Abstract
High-rate production of multicarbon chemicals via the electrochemical CO2 reduction can be achieved by efficient CO2 mass transport. A key challenge for C−C coupling in high-current-density CO2 reduction is how to promote *CO formation and dimerization. Here, we report molecularly enhanced CO2-to-*CO conversion and *CO dimerization for high-rate ethylene production. Nanoconfinement of ascorbic acid by graphene quantum dots enables immobilization and redox reversibility of ascorbic acid in heterogeneous electrocatalysts. Cu nanowire with ascorbic acid nanoconfined by graphene quantum dots (cAA-CuNW) demonstrates high-rate ethylene production with a Faradaic efficiency of 60.7% and a partial current density of 539 mA/cm2, a 2.9-fold improvement over that of pristine CuNW. Furthermore, under low CO2 ratio of 33%, cAA-CuNW still exhibits efficient ethylene production with a Faradaic efficiency of 41.8%. We find that cAA-CuNW increases *CO coverage and optimizes the *CO binding mode ensemble between atop and bridge for efficient C−C coupling. A mechanistic study reveals that ascorbic acid can facilitate *CO formation and dimerization by favorable electron and proton transfer with strong hydrogen bonding.
Subject terms: Electrocatalysis, Electrocatalysis, Heterogeneous catalysis, Carbon capture and storage, Electrocatalysis
Efficiently producing multicarbon chemicals through electrochemical CO2 reduction is essential for achieving economically feasible carbon neutrality. Here, the authors present molecularly enhanced CO2-to-*CO conversion and *CO dimerization for high-rate ethylene production by nanoconfinement of ascorbic acid.
Introduction
The electrochemical CO2 reduction reaction (CO2RR) to form value-added fuels and feedstocks is a promising route to achieve carbon neutrality and long-term energy storage1. The development of CO2RR electrocatalysts has led to advances in selectivity for multicarbon (C2+) chemicals such as ethylene (C2H4)2–4 and ethanol (C2H5OH)5,6 with high energy density and a high market price. However, it is a prerequisite to ensure profitability for the vitalization of CO2RR-based chemical manufacturing. This requires an enhanced production rate for C2+ chemicals7. These chemicals are formed by C−C coupling, which occurs via adsorbed carbon monoxide (*CO) dimerization on the surface of Cu active sites8. Although the product selectivity can be modulated by the design of active materials, including surface morphology, facet, defect, and alloy9, the production rate of CO2RR products, especially for C2+ chemicals, is mainly affected by *CO formation on heterogeneous catalysts; the partial current density of C2H4 (JC2H4) is proportional to the square of *CO coverage 10. In general, at the potential for high current density, the hydrogen evolution reaction (HER) becomes more dominant than CO2RR because of limited *CO coverage. Therefore, it has been a challenge to achieve CO2-to-*CO conversion in high current density CO2 electrolysis for high-rate C2+ chemical production.
CO2-to-*CO conversion is significantly hampered by the limited *CO2– formation, which is one of the main rate-determining steps (RDS) for the CO2RR11. To overcome this bottleneck, gas diffusion electrode (GDE)-based electrolyzers, such as flow cells and membrane-electrode-assembly (MEA), have emerged as an engineering approach to improve CO2 transport to catalysts. GDE enables the supply of a large amount of CO2 by providing gas flow directly to the electrocatalysts over the double- and triple-phase boundaries between gas CO2, liquid electrolytes, and solid catalysts12,13. In the GDE, microenvironment has been optimized in terms of CO2 flow rate14, CO2 partial pressure15, and catalyst architecture8. Also, molecular enhancement of CO2RR by combining molecular additives with heterogeneous catalysts has recently received great attention. They contribute to increasing *CO coverage by enhancing CO2 mass transport (increased local CO2 concentration) or optimizing hydrophobicity2,3,16–24. For instance, ionomers can provide CO2 transport channels that increase the local CO2 concentration near active sites and control the local pH and CO2/H2O ratio via selective ion conduction7,17,18. Furthermore, polymers in molecularly augmented GDEs have been introduced to increase the local CO2 concentration by optimizing porosity or hydrophobicity20,25. However, the strategy to promote proton-coupled electron transfer-based *CO formation from CO2 (g) has not yet been explored significantly for CO2RR with high productivity in heterogeneous electrocatalysts.
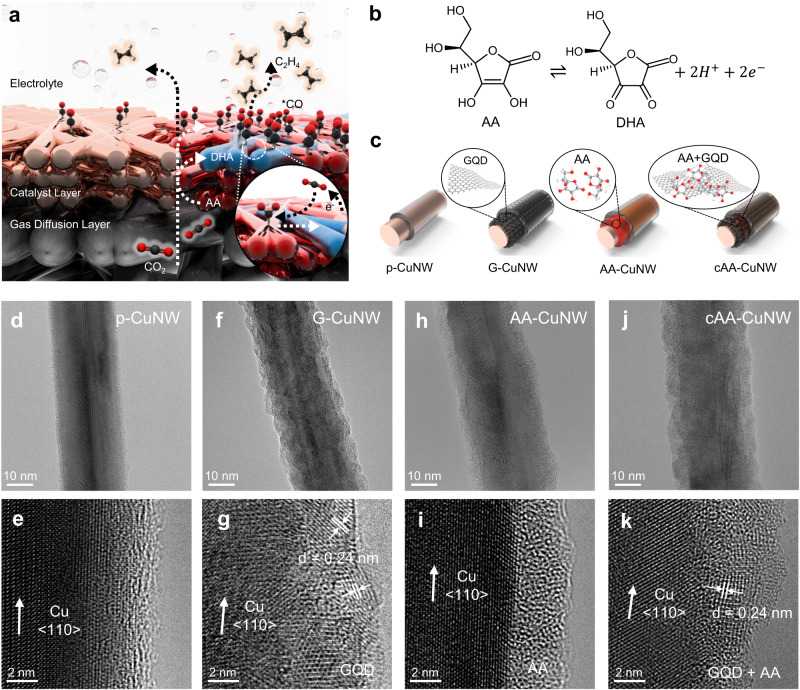
Here, we report molecularly enhanced CO2-to-*CO conversion and *CO dimerization for high-rate C2H4 production using ascorbic acid (AA). AA, also known as vitamin C, has been widely used as a reducing agent and antioxidant in nanomaterial synthesis and biochemical purposes26,27. When we store fruits to preserve AA, maintaining a CO2-deficient environment is essential because AA can react with CO2 and be oxidized to dehydroascorbic acid (DHA) with proton and electron donation28. Furthermore, AA has been utilized for CO2 capture in homogeneous catalysis approaches29–31. Inspired by this AA/DHA redox principle and CO2 capture property, we exploited AA as a promoter to capture CO2 near Cu, increase the *CO coverage and ensuing *CO dimerization on the surface of heterogeneous Cu catalysts.
To employ AA in heterogeneous catalysis with aqueous electrolytes, we pursued a strategy to immobilize water-soluble AA on electrocatalysts and achieve redox reversibility. We designed AA-augmented Cu nanowires (CuNWs) by applying graphene quantum dots (GQDs), which contain −OH and −COOH groups, as a mediator to anchor AA on the Cu surface with an ionomer. This nanoconfined AA on CuNW enhanced the CO2-to-*CO conversion during the CO2RR and resulted in high C2H4 productivity of heterogeneous Cu electrocatalysts. Unlike pristine CuNW (p-CuNW), which mainly produced C2H4 at low potential, CuNW with AA nanoconfined by GQDs (cAA-CuNW) boosted CO production over a similar potential range. As the potential increased for the high-current-density CO2RR, enriched CO formation in cAA-CuNW was dramatically converted to C2H4, while the main electrolysis product of p-CuNW was hydrogen (H2) because of limited CO2 mass transport. We found that this enables efficient CO2RR even in low CO2 concentrations, which can be extended to the CO2RR of flue gas. In situ Raman spectroscopy and operando X-ray absorption spectroscopy (XAS) studies enabled us to verify the effect of nanoconfined AA for inducing a high degree of *CO coverage and binding control between atop-bound CO (COatop) and bridge-bound CO (CObridge) on the reconstructed CuNW during the CO2RR. Grand canonical density functional theory (GC-DFT) revealed that the redox of AA/DHA enabled efficient electron/proton transfer to CO2 and multiple hydrogen bonding sites of AA, thereby improving CO2-to-*CO conversion and *CO dimerization on Cu.
Results
Fabrication of vitamin C-augmented catalysts
AA-augmented CuNW was leveraged for high-rate CO2-to-C2H4 conversion by nanoconfined AA on GQDs; favorable CO2-to-*CO conversion and *CO dimerization on the Cu surface (Fig. 1a). For CO2 capture, we harnessed the redox of AA/DHA; AA was oxidized and converted to DHA with electron and proton donation (Fig. 1b). A major bottleneck for the application of AA in heterogeneous catalysts is that AA is easily dissolved into the electrolyte due to its high solubility in aqueous solutions. Once it dissolves, it is difficult to reduce back to AA due to irreversible dehydration32. This stoichiometric consumption of AA degrades the sustainability of CO2RR systems. Additionally, reversible redox of AA/DHA is required for efficient CO2 capture. We improved the electroactive sustainability of AA by nanoconfined AA on GQDs. The confined AA ensures a redox-reversible environment through the improvement of the reduction reaction by DHA accumulation and stabilization in an aqueous electrolyte32.
Fig. 1. CO2 capture strategy and surface structures of AA-augmented CuNWs.
a Schematic of enhanced CO2-to-*CO conversion and *CO dimerization in cAA-CuNW for high-rate C2H4 production. b Redox of AA and DHA for CO2 capture. c Schematic illustration of surface modification of CuNWs with GQD, AA, and nanoconfined AA on GQDs. An ionomer is coated on the outer surface of CuNWs during the fabrication of the GDE. TEM (top) and HR-TEM (bottom) images of (d, e) p-CuNW, (f, g) G-CuNW, (h, i) AA-CuNW, and (j, k) cAA-CuNW.
