Abstract
Myxococcus xanthus is a gram-negative bacterium which has a complex life cycle. Autochemotaxis, a process whereby cells release a self-generated signaling molecule, may be the principal mechanism facilitating directed motility in both the vegetative swarming and developmental aggregation stages of this life cycle. The process requires the Frz signal transduction system, including FrzZ, a protein which is composed of two domains, both showing homology to the enteric chemotaxis response regulator CheY. The first domain of FrzZ (FrzZ1), when expressed as bait in the yeast two-hybrid system and screened against a library, was shown to potentially interact with the C-terminal portion of a protein encoding an ATP-binding cassette (AbcA). The activation domain-AbcA fusion protein did not interact with the second domain of FrzZ (FrzZ2) or with two other M. xanthus response regulator-containing proteins presented as bait, suggesting that the FrzZ1-AbcA interaction may be specific. Cloning and sequencing of the upstream region of the abcA gene showed the ATP-binding cassette to be linked to a large hydrophobic, potentially membrane-spanning domain. This domain organization is characteristic of a subgroup of ABC transporters which perform export functions. Cloning and sequencing downstream of abcA indicated that the ABC transporter is at the start of an operon containing three open reading frames. An insertion mutation in the abcA gene resulted in cells displaying the frizzy aggregation phenotype, providing additional evidence that FrzZ and AbcA may be part of the same signal transduction pathway. Cells with mutations in genes downstream of abcA showed no developmental defects. Analysis of the proposed exporter role of AbcA in cell mixing experiments showed that the ABC transporter mutant could be rescued by extracellular complementation. We speculate that the AbcA protein may be involved in the export of a molecule required for the autochemotactic process.
The gliding bacterium Myxococcus xanthus has been suggested to utilize autochemotactic and autochemokinetic motility behaviors during its complex life cycle, which involves vegetative swarming to search for food and, in the absence of nutrients, aggregation to form raised multicellular fruiting bodies (39, 40). Both behaviors appear to be dependent on the Frz signal transduction pathway, components of which are highly homologous to the chemotaxis (Che) proteins of the enteric bacteria (25, 27). The Frz system has also been proposed to play a role similar to that of the Che system in that it regulates a mechanism for directional change, modulating the frequency of cell reversals during gliding. Null mutations in most of the frz genes result in cells that rarely reverse direction (6). The Frz proteins have also been shown to possess biochemical properties similar to those of the Che proteins. Methylation is required for activity of the proposed receptor, FrzCD (26), while FrzE has been shown to both autophosphorylate on the CheA-like domain and transfer this phosphate to the CheY-like domain (1). In the enteric bacteria, the response regulator protein CheY has a well-established interaction with the flagella motor apparatus: CheY-P associates with the motor switch complex, which results in motor reversals and reorienting tumbles (3). However, in M. xanthus, no corresponding motility apparatus which could interact with the Frz system has been identified. Additionally, M. xanthus has three CheY homologues within the Frz system. The FrzE protein is a fusion of two of the enteric Che proteins, the N-terminal portion showing homology to CheA, the histidine protein kinase, and the C-terminal portion having homology to CheY (27). Similarly, the FrzZ protein is composed of two domains, linked by an alanine-proline rich region, although both of these domains share homology with CheY (37).
The role of multiple CheY-like response regulators in motility of M. xanthus is unknown. A phenotypic analysis of frzZ mutants has shown the cells to have defects in both aggregation and vegetative swarming. During development in a DZF1 background (which contains a leaky sglA mutation), the cells show a frizzy aggregation phenotype, producing wild-type levels of spores, as is characteristic of other frz mutants in this genetic background (42). Likewise, in the fully motile DZ2 background, vegetative swarming was shown to be reduced, particularly on low-percentage agars. However, the cells were still able to move toward attractant stimuli and away from repellents in a spatial chemotaxis assay (37). Thus, the FrzZ protein has been suggested to be a modulator of chemotactic responses in M. xanthus, rather than being part of the central pathway. Interestingly, the two domains of FrzZ, although both showing homology to CheY, are only 27.5% identical to each other, suggesting that they could play alternative roles or interact with different proteins. While both domains retain the equivalent of CheY Asp-57, the conserved phosphorylation site, and other residues associated with the active site in enteric bacteria (Asp-12, Asp-13, and Lys-109) (36), neither domain shows conservation of the residues suggested to be associated with interactions at the motor switch complex (34). To explore the roles of the CheY-like domains of FrzZ, we used the yeast two-hybrid system to detect protein-protein interactions (13, 31). Since the two domains of FrzZ (FrzZ1 and FrzZ2) are distinct, each was cloned separately into the system as bait to be screened against a library. We present the results of using this system with M. xanthus proteins and suggest that FrzZ may interact with a developmentally important ATP-binding cassette (ABC) transporter, thereby proposing a novel role for a CheY-like protein.
MATERIALS AND METHODS
Strains and culture conditions.
The strains used in this study are listed in Table 1. Plasmids are listed in Table 2. M. xanthus strains were grown in CYE medium (8), and developmental assays were performed on CF starvation media (16). Escherichia coli strains were grown in LB media. Yeast were grown in YPAD rich media or minimal SD media containing appropriate dropout solutions and 2% glucose (Stratagene).
TABLE 1.
