Abstract
BACKGROUND
Mesenchymal stem cells (MSCs) are a type of stem cells that possess relevant regenerative abilities and can be used to treat many chronic diseases. Diabetes mellitus (DM) is a frequently diagnosed chronic disease characterized by hyperglycemia which initiates many multisystem complications in the long-run. DM patients can benefit from MSCs transplantation to curb down the pathological consequences associated with hyperglycemia persistence and restore the function of damaged tissues. MSCs therapeutic outcomes are found to last for short period of time and ultimately these regenerative cells are eradicated and died in DM disease model.
AIM
To investigate the impact of high glucose or hyperglycemia on the cellular and molecular characteristics of MSCs.
METHODS
Human adipose tissue-derived MSCs (hAD-MSCs) were seeded in low (5.6 mmol/L of glucose) and high glucose (25 mmol/L of glucose) for 7 d. Cytotoxicity, viability, mitochondrial dynamics, and apoptosis were deplored using specific kits. Western blotting was performed to measure the protein expression of phosphatidylinositol 3-kinase (PI3K), TSC1, and mammalian target of rapamycin (mTOR) in these cells.
RESULTS
hAD-MSCs cultured in high glucose for 7 d demonstrated marked decrease in their viability, as shown by a significant increase in lactate dehydrogenase (P < 0.01) and a significant decrease in Trypan blue (P < 0.05) in these cells compared to low glucose control. Mitochondrial membrane potential, indicated by tetramethylrhodamine ethyl ester (TMRE) fluorescence intensity, and nicotinamide adenine dinucleotide (NAD+)/NADH ratio were significantly dropped (P < 0.05 for TMRE and P < 0.01 for NAD+/NADH) in high glucose exposed hAD-MSCs, indicating disturbed mitochondrial function. PI3K protein expression significantly decreased in high glucose culture MSCs (P < 0.05 compared to low glucose) and it was coupled with significant upregulation in TSC1 (P < 0.05) and downregulation in mTOR protein expression (P < 0.05). Mitochondrial complexes I, IV, and V were downregulated profoundly in high glucose (P < 0.05 compared to low glucose). Apoptosis was induced as a result of mitochondrial impairment and explained the poor survival of MSCs in high glucose.
CONCLUSION
High glucose impaired the mitochondrial dynamics and regulatory proteins in hAD-MSCs ensuing their poor survival and high apoptosis rate in hyperglycemic microenvironment.
Keywords: Mesenchymal stem cells, High glucose, Mitochondrial dynamics, Apoptosis, Poor survival, Phosphatidylinositol 3-kinase/mammalian target of rapamycin pathway
Core Tip: Mesenchymal stem cells (MSCs) have significant regenerative properties that make them a potential treatment for many chronic diseases. Among these, diabetes mellitus (DM) was found to benefit from MSCs transplantation in which they restored the damaged tissues and prevented hyperglycemia-related complications. However, these therapeutic are short-lived hindering the clinical use of MSCs in the treatment of DM. This study aims to elucidate the mechanisms of hyperglycemia-induced effects on MSCs which will help in improving the therapeutic functions of these cells in this stress environment.
INTRODUCTION
Mesenchymal stem cells (MSCs) are the most commonly studied forms of stem cells as they possess powerful regenerative and immunomodulatory abilities[1]. MSCs have unique molecular machinery that empowers them to modulate many cellular signaling pathways and restore the functionality of damaged tissues rendering them suitable to treat many acute and chronic diseases[1]. Despite their unprecedented abilities to adapt to many stress conditions, they can still behave unexpectedly under certain stress microenvironments. Many preclinical and clinical studies not only demonstrated the positive outcomes after transplanting MSCs to injured sites[2,3], but also reported the short-lived therapeutic results in hostile microenvironments which need to be figured out[4]. When MSCs are undifferentiated, they rely mainly on glycolysis to support their proliferation, expansion, and immunomodulation[5]. When MSCs are programmed to differentiate, they switch to oxidative phosphorylation (OxPhos)[6], thereby, intact mitochondria are also important to maintain their multi-lineage differentiation capacities[6].
In the last decades, diabetes mellitus (DM) incidence has increased rapidly, and it is deemed among top five chronic diseases[7]. DM is associated with serious multisystem complications that impair the life quality of many patients[8]. DM is characterized mainly by chronic hyperglycemic state that causes many devastating, long-term complications[8]. DM patients can benefit remarkably from MSCs therapy to repair and regenerate damaged tissues, restore their functionality, and reduce the severity of DM-related pathological complications[7]. Several studies reported a significant improvement in glycemic parameters and cardiovascular complications after transplanting MSCs in DM patients[7,9,10]; however, other studies reported that DM can accelerate the decline in MSCs quantity and therapeutic quality, and impair their regenerative capabilities[11]. When MSCs turn into a dysfunctioning cells, they may increase the severity of DM-related complications[11]. Although previous studies had addressed the effects of hyperglycemia on the molecular and cellular characteristics of transplanted MSCs, many aspects still need to be elucidated. Thus, it is important to investigate the effect of high glucose on MSCs biological dynamics, which are essential to understand their fate and performance in diabetic microenvironment, and provide new avenues on possible molecular targets to modulate their survival and therapeutic outcomes in DM patients.
MATERIALS AND METHODS
Human MSCs
Human adipose tissue-derived MSCs (hAD-MSCs) were commercially purchased from Lonza (Cat# PT5006, Lot# 21TL138912) and expanded using Dulbecco’s Modified Eagle’s Medium Low Glucose (DMEM-Low Glucose, Euroclone) which contained 5.6 mmol/L glucose and were supplemented with 10% fetal bovine serum (FBS, Gibco)[12], 0.1 mg/mL streptomycin and 100 units/mL penicillin G in standard cell culture incubators (5% CO2/95% air; 37 °C). Medium was changed every 72 h, and cells were sub-cultured when confluence exceeded 60%[13]. For high glucose conditions, cells were cultured in Dulbecco’s Modified Eagle’s Medium High Glucose (DMEM-High Glucose, Euroclone) which contained 25 mmol/L glucose[14] and were supplemented with 10% FBS, 0.1 mg/mL streptomycin and 100 units/mL penicillin G for 3, 7, and 14 d in standard cell culture incubators (5% CO2/95% air; 37 °C). High glucose complete medium was changed every 72 h. MSCs that were cultured in low glucose were considered as our study control. MSCs of passage 5 and 6 were used to perform the experiments. AD-MSCs characterization markers can be found in (Table 1).