Nanoconfinement of AA was achieved by the reaction between AA and GQDs at 95 °C to reduce and functionalize the GQDs33. Note that excess reductant containing diverse oxygenated functional groups can remain on the reduced graphene surface and act as a multidentate hydrogen bonding donor34,35. The reduced GQDs can form 2-dimensional supramolecular systems that could effectively confine AA via physisorption including π interaction or hydrogen bonding. (Supplementary Fig. 1). Then, AA-nanoconfined GQDs were combined with CuNW through mild sonication, and cAA-CuNW uniformly maintained the high-aspect-ratio structure of CuNW (Supplementary Fig. 2).
We fabricated p-CuNW, CuNW with GQD (G-CuNW), CuNW with AA (AA-CuNW) and cAA-CuNW to understand the role of nanoconfined AA on GQDs in the CO2RR by comparing their catalytic reactions (Fig. 1c). Surface functionalization of CuNW was conducted by the interaction between the oxygen-containing functional groups of each reagent and the native oxide surface of the CuNW36. The surface structures of CuNWs were investigated by scanning electron spectroscopy (SEM). Compared to p-CuNWs, surface-modified CuNWs showed a rough surface because of the existence of AA and GQDs. However, they exhibited similar 1-dimensional (1D) structures even after surface modification (Supplementary Fig. 3).
The crystalline structures of CuNWs were investigated by transmission electron spectroscopy (TEM) (Fig. 1d–k, Supplementary Fig. 4). High-resolution (HR)-TEM of p-CuNWs revealed the presence of crystalline Cu (Fig. 1d, e). G-CuNW showed a rough surface morphology because of the polycrystalline GQD assembly (Fig. 1f). This indicates that GQDs uniformly cover the surface of CuNW. The lattice distance of the polycrystalline outer shell in G-CuNW was 0.24 nm, which corresponds to the (100) plane of graphene (Fig. 1g)36,37. AA-CuNW, composed of an amorphous shell with a thickness of ~3 nm, showed a more uniform surface than G-CuNW (Fig. 1h, i). The Cu surface of AA-CuNW was partially oxidized during surface functionalization because of the reaction with oxygen in an organic solvent (Supplementary Fig. 5). In the cAA-CuNW, both polycrystalline and amorphous regions coexisted, indicating that GQDs adequately confined AA on the surface of CuNWs (Fig. 1j, k). In the HR-TEM of CuNWs, lattice distance analysis and fast Fourier transform (FFT) confirmed the presence of pure Cu after surface functionalization (Supplementary Fig. 6).
Chemical states of vitamin C-augmented catalysts
We investigated the chemical states of CuNWs after nanoconfinement to verify the presence of AA and GQD. In the TEM energy-dispersive spectroscopy (EDS) mapping, all CuNWs showed a uniform distribution of C and O along the Cu, implying that the surface of the CuNWs was coated by each reagent (Supplementary Fig. 7). In the EDS spectrum for Cu, C, and O, the atomic fraction of C in G-CuNW was 12.6%, while O was barely detected (Fig. 2a and Supplementary Fig. 8). However, the fraction of O (15.8%) was higher than that of C (7.0%) in AA-CuNW. In cAA-CuNW, the fraction of O was lower than that of C (20.1%) but still showed a significant ratio of 12.8%, suggesting the coexistence of GQDs and AA.
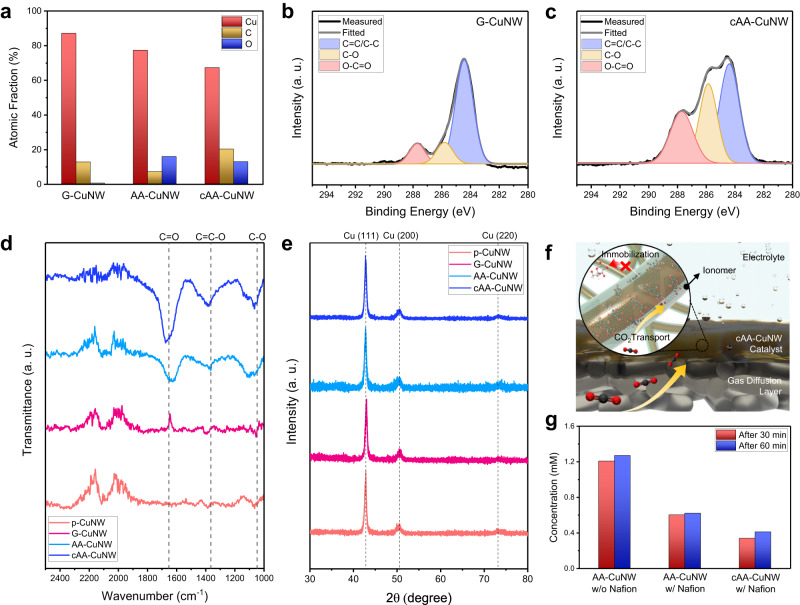
Fig. 2. Chemical states of AA-augmented CuNWs.
a Atomic fractions of Cu, C, and O for G-CuNW, AA-CuNW, and cAA-CuNW. C 1s XPS spectra of (b) G-CuNW and (c) cAA-CuNW. d FT-IR spectra of p-CuNW, G-CuNW, AA-CuNW, and cAA-CuNW. e XRD patterns of p-CuNW, G-CuNW, AA-CuNW, and cAA-CuNW. f Conceptual schematic of the surface components of Nafion ionomer-coated cAA-CuNWs for enhanced CO2-to-*CO conversion and *CO dimerization during the CO2RR. g Comparison of the amount of DHA extracted from AA-CuNW and cAA-CuNW with and without the Nafion ionomer coating. The extracted DHA concentration was analyzed by HPLC.
The presence of AA in cAA-CuNW was verified by X-ray photoelectron spectroscopy (XPS). The C 1s spectra of the G-CuNW showed a strong peak at ~284.5 eV, which corresponds to the sp2 C group of the GQDs (Fig. 2b). In addition, peaks for C−O and O−C=O bonds were observed at ~285.8 and ~287.7 eV, respectively, originating from the oxygenated C functional group at the edge of the GQDs30,37,38. In the C 1s spectra of cAA-CuNW, the intensity of the peaks for C−O and O−C=O bonds increased, indicating that cAA-CuNW contains more oxygenated C groups than G-CuNW (Fig. 2c). Since these peaks were not observed in the C 1s spectra of GQDs separated from cAA-CuNW, the peaks for C−O and O−C=O bonds were attributed to the hydroxyl and carbonyl groups in AA (Supplementary Fig. 9).
In the Fourier transform infrared spectroscopy (FT-IR) analysis, we observed major peaks at approximately 1,666, 1,365, and 1,066 cm−1, which corresponded to the C=C stretching vibration, C=C−O asymmetric stretching vibration of the enol-hydroxyl group, and C−O vibration in the functional groups of AA in AA-CuNW and cAA-CuNW (Fig. 2d)39. With TEM EDS, XPS, and FT-IR, comparison of thermogravimetric analysis (TGA) between G-CuNW and cAA-CuNW supported the presence of AA in cAA-CuNW (Supplementary Fig. 10). In the TGA of cAA-CuNW, an abrupt weight drop was observed at approximately 190 °C, consistent with the decomposition temperature of AA40. The X-ray diffraction (XRD) patterns of CuNWs exhibited a pure metallic Cu phase, indicating that the surface functionalization did not affect the chemical states of the active site in CuNWs (Fig. 2e). Consequently, it was inferred that AA is well confined on the surface of cAA-CuNW without any change in the chemical state of AA and Cu during surface modification.
We investigated the states of CuNWs after CO2RR through XRD, SEM, TEM, and TEM EDS. XRD patterns of all CuNWs showed Cu2O (111) peaks due to the oxidation of metal surface by electrolyte contact after CO2RR (Supplementary Fig. 11). However, the 1D structures were maintained for all CuNWs as shown in SEM images (Supplementary Fig. 12), indicating that there was no significant structural transformation during CO2RR. The crystal structure and atomic distribution of CuNWs were analyzed using TEM and TEM EDS (Supplementary Fig. 13). CuNWs exhibited a rough Cu2O surface after catalysis, which corresponds to the XRD analysis results. In addition, the lattice of GQDs and amorphous nanostructure of AA was still observed at the outer shell of G-CuNW, AA-CuNW, and cAA-CuNW, suggesting that each material was located on the CuNW surface during CO2RR. The elemental distribution in TEM EDS confirmed that the uniform distribution of Cu, C, and O atoms on the entire surface of the CuNW structure was maintained after CO2RR (Supplementary Fig. 14).
The redox reversibility of nanoconfined AA on GQDs was verified by cyclic voltammetry (CV) measured in a 1 M KOH electrolyte without gas supply (Supplementary Fig. 15). CV of nanoconfined AA on GQDs at the surface of a glassy carbon electrode (GCE) showed paired oxidation and reduction peaks after repetitive scans. Early in the cycle, a strong oxidation peak was observed due to the existence of excess AA, and these unconfined AAs were oxidized by repetitive scans. On the other hand, the CVs of intact GCE and GQD-coated GCE showed no significant redox peaks in the same potential window. When redox molecules are confined in nanocavities and their collision frequency with the electrode surface increases, the electrochemical reversibility is improved due to the enhancement of electron transport32. Therefore, we determined that the redox reversibility of AA was enhanced by the nanoconfinement effect through GQDs, ensuring sustainable electron and proton transfer during the CO2RR. Furthermore, we compared the CV of AA and nanoconfined AA on GQDs under N2 and CO2 gas (Supplementary Fig. 16). To minimize changes in CV curve due to pH drop from bicarbonate formation, we proceeded CV with 0.1 M KHCO3 electrolyte. Unlike AA, reversible AA/DHA redox is achieved in nanoconfined AA on GQDs under both N2 and CO2 gas. Therefore, we think that nanoconfined AA on GQDs can promote CO2-to-*CO conversion by enhancing electron and proton transfer from reversible AA/DHA redox during CO2RR (Supplementary Fig. 17).