Strains used in this study
| Strain | Genotype or relevant properties | Reference or source |
|---|---|---|
| M. xanthus | ||
| DZF1 | sglA (leaky) (A+ S−) | 8 |
| DZ2 | Wild type (A+ S+) | 9 |
| DK1300 | sglG (A+ S−), nonaggregating | 19 |
| DK6204 | ΔmglBA (Mot−) | 17 |
| DZF4175 | frzZ sglA | 37 |
| DZF4198 | abcA sglA | This study |
| DZF4199 | ctrA sglA | This study |
| DZ24200 | abcA | This study |
| DZF4204 | orf3 sglA | This study |
| E. coli | ||
| XL1-Blue | General cloning strain | Stratagene |
| Top10 | Cloning strain of choice for pZErO; does not contain lacIq gene, allowing constitutive expression of ccdB | Invitrogen |
| S. cerevisiae | ||
| YRG-2 | Reporter genes: lacZ and HIS3 | Stratagene |
| Transformation markers: LEU2 and TRP1 |
TABLE 2.
Plasmids used in this study
| Plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| pAD-GAL4 | Activation domain cloning vector; AmprLEU2 | Stratagene |
| pBD-GAL4 | Binding domain cloning vector; CmrTRP1 | Stratagene |
| p53 | Murine p53-binding domain fusion; AmprTRP1 | Stratagene |
| pSV40 | Simian virus 40 large-T-antigen–activation domain fusion; AmprLEU2 | Stratagene |
| pLaminC | Human lamin C-binding domain fusion; AmprTRP1 | Stratagene |
| pBDZ1 | FrzZ first domain-binding domain fusion; CmrTRP1 | This study |
| pBDZ2 | FrzZ second domain-binding domain fusion; CmrTRP1 | This study |
| pBDE | FrzE-binding domain fusion; CmrTRP1 | This study |
| pBD-AsgA | AsgA-binding domain fusion; CmrTRP1 | Lynda Plamann, Univer-sity of Missouri |
| pADLIB | Library of random fragments from M. xanthus DZF1-activation domain fusions; AmprLEU2 | This study |
| pADZ165a | C-terminal AbcA-activation domain fusion; AmprLEU2 | This study |
| pUC18 | General cloning vector; Ampr | 41 |
| pZErO-2 | Zero Background cloning vector; Kmr | Invitrogen |
| pZABC | Internal fragment of abcA cloned in pZErO-2; Kmr | This study |
| pZORF3 | Internal fragment of orf3 cloned in pZErO-2; Kmr | This study |
| pZCTR | Internal fragment of ctrA cloned in pZErO-2; Kmr | This study |
| pABCS3.5 | 3.5-kb SacI fragment of abc operon cloned in pUC18; Ampr | This study |
| pABCS1.8 | 1.8-kb SacI fragment of abc operon cloned in pUC18; Ampr | This study |
| pABCS1.3 | 1.3-kb SacI fragment of abc operon cloned in pUC18; Ampr | This study |
| pABCBK2.1 | 2.1-kb BamHI-KpnI fragment of abc operon cloned in pUC18; Ampr | This study |
HybriZAP two-hybrid system.
DNA-binding domain fusion constructs were prepared by PCR amplification of the DNA encoding the bait proteins. PCR was performed with the high-fidelity polymerase Pfu. Primers were designed with 5′ EcoRI restriction sites (underlined in the sequences below) to simplify cloning into the EcoRI site of pBD-GAL4. The following primer pairs were used: FrzZ1 (pBDZ1), forward 5′-ATGAATTCACGATGTCGCGCGTACTGGTCATTGATGACAGCCCG and reverse 5′-ATGAATTCGATGCTCAGGGCGGGGGGGCCAATGAGACCCATGAC; FrzZ2 (pBDZ2), forward 5′-ATGAATTCAAGCCGCGCATCCTCATCGTGGATGAC and reverse 5′-ATGAATTCCTACTCGTTACCGGTGGGCATCAGCTC; and FrzE (pBDE), forward 5′-ATGAATTCCGTACGCCGGCCATGGACACCGAGGCTCTC and reverse 5′-ATGAATTCTCAGGTCAGCCGGTCGATGGCCTGCGCGAG. The insertions within the resultant constructs (pBDZ1, pBDZ2, and pBDE) were sequenced to ensure error-proof amplifications, and then the plasmids were transformed into the Saccharomyces cerevisiae host strain YRG-2. The transformants containing just the binding domain constructs were tested for reporter gene expression prior to retransformation with the activation domain library or test activation domain fusion constructs.
The activation domain library was constructed by using a sonicated sample of sheared genomic DNA (33a). Sonication sufficient to produce fragments of approximately 1 to 2 kb was performed. Recessed ends were filled in with Klenow enzyme and deoxynucleoside triphosphates; then EcoRI and XhoI linkers were randomly ligated onto the free ends, and the fragments were cloned as EcoRI-XhoI fragments into predigested HybriZAP vector arms (as provided in the HybriZAP two-hybrid predigested vector/Gigapack cloning kit; Stratagene). The library was amplified, and pADLIB phagemid vector was excised in vivo. The excised library was reamplified prior to use.
Yeast transformations, the reporter gene assay for HIS3, and the filter lift assay for β-galactosidase activity were performed as stipulated in the Stratagene manual. Other procedures, including the isolation of plasmid DNA from yeast and the verification of specificity of protein-protein interactions, were also performed as specified in the Stratagene manual.
DNA manipulations and PCR.