Table 1.
Cell surface characterization of human adipose tissue-derived mesenchymal stem cells based on the company analysis report
|
Positive markers
|
Negative markers
|
| CD13 | CD14 |
| CD29 | CD31 |
| CD44 | CD45 |
| CD73 | HLA-DR |
| CD90 | |
| CD105 | |
| CD166 |
Cytotoxicity assays
To measure the level of cytotoxicity in human MSCs after being cultured in low and high glucose, we seeded 5 × 104 cells/well in 96 well plate and we measured the lactate dehydrogenase (LDH) which was released from the damaged MSCs (LDH Assay Kit, Abcam, Cat# ab102526) and the absorbance values were obtained using Cytation 5 (BioTek, United States). The viability of MSCs in low and high glucose were also assessed using 0.4% Trypan blue and the percentage of viable cells as well as representative fluorescent images were obtained using Corning® CytoSmart Cell Counter.
Tetramethylrhodamine ethyl ester (MtMP measurement)
Mitochondrial membrane potential was assessed using tetramethylrhodamine ethyl ester (TMRE)-Mitochondrial Membrane Potential Assay Kit (Abcam, Cat # ab113852). Briefly, human AD-MSCs were placed in 24-well plate at 1 × 104 cells per well and allowed to adhere overnight. After that, cells were cultured either in low glucose or high glucose for 7 d. Then, media were aspirated, and cells were stained using 400 nM TMRE in culture media for 30 min in the incubator, and then media were replaced with 200 μL phosphate buffered saline (PBS) per well. Fluorescence intensity was detected using Cytation 5 (BioTek, United States). (λex = 549 nm, λem = 575 nm), and TMRE fluorescent images were captured at Texas red filter using Cytation 5 (BioTek, United States).
Nicotinamide adenine dinucleotide /NADH assay
Nicotinamide adenine dinucleotide (NAD+)/NADH ratio in AD-MSCs that were cultured in low and high glucose media was assessed using NAD/NADH-Glo™ assay kit (Promega, Cat# G9071). Briefly, cells were cultured in low and high glucose media for 7 d. After that, cells were detached and placed in 96 well plate at 5 × 105 in 50 μL of either low or high glucose media and were allowed to adhere overnight. 50 μL of NAD/NADH-Glo™ Detection Reagent was added to each well and the plate was gently shaken to mix and lyse the cells and was incubated with the added reagent for 60 min at room temperature. The luminescence values were recorded using Cytation 5 (BioTek, United States) which were corresponding to the total amount of NAD+ produced from NADH.
Western blotting
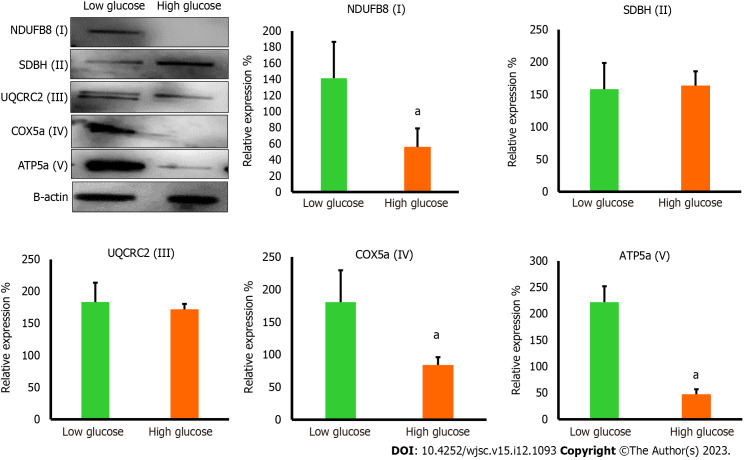
The protein levels for phosphatidylinositol 3-kinase (PI3K) (Santa Cruz Biotechnology Cat # sc-1637), TSC1 (Santa Cruz Biotechnology Cat # sc-377386), and mammalian target of rapamycin (mTOR) (Santa Cruz Biotechnology Cat # sc-293133), NDUFB8 (Abcam, Cat # ab192878), SDHB (Santa Cruz Biotechnology Cat # sc-271548), UQCRC2 (Santa Cruz Biotechnology Cat # sc-390378), COX5a (Santa Cruz Biotechnology Cat # sc-376907), ATP5A (Santa Cruz Biotechnology Cat # sc-136178), and β-actin (Santa Cruz Biotechnology Cat # sc-47778 HRP) were measured by western blot. Briefly, total protein levels were measured using NanoDrop™ Lite Spectrophotometer, and 40 μg of protein was loaded onto sodium-dodecyl sulfate gel electrophoresis. Following electrophoresis, proteins were transferred to polyvinylidene fluoride membrane and were incubated with appropriate primary and secondary antibodies. The membranes were visualized using VILBER FUSION Gel Documentation System, and bands were quantified using ImageJ for densitometry.
Apoptosis assay
To detect apoptosis in hAD-MSCs after being cultured in low and high glucose, we used RealTime-Glo™ Annexin V Apoptosis live assay (Promega, Cat# JA1011, Lot# 0000400486 following the manufacturer’s guidelines. Briefly, cells were seeded 1 × 104 cells/well in 24 well plate and then cultured in low and high glucose for 7 d. The media were aspirated and each well was washed with PBS followed by the addition of 100 μL of fresh medium having Annexin V-LgBiT, Annexin V-SmBiT, CaCl2, and Annexin V NanoBiT Substrate to each well. After 1 h incubation, the fluorescent images of green color which represented cells undergoing apoptosis were detected at GFP filter using Cytation 5 (BioTek, United States).
Statistical analysis
Data were reported as mean ± SD. Comparison of data between multiple groups was performed using one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc multiple comparison test, and analysis between two groups was made using Student’s t-test (two-tailed). Statistical significance is determined as P < 0.05. Each figure represents one of at least three independent quantifiable experiments.