To investigate the redox behavior of AA/DHA in the potential range of CO2RR, we analyzed linear sweep voltammetry (LSV) of nanoconfined AA on GQDs before and after CO2RR (Supplementary Fig. 18). We found that the most AAs were oxidized to DHA during LSV before CO2RR. However, the current density of peak for the oxidation of AA significantly increased after 24 h CO2RR at –1.8 V (vs RHE, non-iR corrected) (Supplementary Fig. 18b). This indicates that DHA was reduced to AA during CO2RR, regenerating AA to continuously promote CO2-to-*CO conversion in the potential range of CO2RR.
An increased local CO2 concentration and immobilization of AA on the CuNW surface could be achieved by a perfluorosulfonic acid (PFSA) ionomer used for GDE fabrication with catalysts (Fig. 2f). When Nafion was coated on the catalyst, hydrophilic functional groups (−SO3−) preferentially interacted with the metal surface and formed hydrophobic domain (−CF2) channels through which electrolytes and gases could be transported18. The effect of Nafion on promoting the immobilization of AA was investigated by quantitative analysis of DHA in aqueous solution extracted from each catalyst. To evaluate the protective role of Nafion via the impermeability of AA and GQD, AA-CuNW and cAA-CuNW were prepared with and without Nafion coating. We quantified the extracted DHA by high-performance liquid chromatography (HPLC). DHA peaks were detected in the chromatographs of all catalysts at a retention time of ~5.8 min because dissolved AA was easily oxidized to form DHA during the extraction process (Supplementary Figs. 19 and 20)32. The concentration of DHA extracted from AA-CuNW without Nafion was 1.27 mM after 1 hour of extraction (Fig. 2g). However, the concentration of DHA dramatically decreased for AA-CuNW (0.62 mM) and cAA-CuNW (0.41 mM) with Nafion. We expect that the immobilization of AA was promoted by the laminar arrangement of Nafion on CuNW, which helps to prevent AA from penetrating into the aqueous electrolyte41.
Electrochemical CO2RR of vitamin C-augmented catalysts
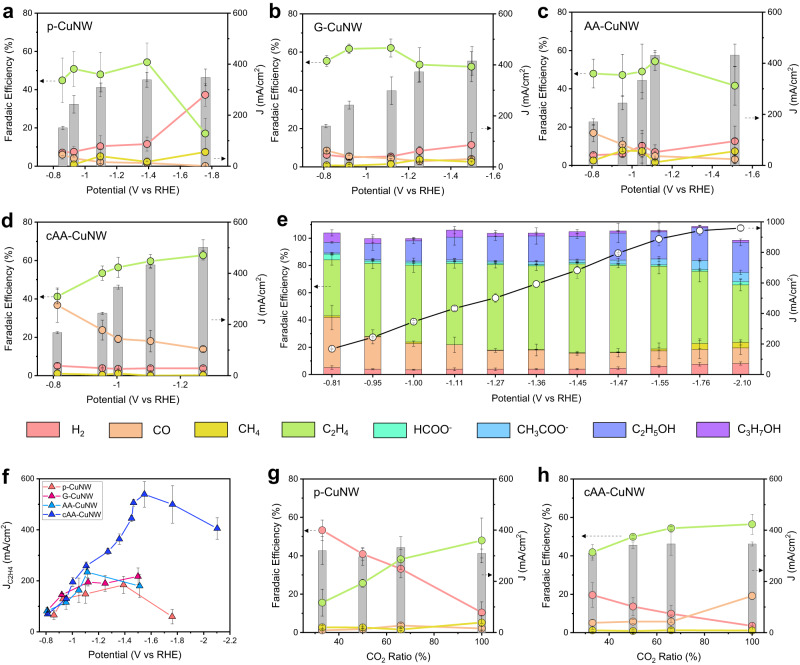
The CO2RR performances of the CuNWs were investigated in a flow cell electrolyzer with a 1 M KOH electrolyte (Fig. 3, Supplementary Figs. 22–25). In the p-CuNW, C2H4 was the main product at low potential ranges with a C2H4 Faradaic efficiency (FE) of 44.9% and CO FE of 6.1% at –0.86 V (Fig. 3a). However, when the potential exceeded –1.39 V (vs RHE) and reached –1.76 V (vs RHE), C2H4 FE decreased from 54.4 to 17.1%, and H2 FE increased from 11.6 to 37.3% by limited CO2 mass transport. G-CuNW showed more efficient C2 product formation with a higher C2H4 FE of more than 50% than p-CuNW at a similar potential range (Fig. 3b). In addition, the maximum total current density of G-CuNW was 465 mA/cm2 (Supplementary Fig. 25), higher than that of p-CuNW (348 mA/cm2). The enhanced total current density of G-CuNW is attributed to the existence of GQDs. Graphitic C shells can stabilize Cu active materials by alleviating reconstruction at a reductive potential4,42. GQDs enhance the electrocatalytic activity, as the electron-donating functional group of GQDs promotes the activity of the CO2RR by reducing the energy barrier of the RDS43. Indeed, intact GQDs participated in the CO2RR (Supplementary Fig. 26). However, we think that hindered gas diffusion44 and hydrophilic nature of GQDs do not show a promotional effect on CO2 mass transport (Supplementary Fig. 27). As a result, G-CuNW exhibited enhanced C2H4 selectivity in the low potential range with a maximum C2H4 FE of 62.1% at –1.12 V (vs RHE), but H2 FE increased over 10%, and C2 selectivity decreased as the potential increased to –1.50 V (vs RHE).
Fig. 3. High-rate C2H4 production of cAA-CuNW by enhanced CO2-to-*CO conversion and *CO dimerization.
Gaseous product FEs and total current densities for (a) p-CuNW, (b) G-CuNW, (c) AA-CuNW, and (d) cAA-CuNW in the CO2RR with 1 M KOH electrolyte. e Total product FEs and total current densities for the cAA-CuNW with applied potentials up to –2.10 V (vs RHE). f Comparison of JC2H4 versus potentials of p-CuNW, G-CuNW, AA-CuNW, and cAA-CuNW. Gaseous product FEs and total current densities of (g) p-CuNW and (h) cAA-CuNW in the CO2RR according to CO2 ratios in CO2 + Ar mixed gas. All the error bars represent standard deviation based on three independent samples.
When the AA was hybridized to CuNW without GQD, we observed increased CO production in the low-potential range, which clearly contrasts with the low CO production in p-CuNW and CuNW hybridized with DHA (DHA-CuNW) (Supplementary Fig. 28). AA-CuNW showed a CO FE of 17.0% at –0.81 V (vs RHE), while p-CuNW and G-CuNW showed CO FEs lower than 10% in a similar potential range (Fig. 3c). However, this increased CO formation did not contribute to *CO dimerization for C2H4 production in the high current density CO2RR. Although CO FE decreased to 3.2% at –1.51 V (vs RHE), the H2 FE increased to 12.6%, and the C2H4 FE decreased to 41.6% (Supplementary Fig. 24c). This may have originated from the absence of a nanoconfinement effect, which helped to prevent the dehydration of the AA and induce reversible AA/DHA redox, even when the Nafion ionomer prevented the AA from being dissociated by the electrolyte.
cAA-CuNW exhibited dramatically elevated CO production with an FE of 36.7% at –0.81 V (vs RHE). As the potential increased to –1.27 V (vs RHE), the CO FE decreased from 36.7 to 13.8%, and the C2H4 FE increased from 41.2 to 62.7% (Fig. 3d). The enhanced CO production implied that a large amount of *CO was formed on the catalyst surface. Since the formation rate of the C2 product was proportional to 2, the evolved CO formation rate in cAA-CuNW could contribute to enhancing CO2-to-*CO conversion for high-rate C2H4 formation. In particular, the ratio of C2H4 FE to methane (CH4) FE (C2H4 FE/CH4 FE) in cAA-CuNW is much higher than that of other CuNWs (Supplementary Fig. 29). This indicates that *CO dimerization is promoted in cAA-CuNW. In addition, cAA-CuNW showed a significantly low HER with an H2 FE of 3.8% at –1.27 V (vs RHE). In cAA-CuNW, highly efficient C2H4 production was well maintained even when the potential reached –2.1 V (vs RHE) with a total current density of 960 mA/cm2 (Fig. 3e).
When we compared partial current densities for H2, CO, and C2H4, cAA-CuNW exhibited the highest C2H4 selectivity and productivity compared to other CuNWs; maximum JC2H4 of 539 mA/cm2 with C2H4 FE of 60.7% at –1.55 V (vs RHE), which is 2.9-fold higher than the highest JC2H4 of p-CuNW with 184 mA/cm2 at –1.39 V (vs RHE) (Fig. 3f and Supplementary Fig. 30). This is one of the highest JC2H4 values among previously reported Cu-based CO2RR electrocatalysts (Supplementary Table 1). cAA-CuNW also showed the highest selectivity and productivity of CO and C2H4 in the electrochemically active surface area (ECSA)-normalized activity (Supplementary Figs. 31 and 32). The highest JC2H4 of cAA-CuNW is confirmed after considering the effect of surface roughness. This reveals that nanoconfined AA on GQDs promotes the intrinsic CO2RR activity of cAA-CuNWs. We found that the overpotential at maximum JC2H4 can be lowered by increasing the concentration of electrolyte such as JC2H4 of 453 mA/cm2, C2H4 FE of 56.3% at –0.57 V (vs RHE) in 2 M KOH electrolyte (Supplementary Fig. 33). However, when GQD and AA mixtures were deposited on the surface of CuNWs without any preceding reaction, there was no trend toward the enhancement of the production of CO and C2H4 (Supplementary Fig. 34). This revealed that the interaction between AA and GQD is key for the nanoconfinement effect, which can promote CO2-to-*CO conversion and *CO dimerization by reversible AA/DHA redox and nanoconfined AA on GQDs.
