the roasting duration should further destabilize the chemical bonds of methylene group [33]. Whilst vitamin B group acts as co-enzymes in various metabolic pathways [1], the vitamin C scavenges reactive species to maintain redox equilibrium [34], and therefore, their presence in T. catappa nuts would help especially in managing metabolic aberrations.
Table 2. Changes in vitamin components of raw and roasted Terminalia catappa nut samples.
| Vitamin contents (mg/100 g) | *Raw nuts | *Roasted nuts | p-values |
#RNI (Adult, 19+ years) References Male Female |
|---|---|---|---|---|
| A (Retinol) | 0.23± 0.20a | 0.28± 0.01b | 0.018 | 0.9 0.7mg/day (Agbai et al., 2021) |
| E (Carotenoid) | 24.77± 2.25a | 26.00± 1.00a | 0.437 | 10 8 mg/day (RDA, 1989) |
| B1 (Thiamin) | 0.32± 0.03b | 0.15± 0.04a | 0.004 | 1.2 1.1 mg/day (FAO/WHO, 2001) |
| B2 (Riboflavin) | 1.14± 0.02b | 0.67± 0.10a | 0.001 | 1.3 1.1 mg/day (FAO/WHO, 2001) |
| B3 (Niacin) | 3.52± 0.10b | 2.52± 0.20a | 0.001 | 16 14 NEs (FAO/WHO, 2001) |
| C (Ascorbic acid) | 0.10± 0.01b | N.R. | 0.000 | 45 45 mg/day (FAO/WHO, 2001) |
Key
*Data of the current study
# Published references; N.R.: No record; RNI: Recommended nutrient intake; NEs: Niacin equivalent; Data represent mean ± standard (SD) of triple measurements. Values with different superscripts in a row are statistically different at p < 0.05.
As shown in Table 3, different minerals were detected, which included selenium (Se), calcium (Ca), copper (Cu), iron (Fe), manganese (Mn), and zinc (Zn). Specifically, only Ca, Mn and Zn composition of raw samples differed noticeably (p < 0.05) from the roasted ones. The obtained minerals compared favorably with the recommended nutrient intake (RNI) values [35]. As co-factor for antioxidant enzymes, Se would protect the human body from oxidative damage [36]. Being somewhat high in the roasted nuts, the Ca alongside lower oxalate, not only confirms an inverse relationship to bring about calcium oxalate, but further enhances the function of membrane permeability, as well as the absorption of Zn and Mg [37]. Vital in the formation of myelin sheaths of nervous system, Cu helps the absorption of Fe into the gastrointestinal tract (GIT) [38]. Herein, the Fe concentration (1.22 ppm) suggests T. catappa nuts with promising anti-anemic potential for red blood cell formation [39], alongside immune function, and cognitive development [40]. Whilst Mn promotes the biosynthesis of proteoglycan in cartilage and useful in pyruvate metabolism and urea formation [38], the Zn aids the biochemical pathways involved in immunity, reproduction, and sexual development [40]. Overall, the above-mentioned minerals help to maintain the biochemical activities, especially those involved in enzyme function as well as tissue homeostasis [36].
Changes in anti-nutritional and total aflatoxin values
As shown in Table 4, raw and roasted T. catappa nut samples contained quantifiable amounts of phytates, oxalate, hydrogen cyanide (HCN), and total aflatoxins, all of which were within the prescribed safety limits [1,41–43]. Specifically, whilst phytates of raw resembled (p > 0.05) those of roasted samples, the latter obtained significantly higher (p < 0.05) oxalate, HCN and total aflatoxins. Above-mentioned post-roasting differences in anti-nutrient (oxalate, HCN and total aflatoxins) might explain their thermolabile nature [15,41], hence, improving both nutrient bioavailability/digestibility and consumer safety of T. catappa nuts. Besides, phytate and oxalate values reported in Passiflora edulis var. flavicarpa (0.47 ± 0.02 mg/100 g) [44] fell below those in T. catappa nuts. Besides, high concentration of Ca2+ facilitating the decreasing phytic acid [15] in roasted T. catappa nuts, the reduced HCN demonstrates the usefulness of high temperatures to progress cellular inactivation of cytochrome oxidase within the mitochondria [44].
Table 4. Changes in anti-nutrient and total aflatoxin contents of raw and roasted Terminalia catappa nut samples.
| Contents | Raw nuts * | Roasted nuts* | p -values | Safety limits# | References of the safety limits # |
|---|---|---|---|---|---|
| Phytate (mg/100 g) | 0.10 ± 0.02a | 0.06 ± 0.02a | 0.070 | < 25 | (Coulibaly et al., 2011) |
| Oxalate (mg/100 g) | 0.08 ± 0.01b | 0.03 ± 0.02a | 0.018 | < 10 | (Agbai et al., 2021) |
| Hydrogen cyanide (mg/100 g) | 0.20 ± 0.50b | 0.10 ± 0.40a | 0.000 | 1 | (FAO/WHO, 2011) |
| Total aflatoxin (μg/kg) | 5.66 ± 0.35b | 2.77 ± 0.26a | 0.000 | < 8 | (EFSA, 2007) |
Key
*Data of the current study
# Published data and references; Data represent mean ± SD of triple determination. Values with different superscripts in a row are statistically different at p < 0.05.
The toxic nature of anti-nutritional factors negate both availability of minerals, and digestibility of proteins [45,46]. As a deprotonated (salt) form of dodecaprotic phytic acid, the phytates capably reduce the absorption/bioavailability of proteins, carbohydrates, lipids, and divalent minerals via electrostatic interactions that involve coordination complexes [45,46]. High temperatures to significantly reduce moisture, and denature the protein contents in roasted T. catappa nut further substantiate the elimination and detoxification of such anti-nutritional contents like HCN [46]. Moreover, the HCN values of T. catappa nut (shown in Table 4) appear comparable with those of raw walnut (0.112 ± 0.10 mg/100 g) [39]. Likewise, the total aflatoxin contents in T. catappa nut (raw = 5.66 ± 0.35 μg/kg; roasted = 2.77 ± 0.26 μg/kg) were below the safety limits (8 μg/kg) established by the European Commission for total aflatoxins in ready-to-eat almonds, hazelnuts, and pistachios [42]. Elsewhere, thermal processing specifically roasting has been shown to reduce the anti-nutritional content to permissible levels [46], which further buttresses the suitability of T. catappa nut for consumption (Table 4). According to Joint FAO/WHO Expert Committee on Food Additives (JECFA), aflatoxins are among well-known mutagenic substances and group 1 carcinogens [22].