All plasmids used in this study were prepared by using a QIAprep spin miniprep kit (Qiagen). Chromosomal DNA was prepared by the following miniprep protocol. A 1.5-ml sample of saturated culture was pelleted and then resuspended in 567 μl of Tris-EDTA (TE); 30 μl of 10% sodium dodecyl sulfate and 3 μl of proteinase K (20 mg/ml) were added, and the mix was incubated for 1 h at 37°C. A 100-μl volume of 5 M NaCl was added, followed by 80 μl of CTAB (cetyltrimethylammonium bromide)-NaCl solution; 50 ml of CTAB-NaCl solution was prepared by mixing 2.05 g of NaCl with 5 g of CTAB in distilled H2O and heating at 65°C until dissolved before use. The mix was then incubated at 65°C for 10 min and then extracted with an equal volume of chloroform-isoamyl alcohol. The aqueous phase was removed and extracted again with an equal volume of phenol-chloroform-isoamyl alcohol. DNA was precipitated by the addition of 0.6 volume of isopropanol, washed in 70% ethanol, dried, and resuspended in TE buffer. Restriction enzyme digests and modifying enzyme protocols were performed as specified by the manufacturers. PCR optimizations and cycling parameters were identified by the protocol of Kramer and Coen (21). In general, glycerol concentrations of 20% were required for high yields of pure products. Taq polymerase (Promega) and Pfu polymerase (Stratagene) were used in amplifications requiring low and high fidelity, respectively.
Cloning and sequencing.
Sequencing upstream of the 0.7-kb fragment cloned in pADZ165a was performed on a 3.5-kb SacI fragment (pABCS3.5) which overlapped the original 0.7-kb insert. Sequencing downstream was performed on two SacI fragments of 1.8 and 1.3 kb, cloned as pABCS1.8 (which also overlapped the original insert) and pABCS1.3 (which lies downstream of the 1.8-kb SacI fragment), respectively. The junction between these two downstream SacI fragments and the final region downstream of the 1.3-kb SacI fragment were sequenced on a 2.1-kb BamHI-KpnI clone (pABCBK2.1). The above pABC clones were all constructed in pUC18, allowing initial sequencing to be performed with the universal forward and reverse primers. Further sequence was obtained by primer walking or construction of further subclones (not listed). All sequencing was performed by the UC Davis Sequencing Facility.
Production of mutants.
Mutants were constructed by cloning internal fragments of the specified genes into the 3.3-kb vector pZErO-2 (Invitrogen). These constructs were then electroporated into M. xanthus strains, and selection on CYE containing kanamycin (25 μg/ml) was used to identify mutants which had the entire vector inserted into the chromosome by homologous recombination.
The abcA gene is 2,130 bp in length, spanning from the start codon at bp 1461 to 1463 to the stop codon at bp 3588 to 3590, within the 6,945-bp sequenced region. The internal fragment used for mutagenesis spanned from bp 2824 to 3356 (see sequences below). The proposed orf3 gene spans from the start codon at bp 3587 to 3589 to the stop codon at bp 4985 to 4987. The 811-bp internal fragment cloned in pZErO-2 (to make pZORF3) spanned from bp 3730 to 4541 within the sequenced region. The proposed ctrA gene spans from the start codon at bp 4984 to 4986 to the stop codon at bp 6514 to 6516. An internal fragment of 669 bp, spanning from bp 5414 to 6083, was used in mutagenesis.
Internal fragments of the genes listed above were prepared by PCR using Taq polymerase (Promega). The following primer combinations were designed with 5′ EcoRI ends (underlined) to facilitate simple cloning into the EcoRI site of pZErO: abcA (pZABC), forward 5′-ATGAATTCGCGCAACAGTTGGGCCTCCG and reverse 5′-ATGAATTCTCCGCGTGGAGGACGTTCGC; orf3 (pZORF3), forward 5′-ATGAATTCATGGAGCGGTTGCCCAGC and reverse 5′-ATGAATTCTCCACCACAACGCCACGC; and ctrA (pZCTR), forward 5′-ATGAATTCATGGAGCGGTTGCCCAGC and reverse 5′-ATGAATTCTCCACCACAACGCCACGC.
Transformations were performed in E. coli Top10 cells, which allow Zero Background cloning (Invitrogen). Plasmids were then denatured with 1 N NaOH prior to electroporation into M. xanthus strains by the method described in reference 20. Chromosomal DNA was prepared from resultant strains, and the sites of insertion were confirmed by Southern blotting. Probes were constructed by PCR incorporating the hapten digoxigenin as digoxigenin-11-dUTP (Boehringer Mannheim), and detection was performed by enzyme immunoassay and an enzyme-catalyzed color reaction (Boehringer Mannheim).
Phenotypic analyses.
Developmental defects were screened for on CF starvation agar by plating 5 μl of cells at 109 CFU/ml directly onto the plate and incubating for 96 h. Spore counts were performed on these aggregates after 4 days of incubation. Cells were removed from the agar and resuspended in TM buffer (10 mM Tris-HCl, 8 mM MgSO4 [pH 7.6]). Spore clumps were dispersed by sonication, and appropriate dilutions were placed in a Petroff-Hausser counting chamber for counting under magnification. Cell mixing experiments were performed on CF agar after cells were concentrated in morpholinepropanesulfonic acid (MOPS)-Mg2+ buffer (10 mM MOPS [pH 7.6], 8 mM MgCl2) and mixed in various ratios of the two cell types. Aggregates were photographed at 24-h intervals for 4 days.
Motion analysis was performed with cells on 1/2 CTT agar (10 mM MOPS [pH 7.6], 1 mM KH2PO4, 1 mM MgSO4, 0.5% Bacto Casitone, 1.5% agar) after 30 min of incubation in CFSC solution (CF salts containing 1 mM CaCl2). Time-lapse video microscopy was performed on fields of 10 to 20 cells for a period of 30 min. Cells were observed with a Nikon Labphot-2 microscope with a 40× objective. Images were recorded with a Dage-MTI CCD-72 series camera and a time-lapse video cassette recorder (120-h speed setting; model GYYR TLC 1800). Data were analyzed manually by tracing the movement of the cells during playback.
Computer analysis.
Genetic analyses, including GC coding predictions, identification of open reading frames (ORFs), translations, sequence alignments, and the identification of membrane-buried regions, were performed with the program Gene Inspector (Textco Inc.).
