Abstract
Gamete development is a fundamental process that is highly conserved from early eukaryotes to mammals. As germ cells develop, they must coordinate a dynamic series of cellular processes that support growth, cell specification, patterning, the loading of maternal factors (RNAs, proteins, and nutrients), differentiation of structures to enable fertilization and ensure embryonic survival, and other processes that make a functional oocyte. To achieve these goals, germ cells integrate a complex milieu of environmental and developmental signals to produce fertilizable eggs. Over the past 50 years, Drosophila oogenesis has risen to the forefront as a system to interrogate the sophisticated mechanisms that drive oocyte development. Studies in Drosophila have defined mechanisms in germ cells that control meiosis, protect genome integrity, facilitate mRNA trafficking, and support the maternal loading of nutrients. Work in this system has provided key insights into the mechanisms that establish egg chamber polarity and patterning as well as the mechanisms that drive ovulation and egg activation. Using the power of Drosophila genetics, the field has begun to define the molecular mechanisms that coordinate environmental stresses and nutrient availability with oocyte development. Importantly, the majority of these reproductive mechanisms are highly conserved throughout evolution, and many play critical roles in the development of somatic tissues as well. In this chapter, we summarize the recent progress in several key areas that impact egg chamber development and ovulation. First, we discuss the mechanisms that drive nutrient storage and trafficking during oocyte maturation and vitellogenesis. Second, we examine the processes that regulate follicle cell patterning and how that patterning impacts the construction of the egg shell and the establishment of embryonic polarity. Finally, we examine regulatory factors that control ovulation, egg activation, and successful fertilization.
Keywords: nutrient storage, oocyte maturation, follicle cell differentiation, eggshell, chorion gene amplification, egg shape, ovulation, egg activation, female reproductive tract, FlyBook
Introduction
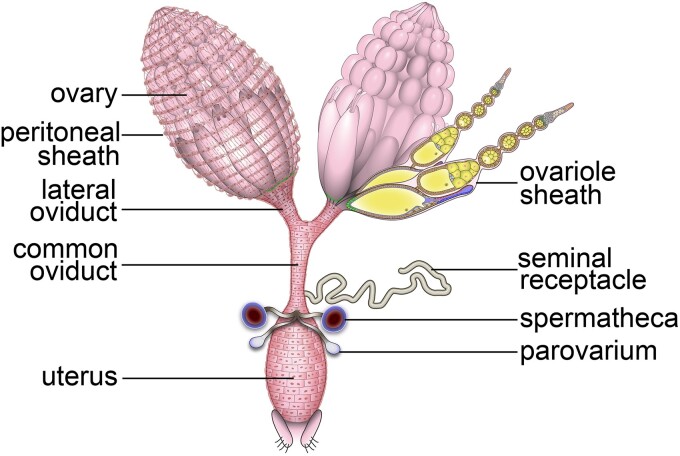
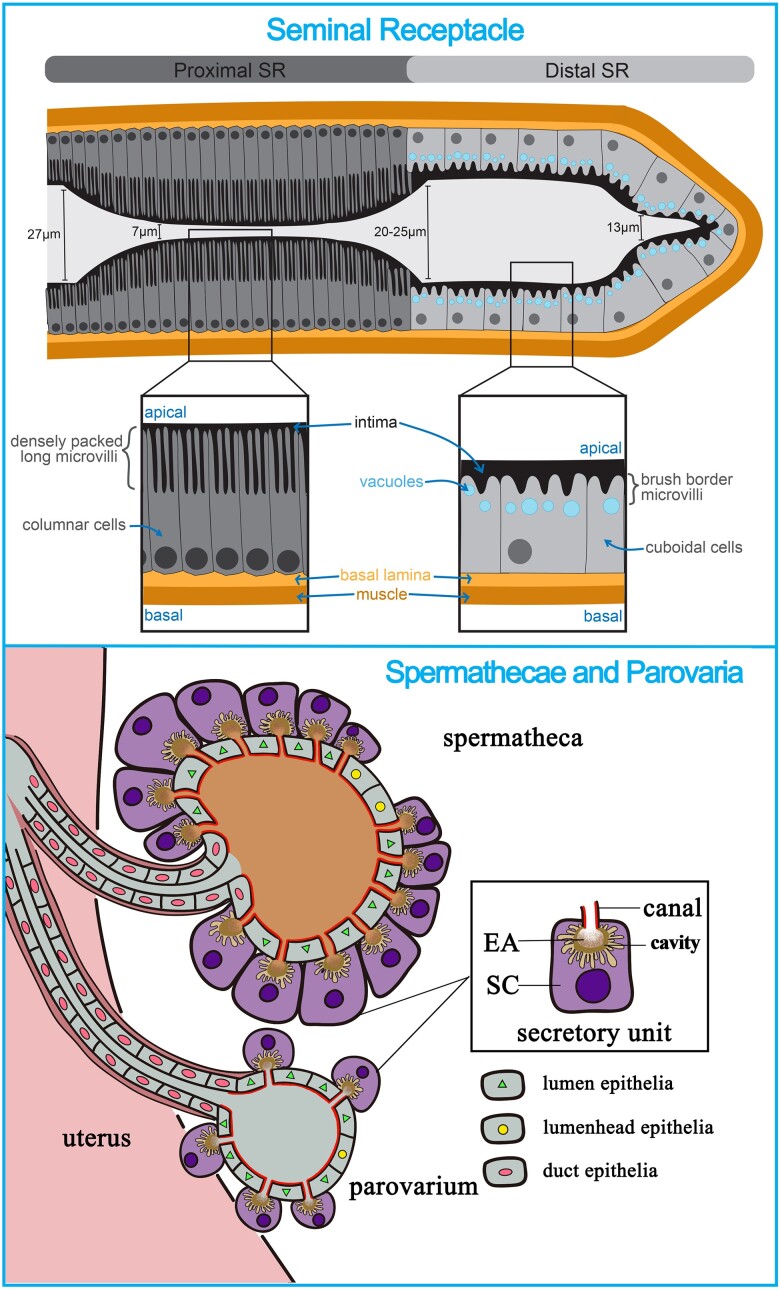
The Drosophila female reproductive system occupies a large portion of the ventral abdomen and consists of 2 ovaries that are linked to bilateral oviducts. These bilateral oviducts are joined at the common oviduct, which in turn connects to the uterus (Fig. 1). The reproductive tract is also connected to several accessory organs, including the seminal receptacle (SR), a pair of spermathecae (SPT), and a pair of parovaria (Fig. 1).
Fig. 1.
Overview of the Drosophila female reproductive system. A schematic drawing (adapted from Deady et al. 2017) of the Drosophila female reproductive system shows the germline-containing ovary, which is comprised of 16 ovarioles that house the germline stem cells and developing egg chambers. The remainder of the reproductive tract (also named the lower reproductive tract) is comprised of somatic tissues such as the lateral oviducts, common oviduct, and the uterus, through which the egg passes during ovulation. The lower reproductive tract has a layer of epithelium separating the lumen and an outer muscle layer. This diagram also shows somatic structures such as the seminal receptacle, spermatheca, and parovarium, which house sperm or produce reproductive secretions that play key roles in ensuring fertilization success.
Each ovary contains roughly 16 ovarioles, which are composed of a string of developing egg chambers wrapped by a thin layer of circular muscle sheath (Fig. 1; Hudson et al. 2008). The anterior end of each ovariole is called the germarium; this structure houses 2 germline stem cells that divide to produce cystoblasts. The cystoblasts undergo 4 rounds of division to produce 16-cell germline cysts; one cell will become the oocyte, while the other 15 will become highly polyploid nurse cells. Interestingly, the germ cell divisions occur via incomplete cytokinesis, leaving the cyst cells connected by intercellular bridges called ring canals. At the same time, follicle stem cells divide and produce daughters that establish a monolayer epithelium of ∼29 cells surrounding the 16-cell germline cyst (Nystul and Spradling 2010). This assemblage then separates from the germarium as a stage-1 (S1) egg chamber or follicle.
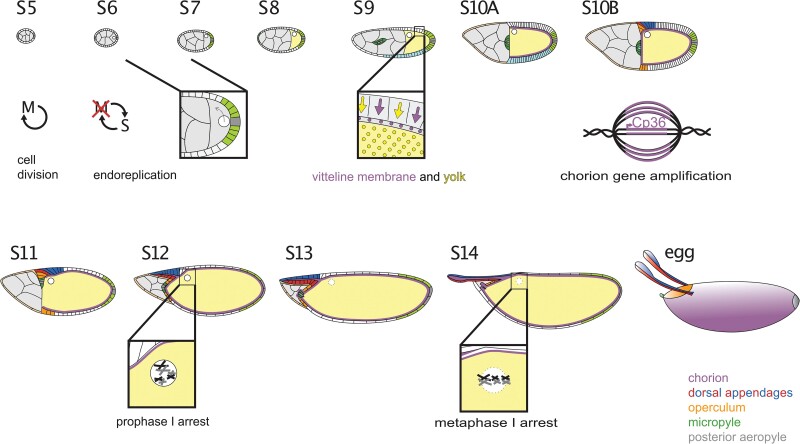
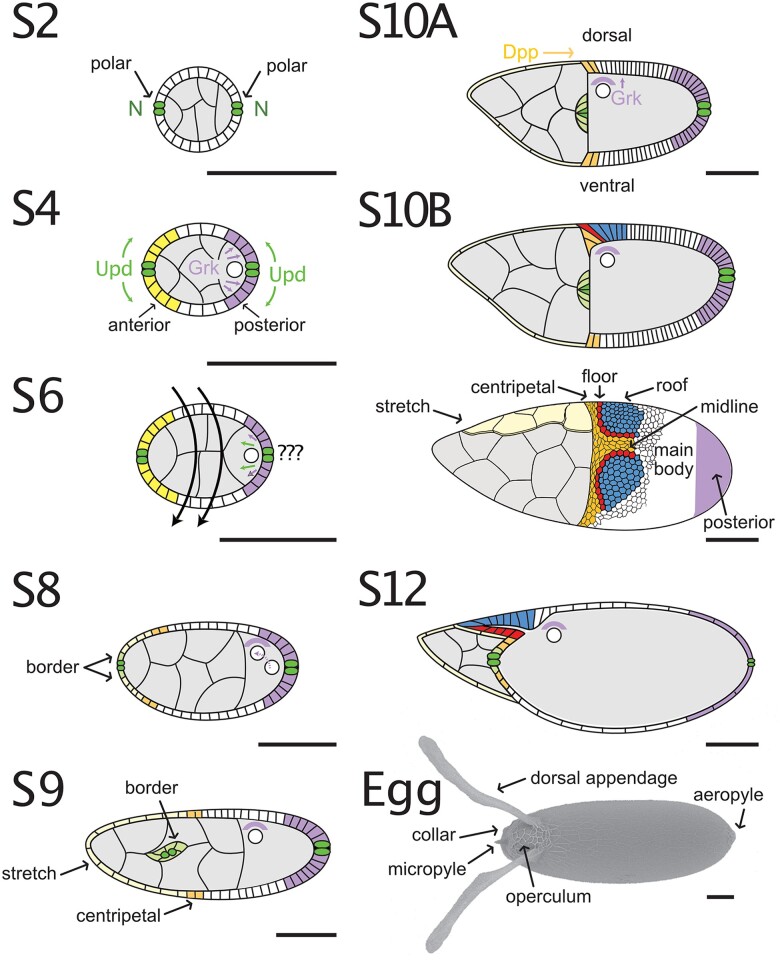
Each egg chamber develops through 14 morphologically distinct stages (S1–S14) to reach maturity (King 1970; Spradling 1993). During this developmental process, which requires ∼3 days, the nurse cells endoreplicate their DNA to facilitate mass production of maternal stores, and the oocyte grows in volume ∼100,000-fold (King 1970). The oocyte itself is essentially transcriptionally quiescent (Mahowald and Tiefert 1970; Jambor et al. 2015); thus, this growth occurs in 3 ways (Fig. 2). First, during early-to-mid stages, nurse cells synthesize RNAs, proteins, ribosomes, and organelles, and they transport these materials into the oocyte through the ring canals (Theurkauf et al. 1992; reviewed by Hudson and Cooley 2002). Transport is selective; e.g. specific mitochondria are selected and amplified and then transferred into the oocyte (Lieber et al. 2019). Second, in the middle stages of oogenesis (S8–S10), the oocyte takes up lipoprotein particles to create a pool of stored materials for use by the embryo (see Nutrient production and storage in oocytes). The transition into S8 is tightly regulated by nutrient availability and is under the control of insulin and ecdysone signaling (reviewed by Peterson et al. 2015). Third, during S11, the nurse cells rapidly transfer all their contents into the oocyte in a process called “dumping”; they then undergo programed cell death (see Nurse cell dumping and degradation). This third phase of oocyte growth is coordinated with morphological processes in the follicle cells (see Terminal patterning and formation of the operculum and micropyle and Dorsal/ventral patterning and dorsal appendage formation).
Fig. 2.
Stages of oogenesis. Germline cysts enveloped by a follicular epithelium emerge from the germarium as S1 egg chambers. This chapter focuses on the events from S5 onward that are needed to finish the egg. At S5, the follicle cells complete their last mitotic cycle and enter an endoreplication cycle at S6. They shut down endocycling at the transition from S10A to 10B, but they continue to amplify regions encoding the chorion genes. At S6, a signal from posterior follicle cells induces a reorganization of the oocyte cytoskeleton, and the oocyte nucleus moves to the anterior. From S8 to S10, the follicle cells synthesize and secrete yolk proteins and vitelline membrane proteins, and from S10 to S14, they synthesize and secrete the layers and specializations of the eggshell. At S9, the border cells delaminate and migrate between the nurse cells while the stretch cells flatten. At S10B, the centripetal cells begin to ingress. At S11, the nurse cells transfer their contents into the oocyte and begin to break down; at the same time, the dorsal appendage cells wrap to make 2 tubes. From S1 to S12, the oocyte chromosomes are held in a prophase I arrest. At S13, the oocyte nuclear envelope breaks down and the chromosomes line up on the metaphase plate. When the egg chamber moves into the oviduct, the follicle cells and nurse cell remnants slough off, revealing the eggshell.
During these periods of nurse cell and oocyte growth, the somatic follicle-cell layer undergoes several cell-cycle transitions (Fig. 2). At first (S1–S5), the follicle cells divide mitotically, sometimes with incomplete cytokinesis (McLean and Cooley 2013), to form a single-cell thick layer of roughly 650–900 cells surrounding the germline cyst (Margolis and Spradling 1995; Kolahi et al. 2009; Chen, Crest et al. 2019). These estimates in follicle cell number may differ due to the use of different methods or strains for quantifying the cell-division process. At S6, the follicle cells exit the mitotic cycle and enter an endocycle in which they skip the G2 and M phases (Lilly and Spradling 1996). Finally, at the transition from S10A to S10B, the follicle cells exit the endocycle but continue to replicate DNA at 6 genomic regions, regions encoding chorion proteins needed to construct the eggshell (see Eggshell composition and Chorion gene amplification). These cell cycle switches are regulated by Notch and ecdysone signaling (reviewed by Klusza and Deng 2011; Jia et al. 2015).
The follicular epithelium plays an important role in controlling egg shape, especially during mid-oogenesis (S5–S10; see Mechanisms of egg elongation), and then later (S9–S14), it produces the eggshell (see Eggshell composition and Chorion gene amplification), including specializations such as the dorsal appendages (see Dorsal/ventral patterning and dorsal appendage formation), operculum, and micropyle (see Terminal patterning and formation of the operculum and micropyle). The follicle cells also exchange signals with the oocyte to establish the polarity of the egg chamber and embryo (see Terminal patterning and formation of the operculum and micropyle and Dorsal/ventral patterning and dorsal appendage formation).