Phytochemical characterisation by GC-FID
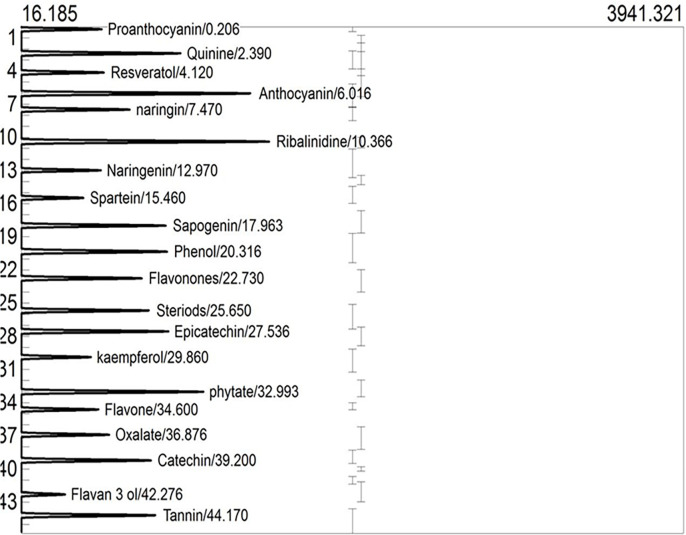
GCMS profiles of raw and roasted nuts are respectively shown in Figs 3 and 4. Phytochemical characteristics of raw and roasted nuts are shown in Table 5. The T. catappa nuts reveal a promising abundance of flavonoids (proanthocyanin, naringin, flavan-3-ol, anthocyanin, naringenin, flavanones, epicatechin, kaempferol, flavone, catechin), phenolics (phenol), stilbenes (resveratrol), polyphenol (tannins), alkaloids (ribalinidine, quinine and sparteine), and saponin (sapogenin) together with phytate, and oxalate. Naringin and its aglycone form naringenin have been explored for their anti-inflammatory, antioxidant, nephroprotective, immunomodulatory, hepatoprotective, antidiabetic, anticancer, and anti-atherosclerotic attributes [47–51]. In addition to being an effective anti-malaria drug, quinine helps in treating diarrhea, whereas ribalinidine, a quinoline alkaloid, possesses radical scavenging capacity [38]. In addition to flavan-3-ol monomers and oligomers with antioxidant potential [52], flavanones possess antioxidant, antihyperlipidemic, and anti-inflammatory effects, whereas flavones have antimicrobial and antifungal properties [38].
Fig 3. GCMS of phytochemicals in raw Terminalia catappa nuts.
Fig 4. GCMS of phytochemicals in roasted Terminalia catappa nuts.
Table 5. Phytochemical characteristics of raw and roasted Terminalia catappa nut samples.
| Peaks | Identified Compounds | Molecular Formulae | Molecular Weights (g/mol) |
Conc. (μg/g) | p-values | Class of compounds | |
|---|---|---|---|---|---|---|---|
| Raw | Roasted | ||||||
| 1 | Proanthocyanin | C31H28O12 | 594.50 | 10.46a | 8.13a | 0.147 | Flavonoid |
| 2 | Naringin | C27H32O14 | 580.54 | 12.71a | 12.57a | 0.919 | Flavonoid |
| 3 | Quinine | C20H24N2O2 | 324.42 | 7.18 b | 0.46a | 0.000 | Alkaloid |
| 4 | Flavan-3-ol | C15H14O2 | 456.39 | 32.67 b | 24.41a | 0.007 | Flavonoid |
| 5 | Anthocyanin | C15H11O | 207.30 | 7.14a | 7.11a | 0.972 | Flavonoid |
| 6 | Ribalinidine | C15H17NO4 | 275.30 | 45.57 b | 17.07a | 0.000 | Alkaloid |
| 7 | Naringenin | C15H12O5 | 272.26 | 8.04a | 8.02a | 0.988 | Flavonoid |
| 8 | Spartein | C15H26N2 | 234.38 | 7.33a | 7.31a | 0.988 | Alkaloid |
| 9 | Sapogenin | C58H94O27 | 490.70 | 22.76 b | 13.45a | 0.007 | Saponin |
| 10 | Phenol | C6H6O | 94.11 | 15.66a | 15.65a | 0.846 | Phenolics |
| 11 | Flavonones | C29O11H27 | 594.50 | 9.97a | 9.96a | 0.991 | Flavonoid |
| 12 | Steroid | C19H28O2 | 288.40 | 17.35a | 17.24a | 0.899 | Steroids |
| 13 | Epicatechin | C15H14O6 | 290.26 | 18.08a | 17.99a | 0.959 | Flavonoid |
| 14 | Kaempferol | C15H10O6 | 286.23 | 7.08a | 7.07 a | 0.994 | Flavonoid |
| 15 | Phytate | C6H18O24P6 | 660.04 | 9.68a | 9.50 a | 0.918 | Anti-nutrient |
| 16 | Flavone | C15H10O2 | 222.24 | 5.44 a | 3.48 a | 0.074 | Flavonoid |
| 17 | Oxalate | C2O4(2-) | 128.09 | 13.67a | 13.65a | 0.988 | Anti-nutrient |
| 18 | Catechin | C15H14O6 | 290.26 | 6.02a | 14.44b | 0.000 | Flavonoid |
| 19 | Resveratrol | C14H12O3 | 228.25 | 6.02a | 5.67a | 0.954 | Stilbenes |
| 20 | Tannin | C76H52O46 | 1701.19 | 27.13 b | 22.54a | 0.024 | Polyphenol |
Values with different superscripts in a row are statistically different at p < 0.05.