Nucleotide sequence accession number.
The DNA sequence presented here has been submitted to the EMBL, GenBank, and DDBJ nucleotide sequence data libraries under accession no. AF047554.
RESULTS
Identification of an activation domain fusion clone that interacts with the FrzZ1 bait.
S. cerevisiae YRG-2 cells carrying the GAL4-binding domain-FrzZ1 fusion construct (pBDZ1) were transformed with a library of randomly sheared M. xanthus DNA activation domain fusion constructs (pADLIB). The resultant transformants were selected for HIS3 reporter gene activity by plating on minimal medium (SD-Leu-Trp-His). Cells growing under these restrictive conditions should contain binding and activation domain constructs which express interacting protein fusions. A second reporter gene assay, for expression of β-galactosidase, was performed on all stably growing transformants. One transformant, which gave positive signals for both reporter gene assays, was studied further to ascertain the specificity of the interaction between the activation domain fusion protein and FrzZ1. The activation domain construct (pADZ165a) was purified from yeast cells and then retransformed into fresh YRG-2 cells containing test or control binding domain constructs (Table 3). The combination of pBDZ1 plus pADZ165a again produced a positive signal in both reporter gene assays. However, the expressed fusion from pADZ165a did not interact with those of the binding domain constructs expressing other M. xanthus response regulator proteins (including the second domain of FrzZ [FrzZ2], FrzE, and AsgA [33]). The control combination of p53 plus pADZ165a also resulted in negative reporter gene assays, although the leaky nature of the HIS3 reporter gene produced low-level growth on the selective media assay in all cases.
TABLE 3.
Two-hybrid reporter gene assays
| Plasmid combination
|
Reporter gene assays resulta
|
||
|---|---|---|---|
| Bait | Test | SD-Leu-Trp-His | β-Gal |
| pBDZ1 | pADZ165a | ++ | + (11 hb) |
| p53 | pADZ165a | + | − |
| pBDZ2 | pADZ165a | + | − |
| pBDE | pADZ165a | + | − |
| pBDAsgA | pADZ165a | + | − |
| p53 | pSV40 | +++ | + (5 h) |
Various bait fusions (Table 2) cloned in pGAL4-BD were screened for reporter gene activity when transformed into S. cerevisiae YRG-2 in combination with the test activation domain fusion expressed from pADZ165a. The control combination p53+pSV40 was used in both assays.
Time when blue color was first observed on filters.
Since the fusion protein expressed by pADZ165a appeared to specifically interact with the first domain of FrzZ, the 0.7-kb M. xanthus DNA insert was sequenced and the fusion was shown to link the GAL4 activation domain to the C-terminal 136 amino acids of a potential protein. The ORF encoding this protein showed appropriate M. xanthus codon usage (35), a good indicator of translational potential due to the high G+C content of M. xanthus DNA (67.5 mol% G+C [30]). The fusion peptide was also shown to have homology to the C-terminal end of a putative ATP-binding cassette, such as are found in ABC transporter proteins. The 0.7-kb fragment, which also encodes the start of a second potential ORF, was therefore used as a probe to clone a larger fragment, containing regions both upstream and downstream, from a lambda-ZAP library (kindly provided by T. Hartzell, University of Idaho). In total, a 6,945-bp region surrounding the original insert was subcloned and sequenced. The sequenced region was shown to contain three complete and two partial ORFs (Fig. 1), all in agreement with M. xanthus codon usage. The three central ORFs are proposed to have overlapping start and stop codons, and we therefore speculate that they may constitute an operon. The first gene of this potential operon encodes the apparent FrzZ1-interacting protein domain. Sequences indicative of Shine-Dalgarno sites were identified upstream of orf3 (GGAGG; 9 bp upstream of the proposed ATG start codon) and orf4 (AGGGG; 10 bp upstream of the proposed ATG start codon). No putative Shine-Dalgarno or promoter site was identified upstream of orf2, and the proposed GTG start codon of this ORF is currently based on codon usage data alone (5).
FIG. 1.
Restriction map of the ORFs identified by sequencing upstream and downstream of the 0.7-kb insert in pADZ165a. The original 0.7-kb EcoRI-XhoI insert cloned in pADZ165a is shown as the resultant GAL4 activation domain protein fusion. Sequencing constructs are shown below the main map. Restriction enzymes: B, BamHI; E, EcoRI; K, KpnI; S, SacI; X, XhoI.
Interacting protein shows homology to a group of bacterial ABC exporter systems.
Gapped BLAST analyses (2) were performed on translations of the three complete and two incomplete ORFs identified within the 7-kb sequenced region. Neither of the translated proteins from the first (orf1) or last (orf5) partial ORF showed any significant homologies with protein sequences within the GenBank-EMBL data bank, and they were not studied further.
The translation of orf2, the first complete ORF, which is suggested to be the start of a transcriptional unit, identified a protein of 709 amino acids based on the codon usage pattern. The protein showed very strong similarity to the ABC transporter proteins (17), and the gene was therefore speculatively named abcA. The AbcA protein showed particularly strong homology to the bacterial ABC exporters and eukaryotic multidrug resistance or P glycoproteins. This subgroup of ABC transporters consists of large proteins (600 to 750 amino acids) which have both the membrane-spanning domains and the ATP-binding cassette on the same protein. In bacteria, these transporters include the repeats-in-toxin (RTX) transporters CyaB and HlyB, which export cyclolysin (CyaA) in Bordetella pertussis (15) and hemolysin (HlyA) in E. coli (12), respectively, along with the nonprotein exporters such as ChvA in Agrobacterium tumefaciens (10). In common with the RTX transporters, the M. xanthus AbcA protein appears to be divided into three domains, a short N-terminal hydrophilic domain (amino acids ∼1 to 130), followed by a large hydrophobic, potentially membrane-spanning domain (amino acids ∼131 to 430) and a C-terminal ATP-binding cassette. Alignment with the CyaB protein (Fig. 2), which has 30% identity to AbcA, showed that the motifs associated with the ATP-binding pocket are also conserved in AbcA. This Walker motif (38) is split into two sites, A and B, with consensus sequences (A/G)xxxxGK(S/T) (amino acids 488 to 496 GETGSGKS in AbcA) and ILILD (amino acids 612 to 616 ILILD in AbcA), respectively (11).