Once the egg chamber matures, it releases the oocyte from the posterior ovariole into the lateral oviduct, a process called ovulation (see Ovulation). Shortly after ovulation, egg activation (see Egg activation) transforms the oocyte into a haploid cell competent for supporting embryogenesis. The egg continues moving through the common oviduct into the uterus; there, a small opening in the eggshell called the micropyle is positioned at the opening of the SR or SPT (2 types of sperm storage organs located at the oviduct–uterus junction; Fig. 1; see Female reproductive tract secretions and reproductive success). If the female has mated, sperm released from the sperm storage organs can enter through the micropyle to fertilize the egg. The egg is then ejected from the uterus to the outside environment, a process named oviposition. The entire sequence from ovulation to oviposition is called egg laying, which is highly coordinated by hormonal, neuronal, and reproductive tract signals and female's mating status to maximize reproductive success.
Similar to mammals, steroid hormones play critical roles in Drosophila oogenesis (Okamoto et al. 2023). Ecdysone and its active form, 20-hydroxyecdysone (20E), which are the only steroid hormones in Drosophila, were first discovered via their critical roles in insect development and metamorphosis (Truman and Riddiford 2002). Ecdysteroid hormones are produced from sterol precursors by a series of cytochrome P-450s called the Halloween genes, including spook, spookier, phantom, disembodied, shadow, and shade, to name a few (Gilbert et al. 2002; Pan et al. 2021). Once produced, 20E binds to ecdysone receptor (EcR), which forms a heterodimer with ultraspiracle (Usp) to regulate target gene expression and control developmental progression and adult physiology (King-Jones and Thummel 2005; Schwedes and Carney 2012; Swevers 2019). Although ecdysteroids have no clear roles in Drosophila sex determination [a process that is mediated by a series of alternative splicing events for Sex lethal (Sxl), transformer (tra), transformer2 (tra2), doublesex (dsx), and fruitless (fru) (Marín and Baker 1998; Pomiankowski et al. 2004; Salz 2011)], they play more profound roles in adult oogenesis than in spermatogenesis. Specifically, ecdysteroid signaling regulates the entire progression of oogenesis from germarium to S14, including germline stem cell proliferation and differentiation; lipid accumulation and S8 quality-control check point (see Nutrient production and storage in oocytes); the transition from endocycle to gene amplification (see Chorion gene amplification); and ovulation in mature follicles (see Ovulation). Therefore, ecdysteroids are considered to be female sex hormones in adult Drosophila (Sieber and Spradling 2015). One unusual feature of ecdysone signaling in the ovary is that it acts locally on individual egg chambers or germaria (Buszczak et al. 1999; Gaziova et al. 2004; Domanitskaya et al. 2014). This local activity contrasts with its global action during larval molting and pupal metamorphosis (Riddiford et al. 2000).
Juvenile hormone (JH), another prominent insect hormone, controls metamorphosis and reproduction. In Drosophila, this pathway regulates follicular development, oviposition, circadian aspects of egg laying, and reproductive dormancy (Saunders et al. 1990; Easwaran et al. 2022; Kurogi et al. 2023). Nevertheless, many aspects of JH function in reproduction remain unclear.
In this chapter, we focus our discussion on the establishment of the mature oocyte, eggshell patterning and synthesis by somatic follicle cells, ovulation and egg activation, as well as the role of female reproductive tract environment on successful fertilization. Due to the page limit, we apologize for being unable to cite the works not included in this review.
Oocyte maturation
Nutrient production and storage in oocytes
Drosophila embryos undergo rapid cell division and differentiation to produce hatching larvae within a day of fertilization. To fuel this exceptional growth and development, mature oocytes are loaded with large amounts of nutrients, including carbohydrates, amino acids, and lipids (Tennessen et al. 2014; Sieber and Spradling 2015; Sieber et al. 2016). These nutrients are stored as large macromolecules [glycogen, yolk proteins (Yps), triacylglycerides (TAGs), and cholesterol ester (CE)] that form distinct membrane-bound particles called neutral lipid droplets and yolk spheres. Two types of yolk spheres (alpha and beta yolk spheres) exist in mature oocytes and exhibit distinct morphologies at the electron micrographic level (Mahowald 1972; Giorgi and Deri 1976; Giorgi and Jacob 1977; Giorgi et al. 1993; Papassideri et al. 2007). Alpha yolk spheres are crystalline in appearance and contain both Yps and stored lipids (Butterworth 1999; Papassideri et al. 2007). Having such a common storage depot may coordinate the mobilization of lipid and Yps and provide the raw material for early embryonic growth. In contrast, beta yolk spheres contain a mixture of Yps and glycogen. The abundance of glycogen in these structures gives the beta yolk spheres a more granular appearance and implies a role in glycogen storage (Butterworth 1999; Papassideri et al. 2007).
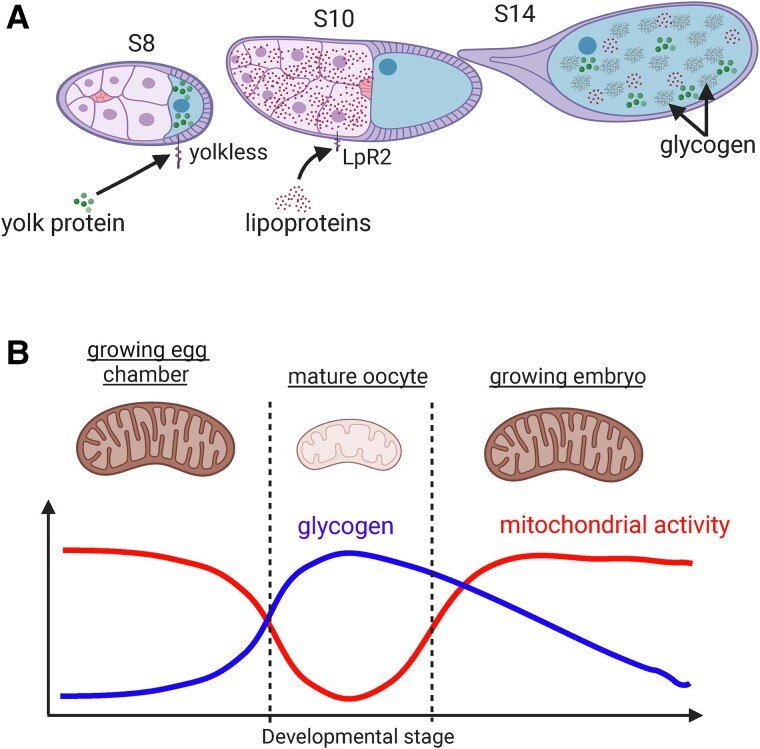
The process of yolk accumulation is called vitellogenesis, which starts at S8. Interestingly, these nutrients are stored in a sequential stepwise manner (Fig. 3a; Sieber and Spradling 2015; Sieber et al. 2016). Amino acids, in the form of Yps, are stored during the early stages of vitellogenesis (S8–S9; Schonbaum et al. 2000). Following Yp accumulation, large amounts of TAGs and CEs are stored in S9 egg chambers (Buszczak et al. 2002; Parra-Peralbo and Culi 2011; Sieber and Spradling 2015). After lipid storage is complete, the developing egg chamber undergoes a massive shift in metabolic state that drives a dramatic increase in stored glycogen as the egg chamber enters cellular quiescence (Sieber et al. 2016). This chronological pattern suggests that the mechanisms driving these shifts in cellular metabolism are mutually exclusive. This idea is consistent with the fact that glycolysis is required to synthesize fatty acids and that fatty acid oxidation feeds cells during the periods of gluconeogenesis. Moreover, this sequential nutrient accumulation implies that a coordinated set of developmental cues regulates the mechanisms that drive nutrient storage.
Fig. 3.
Metabolic transitions drive stepwise nutrient storage during oogenesis. a) Nutrient storage occurs in a stepwise fashion during S8–S14, beginning with Yolk protein uptake during S8–S10, followed by lipoprotein uptake from S9 to S10A, and glycogen storage beginning at S10B. b) A working model of metabolic transitions shows the suppression of mitochondrial respiration that signifies the onset of MRQ (mitochondrial respiratory quiescence) as egg chambers transition from growth in earlier stages to quiescence at S10B. This suppression of mitochondrial metabolism during MRQ helps drive glycogen storage in mature oocytes for use by the developing embryo. Created with BioRender.com.
Yp production, transport, and uptake
The Yps that are stored in both alpha and beta yolk spheres provide the amino acids that feed into the tricarboxylic acid cycle and facilitate high levels of de novo protein synthesis in the early embryo (Papassideri et al. 2007). These Yps, also named Vitellogenins, are encoded by 3 Yp genes (Yp1, Yp2, and Yp3; Gelti-Douka et al. 1974). All 3 Yps contain TAG lipase superfamily domains similar to mammalian acid lipases such as pancreatic lipase (Terpstra and Ab 1988). Sequence comparisons around the catalytic site, however, indicate that Yps are unlikely to retain some TAG lipase activity.
Yps in many species commonly contain lipid-binding domains. These domains may promote the storage of both Yp and lipids required for embryonic development. Yp3 mutant females are weakly fertile and produce oocytes with morphologically abnormal alpha and beta yolk spheres (Butterworth 1999). In contrast, certain temperature-sensitive mutations in Yp1 and Yp2 cause female sterility due to the formation of protein aggregates that accumulate in the space between the follicle cells and oocyte and block assembly of eggshell proteins into the vitelline membrane (Gans et al. 1975; Bownes and Hobson 1980; Butterworth et al. 1992).
Many developmental inputs regulate the expression of Yps, e.g. the steroid hormone ecdysone and its active form 20E. The ecdysone level in adult females increases dramatically in the first few days after eclosion and further increases upon mating (Harshman et al. 1999). This increasing ecdysone level induces Yp1, Yp2, and Yp3 expression in many tissues, including the fat body, heart, head, and somatic follicle cells of the ovary (Isaac and Bownes 1982). The overall effect is to increase the level of Yps in the hemolymph to meet the demands of female reproduction.
The Yp1 and Yp2 genes are located adjacent to each other and are divergently transcribed when ecdysone associates with EcR/Usp. EcR/Usp binds a conserved site in the shared promoter region of Yp1 and Yp2 (Shirk et al. 1983; Bownes et al. 1996). This clustering of functionally related genes around common binding sites is a conserved feature of nuclear receptor target genes. The GATA transcription factor encoded by serpent (srp) also binds a small enhancer region between Yp1 and Yp2. Such binding is required for normal Yp1 and Yp2 expression in follicle cells but not fat body (Lossky and Wensink 1995). Srp, much like other GATA factors, provides tissue specificity to the expression of target genes. In this case, Srp likely functions to ensure that Yps are expressed in tissues with enough biosynthetic and secretory capacity to ensure that adequate levels of Yps are in circulation to support oogenesis.
Predictably, since females make the large gamete that stores nutrients, female flies specifically express Yps. This sex-specific expression is regulated in part by the transcription factor Dsx. dsx mutant flies display significant reductions in Yp gene expression and protein levels in hemolymph (Bownes et al. 1983). This defect in Yp expression is not merely a side effect of the intersexed nature of dsx mutant females. Footprinting studies demonstrate that Dsx protein can directly bind response elements in the promoters of Yp genes to regulate their expression (Burtis et al. 1991). While the female isoform of Dsx promotes Yp expression, the male isoform of Dsx inhibits Yp expression, providing a mechanism that ensures the female-specific expression of Yps. Interestingly, ecdysone levels are significantly higher in female flies, and the hormone behaves like a female sex hormone (Sieber and Spradling 2015) in the adult; ecdysone signaling and dsx likely work together to dictate the sex-specific nature of Yp expression.
Once produced, Yps are secreted into circulation by tissues such as the fat body. Yps pass between the follicle cells in a process called “patency,” are absorbed by germ cells via receptor-mediated endocytosis, and then they are stored in yolk spheres (Richard et al. 2001; Isasti-Sanchez et al. 2021; Row et al. 2021). Both large and small alpha yolk spheres localize to the periphery of the oocyte near micropinocytic invaginations that may influence yolk sphere growth by promoting the uptake of Yps (Mahowald 1972). Yps are taken up by the Yp receptor encoded by yolkless (yl; DiMario and Mahowald 1987; Schonbaum et al. 1995, 2000). Yl belongs to the low-density lipoprotein receptor (LDLR) family of receptors and shares closest homology to the mammalian very-low-density lipoprotein receptor. During vitellogenesis, yl expression levels increase dramatically, and Yl protein is enriched on the oocyte membrane of developing egg chambers (Schonbaum et al. 1995, 2000). Mutants lacking Yl are female sterile and produce vitellogenic oocytes that are severely depleted of Yps (DiMario and Mahowald 1987; Schonbaum et al. 1995). These ultrastructural studies show that yl mutant oocytes also display a significant reduction in clathrin-coated vesicles, clathrin-coated organelles, as well as a dramatic reduction in the number of alpha and beta yolk spheres. In spite of this depletion, oocyte volume at S10B is not obviously diminished, but by S13 or S14, the egg chambers have collapsed. Intriguingly, similarities between Yl and LDLR at the amino acid level support the idea that the mechanism that drives Yp uptake and lipid storage is highly coupled. However, the mechanisms that link lipid storage and Yp uptake at a functional level remain an open question. Interestingly, recent work has implicated Yp uptake in the positioning of mRNAs such as oskar, suggesting nutrient uptake is coupled to germ plasm regulation (Tanaka et al. 2021).
JH also functions in yolk accumulation in many insect species, including Drosophila (Brookes 1969; Bell and Barth 1971; Bownes 1982; Roy et al. 2018). Interfering with JH levels in Drosophila females blocks vitellogenesis, possibly due to disrupted yolk production in the fat body and impaired yolk accumulation in germ cells (Giorgi 1979; Bownes 1982; Saunders et al. 1990; Bownes et al. 1996; Kurogi et al. 2023). Moreover, mutations in the JH receptor gene, Methoprene-tolerant (Met), cause a reduction in egg laying and fewer vitellogenic-stage egg chambers (Wilson and Ashok 1998), consistent with impaired yolk accumulation. The exact mechanisms by which JH acts in yolk accumulation in Drosophila are still unclear.
Lipid accumulation and storage
Following Yp storage, S9 egg chambers begin to absorb lipids from circulation and store large quantities of TAGs and CEs (Sieber and Spradling 2015). These forms of lipid are chemically inert storage depots for free fatty acids and free sterol that allow for safe, long-term sequestration of these metabolites during oogenesis (Parra-Peralbo and Culi 2011). These stored lipids move through the hemolymph as lipoprotein particles, similar to humans, and are absorbed by the cell via Lipophorin receptor 2 (LpR2)-mediated uptake (Fig. 3a; Parra-Peralbo and Culi 2011). In the conventional model for lipoprotein absorption, acylglycerides in the lipoprotein particles are broken down into free fatty acids by lipoprotein lipases after endocytosis, and fatty acids are absorbed by fatty acid transport proteins such as fatty acid transport protein 1 (Fatp1) or apolipoprotein lipid transfer protein particle (Apoltp). This process, however, has not been examined molecularly in detail in the fly.
The lipophorin receptors, LpR1 and LpR2, are homologs of the mammalian LDL receptor and are both expressed at high levels on the nurse cell and oocyte membranes during S9 and S10. Mutants lacking LpR1 display little to no effect on lipid levels in the oocyte, but LpR2 null mutant egg chambers are depleted of stored lipids and eliminated by cell death, suggesting that LpR2 is the primary lipoprotein receptor in the fly ovary (Parra-Peralbo and Culi 2011). Interestingly, LpR2 mediates lipid uptake in part by recruiting the lipid transfer protein Apoltp (Rodriguez-Vazquez et al. 2015). LpR1, LpR2 double mutants arrest during S8 of oogenesis, suggesting that lipid uptake is sustained by the conventional LDL receptor-mediated uptake. These data suggest that lipid levels are tightly monitored by developing egg chambers and that lipid deficiency triggers a S8 developmental checkpoint (Parra-Peralbo and Culi 2011). The severe phenotypes exhibited by LpR2 mutants also suggest that lipid uptake from circulation is the primary source of stored lipids in the ovary, rather than synthesis within the egg chamber itself.