RESULTS
Culturing MSCs in high glucose triggers massive cytotoxicity and poor survival
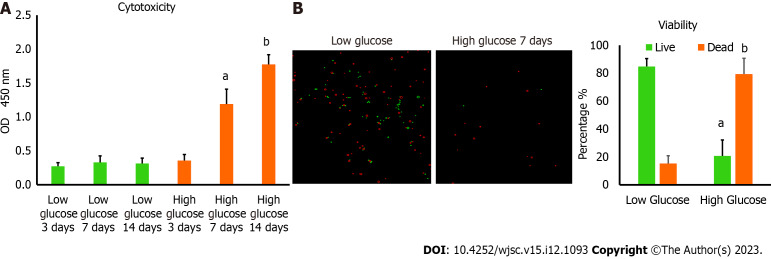
MSCs are known to survive well in low glucose medium which is a physiological requirement to preserve their stemness features. We want to determine the viability of these cells in high glucose microenvironment. hAD-MSCs were cultured in high glucose medium containing 25 mmol/L of glucose for 3, 7, and 14 d. The cytotoxicity was measured by determining the amount of LDH released and was found to be significantly greater (P < 0.01) in hAD-MSCs cultured in high glucose for 7 and 14 d compared with hAD-MSCs cultured in normal low glucose medium containing 5.6 mmol/L of glucose (Figure 1A). To confirm these findings, the viability of hAD-MSCs was measured using 0.4% Trypan blue and found to be remarkably low (P < 0.05) in hAD-MSCs that were cultured in high glucose for 7 d compared to hAD-MSCs cultured in a low glucose medium (Figure 1B).
Figure 1.
Culturing human adipose tissue-derived mesenchymal stem cells in high glucose reduced their viability. A: Lactate dehydrogenase cytotoxicity assay revealed higher level of cytotoxicity in human adipose tissue-derived mesenchymal stem cells (hAD-MSCs) cultured in high glucose for 7 and 14 d compared to cells cultured in low glucose. n = 5, aP < 0.01 compared to low glucose 7 d, bP < 0.01 compared to low glucose 14 d; B: The percentage of cells viability detected by 0.4% Trypan blue showed significant reduction in the number of viable hAD-MSCs after being cultured in high glucose for 7 d. Fluorescent images of live and dead cells were obtained using Corning® CytoSmart Cell Counter. n = 5. n = 5, aP < 0.01 compared to live cells in low glucose 7 d, bP < 0.01 compared to dead cells in low glucose 7 d.
The mitochondrial dynamics of MSCs impaired by high glucose
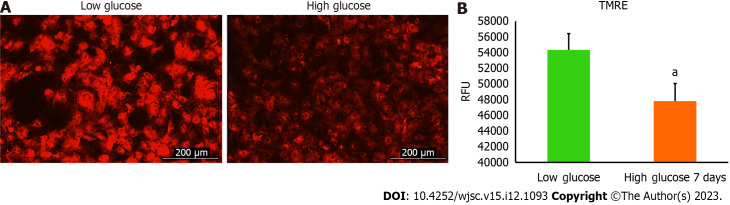
Mitochondria are integral intracellular organelles promoting cells survival and viability. The variance of mitochondrial dynamics depends on the type of microenvironment where cells exist. Previous reports demonstrated that high glucose can induce the fission and fragmentation of mitochondria in glomeruli podocytes[15], but the knowledge surrounding the impact of high glucose on the mitochondrial dynamics of MSCs is still limited. The mitochondrial membrane potential (ΔΨm) generated by proton pumps (complexes I, III and IV) is imperative to ensure proper ATP production by mitochondrial OxPhos. Abnormalities in the mitochondrial membrane potential (ΔΨm) can significantly elicit a decline in cells viability and trigger unwanted signaling pathways. We measured the mitochondrial membrane potential (ΔΨm) in hAD-MSCs after being grown in a high glucose medium for 7 d using TMRE which is sequestered by active mitochondria. Our results illustrated that the fluorescent intensity of TMRE was significantly low (P < 0.05) in hAD-MSCs cultured in a high glucose medium compared to hAD-MSCs cultured in a normal low glucose medium indicating an impairment in the mitochondrial membrane potential in these cells (Figures 2A and B). NAD+ and its reduced form (NADH) regulate many metabolic pathways, including mitochondrial OxPhos. Maintaining NAD+/NADH pool is required to ensure that the influx of electrons is coupled with the translocation of protons to the intermembrane space to generate the required membrane potential (ΔΨm) across the inner mitochondrial membrane to be harnessed by complex V to produce ATP[16]. To investigate if the decrease in the mitochondrial membrane potential (ΔΨm) is correlated with abnormalities in NAD+/NADH pool, we measure the NAD+/NADH ratio in hAD-MSCs cultured in low and high glucose media (Figure 3). The findings revealed a considerable drop (P < 0.01) in NAD+/NADH ratio when hAD-MSCs were cultured in high glucose. This may explain the disturbance in the inner mitochondrial potential detected by TMRE assay (Figures 2A and B).
Figure 2.
High glucose decreased the mitochondrial membrane potential level of human adipose tissue-derived mesenchymal stem cells. The tetramethylrhodamine ethyl ester (TMRE) fluorescence intensity values of human adipose tissue-derived mesenchymal stem cells (hAD-MSCs) cultured in high glucose for 7 d were substantially declined compared to fluorescent intensity values of cells cultured in low glucose. Live fluorescent images showed a weak signal of TMRE stain in hAD-MSCs after being placed in high glucose for 7 d compared to strong TMRE signal in low glucose cultured cells. n = 4, aP < 0.05 compared to low glucose control. Fluroscnet images were captured at Texas Red filter using Cytation 5 (BioTek, United States). TMRE: Tetramethylrhodamine ethyl ester; RFU: Relative fluorescence units.
Figure 3.