Furthermore, we investigated CO2RR of p-CuNW and cAA-CuNW in low CO2 concentration by controlling CO2 ratio in CO2 + Ar mixed gas to further prove the effect of nanoconfined AA on CO2-to-*CO conversion (Fig. 3g, h and Supplementary Fig. 35). As the CO2 ratio decreased in the mixed gas, p-CuNW showed a dramatic increase of H2 selectivity and decrease of C2H4 selectivity (H2 FE of 53.3% and C2H4 FE of 15.6% at the CO2 ratio of 33%). In contrast, cAA-CuNW exhibited H2 FE of 19.6% and C2H4 FE of 41.8% even at the CO2 ratio of 33%. Efficient C2H4 production of cAA-CuNW was maintained by promoted CO2-to-*CO conversion even in low CO2 concentration.
To confirm the stability of nanoconfined AA on GQDs in promoting CO2RR, long-term CO2RR was conducted in a flow cell electrolyzer with a 1 M KOH electrolyte (Supplementary Fig. 36). C2H4 selectivities of p-CuNW and cAA-CuNW were compared according to react ion times at a total current density of 300 mA/cm2. C2H4 FE for cAA-CuNW was maintained over 50% for 8 h, while that of p-CuNW abruptly decreased to 21.3% within 2 h. Also, similar FT-IR spectra of the GDE before and after the CO2RR stability test confirmed that the nanoconfined AA on GQDs was stable in cAA-CuNW during CO2RR (Supplementary Fig. 37). The CO2RR stability of cAA-CuNW was further investigated in a zero-gap membrane electrode assembly (MEA) electrolyzer with 0.1 M KHCO3 anolyte (Supplementary Fig. 38). cAA-CuNW exhibited outstanding stability of C2H4 production for 168 h at a total current density of 150 mA/cm2. These results indicate that the nanoconfined AA is stably immobilized in cAA-CuNW and continuously promote CO2-to-*CO conversion and *CO dimerization to enhance CO2RR productivity.
Real-time analysis to track CO2RR intermediates and Cu reconstruction
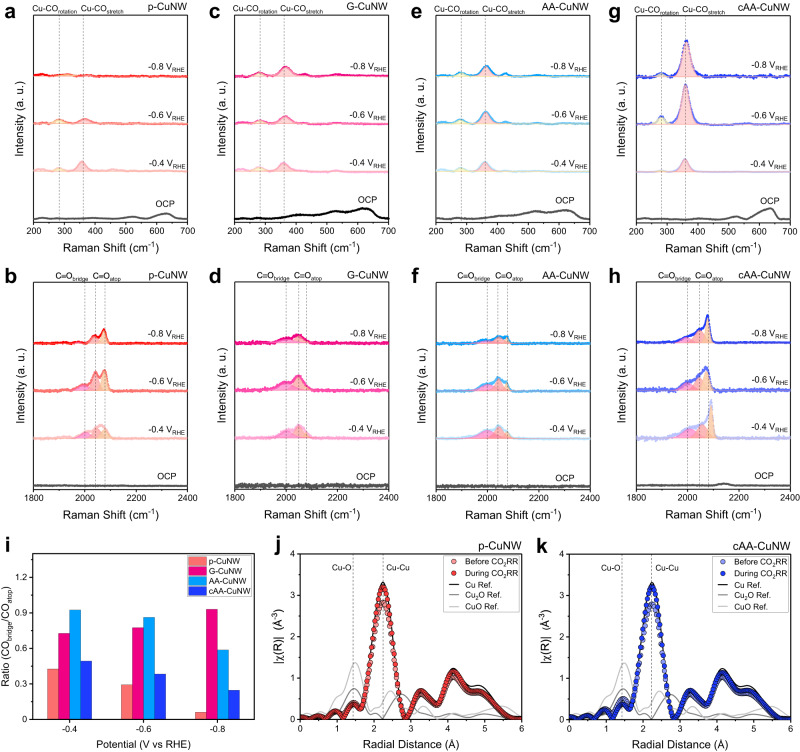
To study the origin of enhanced C2H4 productivity in cAA-CuNW, in situ Raman spectroscopy analysis was performed during the CO2RR with a 1 M KOH electrolyte (Fig. 4, Supplementary Fig. 39). This enabled tracking of the interaction of *CO intermediates with the catalyst surface, as well as the *CO binding mode for C−C coupling. Specific Raman peaks were observed for *CO in the regions of 200–300, 300–400, 1950–2000, and 2050–2100 cm-1, which were related to Cu–CO rotation, Cu–CO stretching, CObridge, and COatop, respectively45,46. The other peaks observed at 525–534 and 615–630 cm−1 of OCP were related to the interactions of the natural oxides with the Cu surface in CuNWs47.
Fig. 4. Real-time analysis of CuNWs with different degrees of surface hybridization during the CO2RR.
In situ Raman spectra of (a, b) p-CuNW, (c, d) G-CuNW, (e, f) AA-CuNW, and (g, h) cAA-CuNW obtained during CO2RR according to the applied potentials in the region of 200-700 cm-1 (top) and 1,800-2,400 cm-1 (bottom). i Comparison of integral area ratios of CObridge and COatop for CuNWs. Operando EXAFS spectra of (j) p-CuNW and (k) cAA-CuNW during the CO2RR.
Securing the *CO intermediate on the active materials is important to maintain C2 selectivity in the high current density CO2RR. The surface *CO coverage of CuNWs was investigated by analyzing the Cu–CO binding peaks at 200–400 cm−1. The origin of the Cu-CO binding peaks from CO2 gas was confirmed through the comparison with in situ Raman spectroscopy under N2 gas (Supplementary Fig. 40). cAA-CuNW, where nanoconfined AA on GQDs exists on the Cu surface, presented a significantly higher peak intensity for Cu–CO rotation and Cu–CO stretching than other CuNWs such as p-CuNW, G-CuNW, and AA-CuNW (Fig. 4a, c, e, g, and Supplementary Fig. 41). However, the peak intensities of p-CuNW were the lowest, which corresponds to the rapid increase of HER in the low-potential range48. Considering that cAA-CuNW exhibited a lower *CO2– peak intensity at 1500–1600 cm−1 compared to p-CuNW (Supplementary Fig. 42)49, high *CO coverage of cAA-CuNW is attributed to the promoted CO2-to-*CO conversion. To further investigate the effect of AA on *CO dimerization, we analyzed the Raman peaks for CObridge and COatop at ~1950–2100 cm-1, representing CO stretching of the adsorbed CO on the metal surface. The ratio between COatop and CObridge was strongly related to C2 product selectivity2,50. In general, the C − C coupling energy barrier decreased in the order of CObridge to CObridge > COatop to COatop > COatop to CObridge. Therefore, maintaining an optimal CObridge/COatop ratio is essential for efficient C2+ chemical production. In the CO stretching region, COatop and CObridge were simultaneously formed in AA-CuNW and cAA-CuNW at all CO2RR potentials ranging from –0.4 to –0.8 V (vs RHE, non-iR corrected), whereas the CObridge peak almost disappeared as the reductive potential increased in p-CuNW (Fig. 4b, d, f, h, and Supplementary Fig. 41). We found that AA-CuNW and cAA-CuNW maintain adequate CObridge/COatop ratio even when the potential increases up to –0.8 V (vs RHE, non-iR corrected), while p-CuNW and G-CuNW exhibit excessive COatop or CObridge as potential increases (Fig. 4i). Note that strong Cu–CO binding peaks and optimal CObridge/COatop ratio in in situ Raman spectroscopy of cAA-CuNW were similarly observed under CO2 + Ar mixed gas (Supplementary Fig. 43). Therefore, cAA-CuNW with C−C coupling between the CObridge and COatop ensemble facilitates *CO dimerization for C2+ chemical formation.
We investigated the reconstruction of Cu active materials in p-CuNW and cAA-CuNW by operando X-ray absorption spectroscopy (XAS) with a flow-cell-type reactor (Fig. 4j, k). We analyzed the oxidation states and coordination number (CN) of Cu during the CO2RR of CuNWs at a cathodic potential of –1.4 V (vs RHE, non-iR corrected) in a 1 M KOH electrolyte. The Cu K-edge X-ray absorption near edge structure (XANES) spectra revealed that both p-CuNW and cAA-CuNW have the oxidation state of metallic Cu0, which was maintained during the CO2RR (Supplementary Fig. 44). The CNs of p-CuNW and cAA-CuNW were investigated by extended X-ray absorption fine structure (EXAFS) spectroscopy (Fig. 4j, k and Supplementary Fig. 45). In the p-CuNW, the Cu−Cu bonding CN increased from 10.269 to 11.557 during the CO2RR, indicating the reconstruction of Cu by reductive potential (Supplementary Table 2). We found that the Cu−Cu bonding CN of cAA-CuNW increased from 10.266 to 11.706, similar to that of p-CuNW. This similar Cu reconstruction between p-CuNW and cAA-CuNW revealed that nanoconfinement of AA on GQDs did not affect the Cu reconstruction behavior and that enhanced CO2-to-*CO conversion and *CO dimerization originated from the augmentation of AA on CuNWs, not active materials.
Mechanistic study on the role of vitamin C in the CO2RR
To understand the effect of AA on improved C2+ production, grand canonical density functional theory (GC-DFT) calculations were performed. We focused on the key steps of the electrochemical CO2RR and compared the energetics and kinetics of Cu (100) with AA-decorated Cu (100), referred to as AA/Cu (100). The main steps include (1) CO2 adsorption, (2) *COOH formation, (3) *CO formation, and (4) *CO dimerization. The competitive HER was additionally investigated. Note that easier *CO formation would increase *CO coverage on the surface, and more facile *CO dimerization would improve the selectivity of C2+ products.