As part of this process, recent work has shown that tricellular junctions in the follicle cell epithelia remodel to open small gaps between cells that allow the transport of lipids and other nutrients to the germ cells, thus facilitating nutrient storage (Isasti-Sanchez et al. 2021; Row et al. 2021). Temporal regulation is tied to the zinc finger transcription factor Tramtrak 69 (Ttk69), which controls the timing of several other processes in oogenesis (see Eggshell gene expression occurs in temporal and spatial patterns), and spatial regulation occurs through the signaling pathways that establish dorsoventral pattern (see Dorsal/ventral patterning and dorsal appendage formation; Row et al. 2021).
Developmental induction of lipid uptake during oogenesis is regulated by ecdysone signaling. Like steroid hormone produced in mammalian ovaries, ecdysone is also locally synthesized in Drosophila ovaries. Although the exact process is not clear, clonal analyses suggest that some enzymatic steps occur in the germline and that those products are passed to the follicle cells for further enzymatic processing (Buszczak et al. 1999; Domanitskaya et al. 2014; Ameku and Niwa 2016). During vitellogenesis, activation of the EcR/Usp receptor by ecdysone drives developing egg chambers to progress into vitellogenesis. The EcR/Usp complex induces a transcriptional shift that triggers many of the critical processes that occur during egg chamber development. Importantly, among these genes induced are many genes involved in lipid uptake, trafficking, and storage (Sieber and Spradling 2015).
EcR/Usp mediates these effects in part by regulating the activity and expression of the highly conserved adipogenic transcription factor sterol regulatory element binding protein (SREBP; Sieber and Spradling 2015). Inactivation of EcR/Usp, using temperature-sensitive mutations, causes modest reductions in SREBP and SCAP (SREBP cleavage activating protein) expression and a complete block in SREBP activation (Sieber and Spradling 2015). It is also possible that insulin signaling functions upstream of SREBP to promote lipid storage given that disruptions in the insulin pathway cause abnormalities in lipid droplet morphology (Vereshchagina and Wilson 2006). Consistent with this hypothesis, studies in mammals show that insulin/Akt signaling promotes SREBP-mediated adipogenesis (Streicher et al. 1996; Matsuda et al. 2001; Owen et al. 2012).
Previous studies have shown that SREBP is a lipid-regulated transmembrane protein that contains a transcriptional activation domain. Mammalian SREBPs are regulated by cellular sterol levels, but the Drosophila SREBP is regulated by fatty acids and phospholipids (Dobrosotskaya et al. 2002). Interestingly, SREBP is activated in nurse cells during vitellogenesis. Germline mutant clones for SREBP cause a dramatic reduction in stored lipid and subsequent developmental arrest (Sieber and Spradling 2015). Intriguingly, SREBP mediates much of these effects on stored lipids by regulating the expression of LpR2 (Sieber and Spradling 2015).
These findings in Drosophila are consistent with numerous studies in mammals that show SREBP-1C binds and regulates the expression of the LDL receptor gene (Hua et al. 1993). Moreover, insulin activation has been implicated in nurse cell lipid accumulation, consistent with conserved regulation of SREBP signaling by insulin/Akt signaling (Mensah et al. 2017). In mammals, SREBPs have been studied primarily in tissues such as the liver and adipose cells. SREBPs, however, are present in yeast and Caenorhabditis elegans (Espenshade 2006; Todd et al. 2006; Nomura et al. 2010), which do not have these types of lipid storage organs. Moreover, studies in C. elegans show that disruption of the worm ortholog of SREBP (SBP-1) decreases egg production, indicating a role in female germline function (Nomura et al. 2010). Taken together, work in Drosophila and C. elegans suggests that SREBPs may have an ancient, conserved role in reproductive processes predating their systemic effects in lipogenic organs. Moreover, these studies highlight the utility of Drosophila as a system to dissect the role of lipid metabolism in oogenesis.
All insects, including Drosophila, circulate massive amounts of diacylglyceride (DAG) in lipoprotein particles. DAG can function as a signaling molecule that can activate and interact with several signaling pathways, whereas TAG is an inactive storage form for fatty acids. As a result, once absorbed by the cell, DAG must be converted into TAG to be stored. The conversion of DAG into TAG requires the esterification of a third fatty acid on to the glycerol backbone of DAG. This fatty acid esterification reaction is facilitated by a family of enzymes called diacylglyceride acyltransferases (DGATs). midway encodes a DGAT family enzyme that shows increasing expression throughout vitellogenesis (Buszczak et al. 2002). Mutations in midway cause severe depletion of stored lipids in developing egg chambers and a subsequent arrest of oocyte development at the S8 nutrient checkpoint (Buszczak et al. 2002). These arrested midway mutant oocytes cannot progress through development and eventually undergo cell death in S9. Recent work in S2 cells has shown that active de novo lipid synthesis drives dramatic increases in lipid droplet size (Wilfling et al. 2013). Lipid droplet size in germ cells is small, however, and despite significant increases in lipid levels in S10 of oogenesis, their size does not increase, consistent with the idea that trafficked DAG provides the majority of lipid to the egg chamber. Together, these observations are consistent with the model that Midway plays a crucial role in the final esterification and storage of these lipids. Interestingly, the lipid droplet protein encoded by Jabba has been implicated in lipid accumulation and the storage of specialized histone proteins (H2AV). This observation suggests that lipid accumulation may be linked to histone storage during oogenesis (Li et al. 2012; McMillan et al. 2018; Stephenson et al. 2021). These data also suggest that lipid metabolism may be linked to genome organization and stability in the early embryo.
Glycogen storage and mitochondrial respiratory quiescence
Developing egg chambers progressing through S10–S14 undergo a massive shift in metabolic state as they enter cellular quiescence (Mermod et al. 1977; Lovett and Goldstein 1977). This shift leads to a 30-fold increase in the levels of glycogen, which is caused by active suppression of glycolysis and allowing the gluconeogenic synthesis of glucose (Fig. 3; Giorgi 1978; Papassideri et al. 2007; Sieber et al. 2016). Stored glycogen is then broken down during embryogenesis to sustain the demands of rapid growth by fueling aerobic glycolysis (Tennessen et al. 2014).
Glycogen storage is controlled by changes in insulin/Akt signaling that occur during oogenesis. Insulin/Akt signaling is active during early germline development and prevents glycogen storage (Sieber et al. 2016). As egg chambers progress into late oogenesis, insulin/Akt signaling decreases, providing a temporal developmental cue that triggers glycogen storage and may help drive the cells into quiescence. Consistent with the role of insulin/Akt signaling as a core sensor of nutritional state and germline development (Drummond-Barbosa and Spradling 2001; Richard et al. 2005; LaFever and Drummond-Barbosa 2005; LaFever et al. 2010; Hsu and Drummond-Barbosa 2011), both starvation and insulin pathway inhibition induce premature glycogen storage and mitochondrial depolarization in germ cells (Sieber et al. 2016). These observations suggest that insulin signaling functions during oocyte development as a nutrient-responsive developmental cue. In this role, insulin coordinates nutritional status with the transitions in the metabolic state that support cellular quiescence and the developmental competence of the oocyte.
Insulin signaling facilitates this metabolic transition in late oogenesis by causing a dramatic shift in mitochondrial function (Fig. 3b; Sieber et al. 2016). During early oogenesis, mitochondrial membrane potential and electron transport chain (ETC) activity are high. Beginning in S10B, however, the mitochondrial membrane potential decreases, and ETC activity drops dramatically in a process called mitochondrial respiratory quiescence (MRQ; Sieber and Spradling 2015). MRQ functions in mature oocytes to prevent nutrient loss and likely to protect the oocyte against oxidative damage before fertilization. Inhibiting insulin/Akt signaling induces a premature onset of MRQ and glycogen accumulation. These data suggest that suppressed mitochondrial activity during MRQ leads to a block in pyruvate utilization and glycolysis, thereby promoting glycogen storage in late oogenesis. Insulin/Akt signaling prevents MRQ in early germ cells by suppressing the serine/threonine kinase glycogen synthesis kinase 3 (GSK3; encoded by shaggy; Sieber and Spradling 2015). When Akt kinase activity decreases, GSK3 stimulates a remodeling of the ETC assembly that leads to suppressed mitochondrial activity and MRQ. During ETC remodeling, complex 1 and adenosine triphosphate (ATP)-synthase are actively disassembled. Inhibiting GSK3 causes mature oocytes to display high levels of mitochondrial activity, decreased levels of nutrients, and compromised oocyte developmental competence.
Recent work has shown that GSK3 mediates this process by phosphorylating targets in the outer mitochondrial membrane, such as the voltage-dependent anion channel (VDAC, encoded by porin), and induces the turnover of outer membrane proteins by the ubiquitin proteasome system (UPS). The phosphorylation of VDAC triggers a massive recruitment of the proteasome to the mitochondrial surface. This recruitment drives the remodeling of the mitochondrial proteome and the suppression of respiration (Yue et al. 2022). Interestingly, this recruitment of the proteasome to the mitochondria is highly conserved in quiescent cells in systems ranging from fungi (Neurospora) to human cell models of cellular quiescence, indicating this process is a highly conserved mechanism that suppresses mitochondrial metabolism and promotes glycogen storage in dormant cells (Yue et al. 2022). Moreover, there is a significant 3-fold increase in cellular UPS activity in these cells that compensates for the sequestration of the proteasome to the mitochondrial surface.
This newfound role for GSK3 in MRQ has subsequently been observed in mammalian quiescent B cells, suggesting GSK3 has a conserved role in regulating mitochondrial function in quiescent cells (Jellusova et al. 2017). Consistent with a role in late oogenesis, previous work has shown that GSK3 also functions to promote the completion of meiosis through regulation of the calcineurin pathway (Takeo et al. 2012). These data suggest that GSK3 may coordinate mitochondrial function with cellular quiescence and progression through meiosis. This hypothesis is consistent with studies in yeast and Drosophila that show that amino acid and glucose metabolism have similar roles in the regulation of early gametogenesis (Wei and Lilly 2014). Interestingly, insulin signaling also functions early in germ cell development to regulate mitochondrial DNA number via Myc, suggesting that insulin signaling has multiple roles in regulating germline mitochondrial function (Wang et al. 2019). Moreover, this early role for insulin signaling is likely coupled to the active selection of high-quality mitochondria that occur in germ cells very early in cyst development (Lieber et al. 2019). These mechanisms likely cooperate to ensure oocyte developmental competence.
Systemic signaling regulating nutrient storage
Considering the tremendous biosynthetic and energetic demands of oocyte production, female flies alter their metabolic state. Newly eclosed male and female flies display similar levels of TAG and glycogen. As the females mature, however, they establish an enhanced metabolic state that supports oocyte development.
Drosophila females are larger and contain roughly 2-fold more stored TAG and glycogen than seen in males (Sieber and Spradling 2015). Females store more TAG and glycogen in part by increasing their feeding rate relative to males. Indeed, metabolic sexual dimorphism is required for female fertility. Mutations and RNAi lines disrupting lipid storage droplet 2 and magro, genes involved with lipid storage and digestion, cause significant reductions in female TAG levels and result in reduced egg laying (Sieber and Spradling 2015).
Females establish metabolic sexual dimorphism by increasing the levels of 20E in mature adults (Sieber and Spradling 2015). In the adult, the ovary is the primary source of steroid production, and production increases after the female mates, leading to significantly higher levels of 20E in female flies (Harshman et al. 1999; Ameku and Niwa 2016). In the female, 20E acts as a female sex hormone to control metabolic sexual dimorphism. Ectopic 20E feeding can stimulate glycogen storage and TAG accumulation in males, demonstrating that ecdysone is sufficient to establish a female metabolic state (Sieber and Spradling 2015). 20E establishes a female metabolic state by acting through EcR/USP in the central nervous system (CNS) to enhance feeding behavior either by altering the neuroendocrine axis [insulin, adipokinetic hormone (Akh), etc.] or directly modulating the neural circuit that dictates feeding in the fly. How exactly 20E establishes a female metabolic state, however, remains an open question. As discussed above, 20E is thought to be predominantly produced by individual late-stage egg chambers. As egg production increases, 20E levels should rise and further enhance feeding when females are producing more oocytes. This feed-forward mechanism allows the female to tune female systemic metabolic state to support the number of eggs she is producing.
While sex and reproductive status can influence the female metabolic state, systemic metabolism has a tremendous impact on the regulation of oogenesis. Studies in Drosophila have shown that female flies fed a diet depleted in amino acids and lipids reduce egg production. Under these conditions, S10B egg chambers complete their development, but young egg chambers arrest at the S8 checkpoint, S8–S10 egg chambers die and are resorbed, and the germline stem cells stop dividing in the germarium (Drummond-Barbosa and Spradling 2001; Buszczak et al. 2002; Parra-Peralbo and Culi 2011). The S8 checkpoint is a nutrient-dependent quality control checkpoint that is under control of hormonal cues and metabolic status (Buszczak et al. 1999; Drummond-Barbosa and Spradling 2001; Parra-Peralbo and Culi 2011). This checkpoint is thought to be present in other insect species and may be a conserved aspect of oogenesis (Chapman et al. 2013). These dietary effects on oogenesis are regulated in part by reduced neuronal production of insulin-like peptides (Britton et al. 2002; LaFever and Drummond-Barbosa 2005). In response to amino acid deficiency, the neural insulin production decreases, thereby causing severe disruptions in development and reproduction (Britton et al. 2002; Rulifson et al. 2002). Under these conditions, insulin signaling functions as a systemic nutrient sensor that monitors metabolic state and adjusts growth, development, and reproductive status to match nutrient availability.
One tissue that integrates systemic metabolism and reproduction on many levels is the fat body. In Drosophila, the fat body combines the function of adipocytes and hepatocytes and plays a significant role in the regulation of systemic metabolism. Fat tissue provides a primary source of several yolk components for developing egg chambers. The fat body synthesizes and stores vast amounts of TAGs that are necessary for egg development. Fat body TAGs are broken down into DAGs, packaged into lipoprotein particles, and secreted to provide the predominant source of the circulating lipoprotein particles in the body. These lipoprotein particles supply the ovary with DAGs and sterols during vitellogenesis (Parra-Peralbo and Culi 2011; Palm et al. 2012). The fat body is also a significant source of Yps for developing oocytes. The fat body produces and secretes Yp1, Yp2, and Yp3 into circulation (Bownes and Hobson 1980). These circulating Yps are abundant in hemolymph, highlighting that the ovary requires peripheral tissue to meet the protein yolk requirements for oogenesis (Isaac and Bownes 1982).
Interestingly, in response to alterations in the diet, many processes change within the fat body. These processes include a diverse array of metabolic pathways including glycolysis, iron transport, and the Kennedy pathway for phospholipid biosynthesis (Matsuoka et al. 2017). Disruption of the Kennedy pathway (eas-RNAi) or iron transport (Fer1HCH-RNAi) in the fat body causes defects in germline stem cell maintenance, whereas disrupting the glycolysis/gluconeogenesis pathway causes defects in early germ cell cyst survival (Matsuoka et al. 2017). These data suggest that specific fat metabolic processes are linked to distinct aspects of oogenesis, either through the regulation of fat body-derived hormones or through the trafficking of downstream metabolites to the ovary.
The fat body also produces and secretes extracellular matrix (ECM) components, such as the type IV collagen encoded by viking (vkg) and collagen type IV alpha 1 (Col4a1), that incorporate into basement membrane throughout the larval body to control organ shape (Pastor-Pareja and Xu 2011; Peng et al. 2022). As discussed below (see Mechanisms of egg elongation), developing egg chambers deposit collagen IV in bands around the egg chamber to help drive elongation and shape the egg (Haigo and Bilder 2011; Weaver and Drummond-Barbosa 2018).
In conjunction with this biosynthetic role, the fat body also plays an essential signaling role in regulating oogenesis. Studies of the adipokine adiponectin in Drosophila have shown that the adiponectin receptor AdipoR functions in the ovary to promote germline stem cell maintenance (Laws et al. 2015). Overexpression of AdipoR protects germaria from stem cell loss (Laws et al. 2015), suggesting that abnormalities in fat body signaling may contribute to the age-associated decline in fertility seen in many systems including Drosophila.