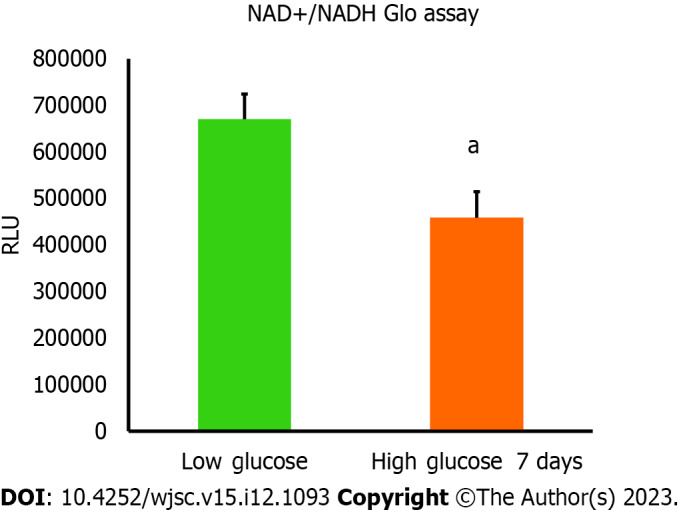
Nicotinamide adenine dinucleotide and NADH amounts were declined in high glucose cultured human adipose tissue-derived mesenchymal stem cells. The amount of nicotinamide adenine dinucleotide (NAD+) produced from NADH was measured using NAD+/NADH Glo assay. Luminescence intensity was significantly less in human adipose tissue-derived mesenchymal stem cells cultured in high glucose for 7 d compared to cells cultured in low glucose. n = 4, aP < 0.01 compared to low glucose control. Luminescence values were measured using Cytation 5 (BioTek, United States). RFU: Relative fluorescence units.
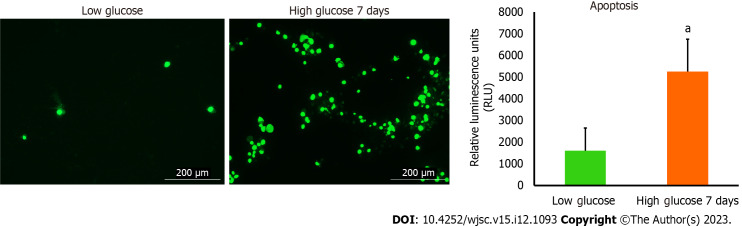
Disturbance of MSCs mitochondria dynamics parameters in high glucose engenders their apoptosis
Apoptosis is a programmed cell death that can be physiological or pathological depending on the triggering factors that enkindle it. Mitochondrial dysfunction is a notable inciting factor which is able to fire up debilitating cellular apoptosis and impact the survival of numerous cells. The noticeable deterioration in the mitochondrial parameters detected in hAD-MSCs after being cultured in high glucose prompted us to examine the level of apoptosis in these cells. To achieve that, we used RealTime-Glo™ Annexin V Apoptosis kit to unveil the presence of apoptosis or not. The luminescence signal which is proportionally linked to apoptosis intensity was considerably higher in hAD-MSCs that were cultured in high glucose (Figure 4). Fluorescent microscopic images showed that apoptosis (marked by green stained cells) was more in high glucose cultured hAD-MSCs (Figure 4).
Figure 4.
The level of apoptosis was higher in human adipose tissue-derived mesenchymal stem cells cultured in high glucose. RealTime-Glo™ Annexin V Apoptosis live assay was used. The level of apoptosis was remarkably increased in human adipose tissue-derived mesenchymal stem cells cultured in high glucose for 7 d. Green fluorescence intensity is corresponding to the number of cells undergoing apoptosis. Fluorescent images were captured at GFP filter using Cytation 5 (BioTek, United States). n = 3, aP < 0.05 compared to low glucose control.
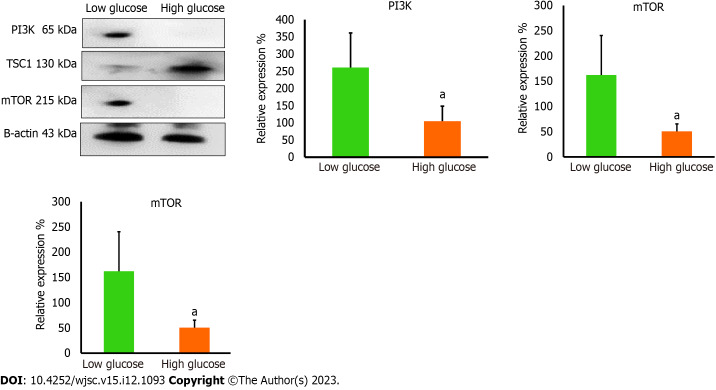
High glucose affects the level of PI3K and mTOR in MSCs
PI3K orchestrates many vital signaling pathways and targets downstream factors that are required to maintain the vivacity of cells. PI3K controls integral proteins that are necessary to preserve mitochondrial dynamics[17,18]. PI3K is a known activator of mTOR which is a central regulator of cell survival and metabolism. It has been reported that mTOR is important to preserve mitochondrial dynamics and generate the required mitochondrial potential to produce ATP. PI3K is required to remove the inhibitory effect of tuberous sclerosis tumor suppressor (TSC1) which binds mTOR and inactivates it[19]. We performed western blotting analysis to measure the expression levels of PI3K, TSC1, and mTOR in high glucose treated hAD-MSCs. Western blotting results demonstrated the loss of PI3K and mTOR in high glucose treated hAD-MSCs (P < 0.05 compared to low glucose), while TSC1 is significantly increased in these cells (P < 0.05 compared to low glucose) (Figure 5). Those findings indicated impaired PI3K/mTOR axis which may explain the disruption in mitochondrial parameters in high glucose cultured MSCs. Taking together, our findings highlight a new mechanism that can be released by high glucose causing the poor survival of MSCs in diabetic microenvironment.
Figure 5.
The protein levels of mitochondrial regulators were reduced in high glucose cultured human adipose tissue-derived mesenchymal stem cells. Western blot protein analysis showed significant reduction in the protein levels of phosphatidylinositol 3-kinase and mammalian target of rapamycin (mTOR) in high glucose cultured cells, while the protein level of mTOR inhibitory protein; TSC1, was elevated in high glucose cultured cells. These proteins are important to maintain proper mitochondrial dynamics. n = 3, aP < 0.05 compared to low glucose control. PI3K: Phosphatidylinositol 3-kinase; mTOR: Mammalian target of rapamycin.