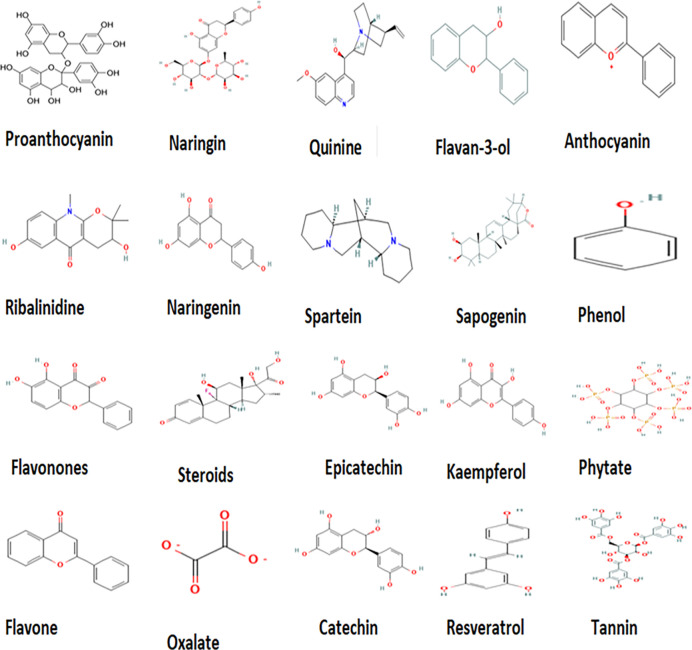
The 2D structures of identified compounds from T. catappa nuts as retrieved from the PubChem database is shown in Fig 5. The presence of hydroxyl, carbonyl, phenyl, amino, and carboxylic acids functional groups might be strengthening the therapeutic efficacy of these phytocompounds, which by acting as reducing agents, would stabilize the redox equilibrium—a precursor for averting the onset of metabolic diseases [28]. Epicatechins possess antimicrobial, antioxidant, cardioprotective, antidiabetic, and anticancer properties [53]. Whilst in vivo platelet anti-aggregation and improved insulin sensitivity have been associated with epicatechin-rich green tea [54], catechins demonstrate anti-obesity, anticancer, hepatoprotective, antidiabetic, antioxidant, and neuroprotective properties [55]. Whereas anthocyanin is an effective agent against the onset of cardiovascular diseases [56], the kaempferol is an aglycone flavonoid with anticancer, antioxidant, anti-inflammatory, and antidiabetic promise [34]. Resveratrol—a stilbene polyphenol, would not only prevent cerebrovascular/cardiovascular diseases, but also resist oxidation, and lower inflammation, obesity, diabetes, and fibrosis [57,58]. The bioactive phytochemicals present in almond nuts, as above-mentioned, should enhance human health after consumption at safety limits. From Table 5 and upon roasting, the concentration of quinine (0.46 μg/g), ribalinidine (17.07 μg/g), sapogenin (13.45 μg/g), flavan-3-ol (24.41 μg/g) and tannin (0.46 μg/g) significantly reduced, whereas catechin (14.44 μg/g) significantly increased. Whilst both quinine and ribalinidine are considered as alkaloids, both sapogenin and flavan-3-ol are deemed respectively as saponins and flavonoids, able to confer a strong bitter taste despite their health-promoting benefits [59,60]. When tannins appear in large quantities, a high astringency/unpleasant flavor would emerge compared to lower amounts easily tolerated [61]. The fact that roasting process of this current study was able to alter the phytoconstituents could depict biochemical change within the food matrix that enhance both flavor and palatability of nut for consumers.
Fig 5. 2D structures of identified compounds from Terminalia catappa as retrieved from PubChem database.
Changes in ADMET properties
ADMET prediction represents a fast yet low-cost approach to evaluate the potential of easily absorbable drug, which should be well-distributed to its target action site, favorably metabolized, and easily eliminated from the body without toxic side effects [62,63]. The predicted drug-likeness of the compounds identified from T. catappa nuts is shown in Table 6. Interestingly but except proanthocyanin, naringin, phytate and tannin, most others appear as available promising oral drugs that do not violate Lipinski, Veber, and Egan drug-likeness parameters having favorable bioavailability score of 0.55. Low polarity of these compounds (TPSA ≤ 140°A) equally portrays a higher interaction with therapeutic targets involved in several diseases [34]. Bioavailability of drug candidates should better predict new drug discovery via the Lipinski “rule of five” (MW ≤ 500; Log P ≤ 5; HBA ≤ 10; HBD ≤ 5) [26], in addition to Nrot ≤ 10 and TPSA ≤ 140°A° as depicted by the rules of Egan et al. [24] as well as Veber et al. [25]. It appears pretty clear that these (above-mentioned) compounds appear not violating these cut-off values, hence, are suggested as excellent oral drug candidates [34]. High rate of clinical failures for orally available drugs are associated with poor availability/efficacy when administered to humans [64].
Table 6. Predicted drug-likeness of the compounds identified from raw and roasted Terminalia catappa nuts.