Nurse cell dumping and degradation
Nurse cell dumping and its regulatory mechanism
Another major source of maternal stores is the highly polyploid nurse cells, which synthesize ribosomes, mitochondria, cytoskeletal subunits, tRNAs, and other products that facilitate rapid development of the embryo. Throughout the early stages of oogenesis, some select material moves from the nurse cells on cytoskeletal tracks through intercellular bridges, the ring canals, and into the oocyte. Recent work has found that cortical dynein also transports microtubules (MTs) through the ring canals as a means to transport bulk cytoplasmic content into the oocyte (Lu et al. 2022). The vast majority of the nurse cell contents, however, is transferred extremely rapidly (20 minutes) at S11 in a process called nurse cell dumping (reviewed by Mahajan-Miklos and Cooley 1994b).
Beginning in stage 10B, the egg chamber prepares for nurse cell dumping by organizing 2 classes of actin filaments in the nurse cells. At the cortex, actin polymerization and myosin activity provide much of the mechanical force that drives the transfer of nurse cell components through the ring canals into the oocyte (Cooley et al. 1992; Cant et al. 1994; Hudson and Cooley 2002; Airoldi et al. 2011). A recent study suggests that the actomyosin contractile force is only responsible for the completion of the dumping, while the initial dumping of the most cytoplasm is mediated by hydraulic transportation (Imran Alsous et al. 2021). In addition, the large polyploid nurse nuclei are anchored in position by actin filaments to prevent these structures from blocking the ring canals and preventing the transfer of nurse cell components (Cooley et al. 1992; Tilney et al. 1996; Guild et al. 1997).
Actin also is a major structural component of the ring canal itself, and actin polymerization and crosslinking play important roles in ring canal growth and pore formation. Given that all the nurse cell-derived factors must be transported into the oocyte via ring canals, studies have focused on ring canal synthesis and maturation as a way of analyzing the regulation of transport into the oocyte (Hudson and Cooley 2002). Disruption of actin-binding proteins such as profilin (chickadee) and villin (quail), as well as actin motor proteins such as nonmuscle myosin II (spaghetti squash, zipper), causes defects in the nurse cell cytoskeleton, defective ring canal formation, and a block in the transfer of nurse cell cytosolic factors to the oocyte, yielding a significant reduction in oocyte size (Cooley et al. 1992; Xue and Cooley 1993; Cant et al. 1994; Knowles and Cooley 1994; Mahajan-Miklos and Cooley 1994a; Edwards and Kiehart 1996). quail and chickadee were named after birds that produce small eggs, and dumpless mutants typically produce small eggs as well. Actin-nucleating factors such as Spire and Cappuccino facilitate the actin mesh network assembly, maintain MT polarity and structure, and help regulate actin and MT dynamics during oogenesis (Manseau and Schüpbach 1989; Dahlgaard et al. 2007; Liu et al. 2009; Yoo et al. 2015).
The dynamic changes in the actin cytoskeleton are regulated by conserved kinases such as Rho kinase (Rok) and protein kinase N (Pkn). Deleting either of these genes in developing germ cells yields S10 egg chambers that display disorganized actin bundles and defective nurse cell dumping. Pkn antagonizes actomyosin activity in S10 egg chambers and, due to excessive actomyosin contractility, Pkn mutants actually delay nurse cell dumping (Ferreira et al. 2014). In contrast, Rok functions in developing egg chambers to promote the formation of actin bundles before dumping (Verdier et al. 2006). In particular, mutant egg chambers lacking Rok display a dramatic reduction in the actin bundles that anchor the nurse cell nuclei. This lack of anchoring leads to a severe dumpless phenotype due to nurse cell nuclei obstructing the ring canals.
Nurse cell dumping is also regulated by a class of active lipid-signaling molecules called prostaglandins. Prostaglandins are synthesized from essential fatty acids through either the cyclooxygenase pathway or the lipoxygenase pathway. Disrupting cyclooxygenase-mediated prostaglandin biosynthesis in Peroxinectin-like (Pxt) mutants leads to defects in egg chamber maturation and severe defects in actin bundle organization (Tootle and Spradling 2008). Interestingly, prostaglandins control actin bundle formation specifically in mid-oogenesis, suggesting a temporally defined role for these molecules (Spracklen et al. 2014). Although prostaglandins mediate these effects on developing germ cells via the actin-bundling protein fascin (Groen et al. 2012; Kelpsch et al. 2016), the mechanism for how prostaglandins affect fascin function remains unknown (Spracklen et al. 2019).
Nurse cell dumping occurs simultaneously with other developmental processes such as chorion gene amplification, centripetal cell migration, and dorsal/ventral patterning. The signals that coordinate these processes, however, remain unclear.
During oogenesis, MTs play key roles in transporting factors that are important for oocyte specification, axis determination, and egg chamber patterning (reviewed by Riechmann and Ephrussi 2001). During S10B, however, the MT network disassembles and short, subcortical MTs form that drive ooplasmic streaming until S13. This ooplasmic streaming ensures adequate mixing of the oocyte cytoplasm during nurse cell dumping. At the same time, ribonuclear protein (RNP) complexes essential for establishing embryonic polarity are captured and anchored at the poles (Forrest and Gavis 2003; Weil et al. 2006; Sinsimer et al. 2011). This phenomenon provides an intriguing system to examine how the cytoskeleton impacts biophysical processes in the cytosol (reviewed by Quinlan 2016).
Nurse cell breakdown
Once nurse cells have transferred their cytosol to the oocyte, their cellular remnants are broken down. Nurse cell turnover requires apoptosis, autophagy, and phagocytosis (reviewed by Lebo and McCall 2021), but many steps in this process remain unclear. The field commonly uses nurse cell nuclear degradation as a readout of the progress of nurse cell breakdown (Yalonetskaya et al. 2020). Nuclear breakdown begins in S12 and is associated with the activation of apoptotic caspases (Peterson and McCall 2013). During S13, nurse cell nuclei become highly acidic and are strongly TUNEL-positive (Terminal deoxynucleotidyl transferase dUTP nick end labeling), suggesting the presence of numerous DNA breaks (Peterson et al. 2003, 2007; Peterson and McCall 2013). Recent work has shown that mouse ovaries also produce nurse cells that support oocyte development and are eliminated, suggesting that nurse cell death is a very highly conserved aspect of oogenesis (Niu and Spradling 2022).
Unlike many other cell death processes, the apoptotic machinery during nurse cell death is not activated by somatic apoptotic inducers Reaper, Hid (Head involution defective), and Grim (Foley and Cooley 1998). Instead, it is activated by degradation of the Drosophila inhibitor of apoptosis Bruce (BIR repeat containing ubiquitin-conjugating enzyme) (Nezis, Shravage, Sagona, Johansen et al. 2010). Furthermore, mutating the key apoptotic caspases encoded by Strica (Ser/Thr-rich caspase), Dronc (Death regulator Nedd2-like caspase), Drice (Death related ICE-like caspase) and Dcp-1 (Death caspase-1) yield oocytes where 1–2 nurse cell nuclei are retained by the mature egg chamber (Baum et al. 2007). Similar mild defects were obtained by overexpressing the apoptotic inhibitors p35 and DIAP (Death-associated inhibitor of apoptosis). This mild phenotype shows that the turnover of nurse cell nuclei is not a strictly apoptotic process and that nurse cell degradation is a unique form of programed cell death.
Consistent with the idea that other processes are required for nurse cell breakdown, autophagy also has a role in nuclei degradation (Bass et al. 2009; Nezis, Shravage, Sagona et al. 2010) and is induced in conjunction with the activation of apoptotic caspases (Peterson and McCall 2013). Mutations that disrupt the autophagy pathway display mild phenotypes, with a fraction of egg chambers containing a small number of persistent nurse cell nuclei. Moreover, disrupting both autophagy and apoptosis in mature germ cells does not enhance the phenotype in the ovary, suggesting that nurse cell death does not require conventional cell death mechanisms. Consistent with this idea, mutations in genes that encode DNaseII and vacuolar-type H+-ATPases (V-ATPases) impair nurse cell turnover, suggesting that enzymatic breakdown of nucleotides and extracellular acidification by lysosomal machinery are critical for nurse cell turnover (Bass et al. 2009; Mondragon et al. 2019). Nevertheless, it remains unclear how these processes biochemically contribute to the breakdown of nurse cells.
Interestingly, phagocytosis of dying nurse cells plays an essential role in their clearance (Cavaliere et al. 1998; Foley and Cooley 1998; Nezis, Shravage, Sagona et al. 2010; Santoso et al. 2018). The phagocytic clearance of apoptotic cells requires specific engulfment receptors to recognize the apoptotic cells; recognition activates at least 2 parallel and conserved cell death (CED) pathways (CED1/CED6/CED7 and CED2/CED5/CED12) to reorganize the cytoskeleton in the nonprofessional phagocytes (stretch follicle cells) and engulf the apoptotic cells (Shklover et al. 2015a, 2015b). The phagocytic receptor Draper (CED-1) and the integrin alphaPS3 (scab) are expressed in stretch follicle cells during S12–13 as nurse cells breakdown. Draper works in parallel with alphaPS3, which is thought to function through CED12 signaling, to facilitate nurse cell degradation. As a result, draper/scab double mutants exhibit a substantial block in nurse cell nuclei breakdown. Draper and aPS3/integrin/CED12 signaling induce the nuclear acidification that is required for the breakdown of nurse cell nuclei (Timmons et al. 2016, 2017). While these phenotypes are consistent with the known role of phagocytosis in clearing cellular debris, it remains unclear how follicle cell phagocytosis impacts the germ cell autonomous aspects of the nurse cell breakdown (Bass et al. 2009; Timmons et al. 2016). Moreover, while insulin, TOR (target of rapamycin), and JNK (Jun kinase) signaling have all been implicated in the regulation of nurse cell turnover at earlier stages of oogenesis (see below and Lipid accumulation and storage), it remains unclear if these pathways coordinate the cell autonomous and noncell autonomous aspects of nurse cell turnover during oocyte maturation. Interestingly, Draper, insulin/TOR, and JNK have all been implicated in other instances of developmentally programed cell death, suggesting that studies of nurse cell death will provide broad insights into development and tissue homeostasis (Tracy and Baehrecke 2013). While nurse cell degradation is a novel form of programed cell death, it remains unclear how germ cell turnover varies from traditional apoptotic or autophagic cell death at a mechanistic level.
In addition to the programed developmental nurse cell death, defects in germ cell physiology and environmental stress can trigger a quality-control check point during S8 of oogenesis. Under these circumstances, vitellogenic egg chambers undergo cell death to prevent suboptimal eggs from progressing further in development; this cell death also allows recovery of nutrients contained within those egg chambers (Pritchett et al. 2009; Jenkins et al. 2013). In contrast to programed developmental germline cell death, starvation-induced egg chamber death relies on the apoptotic machinery, including factors such as Dcp-1. Simple overexpression of apoptotic caspase inhibitors such as DIAP causes a substantial block in egg chamber death in response to starvation (Hou et al. 2021). Autophagy also functions in parallel to facilitate this form of cell death. Like developmental cell death, once egg chamber death is initiated, the follicle cells mediate the phagocytic clearance of the dying cyst via the draper/ced-1 pathway. This process has been discussed at length in several reviews (Buszczak and Cooley 2000; Pritchett et al. 2009; Jenkins et al. 2013). Overall, Drosophila oogenesis provides an excellent system to examine the mechanistic differences in developmental and damage-induced forms of cell death. Studies on these regulatory events may provide critical insight into how these processes could be co-opted to prevent the aggressive ectopic growth seen in cancer.
Eggshell production
The Drosophila eggshell is an outstanding model for studying cell and developmental biology. Section Eggshell composition describes how studies on the eggshell have revealed structural features of the ECM and given insight into its synthesis program (Waring 2000; Cavaliere et al. 2008). Section Chorion gene amplification compares chorion gene amplification and its cell cycle regulation with metazoan DNA replication and highlights the conserved factors that regulate these processes (Tower 2004; Klusza and Deng 2011). Sections Terminal patterning and formation of the operculum and micropyle and Dorsal/ventral patterning and dorsal appendage formation outline the reciprocal signaling pathways between germ cells and follicle cells that lay the foundation for establishing the anterior/posterior (A/P), dorsal/ventral (D/V), and terminal regions of the embryo, thus determining the overall body plan of the fly (Merkle et al. 2020). These sections also examine how these same patterning signals specify follicle cell subtypes that create specialized eggshell structures required for fertilization, embryonic development, and larval hatching (Montell et al. 2012; Duhart et al. 2017; Osterfield et al. 2017; Horne-Badovinac 2020). Section Mechanisms of egg elongation describes how the follicle cells that secrete the eggshell also determine the overall shape of the egg (Cetera and Horne-Badovinac 2015). Thus, in this section, we describe the foundational work in Drosophila melanogaster that established the eggshell as a premier investigative system.
Eggshell composition
The eggshell protects the embryo by preventing dehydration and facilitating gas exchange (Hinton 1981). Two main types of proteins provide structure to the eggshell, vitelline membrane and chorion proteins (reviewed by Waring 2000; Cavaliere et al. 2008), but the construction of the eggshell exhibits a surprising interplay between these 2 constituent classes (Pascucci et al. 1996; Mauzy-Melitz and Waring 2003). The abundance of the vitelline membrane and chorion proteins, and the sex-specific nature of their expression, made analyses of these proteins highly attractive during the early molecular era (e.g. Petri et al. 1976; Waring and Mahowald 1979; Spradling and Mahowald 1980; Spradling et al. 1980; Osheim and Miller 1983; Higgins et al. 1984; Mindrinos et al. 1985; Osheim et al. 1988).
The eggshell consists of the vitelline envelope and chorion
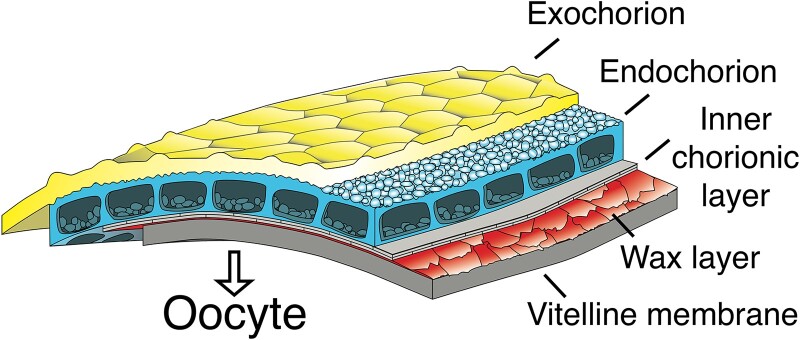
Scanning and transmission electron microscopy studies demonstrate that the eggshell is composed of 5 layers (Fig. 4; reviewed by Waring 2000; Cavaliere et al. 2008). The inner 2 layers are grouped together as the vitelline envelope, while the outer 3 layers are called the chorion.
Fig. 4.
Composition of the Eggshell. Moving from oocyte proximal (bottom) to external surfaces (top), the eggshell consists of a vitelline membrane (thick, solid), wax layer (thin, scalloped), inner chorionic layer (thin, brick-like), endochorion with its floor, roof, and pillars (pebbly windows), and the exochorion (surface craters). (Redrawn with permission from Margaritis et al. 1980.) See also Turner and Mahowald (1976) and Margaritis (1986) for more detail.
The inner most layer, the vitelline membrane, consists of 6 major and numerous minor proteins that are secreted by the columnar follicle cells during S9–S10 (Supplementary Table 1). In subsequent stages, the cleavage of 2 of the most abundant proteins, Vm26Aa and Vm26Ab, together with a 2-step crosslinking process, mediates the formation of a 300-nm envelope that serves as a barrier against the passage of macromolecules (Fargnoli and Waring 1982; Mindrinos et al. 1985). Associated with the vitelline membrane is a thin wax layer that prevents desiccation of the embryo. This layer is produced by the follicle cells in S10–S12, but its composition is unknown (Papassideri et al. 1993).