High glucose disturbs major mitochondrial complexes in MSCs
The reduction in mTOR which is essential for inducing the biogenesis of mitochondrial complexes, most importantly complex I and V[20] prompted us to investigate the expression of mitochondrial complexes in low and high glucose-cultured hAD-MSCs. The results demonstrated that hAD-MSCs that were cultured in high glucose exhibited significant downregulation (P < 0.05) in the level of complex I, IV, and V comparing to hAD-MSCs that were cultured in low glucose (Figure 6), while the expression of complex II and III showed no significant changes (P = 0.85) in both culturing conditions (Figure 6). The disturbance in the level of these major complexes can impact the mitochondrial OxPhos and trigger the mitophagy of mitochondria[21].
Figure 6.
The protein levels of mitochondrial complexes were reduced in high glucose cultured human adipose tissue-derived mesenchymal stem cells. Western blot protein analysis showed significant reduction in the protein levels of NDUF6B (complex I), COX5a (complex IV), and ATP5a (complex V) in high glucose cultured human adipose tissue-derived mesenchymal stem cells comparing to low glucose cultured cells, while the protein levels of SDBH (complex II) and UQCRC2 (complex III) were not significantly changed in both culturing conditions. n = 3, aP < 0.05 compared to low glucose control.
DISCUSSION
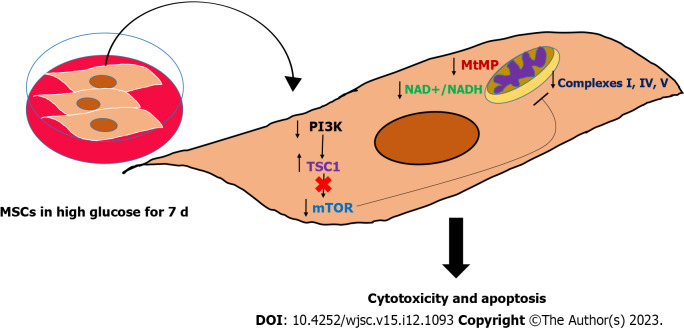
MSCs are the most promising type of stem cells for regenerating damaged tissues and restoring their normal functions which can improve the quality of life of many patients who suffer from debilitating disorders[1]. However, the remarkable improvements in pathological markers reported after transplanting MSCs[22,23] were hindered by the short-lived outcomes and poor survival rate, which need extensive investigation[24,25]. Many pathologies are characterized by stress microenvironments, including hypoxia, inflammation, and nutritional imbalances, which can impact the expected outcomes of transplanting MSCs[26-29], by either inhibiting or activating cellular signaling pathways. DM is a frequent chronic disease that leads to significant multi-system complications[8]. The major hallmark of DM is glucotoxicity, which is featured by the presence of high glucose levels and impaired insulin secretion and function[8]. This glucotoxicity produces massive molecular changes in the residing cells and cause the appearance of DM-related complications, such as cardiovascular and neurological complications[8]. Both animal and clinical studies have demonstrated the great therapeutic efficacy of MSCs transplantation in alleviating chronic hyperglycemia by reversing insulin resistance, improving insulin sensitivity, controlling inflammation, relieving metabolic syndrome symptoms, and ameliorating β-cell destructive abilities[10,30]. Nevertheless, studies also revealed that MSCs exhibited short-lived outcomes due to their poor survival after being transplanted in DM disease model[4]. Several studies have asserted the importance of investigating the mechanisms leading to the poor survival of MSCs in hyperglycemia which is the major insult to DM patients[24,31]. Up to date little is known about the molecular and biological changes in MSCs under hyperglycemia. MSCs are capable of switching their metabolism on to support their regenerative and immunomodulation abilities[32]. MSCs rely primarily on glycolysis when they are undifferentiated as it is important to preserve their stemness and proliferation[33]. Several reports illustrated that glycolysis is required to preserve the immunosuppressive characteristics of MSCs[34]. Upregulation in glycolysis has been found to increase the immunomodulatory abilities of MSCs and promote their capabilities to reprogram immune cells[35]. MSCs use mitochondrial OxPhos when they are induced to differentiate, thus the multi-lineage differentiation capacities of MSCs require intact mitochondria[6]. Maintaining intact mitochondrial dynamics is not only required to support the differentiation capacities of MSCs, but it is also crucial for redox regulation and prevention of excessive production of reactive oxygen species[36]. Recently, it has been proposed by many studies that MSCs are capable of transferring mitochondrial DNA to recipient cells and restoring the normal mitochondrial functions in these cells[37,38]. MSCs-mediated mitochondrial transfer further supports the necessity to conserve normal mitochondrial parameters[38]. Previous reports revealed that the MSCs proliferation decreased in high glucose medium[13,14]. Furthermore, other studies showed that MSCs differentiation capacities might vary when they are cultured in high glucose conditions[39,40]. Our study illustrated that high glucose can reduce the survival rate of MSCs. The reduction in MSCs viability reported in our study is correlated with an impairment in the inner mitochondrial membrane potential (ΔΨm) which is an important parameter and the energy generated from this potential gradient will be utilized by complex V to produce ATP molecules. The maintenance of mitochondrial membrane potential depends on the presence of a plentiful amount of NAD+ molecules[41]. NAD+ serves as an oxidoreductase cofactor that controls many vital energy production pathways. Maintaining normal NAD+/NADH ratio is integral to providing the required electron flux that will be used to generate proper mitochondrial membrane potential (ΔΨm)[41]. High glucose insult causing a substantial drop in NAD+/NADH ratio in hAD-MSCs indicates a needed disturbance in the oxidization power to produce NAD+ and donating electrons that will keep the functionality of mitochondrial complexes. Another important complex that is required to preserve the mitochondrial dynamics is mTOR complex[42]. mTOR is a known regulator of many cellular processes, including the biogenesis of mitochondrial complexes, most importantly complex I and V and physiological mitophagy[42]. The downregulation in the level of mTOR in MSCs cultured in high glucose was associated with significant disturbances in mitochondrial complexes I, IV, and V which can induce mitochondrial dysfunction, mitophagy and massive oxidative stress. The effect of high glucose on modulating mitochondrial functions by targeting mTOR is barely investigated and with controversial results[43]. While other studies found that hyperglycemia activate mTOR pathway in cardiomyocytes[44,45], other evidence showed that insulin can activate mTOR pathway in DM patients and protect cells from developing mitochondrial dysfunction[46], indicating that high glucose can deactivate mTOR and cause pathological consequences[46]. These discrepancies regarding the effect of high glucose on mTOR level and activity seem to differ depending on the type of cells and the upstream molecular regulators. Based on that, the role of high glucose in modulating the level of mTOR needs further investigation in different cell types. In our study, the upstream regulatory protein PI3K decreased remarkably in hAD-MSCs exposed to high glucose. A plentiful number of reports corroborated that PI3K-AKT pathway is required to activate mTOR by removing the inhibitory effect produced by TSC1 or Hamartin protein. We reported in this study that the reduction in PI3K protein level was coupled with marked elevation in TSC1 and downregulation in mTOR in hAD-MSCs cultured in high glucose. Our findings provide a mechanistic explanation of poor survival of MSCs in hyperglycemic microenvironment and suggests new targets that can be modified to enhance the survival rate of MSCs in DM patients. Moreover, our results highlighted the importance of maintaining the desirable pre-transplantation culturing conditions that preserves the salutary properties of MSCs by controlling the level of glucose in the culture media. Schematic summary of the study findings was illustrated in (Figure 7) which highlighted that high glucose microenvironment reduced the level of PI3K and removed its inhibitory effect on TSC1 and caused its upregulation. The increase in the level of TSC1 caused a reduction in mTOR which disturbed many mitochondrial dynamics, mainly MtMB, NAD+/NADH ratio, and mitochondrial complexes that triggered the apoptosis of hAD-MSCs.