| Compounds | MW (g/mol) |
Log P | #HBA | # HBD | Nrot | TPSA | BAS | # Violations | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Lipinski | Veber |
Egan |
||||||||
| Proanthocyanin | 593 | 2.29 | 12 | 9 | 4 | 209.8 | 0.17 | 3 | 1 | 1 |
| Naringin | 581 | 2.38 | 14 | 8 | 6 | 225 | 0.17 | 3 | 1 | 1 |
| Quinine | 324 | 3.36 | 4 | 1 | 4 | 45.59 | 0.55 | 0 | 0 | 0 |
| Flavan-3-ol | 226 | 2.52 | 2 | 1 | 1 | 29.46 | 0.55 | 0 | 0 | 0 |
| Anthocyanin | 207 | -0.76 | 1 | 0 | 1 | 13.14 | 0.55 | 0 | 0 | 0 |
| Ribalinidine | 275 | 2.06 | 4 | 2 | 0 | 71.7 | 0.55 | 0 | 0 | 0 |
| Naringenin | 272 | 1.75 | 5 | 3 | 1 | 86.99 | 0.55 | 0 | 0 | 0 |
| Spartein | 234. | 3.12 | 2 | 0 | 0 | 6.48 | 0.55 | 0 | 0 | 0 |
| Sapogenin | 491 | 4.07 | 5 | 4 | 2 | 90.15 | 0.55 | 0 | 0 | 0 |
| Phenol | 94 | 1.24 | 1 | 1 | 0 | 20.23 | 0.55 | 0 | 0 | 0 |
| Flavonones | 224 | 1.27 | 2 | 0 | 1 | 26.3 | 0.55 | 0 | 0 | 0 |
| Steroid | 392 | 2.26 | 6 | 3 | 2 | 94.8 | 0.55 | 0 | 0 | 0 |
| Epicatechin | 290 | 1.47 | 6 | 5 | 1 | 110.4 | 0.55 | 0 | 0 | 0 |
| Kaempferol | 286 | 1.7 | 6 | 4 | 1 | 111.1 | 0.55 | 0 | 0 | 0 |
| Phytate | 660 | -4.78 | 24 | 12 | 12 | 459 | 0.10 | 3 | 2 | 1 |
| Flavone | 222 | 2.55 | 2 | 0 | 1 | 30.2 | 0.55 | 0 | 0 | 0 |
| Oxalate | 88 | -0.4 | 4 | 0 | 1 | 80.3 | 0.55 | 0 | 0 | 0 |
| Catechin | 290 | 1.47 | 6 | 5 | 1 | 110.4 | 0.55 | 0 | 0 | 0 |
| Resveratrol | 228 | 1.71 | 3 | 3 | 2 | 60.69 | 0.55 | 0 | 0 | 0 |
| Tannin | 729 | 1.89 | 21 | 14 | 12 | 371 | 0.17 | 3 | 2 | 1 |
Key: MW = molecular weight (g/mol); Log P = octanol-water partition coefficient; HBA = hydrogen bond acceptor; HBD = hydrogen bond donor; TPSA = topological polar surface area; Nro = # rotatable hydrogen bond; BAS = bioavailability score.
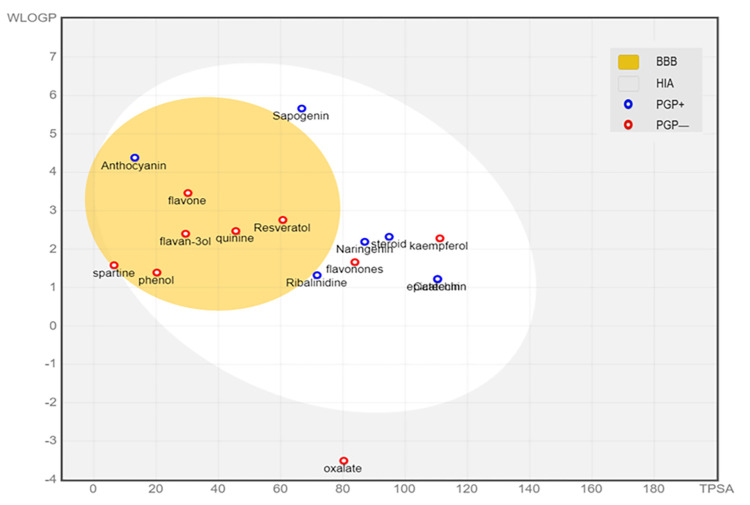
As shown in Fig 6, the BOILED egg model helped to predict the GI absorption and brain permeability of the compounds identified from T. catappa nuts. Most compounds were found to exhibit high GI absorption, with the exception of proanthocyanin, naringin, sparteine, phytate, oxalate and tannin. The blood-brain barrier (BBB) that acts as "physical" and "biochemical" (e.g., p-glycoproteins) barriers would shield the brain from the influx of xenobiotics. Bypassing the barriers would be crucial for drugs that target the neurological system [64]. Also shown in Fig 6, the predicted BBB permeation from WLogP and TPSA revealed anthocyanin, flavone, flavan-3-ol, quinine, resveratrol, phenol, flavonones and sparteine as brain permeants, which further suggested some promise as pharmaceutical agents especially useful in tackling neurological diseases. To ascertain if the potential drug would be orally absorbed, and to either cause minimal/no side effects, or emerge target-tissue(s) specific, should be among the fundamental pharmacokinetics properties [62]. Moreover, two central pharmacokinetics behavior considered as crucial, which are estimated at various stages of the drug discovery process, would include gastrointestinal absorption (GIA) and brain access [34]. Thus, such compounds with a high GIA upon oral administration should be absorbed with ease particularly within the intestinal tract. Rather than the oral administration, the drugs with a low GIA would be best administered through alternative routes.
Fig 6. BOILED Egg model to predict GI absorption and brain permeability of the compounds identified from Terminalia catappa nuts.
Key: BBB; Blood-brain barrier (compounds that can penetrate BBB found inside yellow part of the egg), HIA; Human intestinal absorption (compounds that are absorbed by the human intestine in the white part of the egg), PGP+, P-glycoprotein substrate (blue circle on top of PGP+ substrates) and PGP-; P-glycoprotein inhibitor (red circle on top of PGP- inhibitors).