Moving radially outward into the chorion, the next layer is the inner chorionic layer (Fig. 4); it consists of glycoproteins secreted by the follicle cells beginning at S12. These proteins self-assemble into a 40-nm thick crystalline lattice (Akey and Edelstein 1987; Papassideri and Margaritis 1996). During eggshell synthesis, the inner chorionic layer holds peroxidases that covalently link chorion proteins at the end of oogenesis (Margaritis 1985; Konstandi et al. 2005). Other hypothesized functions for the inner chorionic layer are to anchor the vitelline membrane to the outer chorion layers during hatching and to regulate embryonic water content post egg-laying (Papassideri and Margaritis 1996).
Exterior to the inner chorionic layer is the endochorion, an architecturally sophisticated structure composed of floor, pillars, and roof (Fig. 4). The endochorion, which is 500–700 nm thick, contains 6 major eggshell proteins and over a dozen minor proteins (Supplementary Table 1). Synthesis begins in S10 with the production of Cp36 and Cp38; it then peaks in S12 with the addition of 4 lower molecular weight proteins, Cp15, Cp16, Cp18, and Cp19 (Petri et al. 1976; Waring and Mahowald 1979; reviewed by Waring 2000; Cavaliere et al. 2008). Based on EM immunostaining, it is likely that the chorion proteins are distributed throughout the floor, pillars, and roof; that is, the specific substructures of the endochorion are not formed by unique proteins (Pascucci et al. 1996; Noguerón et al. 2000). Intriguingly, assembly of the endochorion involves transient storage of eggshell proteins within the vitelline membrane (Pascucci et al. 1996).
The outermost eggshell layer, the exochorion, consists of a loose, polysaccharide-rich matrix containing an acidic, alcian blue-positive component, presumably glycosaminoglycan (Fig. 4). This network of protein and carbohydrate forms hexagonal or pentagonal ridges protruding from the main surface of the eggshell, mirroring the shapes of the apical surfaces of the follicle cells that secreted the eggshell layers (Turner and Mahowald 1976; Margaritis et al. 1980).
In addition to these discrete layers, the eggshell has specialized structures: a posterior aeropyle and 2 anterior dorsal appendages, which facilitate gas exchange for the developing embryo; an anterior ventral micropyle, which enables sperm entry; and an anterior operculum and collar, which act like a trap door to facilitate larval hatching (Fig. 2; Duhart et al. 2017). We discuss these specializations below in the sections Terminal patterning and formation of the operculum and micropyle and Dorsal/ventral patterning and dorsal appendage formation.
Several recent studies have exploited high-throughput technologies to identify additional components relevant to eggshell production (Supplementary Table 1). These studies employed genetic, gain-of-function screens (Khokhar et al. 2008); mass spectrometry of eggshell peptides purified from late-stage egg chambers (Fakhouri et al. 2006); microarrays and array-based comparative genomic hybridization to analyze DNA from fluorescent-activated cell sorting (FACS)-sorted follicle cells (Claycomb et al. 2004; Kim et al. 2011); or microarrays to determine genome-wide transcript levels in hand-dissected egg chambers (Tootle et al. 2011). These efforts identified potential new structural components of the eggshell as well as dozens of other genes that likely regulate chorion production or assembly. They also provided markers to facilitate cluster analysis for a single-cell RNA-seq study for assessing the development of the ovary (Jevitt et al. 2020). This scRNA-seq analysis complemented and built upon decades of work analyzing gene expression in follicle cells.
Eggshell gene expression occurs in temporal and spatial patterns
The columnar follicle cells (those lying over the oocyte) follow a precise temporal program to synthesize the major components of the eggshell: during S8–S10, they produce the vitelline membrane proteins; from S10B to S12, they make the higher MW proteins Cp36 and Cp38, and from S12 to S14, they produce the lower MW proteins Cp15, Cp16, Cp18, and Cp19 (reviewed by Waring 2000; Cavaliere et al. 2008). This timing is consistent with the construction of the eggshell layers in zones (Margaritis 1986), but the process is not so simple. For example, both Cp36 and Cp18 reside transiently in the vitelline membrane and are released over time to build up outer layers (Trougakos and Margaritis 1998a). By the end of oogenesis, however, almost all the major proteins are uniformly distributed throughout their respective eggshell layers (reviewed by Waring 2000). Two known exceptions, Vm32E protein and cleavage products of Dec, redistribute at S14, but the functional significance of these movements is not clear (Noguerón et al. 2000; Andrenacci et al. 2001). Two new strains carrying fosmid constructs expressing superfolder green fluorescent protein (GFP)-tagged chorion proteins, Cp18-sfGFP and Cp7Fc-sfGFP, should provide valuable tools for further exploring eggshell synthesis and assembly (Sarov et al. 2016).
Spatially, the major eggshell proteins are present throughout their respective layers, and indeed, genes encoding the late major chorion proteins are expressed uniformly in all columnar follicle cells (Parks and Spradling 1987). The early eggshell genes, however, achieve this homogeneous distribution by using a complex series of spatiotemporal gene expression patterns (Parks and Spradling 1987). For example, Cp36 and Cp38 transcripts appear first in follicle cells residing in the dorsal anterior region over the oocyte and later in the other columnar follicle cells.
Analysis of the Cp36 regulatory region led to the insight that an overall uniform pattern of expression can occur by use of many, redundant, spatially restricted enhancers rather than a single regulatory element that specifies “house-keeping” gene expression (Tolias and Kafatos 1990; Tolias et al. 1993). The Vm32E gene, which unlike other vitelline membrane genes is expressed only at S10, exhibits similar complexity (Cavaliere et al. 1997). Its spatial regulation stems from epidermal growth factor (EGF) and bone morphogenetic protein (BMP) signaling, which together define dorsal anterior eggshell structures (Bernardi et al. 2006, 2007), and its temporal regulation is determined in part by steroid signaling (Bernardi et al. 2009; see below).
Hundreds of other eggshell factors exhibit spatiotemporal regulation (Claycomb et al. 2004; Fakhouri et al. 2006; Tootle et al. 2011) consistent with the patterns exhibited by unique cell types within the follicular epithelium (Yakoby, Bristow et al. 2008). It is not clear, however, whether these patterns are functionally important or simply reflect the evolution of enhancer elements.
The pioneering studies on vitelline membrane gene and chorion gene expression revealed fundamentals of gene regulation, yet relatively little is known about the transcription factors that mediate these outcomes. Molecular or genetic analyses implicate 3 proteins in this process: Chorion factor 1 (now called Usp), Chorion factor 2 (Cf2, Shea et al. 1990; Christianson and Kafatos 1993), and Tramtrack (French et al. 2003).
Usp is a member of the steroid family of nuclear receptors and is the Drosophila ortholog of retinoid X receptors in vertebrates (Oro et al. 1990). Usp forms heterodimers with various EcR isoforms (Riddiford et al. 2000; Yamanaka et al. 2013) and thereby plays a prominent role in regulating major transitions in the ovary (see Nutrient production and storage in oocytes and Ovulation; reviewed by Schwedes and Carney 2012; Bellés and Piulachs 2015; Swevers 2019). Although Usp binds the Cp15 gene ∼60 bp upstream of the start site for transcription (Shea et al. 1990), its role in regulating gene expression is clouded by the observation that a dominant negative form of its binding partner disrupts chorion gene amplification (Hackney et al. 2007).
The Cf2 gene encodes 2 distinct zinc-finger proteins through alternative splicing; analyses of their DNA binding preferences demonstrated that each zinc finger recognizes a specific trinucleotide sequence, thus revealing a key insight into structure–function relationships of this entire class of proteins (Gogos et al. 1992; Hsu et al. 1992). Cf2, which binds upstream of the Cp15 TATA box, responds to patterning signals (see Dorsal/ventral patterning and dorsal appendage formation) and helps establish cell fates along the dorsal-ventral axis (Hsu et al. 1996; Mantrova and Hsu 1998).
tramtrack also encodes 2 distinct zinc-finger proteins. The 69-kD isoform, Ttk69, regulates expression of Cp15, Cp18, and Cp36 and possibly other factors that ensure the integrity of the vitelline membrane (French et al. 2003). In addition, Ttk69 mediates a key temporal transition in the formation of the dorsal eggshell structures by arresting dorsal appendage tube closure and inducing tube elongation (Boyle et al. 2010; Peters et al. 2013). These functions are independent of Ttk69's earlier role in coordinating cell cycle switches in the follicular epithelium (Sun et al. 2008; Boyle and Berg 2009; Huang et al. 2013).
The pleiotropic roles of these transcription factors present challenges for studying their function in late stages of oogenesis. Nevertheless, the application of modern genomic, computational, and comparative methods could identify binding sites, cofactors, and additional regulatory components that coordinate the spatial and temporal programs associated with eggshell synthesis (Papantonis et al. 2015).
Genetic and molecular analyses connect structure with function
Most of our understanding of eggshell proteins is based on genetic studies coupled with biochemical, molecular, and immunological analyses (reviewed by Waring 2000). For example, mutants lacking the major early chorion proteins Cp36 or Cp38 fail to form the elaborate structures of the endochorion, and these females are sterile (Digan et al. 1979; Bauer and Waring 1987; Velentzas et al. 2016, 2018). Similarly, eggs lacking the major vitelline membrane protein Vm26Ab are flaccid, and they collapse shortly after being laid due to defects in the stability of the entire eggshell (Savant and Waring 1989; Pascucci et al. 1996; Manogaran and Waring 2004; Wu et al. 2010).
From these studies, the defective chorion (dec, previously named dec-1) gene stands out as playing a key role in mediating the proper assembly of the eggshell (Bauer and Waring 1987; Komitopoulou et al. 1988; Noguerón et al. 2000; Mauzy-Melitz and Waring 2003). Alternative splicing produces 3 Dec proteins (Hawley and Waring 1988; Waring et al. 1990) that are secreted into the vitelline membrane, where they are later cleaved (Noguerón and Waring 1995). Some processed products are thought to mediate the construction of the floor, pillars, and roof of the endochorion, while others remain in the vitelline membrane or are taken up by the oocyte (Noguerón et al. 2000; Mauzy-Melitz and Waring 2003). Discovering how these proteins create and maintain the stability of the eggshell remains a challenge in the field.
Although Dec proteins are minor constituents of the eggshell, their functions are so important that local loss of gene products in mosaic clones creates abnormal imprints in the eggshell. This feature has made it possible to use recessive mutations in dec as a marker for a cell's genotype, connecting function in the follicle cells with local patterning events in the embryo, long after the follicle cells have sloughed off (Nilson and Schüpbach 1998). Similarly, mutant dec transgenes that lack internal coding regions produce products that interfere with endogenous Dec processing; these proteins cause dominant female sterility (Spangenberg and Waring 2007), again creating a useful tool for mosaic analyses (Lachance et al. 2009).
Several other genes encode proteins that contribute to the integrity of the eggshell, including nudel, female sterile (1) M3 [also called fs (1) polehole], female sterile (1) Nasrat, and closca (Catalan for “turtle shell”) (Degelmann et al. 1990; Hong and Hashimoto 1996; LeMosy and Hashimoto 2000; Cernilogar et al. 2001; Ventura et al. 2010). These genes were originally identified in screens for embryonic patterning mutants, but the dual nature of their protein products became apparent as investigators generated allelic series that affected additional domains or that created null mutations (see Terminal follicle cells initiate head and tail formation in the embryo). Intensive studies attempting to clarify the signaling pathways between the eggshell and embryo predicted the existence of another key protein, Vitelline membrane-like (Vml), and biochemical purification eventually led to its discovery (Zhang, Stevens et al. 2009).
Eggshell production is sufficiently well understood that it now serves as a powerful model for testing drug toxicity in vivo (Keramaris et al. 2020). Nevertheless, much work remains to determine the roles of the dozens of newly identified eggshell components and to ascertain the mechanisms that assemble this beautiful structure.
Chorion gene amplification
To produce the eggshell, Drosophila and many other insects must synthesize a large amount of chorion protein quickly (∼6 h in flies). The silkmoth, Bombyx mori, has solved this problem by duplicating its eggshell genes, so that a single genomic region encodes over 100 related proteins (Chen et al. 2015). In contrast, D. melanogaster follicle cells amplify their few eggshell genes.
Numerous studies demonstrate that chorion gene amplification in Drosophila employs the same mechanisms that regulate DNA replication in higher eukaryotes. Using cytological, molecular, and genomic tools, investigators have measured the extent of DNA amplification, identified distinct cis elements that mediate amplification, and discovered transacting factors that facilitate the process (reviewed by Calvi and Spradling 1999; Tower 2004; Claycomb and Orr-Weaver 2005; Nordman and Orr-Weaver 2012). While early studies focused on identifying the regulatory regions that enable amplification (Amplification Control Element and oriBeta; reviewed by Tower 2004), later studies have concentrated on the molecules that allow escape from the normal cell cycle inhibition of re-replication. Complementary work has revealed the signaling pathways that initiate this cell cycle transition (reviewed by Klusza and Deng 2011).
Since DNA replication is featured in another Fly Book chapter (Hua and Orr-Weaver 2017), here we summarize the basic mechanism of chorion gene amplification, including its regulation and impact on eggshell synthesis.
Cell cycle changes allow DNA amplification at 6 sites
Chorion gene amplification occurs in the context of an altered cell cycle. That is, in young egg chambers, follicle cells divide mitotically (Fig. 2), but at S6, they enter an endocycle in which they replicate their DNA in the absence of either nuclear or cellular division. Thus, the follicle cells have already blocked mitosis and modified other aspects of the cell cycle to allow origin reinitiation without cell division (Klusza and Deng 2011).
By S10, the follicle cells have undergone 3 rounds of DNA replication and achieved a ploidy value of 16C (Lilly and Spradling 1996). At this time, ecdysone signaling in conjunction with the downregulation of Notch activity triggers major changes in follicle cell behavior, including a second switch in the cell cycle (Hackney et al. 2007; Sun et al. 2008; Boyle and Berg 2009; Huang et al. 2013; Ge et al. 2015).
At this transition from S10A to S10B, the follicle cells exit the endocycle but continue to replicate DNA at 6 genomic regions. The region containing the X-linked chorion cluster (Drosophila Amplicon in Follicle Cells, DAFC-7F) amplifies 16-fold, and the region containing the third chromosome chorion cluster (DAFC-66D) amplifies 60-fold (Spradling and Mahowald 1980; Spradling 1981). The 4 other regions, DAFC-22B, DAFC-30B, DAFC-34B, and DAFC-62D, also amplify but to a more modest level of 4- to 6-fold (Claycomb et al. 2004; Kim et al. 2011).
When the follicle cells transition from the endocycle to amplification, replication is shut down in most regions of the genome, probably through persistent expression of cyclin E (Calvi et al. 1998). How these 6 amplicons escape this regulation and continue to replicate their DNA is not clear (Kim and Orr-Weaver 2011), although ecdysone receptor binding might play a role (Kohzaki et al. 2020).
Chorion gene amplification is an elegant model to study metazoan DNA replication
Because chorion gene amplification is temporally regulated, replication initiation and elongation are synchronized, making this process an ideal context to study the phases of DNA replication (Claycomb et al. 2002; Kim et al. 2011). Reinitiation occurs at discrete origins [Fig. 5; e.g. oriβ, which contains an autonomously replicating sequence (ARS) consensus sequence] and is associated with open areas of chromatin (Beall et al. 2002; Aggarwal and Calvi 2004; Hartl et al. 2007; Vorobyeva et al. 2021). In a stepwise manner, factors recruit and build the replication complex, which is activated by cyclin-dependent kinases, leading to bidirectional fork movement (reviewed by Tower 2004; Claycomb and Orr-Weaver 2005; Nordman and Orr-Weaver 2012). Repeated firing creates an onionskin structure in which peak amplification occurs at origins and DNA levels decrease gradually to a distance of ∼ 50 kb in each direction (Park et al. 2007; Kim et al. 2011). The extent of amplification depends on Suppressor of Under-Replication (SuUR), which binds elongating complexes and disrupts their stability, thereby halting fork progression (Nordman et al. 2014).