FIG. 2.
Alignment of the B. pertussis (B.p) CyaB protein (15) with the M. xanthus (M.x) Orf2 translation (AbcA). The two proteins show 30% identity and 53% similarity. The activation domain fusion created in pADZ165a starts at Orf2 amino acid E572. Double blocks above the aligned sequences indicate conserved amino acids, while single blocks define gaps in the alignment.
In order to form a functional complex, ABC transporters which secrete proteins require an accessory factor which is anchored in the inner membrane and spans the periplasm, connecting the inner and outer membranes. Genes encoding such accessory factors have always been found linked to the gene encoding the ATP-binding cassette but may share little sequence homology. For example, in the B. pertussis cyclolysin operon, the gene encoding the accessory factor, cyaD, lies directly downstream of cyaB, and the same gene organization is seen in the E. coli hemolysin operon, where hlyD lies directly downstream of hlyB. While the second ORF of the ABC operon (orf3), which encodes a 466-amino-acid protein, has little identity (20%) to CyaD, it is similar in size to this protein, contains a potentially membrane-spanning region, and could play the role of an accessory factor. However, in the nonprotein exporter systems, a single ATP-binding protein, with associated membrane-spanning domains, has been shown to be sufficient to transport polysaccharides to the outer membrane without the requirement for an accessory factor.
Transporter systems that export macromolecules across both inner and outer membranes have also been shown to require an outer membrane factor (32), which may or may not be linked to the remainder of the operon. The final ORF of the proposed ABC transporter operon (orf4) has no large membrane-associated regions and is unlikely to play this role or that of an accessory factor. In addition, the predicted 510-amino-acid polypeptide shows homology to the carbamoyltransferases involved in signal production in the rhizobia (29). Alignment of Orf4 against the NolO protein from Rhizobium sp. strain NGR234 (14) showed the proteins to have 30% identity and 51% similarity. This reasonable degree of similarity between the proteins suggests that they may have the same enzymatic function, and we have thus speculatively named the orf4 gene ctrA (carbamoyltransferase).
Mutational analyses.
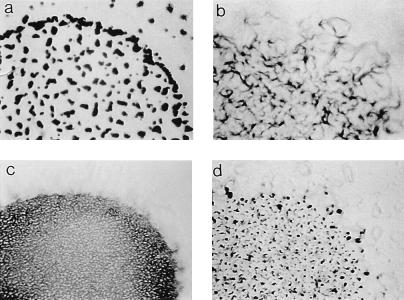
A 532-bp, PCR-derived internal fragment of the abcA gene was cloned into pZErO-2 to provide a kanamycin-resistant (Kmr) construct (pZABC) that could be integrated into the M. xanthus chromosome by homologous recombination at the abcA site, since pZErO-2 cannot replicate in M. xanthus. Using this method, a single crossover event would result in the targeted gene being divided into two truncated parts separated by the vector. The construct pZABC was introduced into the FB strain DZF1 by electroporation, and the resultant Kmr electroporants were screened by Southern blotting to ensure that the insertion had occurred at the correct site. Southern analysis showed that the 3.5-kb SacI genomic fragment, which contains the abcA gene, was missing in all mutants tested and replaced by two bands at approximately 3.1 and 4.1 kb, due to the insertion of pZABC (which contains a single internal SacI site). M. xanthus cells (DZF4198) with the correct insertion mutation were assayed for developmental defects by plating on starvation agar and were shown to display the frizzy aggregation phenotype (Fig. 3b), which is distinct from the parent DZF1 aggregation phenotype (Fig. 3a). Spore counts were performed on the parent DZF1, and frizzy aggregates and spore numbers were shown to be similar, although slightly reduced in the AbcA mutant (data not shown). A preliminary analysis of the reversal frequency of cell gliding, performed to ascertain that the frizzy phenotype of the AbcA mutant was associated with modified motility behavior, showed that the AbcA mutant does display a reduced frequency of cell reversals during gliding (8.2 ± 2.5 reversals/h), as has been observed in other Frz mutants (6), compared to the parent DZF1 reversal frequency (14.9 ± 4.7 reversals/h). While this reduction in reversal frequency is not as dramatic as those observed previously in frz mutants, it does suggest that mutations in the abcA locus have an effect on single cell movements.
FIG. 3.
Developmental aggregation and extracellular complementation by cell mixing in M. xanthus DZF1 (FB parent) (a), DZF4198 (frizzy) (b), DK1300 (nonfruiting) (c), and DZF4198 plus DK1300 (9:1 ratio).
Although in the DZF1 background vegetative swarming is greatly reduced (due largely to a mutation in the sglA locus), an analysis of the cells on 1.5% CYE agar suggested that swarming in the AbcA mutant might be slightly enhanced over that of DZF1 (not shown). To further analyze this potentially enhanced-swarming phenotype, the abcA mutation was reconstructed in the fully motile DZ2 background (see above). In this background, however no defects in aggregation or alterations in swarming behavior on high- or low-percentage CYE agar were apparent.