The pharmacokinetics prediction output of compounds identified from T. catappa nuts is shown in Table 7. Naringin, sparteine, sapogenin, steroid, epicatechin, phytate, oxalate, catechin, and tannin appear unable to inhibit any of the cytochrome P450 (CP450) isoforms, whereas the others were able to inhibit at least 2 of the isoforms. Skin permeation (Log kp) value is key when evaluating drugs that might require transdermal administration, and compounds with Log kp > - 2.5 associated with low skin permeability [65]. Such identified compounds, as found herein, would be suggesting “skin permeants”, given the Log kp values < -2.5, which proffers CYP450 to possess metabolizing enzymes that are involved in drug biotransformation within the hepatocytes. By enhancing the drug-drug interactions, therefore, the inhibition of CYP450 isoforms should bring about unwarranted adverse effects and toxicity, which appear largely linked to either (drug) accumulation or lower clearance [66]. Whilst acute toxicity (LD50) and its classification remain vital in the discovery of doses, there would be harmful effects that emanate from potential drug candidates with lower LD50 (classes 1–3 with higher lethal effects) [67]. In this current work also, the predicted toxicity of compounds was identified from T. catappa nuts, as shown in Table 8. Most compounds that found LD50 > 2000 mg/kg should be considered as non-toxic and relatively harmless (classes 5 and 6). As the lowest predicted LD50 value suggests body weight of 150 mg/kg, a compound like ribalinidine should serve as a p-glycoprotein substrate. Moreover, the efflux protein should prevent the cellular compounds from accumulating and thereby attaining toxic levels [62]. Nonetheless, no identified compound at this current study would predict a hepatotoxic effect, which suggested T. catappa nut likely not able to harm the liver. If the opposite were to be the situation, that is, the ability to deemed to harm the liver, such would be among the major reason(s) for drug rejection, and subsequent withdrawal from the market [68]. Besides, the prediction of carcinogenicity, immunogenicity, mutagenicity, and cytotoxicity helps to ascertain the undesirable effects of a drug compound on the deoxyribonucleic acid (DNA), cells, and immune system [68]. Further, these (above-mentioned) identified compounds found in the T. catappa nuts should not be seen as either completely carcinogenic, immunogenic, mutagenic, or cytotoxic. Both slight optimization and correct dosing should help bypass the toxicity profiles of compounds with low LD50. More so, the synergistic activity of these phyto-constituents might mask the predicted toxic effects of the few identified compounds.
Table 7. Pharmacokinetics prediction output of compounds identified from raw and roasted Terminalia catappa nuts.
| Compounds | P-gp substrate |
Inhibitors of | Log kp | ||||
|---|---|---|---|---|---|---|---|
| CYP1A2 | CYP2C19 | CYP2C9 | CYP2D6 | CYP3A4 | |||
| Proanthocyanin | No | No | No | No | No | Yes | -8.0 |
| Naringin | Yes | No | No | No | No | No | 10.2 |
| Quinine | No | No | No | No | Yes | No | -6.2 |
| Flavan-3-ol | No | No | No | No | Yes | No | -5.7 |
| Anthocyanin | Yes | Yes | No | No | Yes | No | -5.1 |
| Ribalinidine | Yes | Yes | No | No | No | No | -6.9 |
| Naringenin | Yes | Yes | No | No | No | Yes | -6.2 |
| Spartein | No | No | No | No | No | No | -5.9 |
| Sapogenin | Yes | No | No | No | No | No | -6.6 |
| Phenol | No | Yes | No | No | No | No | -5.8 |
| Flavonones | No | Yes | No | No | No | No | -5.4 |
| Steroid | Yes | No | No | No | No | No | -7.3 |
| Epicatechin | Yes | No | No | No | No | No | -7.8 |
| Kaempferol | No | Yes | No | No | Yes | Yes | -6.7 |
| Phytate | Yes | No | No | No | No | No | -17.6 |
| Flavone | No | Yes | Yes | No | No | No | -5.1 |
| Oxalate | No | No | No | No | No | No | -7.0 |
| Catechin | Yes | No | No | No | No | No | -7.8 |
| Resveratrol | No | Yes | No | Yes | No | Yes | -5.5 |
| Tannin | Yes | No | No | No | No | No | -11.7 |
Key: Pgp = P-glycoprotein; CYP450 = Cytochrome P450; Log kp = skin permeation.
Table 8. Toxicity prediction of compounds identified from raw and roasted Terminalia catappa nuts.
| Compounds | LD50 (mg/kg) |
Toxicity class |
Hepato- toxicity |
Carcino- genicity |
Immuno- genicity |
Muta- genicity |
Cyto- toxicity |
|---|---|---|---|---|---|---|---|
| Proanthocyanin | 2500 | 5 | - | - | + | - | - |
| Naringin | 2300 | 5 | - | - | + | - | - |
| Quinine | 263 | 3 | - | - | + | - | - |
| Flavan-3-ol | 2500 | 5 | - | - | - | - | - |
| Anthocyanin | 2500 | 5 | - | + | - | + | - |
| Ribalinidine | 150 | 3 | - | - | + | + | - |
| Naringenin | 2000 | 4 | - | - | - | - | + |
| Spartein | 220 | 3 | - | - | + | - | - |
| Sapogenin | 2500 | 5 | - | + | + | - | - |
| Phenol | 270 | 3 | - | - | - | - | - |
| Flavonones | 2000 | 4 | - | + | - | - | + |
| Steroid | 3000 | 5 | - | - | + | - | - |
| Epicatechin | 10000 | 6 | - | - | - | - | - |
| Kaempferol | 3919 | 5 | - | - | - | - | - |
| Phytate | 1500 | 4 | - | - | - | - | - |
| Flavone | 2500 | 5 | - | + | - | - | - |
| Oxalate | 660 | 4 | - | - | - | - | - |
| Catechin | 10000 | 6 | - | - | - | - | - |
| Resveratrol | 1560 | 4 | - | - | - | - | - |
| Tannin | 2260 | 5 | - | - | - | - | - |
Key: LD50 = lethal median dose; (+) = Active; (—) = inactive.
Conclusions
The changes in nutritional, health benefits, and pharmaceutical potential of raw and roasted T. catappa nuts from Nigeria were investigated. Findings from this current study revealed that T. catappa contains promising bio-nutrients essential for maintaining good health, which places it as a nutritional supplement with pharmaceutical potential able to ameliorate nutrient-related diseases. Not only to influence the fat-soluble vitamin, mineral content and flavor, the roasting processing method would reduce the anti-nutrients that aid absorption and palatability of the nuts. Furthermore, the findings from this current study provided evidence regards the predicted drug-likeness, pharmacokinetic properties, and good safety profile of T. catappa nuts. Nonetheless, the harvest of red almond fruits was of a geographical area as well as from a particular season could be considered a limitation at this current study. Future studies should, therefore, involve T. catappa nuts varieties harvested from different geographical locations in Nigeria, which when submitted to deeper analytically evaluation would provide additional information about the individual phytochemical components supported by in vivo pharmaceutical studies using animal models.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors I.F.C., E.C.O., F.N.N., and E.O.A. funded this study from their personal salary earned as faculty members of the University of Nigeria Nsukka -Nigeria. Covenant University Centre for Research, Innovation, and Discovery (CUCRID) is duly acknowledged for the APC and had no role in study design, data collection/analysis, the decision to publish, or the preparation of the manuscript.