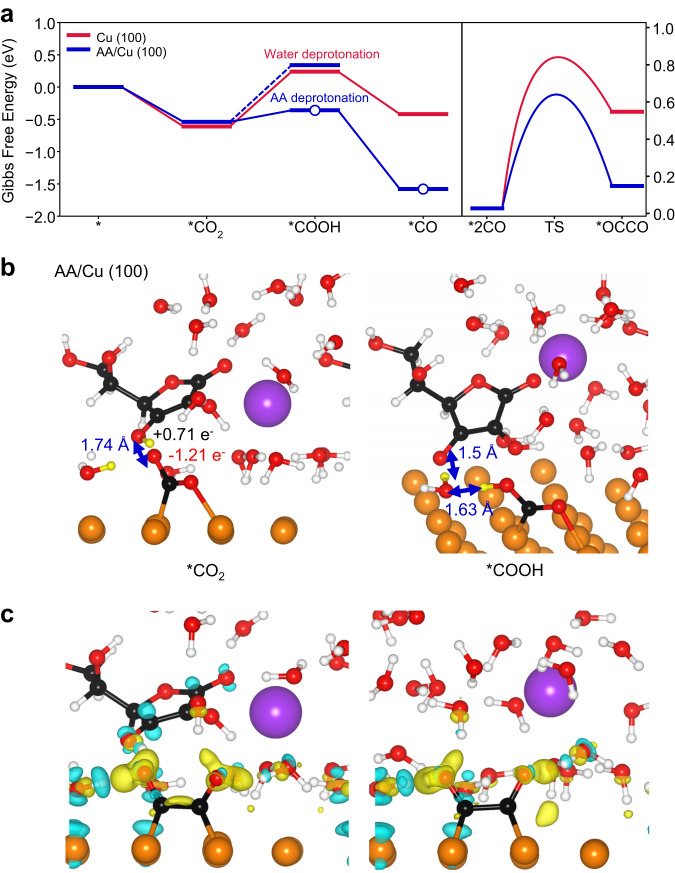
We present the Gibbs free energy diagrams of each step at pH 14 and 0 V (vs RHE) (Fig. 5a). Both Cu (100) and AA/Cu (100) similarly preferred CO2 chemisorption. One C-O bond was oriented parallel to the Cu surface (C-Os) and the other C-O bond was bent toward the solvent (C-Oe), where Os and Oe stand for oxygen atoms near the surface and the electrolyte, respectively (Supplementary Fig. 47). The observed anisotropic configuration of the CO2 adsorption is in consistent with literature51. The Bader charge analysis demonstrates that *CO2 is highly negatively charged in both cases (–1.16 e− on Cu (100) and –1.07 e- on AA/Cu (100)), indicating a strong CO2 adsorption due to the electron transfer (Supplementary Table 3)52.
Fig. 5. Computational modeling of the CO2RR on Cu (100) and AA/Cu (100).
a Gibbs free energy diagram of (left) CO2-to-*CO conversion and (right) *CO dimerization at 0 V (vs RHE). White circles indicate the reaction pathway involving the deprotonation of AA. H2O is the proton source otherwise. b The atomic structure of (left) the initial and (right) the final states of *CO2 protonation from AA on AA/Cu (100) (). c Charge density difference () of *OCCO adsorption on (left) AA/Cu (100) and (right) Cu (100). Color code: black (C), white (H), yellow (H), red (O), purple (K), and orange (Cu). Yellow H atoms are considered for the protonation during the CO2RR. The yellow and blue area represent an electron accumulation and depletion with an isosurface level of 0.005 e/Å3, respectively. The charge density difference is calculated as , where , and correspond to charge densities of the total system, the catalyst surface with adsorbates and solvent layers, respectively.
The adsorbed *CO2 is then protonated to form *COOH. On Cu (100), we considered a water molecule as the protonation source, and the reaction Gibbs energy () was found to be endothermic (0.86 eV). On AA/Cu (100), we evaluated both the water molecule and AA as potential protonation sources (R2b for water and R2c for AA)53. While the protonation from water exhibited a similar endothermic reaction Gibbs energy of 0.88 eV, protonation from AA proved to be thermodynamically more favorable, with a significantly lower reaction Gibbs energy (= 0.18 eV). This enhanced favorability can be attributed to lower pKa value of AA (4.04)54 compared to water (14), leading to a lower electrochemical deprotonation barrier55.
To further elucidate the origin of the enhanced energetics, we inspected the adsorption configurations before and after the protonation. Before the protonation, on Cu (100), the hydrogen in the proton source was positively charged by +0.67 e− and the hydrogen bonding distance (dO-H) between Oe was 1.9 Å (Supplementary Fig. 48). In contrast, on AA/Cu (100), HOX1 exhibited a more positively charged state (+0.71 e−) and a shorter dO-H of 1.74 Å (Fig. 5b). After protonation, the hydrogen bonding network within the solid-electrolyte interface underwent significant reconstruction in the case of AA/Cu (100). The distance between O in water and H in *COOH was 1.63 Å, and the distance between H in water and O in ASC− was 1.5 Å. However, on Cu (100), we did not observe a significant reconstruction following the protonation of *CO2 from water. As a result, *COOH and ASC− ion formed a more robust hydrogen bond network with water, thereby stabilizing the system. This observation suggests that the presence of AA promotes the favorable protonation of *CO2 to *COOH. Further protonation of *COOH to form *CO was energetically favorable in both cases, resulting in higher *CO coverages on AA/Cu.
We subsequently investigated the dimerization of *2CO, a key determinant of the selectivity of C2+ products such as C2H456. The dimerization of *2CO on Cu (100) was found to be endothermic, exhibiting the reaction energy of 0.52 eV and the activation energy of 0.81 eV. The introduction of AA on the surface decreased these energies to 0.13 eV and 0.62 eV, respectively, demonstrating that AA facilitates the dimerization. The charge density difference plot and the Bader charge analysis confirmed more significant electron transfer to *OCCO on AA/Cu (100) (–1.53 e− and +0.18 e− for adsorbate and Cu surface, respectively) compared to Cu (100) (–1.39 e− and +0.08 e−), making the interaction between *OCCO and the surface stronger through a more favorable electrostatic interaction (Fig. 5c)57,58.
We also examined the competitive HER. The energetics of the first protonation step to form the adsorbed *H were found to be less favorable compared to *CO2 adsorption, both with and without AA. Although the introduction of AA lowered the energy barrier of *H formation, the second protonation step remained unfavorable compared to *CO2 adsorption (Supplementary Fig. 49). To sum up, GC-DFT calculations confirmed that the introduction of AA on Cu catalyst surface facilitated the CO2RR, increasing *CO coverage on the surface and lowering the activation barrier of the rate-determining *CO dimerization, leading to higher production of C2+ products.
Discussion
We report vitamin C-induced CO2 capture for effective CO2-to-C2H4 conversion. AA was introduced to CuNWs for high rate C2H4 production by promoting electron and proton transfer and strong hydrogen bonding. By leveraging GQDs as a mediator to anchor water-soluble AA on CuNWs, we immobilized AA with an ionomer and enhanced the redox reversibility of AA, enabling sustainable CO2 capture of AA for high-current density CO2RR. This nanoconfined AA on Cu can steer the pathway toward C2H4 by securing CO2-to-*CO conversion and *CO dimerization at high current density CO2RR. cAA-CuNW exhibited higher CO selectivity than p-CuNW at a similar range of low electrolysis potential, indicating that nanoconfined AA effectively increased *CO coverage during the CO2RR. cAA-CuNW demonstrated CO2RR with a C2H4 FE of 60.7% and JC2H4 of 539 mA/cm2 in 1 M KOH, 2.9-fold higher than the JC2H4 of p-CuNW. In the CO2RR under CO2 + Ar mixed gas (CO2 ratio of 33%), cAA-CuNW exhibited C2H4 FE of 41.8% and H2 FE of 19.6%, while p-CuNW exhibited C2H4 FE of 15.6% and H2 FE of 53.3%. Efficient CO2RR of cAA-CuNW at low CO2 concentration confirms the promoted CO2-to-*CO conversion, applicable to the CO2RR of flue gas. In situ Raman spectroscopy and operando XAS revealed that enhanced *CO coverage and a judiciously controlled CObridge/COatop ratio for efficient C−C coupling was induced by the augmented AA on CuNWs. In GC-DFT, the Gibbs free energy diagram reveals that AA can facilitate *CO formation and dimerization by promoting electron/proton transfer and strong hydrogen bonding on the CuNW surface. This strategy can simultaneously contribute to optimizing *CO coverage for mass production of C2+ chemicals by combining with other molecular strategies for enhancing CO2 mass transport in GDE. We believe that vitamin C-promoted CO2 conversion, enabled by leveraging carbon mediators for bridging homogeneous and heterogeneous catalysis, can provide an avenue for compelling high-rate C2+ chemical manufacturing.
Methods
Nanoconfined AA on GQD preparation
GQDs (Sigma‒Aldrich, 0.1 mg/mL) and L(+)-ascorbic acid (ACROS, 99%, 6 mM) were dissolved in 50 mL of D.I. water. Ammonia solution (Daejung Chemicals & Metals Co., 25%, 0.1 mL) was added to ensure the colloidal stability of the GQDs. The mixtures reacted at 95 °C for 1 h. Then, the solution was evaporated and redispersed in 5 mL of D.I. water.
Synthesis of the CuNWs and surface functionalization
CuNWs were prepared by hydrothermal methods37. Anhydrous copper chloride (CuCl2, Alfa Aesar, 13 mM), D-glucose (Sigma‒Aldrich, 11 mM), and hexadexylamine (TCI, 56 mM) were dissolved in D.I. water (280 mL). The mixtures reacted in a hydrothermal reactor at 120 °C for 24 h and centrifuged with D.I. water. The product was then separated using hexane to collect CuNWs and dispersed in isopropyl alcohol (IPA). To prepare surface-functionalized CuNWs, 0.5 mL of GQDs, AA (5 mg/mL), or nanoconfined AA on GQDs was injected in methanol (20 mL). After adding CuNW solution (3 mg/mL, 2 mL), surface functionalization was conducted with mild sonication for 20 min. After that, the solution was washed with IPA by centrifuging at 2490 g for 15 min and stored in IPA.