Since the AbcA protein appeared to have a DZF1 strain-dependent phenotype, mutational analyses of the roles of the two additional postulated ORFs lying downstream of abcA were performed only in the DZF1 background. To investigate the role of the Orf3 protein as a putative accessory factor required for ABC transporter activity, an 811-bp internal fragment of the orf3 gene was cloned into pZErO-2 to create pZORF3. After electroporation of pZORF3 into M. xanthus DZF1, the stably growing Kmr electroporants were screened for the correct insertion mutation by Southern blotting. In all mutants tested, the 1.1-kb BamHI fragment present in the DZF1 genomic digest was missing and replaced by two bands at approximately 4.5 and 0.8 kb, consistent with insertion of pZORF3 (which contains a single BamHI site) at the orf3 locus. However, screening of the Orf3 mutant cells (DZF4204) under developmental conditions identified no obvious aggregation defect. Fruiting bodies that resembled those formed by the parent strain were apparent after 24 h, and these aggregates were shown to contain a full complement of spores, suggesting that the Orf3 protein is probably not an accessory factor required for AbcA transporter function.
The positioning of a homologue of an enzyme, known to be involved in signal production in the rhizobia, at the end of the ABC operon initially suggested that this protein might either be the exported product itself or be involved in modification of the transported molecule prior to export. To test this hypothesis, a 669-bp internal fragment of the ctrA gene was cloned into pZErO-2. The resultant plasmid (pZCTR) was electroporated into M. xanthus DZF1 and shown by Southern analysis to integrate into the chromosome at the ctrA site. In the parent strain, the ctrA gene is present on an approximately 22-kb BamHI fragment. This fragment was missing in the CtrA mutants, being replaced by two bands, at 1.3 and approximately 30 kb, as would be expected after insertion of pZCTR. However, a phenotypic analysis of the resultant DZF1 ctrA cells (DZF4199) under starvation conditions showed no aberrant aggregation or sporulation phenotype. The wild-type aggregation and sporulation phenotypes of DZF4199 cells suggest that the CtrA protein is not involved in developmental aggregation. In addition, its role as a potential carbamoyltransferase has yet to be proven.
Since neither of the genes positioned downstream of abcA appeared to be involved with developmental aggregation, it seemed unlikely that the frizzy phenotype displayed by the abcA mutant was caused by downstream effects. Rather, we speculate that the AbcA protein alone may either be involved in the export of an aggregation-associated molecule or have an unknown Frz-associated role.
Complementation of the Frz phenotype in DZF4198.
If the ABC transporter does play a role in developmental aggregation by exporting a specific molecule into the extracellular environment, it would be predicted that a developmentally wild-type cell might rescue the DZF4198 AbcA mutant phenotype when presented in a mixed culture. Accordingly, AbcA mutant cells were mixed with DK1300, which is unable to aggregate due to a social motility defect (Fig. 3c) (19), and the mixture was spotted onto starvation plates in order to screen for rescue of development by extracellular complementation. Figure 3d shows rescue of the frizzy phenotype of the DZF4198 cells by DK1300 at a 9:1 ratio, supporting the hypothesis that AbcA could be involved in the export of a developmentally important aggregation-associated molecule. These results were confirmed by using the A− S− mglA motility mutant in a submerged culture assay (22). Again, rescue of the AbcA frizzy phenotype was observed when the same ratio (9:1) of cells was used (not shown).
DISCUSSION
In this study, use of the two-hybrid system has identified a potential interaction between the first domain of the FrzZ response regulator and the cytoplasmic domain of an ABC transporter (AbcA) bearing strong similarity to the bacterial exporters. Although more experimental work is required to fully confirm this interaction, additional evidence that the AbcA transporter is connected to the Frz signal transduction pathway was provided by an analysis of DZF1 cells with insertion mutations at the abcA locus. The frizzy phenotype displayed by these cells indicates that the AbcA protein is required for developmental aggregation and provides secondary evidence for a direct interaction between FrzZ and AbcA. Currently, we have no evidence that the two genes lying downstream of AbcA, suggested to be part of the same operon, have any role in the proposed FrzZ-AbcA interaction or, indeed, in the overall developmental process.
While sequence analysis of the AbcA protein suggested it to be a member of the RTX family of protein exporters, such as CyaB of B. pertussis and HlyB of E. coli, the potential absence of an associated accessory factor indicates that it is more likely to be a member of the nonprotein exporters. Such nonprotein exporters, which include ChvA in A. tumefaciens and NdvA in Rhizobium meliloti (both of which transport β-1,2-glucan), do not utilize accessory factors or specific outer membrane components, since they do not secrete their products directly into the extracellular environment. The ability to extracellularly complement the DZF4198 AbcA frizzy phenotype in a cell mixing experiment provides preliminary evidence that the ABC transporter may indeed perform an exporter role and that the transported molecule could be required for developmental aggregation. Currently, we are exploring methods for purification of the transported molecule, using complementation of the DZF4198 aggregation phenotype as an assay, in order to clarify the role of AbcA as an exporter, analyze the export pathway of the molecule, and facilitate behavioral studies of cells exposed to what could potentially be a developmental aggregation signal.
While analyzing the DZF4198 (abcA sglA) mutant, we noted a vegetative swarming phenotype under nutrient-rich conditions. During growth on CYE agar, DZF4198 appeared to swarm more efficiently than the parent DZF1. However, since the DZF1 strain has a mutation in the sglA gene which results in reduced motility, the abcA mutation was transferred into the fully motile, wild-type strain DZ2. The lack of either a developmental or vegetative motility-associated phenotype in this DZ2 background (DZ24200) may suggest that the SglA protein can complement the abcA mutation, since a major difference between the DZF1 and DZ2 strains is the presence of the sglA1 mutation in DZF1. Previous work by Kashefi and Hartzell (20) has indicated that SglA may activate a function which can substitute for the Frz chemotaxis system during development. However, DZF1 contains other, undefined motility defects with respect to DZ2 (38a) which could play Frz-associated roles. Therefore, we are currently analyzing the potential SglA-AbcA association further.