References
- 1.Agbai CM, Olawuni IA, Ofoedu CE, Ibeabuchi CJ, Okpala COR, Shorstkii I, et al. Changes in anti-nutrient, phytochemical, and micronutrient contents of different processed rubber (Hevea brasiliensis) seed meals. PeerJ. 2021;9: e11327. doi: 10.7717/peerj.11327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apeh V, Agu C, Ogugua V, Uzoegwu P, Anaduaka E, Rex T, et al. Effect of Cooking on Proximate, Phytochemical Constituents and Hematological Parameters of Tetracarpidium conophorum in Male Albino Rats. European J Med Plants. 2014;4: 1388–1399. [Google Scholar]
- 3.Barral-martinez M, Fraga-corral M, Garcia-perez P, Simal-gandara J, Prieto MA. Almond By-Products: Valorization for Sustainability and Competitiveness of the Industry. Foods. 2021;10: 1793. doi: 10.3390/foods10081793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang SK, Alasalvar C, Bolling BW, Shahidi F. Nuts and their co-products: The impact of processing (roasting) on phenolics, bioavailability, and health benefits–A comprehensive review. J Funct Foods. 2016;26: 88–122. [Google Scholar]
- 5.Ros E, Singh A, O’Keefe JH. Nuts: Natural pleiotropic nutraceuticals. Nutrients. 2021;13(9):3269. doi: 10.3390/nu13093269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rocha DM, Caldas AP, e Silva AC, Bressan J, Hermsdorff HH. Nut-enriched energy restricted diet has potential to decrease hunger in women at cardiometabolic risk: a randomized controlled trial (Brazilian Nuts Study). Nutr Res. 2023;109:35–46. doi: 10.1016/j.nutres.2022.11.003 [DOI] [PubMed] [Google Scholar]
- 7.Cuadrado C, Sanchiz Á, Linacero R. Nut Allergenicity: Effect of Food Processing. Allergies. 2021;1(3):150–62. [Google Scholar]
- 8.Masthoff LJ, Hoff R, Verhoeckx KC, van Os‐Medendorp H, Michelsen‐Huisman A, Baumert JL, et al. A systematic review of the effect of thermal processing on the allergenicity of tree nuts. Allergy. 2013;68: 983–993. doi: 10.1111/all.12185 [DOI] [PubMed] [Google Scholar]
- 9.Tu X, Ya BW, Xu XS, Lv X, Wei F, Hong L Du. A comprehensive study of raw and roasted macadamia nuts: Lipid profile, physicochemical, nutritional, and sensory properties. Food Sci Nutr. 2021;9: 1688–1697. doi: 10.1002/fsn3.2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanahuja AB, Perez SEM, Teruel NG, Garcia AV, Moya MSP. Variability of Chemical Profile in Almonds (Prunus dulcis) of Different Cultivars and Origins. Foods. 2021;10: 153. doi: 10.3390/foods10010153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lasekan O, Abbas K. Analysis of volatile flavour compounds and acrylamide in roasted Malaysian tropical almond (Terminalia catappa) nuts using supercritical fluid extraction. Food Chem Toxicol. 2010;48: 2212–2216. doi: 10.1016/j.fct.2010.05.050 [DOI] [PubMed] [Google Scholar]
- 12.Iheagwam FN, Okeke CO, De Campos OC, Adegboye BE, Ogunlana OO, Chinedu SN. Toxicopathological, proinflammatory and stress response evaluation of Terminalia catappa extract in male Wistar rats. Toxicol Rep. 2021;8:1769–76. doi: 10.1016/j.toxrep.2021.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salawu AR, Onyegbula AF, Lawal IO, Akande SA, Oladipo AK. Comparative study of the Nutritional, Phytochemical and Mineral Compositions of the nuts of Tropical Almond (Terminalia catappa) and Sweet Almond (Prunus amygdalus). Ruhuna J Sci. 2018;9: 70. [Google Scholar]
- 14.Jonathan AA. Effects of roasting on the nutritional and anti-nutritional composition of raw Terminalia catappa L (Tropical almond) Kernels. Malaya J Biosci. 2015;2: 119–131. [Google Scholar]
- 15.Folasade MM, Subomi OS. Effects of processing treatments on nutritional quality of raw almond (Terminalia catappa Linn.) kernels. Pelagia Res Libr Adv Appl Sci Res. 2016;7: 1–7. Available: www.pelagiaresearchlibrary.com. [Google Scholar]
- 16.Akpakpan AE, Akpabio UD. Evaluation of proximate composition, mineral element and anti-nutrient in almond (Terminalia catappa) seeds. Research. J Appl Sci. 2012;7: 489–493. [Google Scholar]
- 17.Apeh VO, Njoku OU, Nwodo FOC, Chukwuma IF, Emmanuel AA. In silico drug-like properties prediction and in vivo antifungal potentials of Citrullus lanatus seed oil against Candida albicans. Arab J Chem. 2022;15: 103578. [Google Scholar]
- 18.AOAC. Official Methods of Analysis, Association of Official Analytical Chemists. 19TH ed. Washington D.C; 2012.