Characterization of the CuNWs
The nanostructures of the catalysts were confirmed by an FEI Titan TEM (THEMIS Z, Thermo Fisher Scientific) operated at 300 kV. TEM sampling was performed by drop casting the catalysts on lacey formvar carbon-coated square Au grid. The crystal structures and chemical composition of the catalysts were analyzed by XRD (Miniflex 600 Mini, Horiba) and XPS (ESCALAB 250Xi, Thermo Fisher Scientific). XPS sampling was performed by drop casting of the catalyst on P type Boron doped Si wafer. For the characterizations of CuNWs after CO2RR (XRD, SEM, TEM, TEM EDS), each electrode was operated in a flow cell elctrolyzer at the applied potential of –3.2 V (vs Ag/AgCl) for 1 h (Supplementary Figs. 11–14). The redox reversibility of nanoconfined AA on GQDs was confirmed by an electrochemical analyzer (VSP, Bio-Logic). The electrode was prepared by coating 30 µL of nanoconfined AA on GQDs on a GCE with a radius of 3 mm. Then, 10 µL of Nafion was subsequently coated on the electrode to prevent dissolution into the electrolyte. The CV measurement was conducted using a three-electrode cell consisting of a Pt wire (counter electrode) and Ag/AgCl (reference electrode) with a scan rate of 50 mV/s. The chemical analysis of DHA after elution from the catalysts was performed by HPLC (Vanquish Core HPLC, Thermo Scientific). After sufficient chemical extraction by stirring the film in D.I. water, the extracted DHA was analyzed using an Aminex HPX-87H cation exchange column with a mobile phase of 0.5 mM sulfuric acid aqueous solution. The column temperature was maintained at 25 °C, and the flow rate was set at 0.6 mL/min. The chromatographic peaks were detected by a UV detector at a wavelength of 200 nm. The intermediates of the catalysts during the CO2RR were analyzed by in situ Raman spectroscopy (XploRATM PLUS Raman spectrometer, HORIBA). An altered flow cell with a water immersion objective lens (60×) and 785 nm laser were used. The CO2 gas flow was fixed at 50 sccm by a mass flow controller (MFC). In situ Raman spectroscopy was measured up to the potentials of –0.8 V (vs RHE, non-iR corrected) due to laser focusing interference induced by gaseous products. The data were collected with an acquisition time of 10 s and acquired ten times in all measurements. Real-time analysis of the chemical states and bonding nature was performed by means of operando XANES and EXAFS. The XAS measurements were conducted at the 10 C X-ray beamline of Pohang Accelerator Laboratory (Pohang, Republic of Korea).
Electrode preparation and electrochemical CO2RR measurement
Fifteen milligrams of CuNW were prepared as dispersed in IPA. Sixty microliters of Nafion ionomer (Sigma‒Aldrich, 5 wt%) was mixed to increase both the immobilization of the catalysts and the CO2 transport capacity. After vortex mixing, the solution was spray-coated on a porous polytetrafluoroethylene substrate (PTFE, Sterlitech) with an airbrush gun using N2 gas to fabricate the GDE. To ensure uniform layer formation, spray coating was conducted on a 60 °C hot plate.
For the CO2RR experiment, Ag/AgCl was used as the reference electrode, and Ni foam was used as the counter electrode. The anolyte and catholyte were separated by the anion exchange membrane. The electrocatalytic CO2RR performances of CuNWs were investigated at different cathodic potentials in a flow cell electrolyzer with a 1 M KOH electrolyte. The potential of Ag/AgCl was converted to RHE and the RHE conversion equation is as follows:
| 1 |
We performed the calculation of 80% iR compensation losses between the Ag/AgCl and working electrode through electrochemical impedance spectroscopy (EIS) analysis.
The gas products of CO2RR were analyzed by gas chromatography (GC, Micro GC Fusion, INFICON Inc.) equipped with two thermal conductivity detectors (TCDs) employing different carrier gases (argon and helium). The liquid products were analyzed by nuclear magnetic resonance spectrometry (NMR, Bruker). The mixture of catholyte, D2O and dimethyl sulfoxide as an internal standard was collected in NMR tubes. The Faradaic efficiency of each product was calculated by the following equation:
| 2 |
where z and n are the number of electrons exchanged and moles of products, respectively; F is the Faradaic constant; and Q is the input charge. Gas product GC data per each sample were collected as the average value measured at 5, 30, and 55 min intervals. The error bars for CO2RR data (gas and liquid products) represent standard deviation based on three independent samples. In the CO2RR under CO2 + Ar mixed gas, all measurements were conducted at the applied potential of –2.2 V (vs RHE, non-iR corrected) with 1 M KOH electrolyte. Gas flow rate was fixed to 60 sccm.
Grand canonical density functional theory (GC-DFT) calculations
The conventional calculation approach based on computational hydrogen electrode (CHE) method59 simulates all systems at constant charge. Although this method is advantageous in understanding electrochemical processes with only a few DFT calculations60,61, it could mislead the fundamental misunderstanding since the actual processes take place at a constant potential. This is because the systems described with the CHE method remain neutral during the electrochemical reactions, resulting in Fermi level fluctuations62,63. The GC-DFT method adjusts the number of electrons in the systems, thus maintaining the Fermi level for all electrochemical reaction steps. This method essentially provides an accurate description of the electrode-electrolyte interface at a constant electrode potential64–66.
To account for the combined effect of solvation and applied potentials, we treated the liquid-metal interface as a polarizable continuum using the linearized Poisson-Boltzmann equation, as implemented in VASPsol67, where the Debye length was set to 3 Å, corresponding to a 1 M concentration of electrolytes with a relative permittivity of 78.4.
In this computational framework, we could tune the potential of the system by changing the number of electrons. The potential versus standard hydrogen electrode () was calculated as
| 3 |
where and correspond to the chemical potential of an electron and the work function of the standard hydrogen electrode (SHE), respectively. is defined as the Fermi level () compared to the electrostatic potential of the bulk electrolyte () (Supplementary Fig. 50), and was calculated to be 4.43 by RPBE68. By changing the number of electrons, one can equate the of many states during the electrochemical reactions, thus maintaining the potential. We set the convergence criteria for U to be 10-3 V.
The grand canonical electronic energy is calculated as
| 4 |
where is the number of electrons adjusted. Thus, is positive (negative) when electrons are added (subtracted)58,69.
Computational details
Spin polarized DFT calculations were performed using the Vienna Ab initio Simulation Package (version 5.4.4)70. The generalized gradient approximation with the revised Perdew-Burke-Ernzerhof (GGA-RPBE) functional71,72 was used to describe the exchange-correlation interaction, and the D3 method of Grimme with a zero-damping function73 was applied to include van der Waals interactions. The cutoff energy was set to 400 eV, and the convergence tolerances of energy and force were set to 10-4 eV and 0.05 eV/Å, respectively. (221) Monkhorst-Pack mesh of k-points74 was sampled. We generated the initial guess of the transition state (TS) of *CO dimerization using the climbing image nudged elastic band (CI-NEB) method75 with five intermediate images. Subsequently, we employed the dimer method76 to converge the TS to the saddle point at the specific potential by adjusting the number of electrons.
To simulate the Cu catalyst surfaces, we fully relaxed a bulk structure of face-centered cubic (FCC) Cu. Subsequently, we constructed a three-layered (44) Cu (100) surface, with the bottommost layer fixed to the bulk positions. To model the liquid-metal interfaces, we prepared 25 water molecules with one K+ ion11,77. We then included a vacuum region of ~12 Å in the z-direction to prevent any spurious interactions between repeating images. To obtain the liquid configuration, we conducted Ab-initio molecular dynamics (AIMD) simulations of a *CO2 adsorbed Cu surface in the NVT ensemble using the Nose-Hoover thermostat for 5 ps, with a time step of 1 fs at 300 K. After confirming that the system had equilibrated, we optimized the last snapshot from the simulation and used it as the starting structural configuration. To model AA/Cu (100), we initially positioned AA approximately 3.5 Å above the surface according to the previous work on AA/Pd (100)78, and then performed the AIMD simulations. Note that the optimized position of K+ is approximately 6 Å above the surface for both Cu (100) and AA/Cu (100). The AIMD simulations demonstrate that HOX1 is most closely positioned to the adsorbed *CO2, suggesting that it is reasonable to assume the proton transfer of HOX1 in AA to adsorbates during the CO2RR (Supplementary Fig. 51).
The grand canonical Gibbs free energy () was calculated by adding Gibbs free energy correction values () to . The correction values for adsorbates (gaseous molecules) were calculated using harmonic oscillator (ideal gas) approximations at 298.15 K in neutral systems as implemented in the Atomic Simulation Environment (ASE) (Supplementary Table 4)79. To correct the gas-phase errors originating from the RPBE functional, we added +0.46 eV to the DFT energy of the CO2 molecule80.
Supplementary information
Source data
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2021R1C1C1013784 and 2021M3D1A2047041, D.-H.N.). S.B. acknowledges the support from the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2015M3D3A1A01064929, S.B.) and generous supercomputing time from KISTI. S.B. thanks Dr. Changhyeok Choi (University of Toronto) for helpful discussions on grand canonical DFT method. Y.L. acknowledges the support from Basic Science Research Program through the National Research Foundation (NRF) funded by the Ministry of Science and ICT (NRF-2018R1A5A1025594 and 2023R1A2C1003194, Y.L.). Y.L. also acknowledges the support from the BK21 FOUR program through the National Research Foundation (NRF) funded by the Ministry of Education of Korea. Experiments at PLS-II were supported in part by MSIT and POSTECH.
Author contributions
D.-H.N., Youngu L., and S.B. conceived and supervised the project. J.K. carried out sample synthesis and characterization. M.K. and B.K. assisted sample preparation. T.L. performed electrochemical CO2RR, in situ Raman and operando XAS analysis. J.E. assisted electrochemical CO2RR measurement. W.K., D.B., Yujin L., S.P. contributed to the real time analysis. H.J. and J.P. participated in data collection. S.B. and H.D.J. contributed to the DFT calculation and analysis. All authors discussed the results and contributed to writing the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data that support the findings of this study are available from the corresponding authors upon request. Source data for the figures in the main text are provided with this paper. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jongyoun Kim, Taemin Lee, Hyun Dong Jung.
Contributor Information
Seoin Back, Email: sback@sogang.ac.kr.