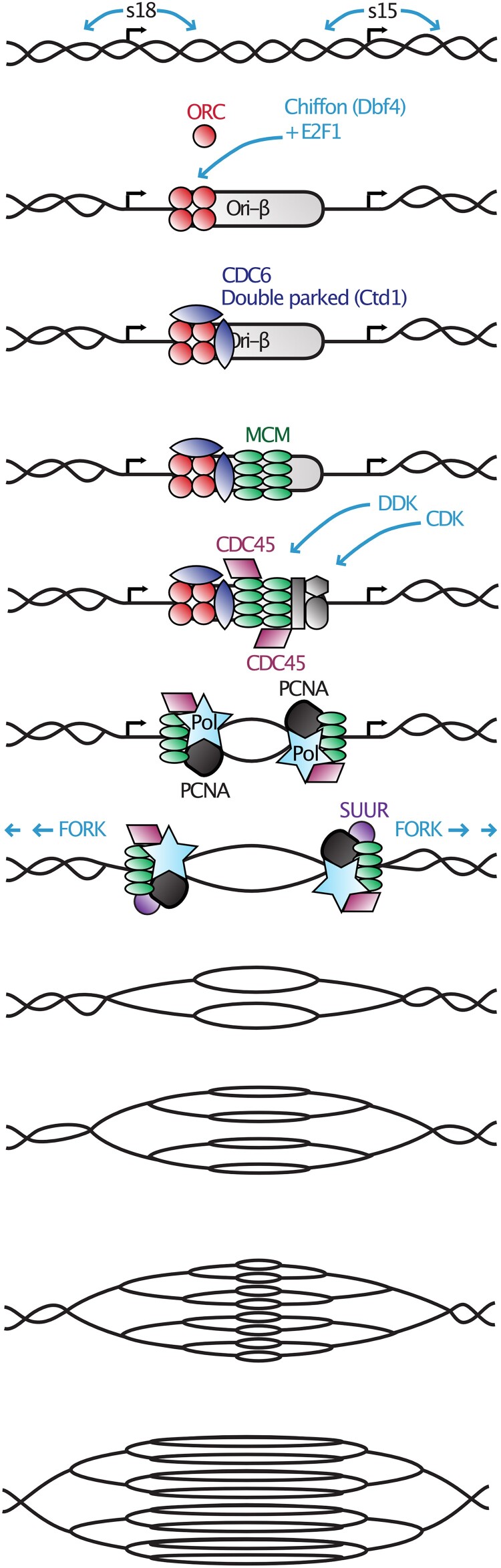
Fig. 5.
Chorion gene amplification. Six locations in the genome amplify the DNA encoding major and minor chorion proteins. Shown here is the portion of DAFC-66D that contains s18 and s15, genes encoding the late chorion proteins Cp18 and Cp15. DNA replication initiation occurs at discrete origins associated with open areas of chromatin, which are indicated by curved arrows bracketing each gene. The winged helix-turn-helix E2F transcription factor and the Dbf4-like zinc finger protein encoded by chiffon (so named for the translucent eggshells of mutants; Landis and Tower 1999) facilitate the binding of origin recognition complex (ORC), which in turn recruits several additional winged helix-turn-helix proteins (Cdc6, double-parked/Cdt1) and the mini-chromosome maintenance (MCM) DNA helicase complex, allowing association of the helicase CDC45. Two kinases, Cyclin-dependent kinase (Cdk2), with its partner Cyclin E, and Dbf4-dependent kinase (Cdc7; Stephenson et al. 2015), activate this prereplication complex. Activation facilitates binding of proliferating cell nuclear antigen (PCNA) and the DNA polymerase complex (Pol), thereby initiating bidirectional fork movement. SUUR limits fork movement to ∼50 kb in each direction. Several rounds of reinitiation create branched duplexes such that DNA levels are highest at the origins and gradually decrease on each side (reviewed by Tower 2004; Claycomb and Orr-Weaver 2005; Nordman and Orr-Weaver 2012).
Transcript abundance is not always correlated with the magnitude of DNA amplification (Kim et al. 2011). Although the expression of the major chorion genes correlates well with the level of DNA amplification (Griffin-Shea et al. 1982), mRNA levels from other genes in these regions vary. Some genes are expressed highly but do not reside in amplified regions, while other genes are amplified but are expressed at a low level (Kim et al. 2011). These inconsistencies may simply reflect the independent evolution of genes in each cluster and the existence of selective pressures on some genes but not others.
Many features of chorion gene amplification are conserved in other species. For example, other Drosophilids employ similar timing, chromatin modifications, and sequence-specific binding complexes to coordinate DNA amplification with eggshell synthesis (Calvi et al. 2007). Studies in the gnat Sciara coprophila, which amplifies pupal case genes in an ecdysone-dependent manner (Liew et al. 2013), reveal parallels with Drosophila chorion gene amplification, including an association with open chromatin (Lunyak et al. 2002; Urnov et al. 2002) and sequence or structural similarities at origins that allow binding of Sciara and Drosophila ORC machineries (Bielinsky et al. 2001; Yamamoto et al. 2021). In humans, orthologs of chorion gene amplification factors, such as E2F, are involved in bypassing mechanisms that restrict growth in tumor cells. Moreover, amplification of oncogenes is itself a common mechanism to promote tumor progression (Matsui et al. 2013). Thus, analyses of chorion gene amplification reveal fundamental mechanisms of DNA replication and give insight into aberrant processes that impact human health.
Thin eggshell phenotypes help identify new amplification factors
The genes involved in chorion gene amplification are required for DNA replication in other developmental contexts, and as a result, null mutations are lethal. Partial loss-of-function mutations produce phenotypes when protein levels drop below 10% of wild type (Orr et al. 1984; Komitopoulou et al. 1988; Trougakos and Margaritis 1998b), and this observation allowed genetic screens for female sterile mutants that produce eggs with thin eggshells (Underwood et al. 1990; Schüpbach and Wieschaus 1991).
The ease of scoring eggshell defects encouraged investigation of other conserved proteins, to ask if they play a role in chorion gene amplification. For example, the RecQ family helicases are considered “guardians of the genome” (Croteau et al. 2014), and mutations in the human genes cause rare autosomal recessive disorders associated with defective bone growth, premature aging, and cancer (Lu et al. 2014). Genetic analyses of RecQ4 helicase mutations suggest that chorion gene amplification requires this protein for initiation but not for elongation (Wu et al. 2008). In contrast, mutations in mutagen-sensitive 308, which encodes Polymerase theta, reveal a role for this microhomology-mediated end-joining protein in fork progression (Alexander et al. 2016). The checkpoint protein Claspin is also needed for elongation, while Claspin and Mutagen sensitive 101, a BRCA1 domain-containing protein, are required for initiation as well (Choi et al. 2017).
Terminal patterning and formation of the operculum and micropyle
Patterning of the follicular epithelium along the A/P axis establishes cell types that create specialized eggshell structures (Fig. 6). Formation of the anterior structures, the micropyle, operculum, and collar, involves elaborate cell movements that have merited intensive study (reviewed by Duhart et al. 2017; Horne-Badovinac 2020). Less well characterized is the posterior aeropyle, a raised placode with large pores that facilitate gas exchange. Both eggshell termini are reservoirs for signaling molecules that specify head and tail structures in the embryo. Here, we discuss the patterning, cell migration, and eggshell features that characterize the terminal regions of the egg chamber.
Fig. 6.
Patterning and morphogenesis. Representative stages showing progressive patterning and movements of follicle cells. All drawings are lateral cross sections, except the lower S10B example, which is a dorsolateral surface view. By S2, Notch signaling has defined 2 polar cells (dark green) at the anterior and posterior of the egg chamber. During S1–S5, the polar follicle cells secrete the JAK/STAT ligand Upd to pattern terminal regions. At the anterior (yellow), the gradient will specify border cells (light green), squamous stretch cells (yellow), and centripetal cells (orange) (see S8 and S9), while at the posterior, Gurken (Grk, EGF) signaling converts these cells to a posterior fate (purple). At S6, an unknown signal from posterior cells to the oocyte induces a microtubule rearrangement that moves the oocyte nucleus to the anterior. grk RNA and protein (crescent) move with the oocyte nucleus. Rotation of the egg chamber by migration of follicle cells on the ECM, which begins slowly at S1 but speeds up at S6, alters egg chamber shape from round to elongated, particularly during S6–S8. At S9, the border cells move between nurse cells toward the oocyte, carrying the polar cells, while the stretch cells flatten. At the transition from S10A to S10B, dorsal anterior follicle cells begin to express markers responding to Grk (EGF) and Dpp (BMP) signals (red, floor cells; blue, roof cells; orange, midline cells). At S10B, the centripetal and midline cells move inward. At S11, the dorsal appendage-forming cells wrap to make 2 tubes while the nurse cells dump their contents into the oocyte, and at S12–S13 the dorsal appendage-forming cells move out over the stretch cells, which envelope the degenerating nurse cells. Scanning electron microscope image of a laid egg (wild-type, Oregon R) reveals structures synthesized by the anterior cells (operculum, micropyle, collar), roof and floor cells (dorsal appendages), main body cells (majority of eggshell), and posterior cells (aeropyle). Image courtesy of Dr. Miriam Osterfield. All black bars = 50 µ.
Notch and JAK/STAT signaling define the termini of the egg chamber
Early in egg chamber development (region 2b-S2), after the follicle cells have created a monolayer epithelium around the germ cells, Notch signaling defines 2 unique cells at each pole of the egg chamber (reviewed by Nystul and Spradling 2010). These polar cells facilitate establishment of the stalk cells, which separate individual egg chambers, and they influence the overall architecture of the egg chamber through an unusual, Notch-dependent relay system that helps position the oocyte at the posterior of the egg chamber via differential E-cadherin expression (reviewed by Huynh and Johnston 2004).
Polar cells also influence cell fates within the follicular epithelium by expressing Unpaired (Upd) 1 and Upd3, 2 Drosophila cytokines that bind the receptor Domeless to activate Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling in a graded fashion (McGregor et al. 2002; Xi et al. 2003; Wang et al. 2014). Based on expression of lacZ reporter constructs, JAK/STAT signaling defines the termini by S6, but distinct cell type markers do not begin to appear until later in development, at S8 (Grammont and Irvine 2002; Xi et al. 2003). In the anterior of the egg chamber, the Upd signaling gradient defines 3 cell types (Fig. 6): high levels establish a ring of cells surrounding the polar cells called the border cells; moderate levels define the stretch cells, and lower levels determine the centripetal cells. As discussed below, each of these cell types exhibit distinct behaviors that contribute to finishing the egg. At the posterior of the egg chamber, however, Gurken (Grk, EGF) signaling from the oocyte (Fig. 6; see Dorsal/ventral patterning and dorsal appendage formation) converts the posterior terminal domain to a uniquely posterior cell type. Overall, the net effect of JAK/STAT signaling in this context is to distinguish the mid-body follicle cells from those at the termini (reviewed by Duhart et al. 2017; Merkle et al. 2020).
As discussed in the next 2 sections, the border cells and centripetal cells create the anterior face of the eggshell. Although the stretch cells do not secrete eggshell proteins, they do play important structural roles in ensuring the proper morphogenesis of the egg. For example, their affinity for the nurse cells may help position the centripetal cells relative to the oocyte (Weichselberger et al. 2022). The stretch cells provide a substrate for the anterior movement of the dorsal appendage-forming cells (see Dorsal appendage morphogenesis involves wrapping and tube elongation; Tran and Berg 2003), and if they fail to engulf the dying nurse cells (see Nurse cell breakdown; Timmons et al. 2016), this anterior movement is disrupted (Dorman et al. 2004). The stretch cells also express signals that modulate the shape of the dorsal appendages (Zimmerman et al. 2017; Sustar et al. 2023). Thus, stretch cells contribute to essential functions in finishing the egg.
Border cells migrate collectively and facilitate construction of a sperm entry point
The border cells have emerged as a premier system for studying collective cell migration (reviewed by Montell et al. 2012; Saadin and Starz-Gaiano 2016; Duhart et al. 2017). During S9, 6–10 anterior cells, including the 2 polar cells, delaminate from the follicular epithelium and migrate as a cluster between the nurse cells; by S10, they reach the border with the oocyte, hence their name (King 1970). Analyses of these cells have revealed insights into other types of invasive cell behaviors, including tumor cell metastasis (reviewed by Cai and Montell 2014; Rosales-Nieves and González-Reyes 2014; Cheung and Ewald 2016). Here, we summarize the key features of this model system.
In addition to Notch and JAK/STAT signaling as discussed above, the Hippo pathway facilitates cell fate specification of the border cells by regulating polar cell expression of Upd (Lin et al. 2014). Activation of STAT by Upd induces the expression of and modulates the activity of Slbo, a C/EBP transcription factor encoded by slow border cells (Montell et al. 1992). Precise levels of Slbo and JAK/STAT signaling are important both for defining the correct number of border cells and for controlling their migration. As a result, the gene regulatory network has evolved positive and negative feedback mechanisms, including input from other growth control pathways, to maintain proper regulation of these factors (reviewed by Saadin and Starz-Gaiano 2016; Kang et al. 2018; Sharma et al. 2018; Ogienko et al. 2020).
Mosaic analyses demonstrate that delamination of the border cells at S9 depends on local production of the steroid hormone ecdysone in the follicle cells (Domanitskaya et al. 2014). Steroid receptor activation (Bai et al. 2000; Cherbas et al. 2003) regulates target genes (Manning et al. 2017) specifically in border cells through JAK/STAT-mediated downregulation of Abrupt, a negative regulator of the steroid receptor coactivator Taiman (Jang et al. 2009; reviewed by Bellés and Piulachs 2015). One important outcome of this hormonal regulation is to modulate E-cadherin such that the central polar cells maintain high levels, the outer border cells express modest levels, and nurse cells express the lowest level (Hackney et al. 2007; Graeve et al. 2012). These differences in adhesive properties facilitate movement between the nurse cells toward the oocyte (Cai et al. 2014). High E-cadherin levels also prevent formation of a tubular lumen during cell migration (Wang, Wang, Liu et al. 2020).
As the border cells dissociate from their sister follicle cells, i.e. the cells that will become the stretch cells, their movement toward the posterior coincides with the flattening of the stretch cells into a squamous epithelium (Kolahi et al. 2009; reviewed by Duhart et al. 2017). Although the mechanism that achieves this coordination is not clear, it is known that stretch cell flattening is regulated by BMP, Hippo, and ecdysone signaling (Brigaud et al. 2015; Fletcher et al. 2018; Borreguero-Muñoz et al. 2019; Jia et al. 2022).
In addition, border cells maintain an epithelial architecture when moving between nurse cells (Niewiadomska et al. 1999). The cluster has a leader cell that extends protrusions between the nurse cells, follower cells that surround and transport the 2 central polar cells, and apicobasal polarity as revealed by differential E-cadherin localization (Montell et al. 2012). The border cells communicate with each other and with the polar cells through planar cell polarity (PCP), Jun-kinase, and G protein-coupled receptor signaling (Bastock and Strutt 2007; Llense and Martín-Blanco 2008; Anllo and Schüpbach 2016). These signals regulate cell polarity proteins, motor proteins, and adhesion molecules, thereby ensuring epithelial cohesiveness during delamination and migration (reviewed by Montell et al. 2012; Saadin and Starz-Gaiano 2016; Aranjuez et al. 2016; Cha et al. 2017; Chen, Kotian, et al. 2019; Mishra et al. 2019; Kotian et al. 2021; Campanale et al. 2022). Live image analyses show that the initial phase of migration is rapid and depends heavily on maintenance of cluster architecture. Nevertheless, the border cells do alter their position in the cluster. In the later, slower phase, this reorganization is more dramatic, and the entire assemblage tumbles as it moves to the posterior (Bianco et al. 2007; Prasad and Montell 2007; reviewed by Sano et al. 2007; Cliffe et al. 2017).