- 19.Achikanu CE, Ude CM, Ugwuokolie OC. Determination of the vitamin and mineral composition of common leafy vegetables in south eastern Nigeria. Int J Curr Microbiol App Sci. 2016;2: 347–353. [Google Scholar]
- 20.Day R., Underwood AL. Quantification of oxalate, Laboratory manual information center for Agriculture research in the dry Areas, Aleppo Syria; 1986, pg 12. [Google Scholar]
- 21.Lucas GM, Markaka P. Phytic acid and other phosphorus compound of bean (Phaseolus vugaris). J Agric Educ Chem. 1975;23: 13–15. [Google Scholar]
- 22.Reza SSM, Masoud A, Ali T, Faranak G, Mahboob N. Determination of aflatoxins in nuts of Tabriz confectionaries by ELISA and HPLC methods. Adv Pharm Bull. 2012;2: 123–126. doi: 10.5681/apb.2012.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bezerra KS, Filho NRA. Characterization and Quantification by Gas Chromatography of Free Steroids in Unsaponifiable Matter of Vegetable Oils. J Braz Chem Soc,. 2014;25: 238–245. [Google Scholar]
- 24.Egan WJ, Merz KM, Baldwin JJ. Prediction of drug absorption using multivariate statistics. J Med Chem. 2000;43: 3867–3877. doi: 10.1021/jm000292e [DOI] [PubMed] [Google Scholar]
- 25.Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem. 2002;45: 2615–2623. doi: 10.1021/jm020017n [DOI] [PubMed] [Google Scholar]
- 26.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced Drug Delivery Reviews. 2012, 64, 4–17. [DOI] [PubMed] [Google Scholar]
- 27.Iheagwam FN, Odiba JK, Iheagwam OT, Ogunlana OO, Chinedu SN. Type 2 diabetes mellitus mediation by the disruptive activity of environmental toxicants on sex hormone receptors: In silico evaluation. Toxics. 2021;9(10):255. doi: 10.3390/toxics9100255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chukwuma IF, Nkwocha CC, Ezeanyika LUS, Ogugua VN. Phytochemical investigation and in vitro antioxidant potency of root bark of Brenania brieyi fractions. Trop J Nat Prod Res. 2020;4: 970–975. [Google Scholar]
- 29.Aguchem RN, Okagu IU, Okagu OD, Ndefo JC, Udenigwe CC. A Review on the Techno-Functional, Biological, and Health-Promoting Properties of Hempseed-Derived Proteins and Peptides. J Food Biochem. 2022;46: e14127. doi: 10.1111/jfbc.14127 [DOI] [PubMed] [Google Scholar]
- 30.Tenyang N, Ponka R, Tiencheu B, Djikeng FT, Azmeera T, Karuna MS, et al. Effects of boiling and roasting on proximate composition, lipid oxidation, fatty acid profile and mineral content of two sesame varieties commercialized and consumed in Far-North Region of Cameroon. Food Chem. 2017;221:1308–16. doi: 10.1016/j.foodchem.2016.11.025 [DOI] [PubMed] [Google Scholar]
- 31.Tenyang N, Ponka R, Tiencheu B, Tonfack Djikeng F, Womeni HM. Effect of boiling and oven roasting on some physicochemical properties of sunflower seeds produced in Far North, Cameroon. Food Science & Nutrition. 2022;10(2):402–11. doi: 10.1002/fsn3.2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumawat KL, Raja WH, Chand L, Rai KM. Nutritional value and health benefits of nuts. Indian Farmer. 2017;4: 627–637. [Google Scholar]
- 33.Kim DS., Kim H.S., Hong S.J. et al. Comparison of the retention rates of thiamin, riboflavin, and niacin between normal and high-oleic peanuts after roasting. Applied Biological Chemistry 2018; 61: 449–458. [Google Scholar]
- 34.Chukwuma IF, Nworah FN, Apeh VO, Omeje KO, Nweze EJ, Asogwa CD, et al. Phytochemical Characterization, Functional Nutrition, and Anti-Diabetic Potentials of Leptadenia hastata (pers) Decne Leaves: In Silico and In Vitro Studies. Bioinform Biol Insights. 2022;16: 1–17. doi: 10.1177/11779322221115436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.RDA. Recommended Dietary Allowances. 10th Editi. Washington, D.C.: National Academies Press; 1989. [PubMed] [Google Scholar]
- 36.Dubey P, Thakur V, Chattopadhyay M. Role of minerals and trace elements in diabetes and insulin resistance. Nutrients. 2020. 12(6), 1864. doi: 10.3390/nu12061864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ofoedu CE, Osuji CM, Omeire GC, Ojukwu M, Okpala COR, Korzeniowska M. Functional properties of syrup from malted and unmalted rice of different varieties: A comparative study. J Food Sci. 2020;85: 3081–3093. doi: 10.1111/1750-3841.15446 [DOI] [PubMed] [Google Scholar]
- 38.Duru CE. Mineral and phytochemical evaluation of Zea mays husk. Sci African. 2020;7: e00224. [Google Scholar]
- 39.Agwupuye AP, Akwagiobe J. Proximate Composition, Mineral Content, Phytochemical Screening and Anti-Nutritional Constituents of Walnut (Tetracarpidium conophorum OR Plukenetia conophora) Seeds. Int J Sci Res. 2019;9: 969–976. [Google Scholar]
- 40.Suleiman M.S., Olajide JE, Omale JA, Abbah OC, Ocholi DE. Proximate composition, mineral and some vitamin contents of tigernut (Cyperus esculentus). Clinical-Investigation. 2018;8: 161–165. [Google Scholar]
- 41.Samtiya M, Aluko RE, Dhewa T. Plant food anti-nutritional factors and their reduction strategies: an overview. Food Production, Processing and Nutrition. 2020;2:1–4. [Google Scholar]
- 42.EFSA. Opinion of the Scientific Panel on Contaminants in the Food chain on a request from the commission related to the potential increase of consumer health risk by a possible increase of the existing maximum levels for aflatoxins in almonds hazelnuts and. EFSA J. 2007;446: 1–127. [Google Scholar]
- 43.FAO/WHO. Cyanogenic glycosides: evaluations of joint FAO/WHO expert committee on food additives (JECFA)—food and nutrition division. Rome: FAO,; 2011. [Google Scholar]
- 44.Rsu W. Comparative Chemical Analysis, Phytochemical Screening and Antimicrobial Activities of the Rinds, Seeds and Juice of (Passiflora edulis var. flavicarpa) Passion Fruit. J Nat Sci Res. 2016;6: 138–143. [Google Scholar]