Figure 7.
Graphical summary of the study findings. High glucose impaired the mitochondrial function in mesenchymal stem cells (MSCs) by perturbing many mitochondrial regulatory dynamics and factors, particularly mitochondrial membrane potential, nicotinamide adenine dinucleotide (NAD+)/NADH pool, and mammalian target of rapamycin protein. This mitochondrial dysfunction is associated with triggering apoptosis in MSCs, which mechanistically explained the poor survival of MSCs in hyperglycemia. MSCs: Mesenchymal stem cells; PI3K: Phosphatidylinositol 3-kinase; mTOR: Mammalian target of rapamycin; NAD+: Nicotinamide adenine dinucleotide.
CONCLUSION
MSCs have the potential to regenerate damaged tissues and restore their functions. DM patients can hugely benefit from MSCs transplantation to abrogate the pathological consequences and improve their quality of life. The poor survival and short-lived positive outcomes following MSCs transplantation in DM patients are considered stumbling blocks. High glucose impaired mitochondrial function in MSCs by perturbing mitochondrial regulatory factors, particularly mitochondrial membrane potential, NAD+/NADH pool, and mTOR protein. This mitochondrial dysfunction is associated with triggering apoptosis in MSCs. Preserving these factors may help in improving the survival rate of MSCs in diabetic microenvironment and initiate long-lasting therapeutic outcomes. Future studies should focus on providing new strategies to overcome the poor survival of MSCs in high glucose using genetic modification or biomaterials and nanoparticles. Moreover, the impact of other stressors existed in DM patients other than hyperglycemia should be studied.
ARTICLE HIGHLIGHTS
Research background
Mesenchymal stem cells (MSCs) have the ability to cure many chronic diseases, including diabetes mellitus (DM) and its related multisystem complications.
Research motivation
The therapeutic outcomes of MSCs transplantation in DM are short-lived which require thorough investigation.
Research objectives
This study aimed to determine the effects of hyperglycemia microenvironment on various mitochondrial-related parameters in MSCs to better understand their fate in DM patients.
Research methods
Adipose tissue-derived MSCs were exposed to low and high glucose media and mitochondrial dynamics and regulators were measured and analyzed.
Research results
High glucose induces the apoptosis of adipose tissue-derived MSCs by disturbing many mitochondrial parameters, including mitochondrial membrane potential, nicotinamide adenine dinucleotide (NAD+)/NADH pool, mammalian target of rapamycin, and mitochondrial complexes I, IV, and V.
Research conclusions
Hyperglycemia decreases the survival of MSCs by triggering mitochondrial dysfunction in these cells causing their short-lived therapeutic outcomes.
Research perspectives
New strategies to improve the survival rate of MSCs in hyperglycemia should be the focus of future studies which can help in increasing the chances of using these cells clinically for the treatment of DM.
ACKNOWLEDGEMENTS
This work was conducted in the Applied and Basic Medical Research Lab in the Faculty of Medicine at Yarmouk University. We would like to thank Mr. Muhammad Abu-El-Rub for his efforts in proof-editing and correcting the manuscript.
Footnotes
Institutional review board statement: The ethical approval was not needed in this study. The human cell lines used for this study were commercially purchased.
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: September 28, 2023
First decision: October 23, 2023
Article in press: November 24, 2023
Specialty type: Cell and tissue engineering
Country/Territory of origin: Jordan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Luo ZW, China; Shen Y, China; Tanabe S, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Zhao S
Contributor Information
Ejlal Abu-El-Rub, Department of Basic Medical Sciences, Faculty of Medicine, Yarmouk University, Irbid 21163, Jordan. ejlal.abuelrub@yu.edu.jo.
Fatimah Almahasneh, Department of Basic Medical Sciences, Faculty of Medicine, Yarmouk University, Irbid 21163, Jordan.
Ramada R Khasawneh, Department of Basic Medical Sciences, Faculty of Medicine, Yarmouk University, Irbid 21163, Jordan.
Ayman Alzu'bi, Department of Basic Medical Sciences, Faculty of Medicine, Yarmouk University, Irbid 21163, Jordan.
Doaa Ghorab, Department of Pathology, Faculty of Medicine, Mansoura University, Mansoura 35516, Egypt.
Rawan Almazari, Department of Basic Medical Sciences, Faculty of Medicine, Yarmouk University, Irbid 21163, Jordan.
Huthaifa Magableh, Department of Basic Medical Sciences, Faculty of Medicine, Yarmouk University, Irbid 21163, Jordan.
Ahmad Sanajleh, Department of Basic Medical Sciences, Faculty of Medicine, Yarmouk University, Irbid 21163, Jordan.
Haitham Shlool, Department of Basic Medical Sciences, Faculty of Medicine, Yarmouk University, Irbid 21163, Jordan.
Mohammad Mazari, Department of Basic Medical Sciences, Faculty of Medicine, Yarmouk University, Irbid 21163, Jordan.