The cell located at the leading edge of the cluster exhibits protrusive behavior, which depends on tight regulation of actin and myosin (reviewed by Montell et al. 2012; Saadin and Starz-Gaiano 2016; Roberto and Emery 2021). Elegant in vivo studies using light-activated Rac, a GTPase that regulates the actin cytoskeleton, demonstrate that directionality and speed of movement depend on the location and strength of Rac activity (Wang et al. 2010). Membrane trafficking contributes to this regulation by ensuring that activated Rac remains localized to the leading edge where other GTPase regulators control its ability to induce protrusive behavior (Fernández-Espartero et al. 2013; Ramel et al. 2013; Chang et al. 2018). Follower cells also require Rac to modulate actin dynamics, maintain cluster cohesion, and promote crawling (Campanale et al. 2022). During migration, 3 distinct polarity complexes coordinate the behavior of cells within the cluster through localization of atypical protein kinase C (aPKC) and a guanine nucleotide exchange factor, chondrocyte-derived ezrin-like domain-containing protein (Cdep) (Wang et al. 2018; Campanale et al. 2022). Additional in vivo studies using an E-cadherin tension sensor provide unparalleled views into how cells perceive signals and coordinate their movements (Cai et al. 2014). These and other powerful genetic and imaging tools have allowed detailed characterizations of the regulatory pathways that control cellular dynamics at the leading and trailing edges of the cluster (Plutoni et al. 2019; Wang, Guo et al. 2020; Fox et al. 2020; Badmos et al. 2021; Lamb et al. 2021).
The border cells delaminate from the follicular epithelium and migrate medially, in between the nurse cells, from the anterior toward the posterior. To identify the signals that provide spatial direction to the border cells, an overexpression screen of random genes in the genome found 2 signaling molecules that could confuse border cells during their migration. These ligands were Vein (1 of 4 fly EGF homologs) and PDGF- and VEGF-related factor 1 (Pvf1, which is related to vertebrate Platelet-derived growth factor, PDGF, and Vascular endothelial growth factor, VEGF; Duchek et al. 2001). This gain-of-function approach was instrumental in identifying these guidance cues since loss of either molecule alone produced only minor delays in border cell movement. Other approaches clarified the process in part by developing tools to locally misexpress ligands and thereby redirect border cells to new sites (McDonald et al. 2003, 2006). These studies showed that 2 other EGF ligands, Keren and Spitz, together with Pvf1, guide the border cells to the oocyte. The tumor suppressor gene abnormal wing discs (human Nme1/Nm23) must be downregulated to optimize integration of these guidance cues with JAK/STAT signaling and allow forward movement (Nallamothu et al. 2008). In addition to these chemical cues, the border cells interpret the cellular geometry and packing of the nurse cells to choose a path of least resistance as they move forward (Mekus et al. 2018; Peercy and Starz-Gaiano 2020; Dai et al. 2020).
When the border cells arrive at the oocyte at S10A, they move dorsally under the influence of Grk signaling (Duchek and Rørth 2001). This movement is transient, however, since by S11 the cells occupy a ventral position at the anterior of the egg. There they establish new contacts with the oocyte in an innexin- and MT-dependent manner (Miao et al. 2020) and then collaborate with centripetally migrating cells to generate the micropyle (see Centripetal and midline cells migrate inward to create the operculum and micropyle; reviewed by Horne-Badovinac 2020). Electron and light microscopy studies show that the polar cells extend MT- and actin-rich processes toward the oocyte; these processes embed into the vitelline membrane and block coalescence of chorion proteins secreted by neighboring cells (Zarani and Margaritis 1986; Edwards et al. 1997). As a result, the micropyle is a small cone-shaped structure, 20 μM long and 10 μM in diameter at its base, with a central hole for sperm entry leading to a specialized vitelline membrane that must be penetrated during fertilization (Turner and Mahowald 1976). Delay or failure of border cell migration results in a micropyle that lacks this hole, and thus, border cell mutants are sterile (Montell et al. 1992).
Centripetal and midline cells migrate inward and create the operculum, collar, and micropyle
Two populations of follicle cells, the centripetal cells and the midline cells, cooperate to synthesize several eggshell structures: the anterior face of the eggshell or operculum, a surrounding collar, and the micropyle (reviewed by Duhart et al. 2017). These cells are defined in part by the initial gradient of Upd that distinguishes main body cells from termini (Xi et al. 2003; see also Notch and JAK/STAT signaling define the termini of the egg chamber); 2 other pathways, Decapentaplegic (Dpp, BMP) and Gurken (Grk, EGF), subsequently contribute to their specification (Dobens et al. 2000; Yakoby, Lembong et al. 2008; see also Gurken is a morphogen that cooperates with other signals to specify fates). High levels of these latter signals define a 2-row ring of cells at the anterior-most region of columnar follicle cells (Dobens et al. 2005; Chen and Schüpbach 2006; Shravage et al. 2007; Charbonnier et al. 2015) as well as a thin stripe of cells on the dorsal midline that separates the 2 dorsal appendage-forming primordia (Lachance et al. 2009; Zartman, Kanodia, Cheung et al. 2009). One key feature of this process is the downregulation of Bunched, a member of the TSC22 family of leucine zipper transcription factors (Dobens et al. 2000). Bunched acts in a negative feedback loop with the C/EBP ortholog Slbo to maintain distinct cell fates within the epithelium; this interaction resembles a regulatory network that controls fat cell differentiation in mammals (Levine et al. 2007).
Centripetal migration is a 2-phase process (Parsons et al. 2023). Beginning at S10B with cells on the dorsal side of the egg chamber, leading centripetal cells invade the space between the nurse cells and oocyte (King 1970). One hypothesis suggests that by localizing actin and myosin at their apical (inward facing) surfaces, these cells create a purse string structure that contracts to drive the movement of the cells toward the interior of the egg chamber until the entire anterior face of the oocyte is covered (Edwards and Kiehart 1996). Consistent with this hypothesis, capping protein beta is required for centripetal migration (Ogienko et al. 2013). Based on the behaviors of the centripetal cells and on studies of actin dynamics in the border cells or in the embryo, this actomyosin complex could also be involved in remodeling of centripetal cell shapes (e.g. apical constriction of the leading cells), generating the cortical tension needed to squeeze between the germ cells, or producing filopodia or lamellipodia (Aranjuez et al. 2016; Kiehart et al. 2017; Parsons et al. 2023).
In the second phase of centripetal migration, more posterior following cells change their orientation as they tilt downward to invade, but their aspect ratio does not change (Parsons et al. 2023). During ingression, ∼2 squamous cells move inward with the centripetal cells (Tran and Berg 2003; Parsons et al. 2023); the function of these accompanying cells is not known. At the basal surface, both the centripetal cells and the midline cells constrict, and these cells exchange edges with lateral neighbors to allow their reorganization and to dissociate from the ECM, the dorsal appendage-forming floor cells, and other main-body follicle cells (Levine et al. 2010; Osterfield et al. 2013; Parsons et al. 2023). These studies demonstrate that centripetal migration in D. melanogaster is actively invasive, unlike the process in some other insects, which lack centripetal migration or use a simple folding mechanism to achieve ingression (reviewed by Jaglarz et al. 2008, 2010; Garbiec and Kubrakiewicz 2012; Garbiec et al. 2016).
Several types of cell adhesion molecules play key roles in mediating centripetal migration. The homophilic cell adhesion molecule E-cadherin (encoded by shotgun) is required in both the germline and soma for inward movement (Niewiadomska et al. 1999; Parsons et al. 2023), while 18-wheeler, which encodes a Toll-like receptor, is needed in centripetal and midline cells (Kleve et al. 2006). Other cell adhesion molecules, such as Fasciclin 3 (Ward and Berg 2005; Shravage et al. 2007) and Cadherin 99C (D’Alterio et al. 2005; Zartman, Kanodia, Yakoby et al. 2009), are also expressed in these cells and likely mediate their activities.
The onset of centripetal migration marks the decision by the egg chamber to complete oogenesis. Prior to this time, egg chambers maturing in culture demand nutritional inputs and growth factors (e.g. insulin and fetal bovine serum) for their continued development. At S10B, however, egg chambers will mature to S14 in a chemically defined medium (Robb's R14 medium; reviewed by Peters and Berg 2016a).
Like other changes that occur at this time (see Eggshell composition and Chorion gene amplification), centripetal migration begins following a pulse of ecdysone signaling (Hackney et al. 2007). BMP signaling may also be required since ligand, receptors, and transcription factors are all expressed highly in these cells (Twombly et al. 1996; Jékely and Rørth 2003; Shravage et al. 2007; Yakoby, Lembong et al. 2008). Distinguishing between a role for BMP in cell fate specification and morphogenesis is difficult, however, since no alleles or constructs exist that disrupt the function only during migration.
In addition to these temporal cues, the spatial positioning of the centripetal cells relative to the oocyte might impact their ability to migrate (Schüpbach and Wieschaus 1991; Mahone et al. 1995; Swan and Suter 1996; Keyes and Spradling 1997). For example, in mutants that produce open-ended eggshells, such as chalice or cup, oocyte growth is retarded relative to the maturation of the follicular epithelium. As a result, the centripetal cells reside too far to the anterior, and they either fail to initiate inward movement or they migrate aberrantly between nurse cells.
At the transcriptional level, the C/EBP homolog Slbo is highly expressed in centripetal cells, and although not essential for centripetal migration (Montell et al. 1992), it coordinates cell movements with nurse cell dumping by regulating expression of E-cadherin and other surface proteins (Levine et al. 2007). Transcriptional profiling of Slbo-expressing cells (border cells and centripetal cells) (Wang et al. 2006) and comparisons of wild-type egg chambers with those in which EGF or BMP signaling is manipulated uncover additional genes with intriguing expression patterns and potential roles in centripetal and midline cell migration (Yakoby, Bristow et al. 2008).
Scanning and transmission electron microscopy reveal regional differences in operculum structure (Turner and Mahowald 1976; Margaritis et al. 1980), presumably due to the input of the 3 cell types that affect its assembly (border cells, centripetal cells, and midline cells). The size and shape of follicle cell imprints differ: ventrally, surrounding the micropyle, they are small hexagons, while dorsally they are larger, more elongated rhomboids. Because egg chambers that lack border cells still produce a micropyle, albeit misshapen and lacking a sperm entry hole (Montell et al. 1992), the assumption is that the centripetal cells are the major source of vitelline membrane and chorion proteins that make up that structure. The most specialized region is the collar, which exhibits a patchy vitelline membrane covered by 2 separate layers of endochorion, so constructed to allow ready disintegration upon larval hatching (Margaritis et al. 1980). As discussed above, defects in centripetal cell migration lead to open-ended eggshells, and females harboring mutations affecting this process are sterile (Schüpbach and Wieschaus 1991; Twombly et al. 1996; Edwards and Kiehart 1996).
Terminal follicle cells initiate head and tail formation in the embryo
Terminal patterning of the egg chamber is important not only for creating specialized structures of the eggshell but also for establishing the unsegmented terminal regions of the embryo. This process exhibits many parallels with D/V patterning of the embryo (see Gurken is a morphogen that cooperates with other signals to specify fates) and indeed shares some genes. The downstream components of the pathway are well known and constitute a premier system for exploring quantitative behavior of mitogen activated protein kinase (MAPK) signaling (Goyal et al. 2018; Smits and Shvartsman 2020).
Briefly, Trunk, a cysteine knot protein related to vertebrate Noggin (Duncan et al. 2013), activates a uniformly distributed receptor tyrosine kinase, Torso, only at the embryo poles, as discussed below. Signal transduction through Ras, Raf, MEK, and MAPK leads to regional degradation of the transcriptional repressor Capicua (Catalan for “head and tail”), thereby allowing expression of the zygotic genes tailless and huckebein (reviewed by Li 2005; Mineo, Furriols et al. 2018). Intriguingly, cell cycle-dependent degradation of Torso protects the germ cells, which reside at the posterior pole within the domain of activated Torso, from being specified as somatic terminal cells (Pae et al. 2017; Colonnetta et al. 2021).
Through numerous challenging experiments, the upstream mechanisms of the Torso pathway are becoming clear. Transcripts encoding the Trunk ligand are loaded maternally into the embryo, but the protein is cleaved and secreted only at the termini (Casali and Casanova 2001; Henstridge et al. 2014; Johnson et al. 2015). This localized activation of the pathway occurs through the earlier production of Torso-like by small groups of terminal follicle cells (Stevens et al. 1990; Savant-Bhonsale and Montell 1993; Martin et al. 1994). Torso-like, which is related to perforin proteins that poke holes in membranes (Johnson, Henstridge et al. 2017), is secreted into the vitelline membrane (Stevens et al. 2003) and is stored there until egg activation, when it translocates to the embryo plasma membrane (Mineo et al. 2015). Cell culture experiments suggest that Torso-like facilitates activation of Torso by mediating or stabilizing dimerization of the receptor upon binding to its ligand (Amarnath et al. 2017). How a perforin-like protein might facilitate this interaction is unclear, although one possibility is that it alters the structure of the plasma membrane near Torso (reviewed by Santos et al. 2015). Consistent with this hypothesis, physical perturbation of the membrane can mimic Torso-like activity (Mineo, Fuentes et al. 2018), and a screen for new components of the Torso pathway identified Tetraspanin 3A, which might also act by altering local membrane structure (Johns et al. 2018).
Mechanistically, the localized activation of the Torso signaling pathway by an eggshell-associated protein, Torso-like, resembles the process that establishes D/V patterning in the embryo (see Gurken is a morphogen that cooperates with other signals to specify fates; reviewed by Stein and Stevens 2014; Merkle et al. 2020). Both pathways use autocrine signaling mechanisms, and Trunk, the ligand for Torso, shares structural features with Spätzle, the ligand for Toll (Morisato and Anderson 1994; Casanova et al. 1995). Both pathways require the activities of Polehole, Nasrat, and Closca, which are made in the oocyte and secreted into the vitelline membrane (Jiménez et al. 2002; Ventura et al. 2010). There they facilitate late steps in the processing of Nudel protein, a component of the protease cascade that activates Spätzle, and they stabilize Torso-like at the embryo poles (Mineo et al. 2017). A recent study also shows that Torso-like activity at the poles coordinates cell shape changes during ventral furrow formation (Johnson, Moore et al. 2017), a morphogenetic process induced by D/V patterning.
These striking features of Drosophila terminal patterning involve the likely co-option of Torso signaling from a molting pathway and are recent evolutionary innovations (reviewed by Duncan et al. 2013; Weisbrod et al. 2013; Auman and Chipman 2017; Skelly et al. 2019; Taylor et al. 2019).
Dorsal/ventral patterning and dorsal appendage formation
The dorsal appendages are the most prominent eggshell specializations. Studies on these structures led to the exciting discovery that gurken (grk) (German for “cucumber”) regulates D/V patterning of both the eggshell and embryo (Schüpbach 1987). grk encodes a homolog of vertebrate EGF (Neuman-Silberberg and Schüpbach 1993) and activates the EGF receptor (EGFR; encoded by torpedo; Price et al. 1989; Schejter and Shilo 1989). Subsequent screens identified additional components of the EGF signaling pathway and other factors that regulate grk in some way (reviewed by Berg 2005; Schüpbach 2016, 2019; Merkle et al. 2020).
In the next sections, we summarize the essential steps associated with D/V axis formation and dorsal appendage morphogenesis, and we show how Grk signaling also regulates A/P patterning. We direct the reader to timely reviews for more in-depth discussions of the key findings (Roth 2003; Horne-Badovinac and Bilder 2005; Stein and Stevens 2014; Osterfield et al. 2017; Merkle et al. 2020).
Gurken (EGF) signaling creates asymmetry
Although D/V patterning mutants such as grk exhibit striking changes in dorsal eggshell structures, strong loss-of-function mutations also generate eggs with 2 anterior ends, i.e. the posterior consists of an operculum, collar, and micropyle rather than an aeropyle (Schüpbach 1987). These phenotypes showed that EGF signaling regulates both D/V and A/P patterning of the egg chamber. Subsequent molecular studies showed that grk mRNA and Grk protein are first localized to the posterior of the egg chamber and then move to the outer anterior cortex of the oocyte, and this shift in subcellular localization regulates distinct signaling processes (Neuman-Silberberg and Schüpbach 1993). The current hypothesis is that early Grk signaling to posterior follicle cells at S1–S5 establishes posterior cell fates. These cells then signal back to the oocyte at S6, inducing a relocalization of grk products to the anterior, where subsequent signaling at S10 establishes dorsal follicle cell fates (González-Reyes et al. 1995; Roth et al. 1995; González-Reyes and Johnston 1998; reviewed by Merkle et al. 2020).