Youngu Lee, Email: youngulee@dgist.ac.kr.
Dae-Hyun Nam, Email: dhnam@dgist.ac.kr.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-44586-0.
References
- 1.Ross MB, et al. Designing materials for electrochemical carbon dioxide recycling. Nat. Catal. 2019;2:648–658. doi: 10.1038/s41929-019-0306-7. [DOI] [Google Scholar]
- 2.Li F, et al. Molecular tuning of CO2-to-ethylene conversion. Nature. 2020;577:509–513. doi: 10.1038/s41586-019-1782-2. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, et al. Electrochemical CO2-to-ethylene conversion on polyamine-incorporated Cu electrodes. Nat. Catal. 2021;4:20–27. doi: 10.1038/s41929-020-00547-0. [DOI] [Google Scholar]
- 4.Kim J-Y, et al. Quasi-graphitic carbon shell-induced Cu confinement promotes electrocatalytic CO2 reduction toward C2+ products. Nat. Commun. 2021;12:3765. doi: 10.1038/s41467-021-24105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li F, et al. Cooperative CO2-to-ethanol conversion via enriched intermediates at molecule–metal catalyst interfaces. Nat. Catal. 2020;3:75–82. doi: 10.1038/s41929-019-0383-7. [DOI] [Google Scholar]
- 6.Wang X, et al. Efficient electrically powered CO2-to-ethanol via suppression of deoxygenation. Nat. Energy. 2020;5:478–486. doi: 10.1038/s41560-020-0607-8. [DOI] [Google Scholar]
- 7.Zhao Y, et al. Industrial-current-density CO2-to-C2+ electroreduction by anti-swelling anion-exchange ionomer-modified oxide-derived Cu nanosheets. J. Am. Chem. Soc. 2022;144:10446–10454. doi: 10.1021/jacs.2c02594. [DOI] [PubMed] [Google Scholar]
- 8.Xiao C, Zhang J. Architectural design for enhanced C2 product selectivity in electrochemical CO2 reduction using Cu-based catalysts: a review. ACS Nano. 2021;15:7975–8000. doi: 10.1021/acsnano.0c10697. [DOI] [PubMed] [Google Scholar]
- 9.Nitopi S, et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 2019;119:7610–7672. doi: 10.1021/acs.chemrev.8b00705. [DOI] [PubMed] [Google Scholar]
- 10.Gao D, et al. Activity and selectivity control in CO2 electroreduction to multicarbon products over CuOx catalysts via electrolyte design. ACS Catal. 2018;8:10012–10020. doi: 10.1021/acscatal.8b02587. [DOI] [Google Scholar]
- 11.Monteiro MCO, et al. Absence of CO2 electroreduction on copper, gold and silver electrodes without metal cations in solution. Nat. Catal. 2021;4:654–662. doi: 10.1038/s41929-021-00655-5. [DOI] [Google Scholar]
- 12.Ren S, et al. Molecular electrocatalysts can mediate fast, selective CO2 reduction in a flow cell. Science. 2019;365:367–369. doi: 10.1126/science.aax4608. [DOI] [PubMed] [Google Scholar]
- 13.Weekes DM, et al. Electrolytic CO2 reduction in a flow cell. Acc. Chem. Res. 2018;51:910–918. doi: 10.1021/acs.accounts.8b00010. [DOI] [PubMed] [Google Scholar]
- 14.Tan YC, Lee KB, Song H, Oh J. Modulating local CO2 concentration as a general strategy for enhancing C−C coupling in CO2 electroreduction. Joule. 2020;4:1104–1120. doi: 10.1016/j.joule.2020.03.013. [DOI] [Google Scholar]
- 15.Bondue CJ, Graf M, Goyal A, Koper MTM. Suppression of hydrogen evolution in acidic electrolytes by electrochemical CO2 reduction. J. Am. Chem. Soc. 2021;143:279–285. doi: 10.1021/jacs.0c10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nam D-H, et al. Molecular enhancement of heterogeneous CO2 reduction. Nat. Mater. 2020;19:266–276. doi: 10.1038/s41563-020-0610-2. [DOI] [PubMed] [Google Scholar]
- 17.Kim C, et al. Tailored catalyst microenvironments for CO2 electroreduction to multicarbon products on copper using bilayer ionomer coatings. Nat. Energy. 2021;6:1026–1034. doi: 10.1038/s41560-021-00920-8. [DOI] [Google Scholar]
- 18.García de Arquer FP, et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science. 2020;367:661–666. doi: 10.1126/science.aay4217. [DOI] [PubMed] [Google Scholar]
- 19.Wagner A, Sahm CD, Reisner E. Towards molecular understanding of local chemical environment effects in electro- and photocatalytic CO2 reduction. Nat. Catal. 2020;3:775–786. doi: 10.1038/s41929-020-00512-x. [DOI] [Google Scholar]
- 20.Wang J, et al. Selective CO2 electrochemical reduction enabled by a tricomponent copolymer modifier on a copper surface. J. Am. Chem. Soc. 2021;143:2857–2865. doi: 10.1021/jacs.0c12478. [DOI] [PubMed] [Google Scholar]
- 21.Wei X, et al. Highly selective reduction of CO2 to C2+ hydrocarbons at copper/polyaniline interfaces. ACS Catal. 2020;10:4103–4111. doi: 10.1021/acscatal.0c00049. [DOI] [Google Scholar]
- 22.Liang H-Q, et al. Hydrophobic copper interfaces boost electroreduction of carbon dioxide to ethylene in water. ACS Catal. 2021;11:958–966. doi: 10.1021/acscatal.0c03766. [DOI] [Google Scholar]
- 23.Wakerley D, et al. Bio-inspired hydrophobicity promotes CO2 reduction on a Cu surface. Nat. Mater. 2019;18:1222–1227. doi: 10.1038/s41563-019-0445-x. [DOI] [PubMed] [Google Scholar]
- 24.Lin Y, et al. Tunable CO2 electroreduction to ethanol and ethylene with controllable interfacial wettability. Nat. Commun. 2023;14:3575. doi: 10.1038/s41467-023-39351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xing Z, Hu X, Feng X. Tuning the microenvironment in gas-diffusion electrodes enables high-rate CO2 electrolysis to formate. ACS Energy Lett. 2021;6:1694–1702. doi: 10.1021/acsenergylett.1c00612. [DOI] [Google Scholar]
- 26.Xiong J, Wang Y, Xue Q, Wu X. Synthesis of highly stable dispersions of nanosized copper particles using L-ascorbic acid. Green Chem. 2011;13:900–904. doi: 10.1039/c0gc00772b. [DOI] [Google Scholar]
- 27.Song J, et al. Synthesis of Au/graphene oxide composites for selective and sensitive electrochemical detection of ascorbic acid. Sci. Rep. 2014;4:7515. doi: 10.1038/srep07515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agar IT, Streif J, Bangerth F. Effect of high CO2 and controlled atmosphere (CA) on the ascorbic and dehydroascorbic acid content of some berry fruits. Postharvest Biol. Technol. 1997;11:47–55. doi: 10.1016/S0925-5214(97)01414-2. [DOI] [Google Scholar]
- 29.Arayachukiat S, et al. Ascorbic acid as a bifunctional hydrogen bond donor for the synthesis of cyclic carbonates from CO2 under ambient conditions. ACS Sustain. Chem. Eng. 2017;5:6392–6397. doi: 10.1021/acssuschemeng.7b01650. [DOI] [Google Scholar]
- 30.Pastero L, et al. CO2 capture and sequestration in stable Ca-oxalate, via Ca-ascorbate promoted green reaction. Sci. Total Environ. 2019;666:1232–1244. doi: 10.1016/j.scitotenv.2019.02.114. [DOI] [PubMed] [Google Scholar]
- 31.Pastero L, Marengo A, Boero R, Pavese A. Non-conventional CO2 sequestration via vitamin C promoted green reaction: yield evaluation. J. CO2 Util. 2021;44:101420. doi: 10.1016/j.jcou.2020.101420. [DOI] [Google Scholar]
- 32.Choi S, et al. Redox-transition from irreversible to reversible vitamin C by pore confinement in microporous carbon network. ACS Appl. Mater. Interfaces. 2022;14:36557–36569. doi: 10.1021/acsami.2c07719. [DOI] [PubMed] [Google Scholar]
- 33.De Silva KKH, Huang H-H, Joshi RK, Yoshimura M. Chemical reduction of graphene oxide using green reductants. Carbon. 2017;119:190–199. doi: 10.1016/j.carbon.2017.04.025. [DOI] [Google Scholar]
- 34.Kwon Y-B, et al. Simultaneous reduction and functionalization of graphene oxide sheets with tannic acid for a strong composite material with multi-modally interactive interfaces. Diam. Relat. Mater. 2021;119:108565. doi: 10.1016/j.diamond.2021.108565. [DOI] [Google Scholar]
- 35.Kwon Y-B, Lee S-R, Seo TH, Kim Y-K. Fabrication of a strong artificial nacre based on tannic acid-functionalized graphene oxide and poly(vinyl alcohol) through their multidentate hydrogen bonding. Macromol. Res. 2022;30:279–284. doi: 10.1007/s13233-022-0031-y. [DOI] [Google Scholar]
- 36.Kim J, et al. Ultrastable 2D material-wrapped copper nanowires for high-performance flexible and transparent energy devices. Nano Energy. 2023;106:108067. doi: 10.1016/j.nanoen.2022.108067. [DOI] [Google Scholar]
- 37.Ahn Y, Jeong Y, Lee D, Lee Y. Copper nanowire–graphene core–shell nanostructure for highly stable transparent conducting electrodes. ACS Nano. 2015;9:3125–3133. doi: 10.1021/acsnano.5b00053. [DOI] [PubMed] [Google Scholar]
- 38.Tang L, et al. Bottom-up synthesis of large-scale graphene oxide nanosheets. J. Mater. Chem. 2012;22:5676–5683. doi: 10.1039/c2jm15944a. [DOI] [Google Scholar]
- 39.Pan G-H, et al. Photochemical reaction of vitamin C with silicon nanocrystals: polymerization, hydrolysis and photoluminescence. J. Mater. Chem. C. 2013;1:5856–5865. doi: 10.1039/c3tc31082e. [DOI] [Google Scholar]