Noor S Bader, Department of Basic Medical Sciences, Yarmouk University, Irbid 21163, Jordan.
Joud Al-Momani, Department of Basic Medical Sciences, Yarmouk University, Irbid 21163, Jordan.
Data sharing statement
No additional data are available.
References
- 1.Merimi M, El-Majzoub R, Lagneaux L, Moussa Agha D, Bouhtit F, Meuleman N, Fahmi H, Lewalle P, Fayyad-Kazan M, Najar M. The Therapeutic Potential of Mesenchymal Stromal Cells for Regenerative Medicine: Current Knowledge and Future Understandings. Front Cell Dev Biol. 2021;9:661532. doi: 10.3389/fcell.2021.661532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markov A, Thangavelu L, Aravindhan S, Zekiy AO, Jarahian M, Chartrand MS, Pathak Y, Marofi F, Shamlou S, Hassanzadeh A. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res Ther. 2021;12:192. doi: 10.1186/s13287-021-02265-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Mukai T, Tojo A, Nagamura-Inoue T. Mesenchymal stromal cells as a potential therapeutic for neurological disorders. Regen Ther. 2018;9:32–37. doi: 10.1016/j.reth.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan XL, Zhang Y, Li X, Fu QL. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci. 2020;77:2771–2794. doi: 10.1007/s00018-020-03454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rigaud VOC, Hoy R, Mohsin S, Khan M. Stem Cell Metabolism: Powering Cell-Based Therapeutics. Cells. 2020;9 doi: 10.3390/cells9112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shyh-Chang N, Ng HH. The metabolic programming of stem cells. Genes Dev. 2017;31:336–346. doi: 10.1101/gad.293167.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arroyave F, Montaño D, Lizcano F. Diabetes Mellitus Is a Chronic Disease that Can Benefit from Therapy with Induced Pluripotent Stem Cells. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21228685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H, Martín C. Pathophysiology of Type 2 Diabetes Mellitus. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21176275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Badri N, Ghoneim MA. Mesenchymal stem cell therapy in diabetes mellitus: progress and challenges. J Nucleic Acids. 2013;2013:194858. doi: 10.1155/2013/194858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Wang F, Liang H, Tang D, Huang M, Zhao J, Yang X, Liu Y, Shu L, Wang J, He Z. Efficacy of mesenchymal stem cell transplantation therapy for type 1 and type 2 diabetes mellitus: a meta-analysis. Stem Cell Res Ther. 2021;12:273. doi: 10.1186/s13287-021-02342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J, Zuo C. The Fate Status of Stem Cells in Diabetes and its Role in the Occurrence of Diabetic Complications. Front Mol Biosci. 2021;8:745035. doi: 10.3389/fmolb.2021.745035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khasawneh RR, Al Sharie AH, Abu-El Rub E, Serhan AO, Obeidat HN. Addressing the impact of different fetal bovine serum percentages on mesenchymal stem cells biological performance. Mol Biol Rep. 2019;46:4437–4441. doi: 10.1007/s11033-019-04898-1. [DOI] [PubMed] [Google Scholar]
- 13.Al-Qarakhli AMA, Yusop N, Waddington RJ, Moseley R. Effects of high glucose conditions on the expansion and differentiation capabilities of mesenchymal stromal cells derived from rat endosteal niche. BMC Mol Cell Biol. 2019;20:51. doi: 10.1186/s12860-019-0235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang TC, Hsu MF, Wu KK. High glucose induces bone marrow-derived mesenchymal stem cell senescence by upregulating autophagy. PLoS One. 2015;10:e0126537. doi: 10.1371/journal.pone.0126537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Y, Chen Z, Tao Y, Zhu J, Yang H, Liang W, Ding G. Increased mitochondrial fission of glomerular podocytes in diabetic nephropathy. Endocr Connect. 2019;8:1206–1212. doi: 10.1530/EC-19-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein LR, Imai S. The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol Metab. 2012;23:420–428. doi: 10.1016/j.tem.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trotta AP, Chipuk JE. Mitochondrial dynamics as regulators of cancer biology. Cell Mol Life Sci. 2017;74:1999–2017. doi: 10.1007/s00018-016-2451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iershov A, Nemazanyy I, Alkhoury C, Girard M, Barth E, Cagnard N, Montagner A, Chretien D, Rugarli EI, Guillou H, Pende M, Panasyuk G. The class 3 PI3K coordinates autophagy and mitochondrial lipid catabolism by controlling nuclear receptor PPARα. Nat Commun. 2019;10:1566. doi: 10.1038/s41467-019-09598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, Cicchetti G, Onda H, Koon HB, Asrican K, Bajraszewski N, Vazquez F, Carpenter CL, Kwiatkowski DJ. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest. 2003;112:1223–1233. doi: 10.1172/JCI17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morita M, Gravel SP, Hulea L, Larsson O, Pollak M, St-Pierre J, Topisirovic I. mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell Cycle. 2015;14:473–480. doi: 10.4161/15384101.2014.991572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo C, Sun L, Chen X, Zhang D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res. 2013;8:2003–2014. doi: 10.3969/j.issn.1673-5374.2013.21.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ocansey DKW, Pei B, Yan Y, Qian H, Zhang X, Xu W, Mao F. Improved therapeutics of modified mesenchymal stem cells: an update. J Transl Med. 2020;18:42. doi: 10.1186/s12967-020-02234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan L, Liu X, Dou H, Hou Y. Characteristics and regulation of mesenchymal stem cell plasticity by the microenvironment - specific factors involved in the regulation of MSC plasticity. Genes Dis. 2022;9:296–309. doi: 10.1016/j.gendis.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deschepper M, Oudina K, David B, Myrtil V, Collet C, Bensidhoum M, Logeart-Avramoglou D, Petite H. Survival and function of mesenchymal stem cells (MSCs) depend on glucose to overcome exposure to long-term, severe and continuous hypoxia. J Cell Mol Med. 2011;15:1505–1514. doi: 10.1111/j.1582-4934.2010.