The identification of a potential interaction between a component of the Frz signal transduction system and an ABC transporter involved in developmental aggregation supports the hypothesis that M. xanthus may export specific motility-associated molecules into the extracellular environment during starvation-induced development, in agreement with the autochemotactic model (40). Such autochemotactic situations are unusual, but not unknown, in bacteria. For example, both E. coli and Salmonella typhimurium have been shown to secrete a strong attractant (aspartate) when grown on components of the tricarboxylic acid cycle, which are themselves poor attractants (4, 7). The bacteria are then attracted to the secreted aspartate, resulting in the cells accumulating into aggregates with complex and intricate patterns. While the rationale for the enteric bacteria forming these patterns is unknown, the myxobacteria clearly use the aggregates in the construction of fruiting bodies.
M. xanthus has for many years been known to utilize extracellular signaling during starvation-induced development (23). Previous studies have also suggested that M. xanthus could generate signaling molecules involved in directed motility functions (24, 28). The work presented in this study complements such suggestions and provides a potential mechanism for export of the signals, as well as providing a potentially useful assay for identification of a potential signaling molecule. While the Frz signal transduction pathway has many similarities to the Che system of the enteric bacteria, our results suggest that Frz regulation of motility behavior is more complex than the enteric paradigm. In particular, we suggest that the FrzZ protein may be involved in a novel role for a CheY homologue, not interacting at the gliding motor but regulating the export of a developmentally active molecule. Future work will be aimed at further characterization of this system and an analysis of the motility-associated signaling molecule(s) used by M. xanthus during its complex life cycle.
ACKNOWLEDGMENTS
We particularly thank Bob Osborne for help in construction of the two-hybrid library. We also thank Lynda Plamann and Trish Hartzell for kindly providing other useful tools for this project. In addition, we are grateful to Stacia Hoover and Eric Bowman at the UC Davis Sequencing Facility for all of the sequence data published in this report.
Research in our laboratory is supported by Public Health Service grant GM20509 from the National Institutes of Health. D.P.A. was supported in part by a training grant (GM 07232) from the National Institutes of Health.
REFERENCES
- 1.Acuña G, Shi W, Trudeau K, Zusman D R. The ‘CheA’ and ‘CheY’ domains of Myxococcus xanthus FrzE function independently in vitro as an autokinase and a phosphate acceptor, respectively. FEBS Lett. 1995;358:31–33. doi: 10.1016/0014-5793(94)01389-i. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amsler C D, Matsumura P. Chemotactic signal transduction in Escherichia coli and Salmonella typhimurium. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C: American Society for Microbiology; 1995. pp. 89–103. [Google Scholar]
- 4.Berg H C. Symmetries in bacterial motility. Proc Natl Acad Sci USA. 1996;93:14225–14228. doi: 10.1073/pnas.93.25.14225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bibb M J, Findlay P R, Johnson M W. The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding regions. Gene. 1984;30:157–166. doi: 10.1016/0378-1119(84)90116-1. [DOI] [PubMed] [Google Scholar]
- 6.Blackhart B D, Zusman D R. ‘Frizzy’ genes of Myxococcus xanthus are involved in control of frequency of reversals of gliding motility. Proc Natl Acad Sci USA. 1985;82:8767–8770. doi: 10.1073/pnas.82.24.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budrene E O, Berg H C. Complex patterns formed by motile cells of Escherichia coli. Nature. 1991;349:630–633. doi: 10.1038/349630a0. [DOI] [PubMed] [Google Scholar]
- 8.Campos J M, Geisselsoder J, Zusman D R. Isolation of bacteriophage Mx4, a generalized transducing phage for Myxococcus xanthus. J Mol Biol. 1978;119:167–178. doi: 10.1016/0022-2836(78)90431-x. [DOI] [PubMed] [Google Scholar]
- 9.Campos J M, Zusman D R. Regulation of development in Myxococcus xanthus: effect of 3′:5′ cyclic AMP, ADP and nutrition. Proc Natl Acad Sci USA. 1975;72:518–522. doi: 10.1073/pnas.72.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cangelosi G A, Martinetti G, Leigh J A, Lee C C, Theines C, Nester E W. Role of Agrobacterium tumefaciens ChvA protein in export of β-1,2-glucan. J Bacteriol. 1989;171:1609–1615. doi: 10.1128/jb.171.3.1609-1615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fath M J, Kolter R. ABC transporters: bacterial exporters. Microbiol Rev. 1993;57:995–1017. doi: 10.1128/mr.57.4.995-1017.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felmlee T, Pellett S, Welch R A. Nucleotide sequence of an Escherichia coli chromosomal haemolysin. J Bacteriol. 1985;163:94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 14.Freiberg C, Fellay R, Bairoch A, Broughton W J, Rosenthal A, Perret X. Molecular basis of symbiosis between Rhizobium and legumes. Nature. 1997;387:394–401. doi: 10.1038/387394a0. [DOI] [PubMed] [Google Scholar]
- 15.Glaser P, Sakamoto H, Bellalou J, Ullmann A, Danchin A. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-haemolysin bifunctional protein of Bordetella pertussis. EMBO J. 1988;7:3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagen D C, Bretscher A P, Kaiser D. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev Biol. 1978;64:284–296. doi: 10.1016/0012-1606(78)90079-9. [DOI] [PubMed] [Google Scholar]