- 40.Jingyan S, Yuwen L, Zhiyong W, Cunxin W. Investigation of thermal decomposition of ascorbic acid by TG-FTIR and thermal kinetics analysis. J. Pharm. Biomed. Anal. 2013;77:116–119. doi: 10.1016/j.jpba.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 41.Gerhardt GA, et al. Nafion-coated electrodes with high selectivity for CNS electrochemistry. Brain Res. 1984;290:390–395. doi: 10.1016/0006-8993(84)90963-6. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, et al. Structure-sensitive CO2 electroreduction to hydrocarbons on ultrathin 5-fold twinned copper nanowires. Nano Lett. 2017;17:1312–1317. doi: 10.1021/acs.nanolett.6b05287. [DOI] [PubMed] [Google Scholar]
- 43.Zhang T, et al. Regulation of functional groups on graphene quantum dots directs selective CO2 to CH4 conversion. Nat. Commun. 2021;12:5265. doi: 10.1038/s41467-021-25640-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torrisi L, Cutroneo M, Torrisi A, Silipigni L. Nitrogen diffusion in graphene oxide and reduced graphene oxide foils. Vacuum. 2021;194:110632. doi: 10.1016/j.vacuum.2021.110632. [DOI] [Google Scholar]
- 45.Chen C, et al. Highly efficient electroreduction of CO2 to C2+ alcohols on heterogeneous dual active sites. Angew. Chem. Int. Ed. 2020;59:16459–16464. doi: 10.1002/anie.202006847. [DOI] [PubMed] [Google Scholar]
- 46.Chernyshova IV, Somasundaran P, Ponnurangam S. On the origin of the elusive first intermediate of CO2 electroreduction. Proc. Natl Acad. Sci. USA. 2018;115:E9261–E9270. doi: 10.1073/pnas.1802256115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deng Y, Yeo BS. Characterization of electrocatalytic water splitting and CO2 reduction reactions using in situ/operando Raman spectroscopy. ACS Catal. 2017;7:7873–7889. doi: 10.1021/acscatal.7b02561. [DOI] [Google Scholar]
- 48.Tadesse LF, et al. Toward rapid infectious disease diagnosis with advances in surface-enhanced Raman spectroscopy. J. Chem. Phys. 2020;152:240902. doi: 10.1063/1.5142767. [DOI] [PubMed] [Google Scholar]
- 49.Zhao Y, et al. Elucidating electrochemical CO2 reduction reaction processes on Cu(hkl) single-crystal surfaces by in situ Raman spectroscopy. Energy Environ. Sci. 2022;15:3968–3977. doi: 10.1039/D2EE01334G. [DOI] [Google Scholar]
- 50.Zhan C, et al. Revealing the CO coverage-driven C-C coupling mechanism for electrochemical CO2 reduction on Cu2O nanocubes via operando Raman spectroscopy. ACS Catal. 2021;11:7694–7701. doi: 10.1021/acscatal.1c01478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng T, Xiao H, Goddard WA., III Reaction mechanisms for the electrochemical reduction of CO2 to CO and formate on the Cu(100) surface at 298 K from quantum mechanics free energy calculations with explicit water. J. Am. Chem. Soc. 2016;138:13802–13805. doi: 10.1021/jacs.6b08534. [DOI] [PubMed] [Google Scholar]
- 52.Ye Y, et al. Dramatic differences in carbon dioxide adsorption and initial steps of reduction between silver and copper. Nat. Commun. 2019;10:1875. doi: 10.1038/s41467-019-09846-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen J, et al. Ascorbate oxidation by iron, copper and reactive oxygen species: review, model development, and derivation of key rate constants. Sci. Rep. 2021;11:7417. doi: 10.1038/s41598-021-86477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams NH, Yandell JK. Outer-sphere electron-transfer reactions of ascorbate anions. Aust. J. Chem. 1982;35:1133–1144. doi: 10.1071/CH9821133. [DOI] [Google Scholar]
- 55.Fan L, et al. Proton sponge promotion of electrochemical CO2 reduction to multi-carbon products. Joule. 2022;6:205–220. doi: 10.1016/j.joule.2021.12.002. [DOI] [Google Scholar]
- 56.Montoya JH, Shi C, Chan K, Nørskov JK. Theoretical insights into a CO dimerization mechanism in CO2 electroreduction. J. Phys. Chem. Lett. 2015;6:2032–2037. doi: 10.1021/acs.jpclett.5b00722. [DOI] [PubMed] [Google Scholar]
- 57.Calle-Vallejo F, Koper MTM. Theoretical considerations on the electroreduction of CO to C2 species on Cu (100) electrodes. Angew. Chem. Int. Ed. 2013;52:7282–7285. doi: 10.1002/anie.201301470. [DOI] [PubMed] [Google Scholar]
- 58.Lin S-C, et al. Operando time-resolved X-ray absorption spectroscopy reveals the chemical nature enabling highly selective CO2 reduction. Nat. Commun. 2020;11:3525. doi: 10.1038/s41467-020-17231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nørskov JK, et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B. 2004;108:17886–17892. doi: 10.1021/jp047349j. [DOI] [Google Scholar]
- 60.Peterson AA, et al. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 2010;3:1311–1315. doi: 10.1039/c0ee00071j. [DOI] [Google Scholar]
- 61.Back S, Kim J-H, Kim Y-T, Jung Y. Bifunctional interface of Au and Cu for improved CO2 electroreduction. ACS Appl. Mater. Interfaces. 2016;8:23022–23027. doi: 10.1021/acsami.6b05903. [DOI] [PubMed] [Google Scholar]
- 62.Kim D, Shi J, Liu Y. Substantial impact of charge on electrochemical reactions of two-dimensional materials. J. Am. Chem. Soc. 2018;140:9127–9131. doi: 10.1021/jacs.8b03002. [DOI] [PubMed] [Google Scholar]
- 63.Melander MM, Kuisma MJ, Christensen TEK, Honkala K. Grand-canonical approach to density functional theory of electrocatalytic systems: thermodynamics of solid-liquid interfaces at constant ion and electrode potentials. J. Chem. Phys. 2019;150:041706. doi: 10.1063/1.5047829. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y, Liu T, Li Y. Why heterogeneous single-atom catalysts preferentially produce CO in the electrochemical CO2 reduction reaction. Chem. Sci. 2022;13:6366–6372. doi: 10.1039/D2SC01593E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alsunni YA, Alherz AW, Musgrave CB. Electrocatalytic reduction of CO2 to CO over Ag(110) and Cu(211) modeled by grand-canonical density functional theory. J. Phys. Chem. C. 2021;125:23773–23783. doi: 10.1021/acs.jpcc.1c07484. [DOI] [Google Scholar]
- 66.Choi C, et al. Understanding potential-dependent competition between electrocatalytic dinitrogen and proton reduction reactions. Nat. Commun. 2021;12:4353. doi: 10.1038/s41467-021-24539-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mathew K, et al. Implicit self-consistent electrolyte model in plane-wave density-functional theory. J. Chem. Phys. 2019;151:234101. doi: 10.1063/1.5132354. [DOI] [PubMed] [Google Scholar]
- 68.Jinnouchi R, Anderson AB. Aqueous and surface redox potentials from self-consistently determined Gibbs energies. J. Phys. Chem. C. 2008;112:8747–8750. doi: 10.1021/jp802627s. [DOI] [Google Scholar]
- 69.Garza AJ, Bell AT, Head-Gordon M. Mechanism of CO2 reduction at copper surfaces: pathways to C2 products. ACS Catal. 2018;8:1490–1499. doi: 10.1021/acscatal.7b03477. [DOI] [Google Scholar]
- 70.Kresse G, Furthmüller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996;6:15–50. doi: 10.1016/0927-0256(96)00008-0. [DOI] [PubMed] [Google Scholar]
- 71.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996;77:3865. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 72.Hammer B, Hansen LB, Nørskov JK. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B. 1999;59:7413. doi: 10.1103/PhysRevB.59.7413. [DOI] [Google Scholar]
- 73.Grimme S, Ehrlich S, Goerigk L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011;32:1456–1465. doi: 10.1002/jcc.21759. [DOI] [PubMed] [Google Scholar]
- 74.Monkhorst HJ, Pack JD. Special points for Brillouin-zone integrations. Phys. Rev. B. 1976;13:5188. doi: 10.1103/PhysRevB.13.5188. [DOI] [Google Scholar]
- 75.Henkelman G, Uberuaga BP, Jónsson H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000;113:9901–9904. doi: 10.1063/1.1329672. [DOI] [Google Scholar]
- 76.Henkelman G, Jónsson H. A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives. J. Chem. Phys. 1999;111:7010–7022. doi: 10.1063/1.480097. [DOI] [Google Scholar]
- 77.Shin S-J, et al. A unifying mechanism for cation effect modulating C1 and C2 productions from CO2 electroreduction. Nat. Commun. 2022;13:5482. doi: 10.1038/s41467-022-33199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yue J, Du Z, Shao M. The role of citric acid and ascorbic acid in morphology control of palladium nanocrystals: a molecular dynamics and density functional theory study. Chem. Phys. Lett. 2016;659:159–163. doi: 10.1016/j.cplett.2016.07.019. [DOI] [Google Scholar]
- 79.Larsen AH, et al. The atomic simulation environment—a Python library for working with atoms. J. Phys. Condens. Matter. 2017;29:273002. doi: 10.1088/1361-648X/aa680e. [DOI] [PubMed] [Google Scholar]
- 80.Granda-Marulanda LP, et al. A semiempirical method to detect and correct DFT-based gas-phase errors and its application in electrocatalysis. ACS Catal. 2020;10:6900–6907. doi: 10.1021/acscatal.0c01075. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon request. Source data for the figures in the main text are provided with this paper. Source data are provided with this paper.