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of mesenchymal stem cells. Front Immunol. 2014;5:148. doi: 10.3389/fimmu.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu C, Li L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J Cell Mol Med. 2018;22:1428–1442. doi: 10.1111/jcmm.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA, Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 28.Planat-Benard V, Varin A, Casteilla L. MSCs and Inflammatory Cells Crosstalk in Regenerative Medicine: Concerted Actions for Optimized Resolution Driven by Energy Metabolism. Front Immunol. 2021;12:626755. doi: 10.3389/fimmu.2021.626755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kornicka K, Houston J, Marycz K. Dysfunction of Mesenchymal Stem Cells Isolated from Metabolic Syndrome and Type 2 Diabetic Patients as Result of Oxidative Stress and Autophagy may Limit Their Potential Therapeutic Use. Stem Cell Rev Rep. 2018;14:337–345. doi: 10.1007/s12015-018-9809-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izadi M, Sadr Hashemi Nejad A, Moazenchi M, Masoumi S, Rabbani A, Kompani F, Hedayati Asl AA, Abbasi Kakroodi F, Jaroughi N, Mohseni Meybodi MA, Setoodeh A, Abbasi F, Hosseini SE, Moeini Nia F, Salman Yazdi R, Navabi R, Hajizadeh-Saffar E, Baharvand H. Mesenchymal stem cell transplantation in newly diagnosed type-1 diabetes patients: a phase I/II randomized placebo-controlled clinical trial. Stem Cell Res Ther. 2022;13:264. doi: 10.1186/s13287-022-02941-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saki N, Jalalifar MA, Soleimani M, Hajizamani S, Rahim F. Adverse effect of high glucose concentration on stem cell therapy. Int J Hematol Oncol Stem Cell Res. 2013;7:34–40. [PMC free article] [PubMed] [Google Scholar]
- 32.Dabrowska S, Del Fattore A, Karnas E, Frontczak-Baniewicz M, Kozlowska H, Muraca M, Janowski M, Lukomska B. Imaging of extracellular vesicles derived from human bone marrow mesenchymal stem cells using fluorescent and magnetic labels. Int J Nanomedicine. 2018;13:1653–1664. doi: 10.2147/IJN.S159404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol. 2014;15:243–256. doi: 10.1038/nrm3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burnham AJ, Foppiani EM, Horwitz EM. Key Metabolic Pathways in MSC-Mediated Immunomodulation: Implications for the Prophylaxis and Treatment of Graft Versus Host Disease. Front Immunol. 2020;11:609277. doi: 10.3389/fimmu.2020.609277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zegallai HM, Abu-El-Rub E, Cole LK, Field J, Mejia EM, Gordon JW, Marshall AJ, Hatch GM. Tafazzin deficiency impairs mitochondrial metabolism and function of lipopolysaccharide activated B lymphocytes in mice. FASEB J. 2021;35:e22023. doi: 10.1096/fj.202100811RR. [DOI] [PubMed] [Google Scholar]
- 36.Tan YL, Eng SP, Hafez P, Abdul Karim N, Law JX, Ng MH. Mesenchymal Stromal Cell Mitochondrial Transfer as a Cell Rescue Strategy in Regenerative Medicine: A Review of Evidence in Preclinical Models. Stem Cells Transl Med. 2022;11:814–827. doi: 10.1093/stcltm/szac044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohammadalipour A, Dumbali SP, Wenzel PL. Mitochondrial Transfer and Regulators of Mesenchymal Stromal Cell Function and Therapeutic Efficacy. Front Cell Dev Biol. 2020;8:603292. doi: 10.3389/fcell.2020.603292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Liu Q, Hu H, Zhao L, Zhu K. Progress in mesenchymal stem cell mitochondria transfer for the repair of tissue injury and treatment of disease. Biomed Pharmacother. 2022;153:113482. doi: 10.1016/j.biopha.2022.113482. [DOI] [PubMed] [Google Scholar]
- 39.Dhanasekaran M, Negi S, Sugiura Y. Designer zinc finger proteins: tools for creating artificial DNA-binding functional proteins. Acc Chem Res. 2006;39:45–52. doi: 10.1021/ar050158u. [DOI] [PubMed] [Google Scholar]
- 40.Li YM, Schilling T, Benisch P, Zeck S, Meissner-Weigl J, Schneider D, Limbert C, Seufert J, Kassem M, Schütze N, Jakob F, Ebert R. Effects of high glucose on mesenchymal stem cell proliferation and differentiation. Biochem Biophys Res Commun. 2007;363:209–215. doi: 10.1016/j.bbrc.2007.08.161. [DOI] [PubMed] [Google Scholar]
- 41.Zorova LD, Popkov VA, Plotnikov EY, Silachev DN, Pevzner IB, Jankauskas SS, Babenko VA, Zorov SD, Balakireva AV, Juhaszova M, Sollott SJ, Zorov DB. Mitochondrial membrane potential. Anal Biochem. 2018;552:50–59. doi: 10.1016/j.ab.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de la Cruz López KG, Toledo Guzmán ME, Sánchez EO, García Carrancá A. mTORC1 as a Regulator of Mitochondrial Functions and a Therapeutic Target in Cancer. Front Oncol. 2019;9:1373. doi: 10.3389/fonc.2019.01373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mao Z, Zhang W. Role of mTOR in Glucose and Lipid Metabolism. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19072043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pandey S, Madreiter-Sokolowski CT, Mangmool S, Parichatikanond W. High Glucose-Induced Cardiomyocyte Damage Involves Interplay between Endothelin ET-1/ET(A)/ET(B) Receptor and mTOR Pathway. Int J Mol Sci. 2022;23 doi: 10.3390/ijms232213816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sen S, Kundu BK, Wu HC, Hashmi SS, Guthrie P, Locke LW, Roy RJ, Matherne GP, Berr SS, Terwelp M, Scott B, Carranza S, Frazier OH, Glover DK, Dillmann WH, Gambello MJ, Entman ML, Taegtmeyer H. Glucose regulation of load-induced mTOR signaling and ER stress in mammalian heart. J Am Heart Assoc. 2013;2:e004796. doi: 10.1161/JAHA.113.004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galizzi G, Di Carlo M. Insulin and Its Key Role for Mitochondrial Function/Dysfunction and Quality Control: A Shared Link between Dysmetabolism and Neurodegeneration. Biology (Basel) 2022;11 doi: 10.3390/biology11060943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.