- 17.Hartzell P, Kaiser D. Function of MglA, a 22-kilodalton protein essential for gliding in Myxococcus xanthus. J Bacteriol. 1991;173:7625–7635. doi: 10.1128/jb.173.23.7615-7624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins C F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 19.Hodgkin J, Kaiser D. Genetics of gliding motility in Myxococcus xanthus (Myxobacterales): two gene systems control movement. Mol Gen Genet. 1979;171:177–191. [Google Scholar]
- 20.Kashefi K, Hartzell P L. Genetic suppression and phenotypic masking of a Myxococcus xanthus frzF− defect. Mol Microbiol. 1995;15:483–494. doi: 10.1111/j.1365-2958.1995.tb02262.x. [DOI] [PubMed] [Google Scholar]
- 21.Kramer M F, Coen D M. Enzymatic amplification of DNA by PCR: standard procedures and optimization, unit 15.1. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1995. [DOI] [PubMed] [Google Scholar]
- 22.Kuner J M, Kaiser D. Fruiting body morphogenesis in submerged cultures of Myxococcus xanthus. J Bacteriol. 1982;151:458–461. doi: 10.1128/jb.151.1.458-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuspa A, Kroos L, Kaiser D. Intercellular signaling is required for developmental gene expression in Myxococcus xanthus. Dev Biol. 1986;117:267–276. doi: 10.1016/0012-1606(86)90369-6. [DOI] [PubMed] [Google Scholar]
- 24.Lev M. Demonstration of a diffusible fruiting factor in myxobacteria. Nature. 1954;173:501. [Google Scholar]
- 25.McBride M J, Weinberg R A, Zusman D R. ‘Frizzy’ aggregation genes of the gliding bacterium Myxococcus xanthus show sequence similarities to the chemotaxis genes of enteric bacteria. Proc Natl Acad Sci USA. 1989;86:424–428. doi: 10.1073/pnas.86.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCleary W R, McBride M J, Zusman D R. Developmental sensory transduction in Myxococcus xanthus involves methylation and demethylation of FrzCD. J Bacteriol. 1990;172:4877–4887. doi: 10.1128/jb.172.9.4877-4887.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCleary W R, Zusman D R. FrzE of Myxococcus xanthus is homologous to both CheA and CheY of Salmonella typhimurium. Proc Natl Acad Sci USA. 1990;87:5898–5902. doi: 10.1073/pnas.87.15.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McVittie A, Zahler S A. Chemotaxis in Myxococcus. Nature. 1962;194:1299–1300. [Google Scholar]
- 29.Mergaert P, Van Montagu M, Holsters M. Molecular mechanisms of Nod factor diversity. Mol Microbiol. 1997;25:811–817. doi: 10.1111/j.1365-2958.1997.mmi526.x. [DOI] [PubMed] [Google Scholar]
- 30.Mesbah M, Premachandran U, Whitman W B. Precise measurement of the G+C content of deoxyribonucleic acid by high-performance liquid chromatography. Int J Syst Bacteriol. 1989;39:159–167. [Google Scholar]
- 31.Mullinax R L, Sorge J A. HybriZAP™ two-hybrid vector system for detecting protein-protein interactions. Strategies. 1995;8:3–5. [Google Scholar]
- 32.Paulsen I T, Park J H, Choi P S, Saier M H. A family of Gram-negative bacterial outer membrane factors that function in the export of proteins, carbohydrates, drugs and heavy metals from Gram-negative bacteria. FEMS Microbiol Lett. 1997;156:1–8. doi: 10.1111/j.1574-6968.1997.tb12697.x. [DOI] [PubMed] [Google Scholar]
- 33.Plamann L, Li Y, Cantwell B, Mayor J. The Myxococcus xanthus asgA gene encodes a novel signal transduction protein required for multicellular development. J Bacteriol. 1995;177:2014–2020. doi: 10.1128/jb.177.8.2014-2020.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33a.Osborne, R. Personal communication.
- 34.Roman S J, Meyers M, Volz K, Matsumura P. A chemotactic signaling surface on CheY defined by suppressors of flagellar switch mutations. J Bacteriol. 1992;174:6247–6255. doi: 10.1128/jb.174.19.6247-6255.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimkets L J. The myxobacterial genome. In: Dworkin M, Kaiser D, editors. Myxobacteria II. Washington, D.C: American Society for Microbiology; 1993. pp. 85–107. [Google Scholar]
- 36.Stock A M, Mottonen J M, Stock J B, Schutt C E. Three-dimensional structure of CheY, the response regulator of bacterial chemotaxis. Nature. 1989;337:745–749. doi: 10.1038/337745a0. [DOI] [PubMed] [Google Scholar]
- 37.Trudeau K G, Ward M J, Zusman D R. Identification and characterization of FrzZ, a novel response regulator, necessary for swarming and fruiting-body formation in Myxococcus xanthus. Mol Microbiol. 1996;20:645–655. doi: 10.1046/j.1365-2958.1996.5521075.x. [DOI] [PubMed] [Google Scholar]
- 38.Walker J E, Saraste M, Runswick M J, Gay N J. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38a.Wall, D. Personal communication.
- 39.Ward M J, Mok K C, Zusman D R. Myxococcus xanthus displays Frz-dependent chemokinetic behavior during vegetative swarming. J Bacteriol. 1998;180:440–443. doi: 10.1128/jb.180.2.440-443.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward M J, Zusman D R. Regulation of directed motility in Myxococcus xanthus. Mol Microbiol. 1997;24:885–893. doi: 10.1046/j.1365-2958.1997.4261783.x. [DOI] [PubMed] [Google Scholar]
- 41.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 42.Zusman D R. “Frizzy” mutants: a new class of aggregation-defective developmental mutants of Myxococcus xanthus. J Bacteriol. 1982;150:1430–1437. doi: 10.1128/jb.150.3.1430-1437.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]