- 45.Ukwo P. S, Ntukidem, Victorand Udoh IE. Effect of Roasting On Proximate Composition and Anti- Nutritional Content of Skinned and Unskinned Roasted Groundnut (Arachis hypogeae) Varieties in Nigeria. IOSR J Environ Sci Toxicol Food Technol 2019;13: 59–64. [Google Scholar]
- 46.Ndidi U.S., Ndidi C.U., Olagunju A., Muhammad A., Billy F.G., Okpe O. Proximate, antinutrients and mineral composition of raw and processed (Boiled and Roasted) Sphenostylis stenocarpa seeds from Southern Kaduna, Northwest Nigeria. ISRN Nutrition, 2014; Article ID 280837, pp. 1–9. doi: 10.1155/2014/280837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alam MA, Subhan N, Rahman MM, Uddin SJ, Reza HM, Sarker SD. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv Nutr. 2014;5: 404–417. doi: 10.3945/an.113.005603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhia M, Motallebi M, Abadi B, Zarepour A, Pereira-silva M. Naringenin Nano-Delivery Systems and Their Therapeutic Applications. Pharmaceutics. 2021;13: 291. doi: 10.3390/pharmaceutics13020291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hartogh D. D, Tsiani E. A ntidiabetic Properties of Naringenin: A Citrus Fruit Polyphenol. Biomolecules. 2019;9: 99–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Imran M, Salehi B, Sharifi-Rad J, Gondal TA, Saeed F, Imran A, et al. Kaempferol: A key emphasis to its anticancer potential. Molecules. 2019, 24(12), 2277. doi: 10.3390/molecules24122277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rotimi SO, Adelani IB, Bankole GE, Rotimi OA. Naringin enhances reverse cholesterol transport in high fat/low streptozocin induced diabetic rats. Biomed Pharmacother. 2018;101:430–7. doi: 10.1016/j.biopha.2018.02.116 [DOI] [PubMed] [Google Scholar]
- 52.Meng XH, Liu C, Fan R, Zhu LF, Yang SX, Zhu HT, et al. Antioxidative Flavan-3-ol Dimers from the Leaves of Camellia fangchengensis. J Agric Food Chem. 2018;66: 247–254. [DOI] [PubMed] [Google Scholar]
- 53.Prakash M, Basavaraj B V., Chidambara Murthy KN. Biological functions of epicatechin: Plant cell to human cell health. J Funct Foods. 2019;52: 14–24. [Google Scholar]
- 54.Ugoeze CK, Oluigbo EK, Chinko CBB. Phytomedicinal and Nutraceutical Benefits of the GC-FID Quantified Phytocomponents of the Aqueous Extract of Azadirachta indica leaves. J Pharm Pharmacol Res. 2020;04. [Google Scholar]
- 55.Isemura M. Catechin in human health and disease. Molecules. 2019;24: 528–532. doi: 10.3390/molecules24030528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ranjha MMAN, Shafique B, Wang L, Irfan S, Safdar MN, Murtaza MA, et al. A comprehensive review on phytochemistry, bioactivity and medicinal value of bioactive compounds of pomegranate (Punica granatum). Advances in Traditional Medicine. Springer; Singapore; 2021. [Google Scholar]
- 57.Salehi B, Mishra AP, Nigam M, Sener B, Kilic M. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines. 2018;6: 91–110. doi: 10.3390/biomedicines6030091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu D, Tang Z, Li B, Yu J, Li W, Liu Z, et al. Resveratrol against cardiac fibrosis: Research progress in experimental animal models. Molecules. 2021, 26(22), 6860. doi: 10.3390/molecules26226860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Badyal S, Singh H, Yadav AK, Sharma S, Bhushan I. Plant secondary metabolites and their uses. Plant Archives. 2020;20(2):3336–40. [Google Scholar]
- 60.Aduama-Larbi MS, Amoako-Attah I, Kumi WO, Lowor ST. Post-harvest quality assessment of freshly harvested and processed kola nuts [Cola nitida (Vent.) Schott & Endl.] from selected growing regions in Ghana. Cogent Food & Agriculture. 2022, 8(1):2054565. [Google Scholar]
- 61.Pereira JA, Berenguer CV, Andrade CF, Câmara JS. Unveiling the Bioactive Potential of Fresh Fruit and Vegetable Waste in Human Health from a Consumer Perspective. Applied Sciences. 2022,12(5):2747. [Google Scholar]
- 62.Johnson TO, Adegboyega AE, Ojo OA, Yusuf AJ, Iwaloye O, Ugwah-Oguejiofor C.J. et al. A Computational Approach to Elucidate the Interactions of Chemicals From Artemisia annua Targeted Toward SARS-CoV-2 Main Protease Inhibition for COVID-19 Treatment. Front Med. 2022;9: 907583. doi: 10.3389/fmed.2022.907583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iheagwam FN, Rotimi SO. Computer-aided analysis of multiple SARS-CoV-2 therapeutic targets: Identification of potent molecules from African medicinal plants. Scientifica. 2020; Article ID 1878410. doi: 10.1155/2020/1878410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iwaloye O, Elekofehinti OO, Momoh AI, Babatomiwa K, Ariyo EO. In silico molecular studies of natural compounds as possible anti-Alzheimer’s agents: ligand-based design. Netw Model Anal Heal Informatics Bioinforma. 2020;9: 54–67. [Google Scholar]
- 65.Naspiah N, Pratama MRF, Sukardiman. Xanthine oxidase inhibition activity and ADMET properties of terap (Artocarpus odoratissimus Blanco) leaves metabolites: Phytochemical screening and in silico studies. Pharmacogn J. 2021;13: 1150–1160. [Google Scholar]
- 66.Daina A, Michielin O, Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aja PM, Agu PC, Ezeh EM, Awoke JN, Ogwoni HA, Deusdedit T, et al. Prospect into therapeutic potentials of Moringa oleifera phytocompounds against cancer upsurge: de novo synthesis of test compounds, molecular docking, and ADMET studies. Bull Natl Res Cent. 2021; 45. [Google Scholar]
- 68.Banerjee P, Eckert AO, Schrey AK, Preissner R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018;46: W257–W263. doi: 10.1093/nar/gky318 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.