Two other findings support this hypothesis. First, MTs undergo a major reorganization during S6–S8 (Theurkauf et al. 1992; Gillespie and Berg 1995; Pokrywka and Stephenson 1995). This reorganization repositions the plus ends of MTs at the posterior of the oocyte and the minus ends at the anterior, pushing the oocyte nucleus to a random position at the anterior of the oocyte and relocalizing grk transcripts and protein in the process (reviewed by Bernard et al. 2017). Importantly, other embryonic patterning molecules, e.g. those encoded by bicoid and oskar, localize to the anterior or posterior of the oocyte due to their dependence on different classes of MT motor proteins (reviewed by Merkle et al. 2020).
The second finding was that Notch is required in the follicle cells for proper localization of patterning molecules in the oocyte (Ruohola et al. 1991). We now know that Notch signaling is required to differentiate terminal follicle cells (see Notch and JAK/STAT signaling define the termini of the egg chamber) and thus works in conjunction with EGF signaling to establish posterior follicle cell fates.
Since these initial discoveries, several groups have set out to identify the signal sent from posterior follicle cells back to the oocyte (Deng and Ruohola-Baker 2000; MacDougall et al. 2001; Deng et al. 2003; Poulton and Deng 2006; Meignin et al. 2007; Polesello and Tapon 2007; Yu et al. 2008; Sun et al. 2011; Wittes and Schüpbach 2018; and others cited in Merkle et al. 2020). The assumption is that the molecule(s) and its pathway are involved in other biological processes, and therefore, the screening strategies have exploited methods to bypass earlier requirements in development. Although these studies revealed contributing factors, the signaling mechanism that initiates the dramatic cytoskeletal changes in the oocyte is still unknown. One unexplored possibility, hinted at by studies on the maintenance of oocyte polarity in S10 egg chambers (Milas et al. 2022), is that mechanical contact between posterior follicle cells and the oocyte might facilitate this critical reorganization of the oocyte MT network.
Gurken is highly regulated
Since Grk signaling initiates patterning of the entire body plan, it is under tight control (reviewed by Johnstone and Lasko 2001; Kugler and Lasko 2009; Lasko 2012; Derrick and Weil 2017). In fact, Grk is so important that hundreds of genes impinge on its function, including inputs from growth regulatory pathways and meiotic checkpoints (reviewed by Merkle et al. 2020).
Mosaic studies reveal that grk mRNA is made in the nurse cells, packaged into RNP particles, and transported rapidly into the oocyte via dynein motors (Duncan and Warrior 2002; MacDougall et al. 2003; Cáceres and Nilson 2005; Rom et al. 2007). During transport, limiting amounts of the poly-A binding protein Orb (and therefore the poly-A polymerase Wispy) ensure that grk transcripts maintain short poly-A tails, repressing translation until the RNA is localized properly in the oocyte (Tan et al. 2001; Wong et al. 2011; Norvell et al. 2015; Davidson et al. 2016). Translational repressors (e.g. Squid; Cáceres and Nilson 2009) contribute to this restricted expression.
Localization of grk RNA within the oocyte requires the activity of over a dozen of RNA binding proteins or MT-associated proteins. Unlike most other localized transcripts in which sequences in the 3′ UTR are sufficient to target the mRNA to a locale, grk transcripts contain regions in the 5′ UTR and coding sequence that also contribute to localization (Bor et al. 2005; Thio et al. 2000; Lan et al. 2010).
Once grk transcripts are localized to the dorsal anterior corner of the oocyte, Squid facilitates a switch in the behavior of the transport particles; this switch creates static ribonucleoprotein particles called “sponge bodies” that anchor grk message and prevent its diffusion throughout the oocyte (Delanoue et al. 2007). Within these endoplasmic reticulum-associated sponge bodies, translational repressors are displaced, and the DEAD box helicase Vasa facilitates translation (reviewed by Lasko 2012). Subsequent Grk secretion requires cleavage by the intramembrane protease Rhomboid (Strisovsky et al. 2009). Once secreted into the extracellular space between the oocyte and follicle cells, glycosphingolipids present on the outer leaflet of the oocyte plasma membrane modulate Grk diffusion and thereby shape the gradient of Grk protein (Pizette et al. 2009).
Grk's key role in fly development is underscored by the fact that production of the protein depends on the integrity of other critical processes in the egg chamber. For example, meiotic recombination facilitates DNA exchange and segregation of homologs during oocyte development (reviewed by Lake and Hawley 2012), but a failure to repair the double-strand breaks that mediate recombination elicits a checkpoint response that blocks translation of grk RNA into protein (reviewed by Merkle et al. 2020). Similarly, mutations that disrupt production of Piwi-interacting RNAs (piRNAs, also called repeat associated small interfering RNAs or rasiRNAs) allow expression and mobilization of transposable elements, inducing double-strand breaks and activating checkpoints that regulate the amount of Grk protein (reviewed by Saito 2013; Hsu et al. 2020). Grk levels can also be affected by competition for RNA-binding or transport proteins; for example, retrotransposons such as I factor, G2, and Jockey carry cis elements that produce secondary structures mimicking grk transcripts and interfering with grk mRNA regulation (Bor et al. 2005; Hamilton et al. 2009). In general, when the oocyte suffers irreparable DNA damage, egg chamber maturation continues, but the grk “quality-control checkpoint” prevents transmission of that mutant DNA into progeny by inhibiting axis specification and thereby disrupting embryonic development. Thus, grk is a hub that integrates information from numerous processes to ensure robust gamete production.
In contrast, if oocyte development is normal but female flies suddenly face a reduction in nutritional resources, a bypass mechanism allows synthesis of Grk protein (Ferguson et al. 2012) even though most other transcripts experience a block in translation initiation (Richter and Sonenberg 2005; reviewed by Jackson et al. 2010). This response may increase survival of the resulting progeny (Burn et al. 2015).
Gurken is a morphogen that cooperates with other signals to specify fates
In early stages of oogenesis, low levels of Grk specify posterior follicle cell fates, while at later stages, moderate or high levels of signaling specify dorsal appendage- or operculum-forming cell fates, respectively. Signaling levels that lie below a minimum threshold result in cells with a “default” main body or ventral fate. Although in each context signaling occurs through the Ras/Raf/MAPK pathway (reviewed by Berg 2005), the concentration of Grk ligand determines the outcome (Goentoro et al. 2006; Chang et al. 2008; Lachance et al. 2009; Zartman, Kanodia, Cheung et al. 2009; Wang and Pai 2011).
Two other pathways function with grk in follicle cell specification. In posterior follicle cells, JAK/STAT signaling (see Notch and JAK/STAT signaling define the termini of the egg chamber) facilitates expression of 2 paralogous T-box transcription factors, Midline and H15, in response to early Grk signals, and these factors then prevent posterior follicle cells from responding to later higher levels of EGF signaling (Lomas et al. 2013, 2016). In the anterior, graded levels of Dpp (BMP) signaling from stretch follicle cells combine with Grk signaling to define unique cell types along the anterior-posterior axis (Twombly et al. 1996; Deng and Bownes 1997; reviewed by Dobens and Raftery 2000; Dobens et al. 2000; Peri and Roth 2000; Chen and Schüpbach 2006; Shravage et al. 2007; Yakoby, Lembong et al. 2008; Charbonnier et al. 2015; Duhart et al. 2017). Together these pathways create diverse patterns of gene expression (Yakoby, Bristow et al. 2008; Zartman, Kanodia, Yakoby et al. 2009; Cheung et al. 2011). Thus, the follicle cells integrate information from multiple signaling pathways to specify subpopulations that create distinct eggshell structures, including dorsal appendage-forming cells marked by expression of the BTB zinc finger transcription factors encoded by broad (reviewed by Duhart et al. 2017; Osterfield et al. 2017).
Several mathematical models have reshaped our interpretation of the underlying molecular mechanisms by quantifying the initial Grk and Dpp gradients (Goentoro et al. 2006; Lembong et al. 2008). Other models show how those gradients feed into gene regulatory networks (Lembong et al. 2009; Zartman, Kanodia, Cheung et al. 2009) that specify subpopulations within the epithelium (Simakov et al. 2012; Fauré et al. 2014). These approaches provide predictive models to explain alternative patterning processes in other species (Zartman et al. 2011).
One key function of Grk signaling is to regulate D/V patterning in the embryo. More than a dozen genes are required for the downstream steps, but the upstream initiating event occurs when Grk-activated EGFR causes dorsal follicle-cell repression of pipe, which encodes 10 related glycosaminoglycan-modifying enzymes (Sen et al. 1998; Sergeev et al. 2001). Pipe activity in ventral follicle cells modifies components of the vitelline membrane (Zhang, Stevens et al. 2009; Zhang, Zhu et al. 2009), creating a region of sulfated eggshell that triggers localized cleavage of the ligand Spätzle, which was made in the embryo and secreted uniformly into the perivitelline space. Cleaved Spätzle activates the Toll signaling pathway, leading to translocation of the Dorsal transcription factor (a homologue of vertebrate Nuclear factor kappa-light-chain-enhancer of activated B cells, NF-κB) into nuclei on the ventral side of the embryo (reviewed by Stein and Stevens 2014; Schloop et al. 2020; Merkle et al. 2020). Intriguingly, the Toll pathway is quite ancient and regulates innate immunity in all metazoans (Lemaitre et al. 1996; Kimbrell and Beutler 2001). Through evolutionary mechanisms that are not yet clear, arthropods have co-opted this pathway to establish D/V polarity (Lynch and Roth 2011; Vreede et al. 2013; Bressan and Araujo 2021).
Dorsal appendage morphogenesis involves wrapping and tube elongation
The 2 dorsal appendages of the eggshell consist of cross-linked chorion protein complexes interspersed with air-filled “plastrons” that facilitate gas exchange for the embryo (Hinton 1960). They are made by 2 patches of follicle cells that create elongated tubes, secrete eggshell proteins into the lumens of the tubes, and then slough off during ovulation. Thus, the dorsal appendages on the laid egg serve as readout for proper tube formation, similar to the way that a Jell-o's shape reveals its mold. In D. melanogaster, these respiratory structures resemble oars, with a long, rounded stalk and a flat paddle, but the number and shape of appendages vary widely among Drosophilids, offering an outstanding system for comparative studies (Throckmorton 1962; reviewed by Osterfield et al. 2017).
Analysis of markers in fixed tissues (Dorman et al. 2004; Ward and Berg 2005; Osterfield et al. 2013) and live imaging of egg chambers developing in culture (Dorman et al. 2004; Osterfield et al. 2013; reviewed by Peters and Berg 2016a) demonstrate that the 2 patches of cells specified by EGF and BMP signaling first “wrap” to make a cone-shaped structure parallel to the follicular epithelium sheet. Wrapping is one of a few highly conserved tube-forming mechanisms and produces the neural tube in most vertebrates, the ventral furrow during gastrulation of the fly embryo, and other foundational rudiments during organ formation (reviewed by Hogan and Kolodziej 2002; Lubarsky and Krasnow 2003; Andrew and Ewald 2010). Wrapping during dorsal appendage tube formation involves distinct behaviors by 2 subpopulations in each patch of cells: the roof cells constrict their apical surfaces (which face the oocyte), bending the epithelium up and out of the flat plane (Dorman et al. 2004), and the floor cells dive beneath the roof cells, exchanging neighbors to zip up and seal the floor (Dorman et al. 2004; Osterfield et al. 2013). The mechanical forces that drive these movements likely arise from myosin-based apical tension. Mathematical vertex models support this hypothesis and suggest that forces within the floor cells are particularly important in creating the tube (Osterfield et al. 2013).
In the next phase of dorsal appendage formation, lateral roof cells move toward the dorsal midline; this intercalation narrows and lengthens the tubes (Dorman et al. 2004; Ward and Berg 2005). The roof cells expand their apical surfaces in a biased fashion (Peters and Berg 2016b; Espinoza and Berg 2020), and the basal surfaces of both roof and floor cells extend filopodia and lamellapodia while crawling anteriorly (Dorman et al. 2004; Ward and Berg 2005; Boyle and Berg 2009). Continued cell shape changes and rearrangements create the rounded stalk and flattened paddle that exemplify the dorsal appendages of this species (reviewed by Osterfield et al. 2017).
Mosaic analyses and laser ablation studies demonstrate that signals from within and outside the tubes regulate tube shape. These studies show that the floor cells and first row of roof cells control tube elongation (Boyle and Berg 2009; Boyle et al. 2010), while signals from the nurse cells and stretch follicle cells control tube closure and tube shape (Rittenhouse and Berg 1995; Tran and Berg 2003). Proteomic analyses of purified stretch cells identified a novel class of growth factors, the Imaginal disc growth factors, that when overexpressed induce dramatic changes in cell shape and cell adhesion, creating short, broad, open tubes (Zimmerman et al. 2017). In contrast, the complete loss of all 6 family members (the first example of gene editing of a dispersed gene family) reveals a requirement for these factors in maintaining dorsal appendage tube architecture. The entire epithelium is disrupted, particularly in response to a brief pulse of CO2 (Sustar et al. 2023). How these factors modulate tube behaviors is not yet understood.
Tube formation in other species resembles that in D. melanogaster with 2 striking exceptions. First, in D. willistoni, enhanced Grk signaling creates a dorsal ridge in addition to the 2 appendages. Amazingly, introduction of D. willistoni grk into D. melanogaster is sufficient to create a dorsal ridge (Niepielko and Yakoby 2014). The second exception occurs in Scaptodrosophila; there, females produce eggs with a variable number of long, thin dorsal appendages, and they do so by skipping the wrapping phase and simply elongating pairs of floor cells, which pull roof cells behind them. Several factors hint at possible molecular mechanisms for this remarkable divergence in tube formation, including differences in the localization patterns of myosin and the apical basal polarity protein Bazooka (Par3) (Osterfield et al. 2015), and a heterochronic shift in the transcriptional regulatory pathway governing tube elongation (O’Hanlon et al. 2018).
Thus, dorsal appendage formation provides a relatively simple model to explore the processes that regulate the mechanisms and evolution of tube formation.
Mechanism of egg elongation
S1 egg chambers are round, but the finished egg is elongated along the anterior-posterior axis. Early analyses of mutants that fail to elongate, such as kugelei (German for ball egg) and bola (Portuguese for ball), demonstrated that interactions between basal cytoskeletal networks and the ECM are required for egg morphogenesis (Gutzeit et al. 1991; Bateman et al. 2001; Frydman and Spradling 2001). Later live imaging studies revealed roles for pulsed actin-based contractions and a physical rotation of the egg chamber along the A/P axis, implicating mechanical forces in the process (He et al. 2010; Haigo and Bilder 2011; reviewed by Gates 2012; Isabella and Horne-Badovinac 2015a; Cetera and Horne-Badovinac 2015). Here, we describe how egg chamber elongation offers a simple system to study the biophysical and cell biological aspects of cellular morphogenesis.
Actin-based contractions and collective cell migration shape the egg chamber
Egg chamber elongation involves at least 4 distinct follicle cell behaviors (Fig. 7): pulsed apical constrictions at the poles of the egg chamber; collective cell migration that rotates the entire egg chamber around the A/P axis; pulsed basal contractions centered in the middle of the egg chamber; and follicle cell–matrix interactions that sustain the elongation process. These behaviors create mechanical forces that drive egg shape changes during oogenesis.
Fig. 7.
Mechanisms of egg elongation. The round egg chamber elongates through distinct follicle cell behaviors. During S3–S7 (represented by the S5 egg chamber), a gradient of JAK/STAT signaling at the poles induces apical constriction of the follicle cells, pulling the germ cells outward. JAK/STAT signaling also induces expression of proteases (AdamTS-A and MMP1) that modify the ECM (outer, thick, light green lines). As shown by the S7 egg chamber, the main force driving elongation is the rotation of the egg chamber along its long axis. The follicle cells (middle two egg chambers, dark green) walk in the same direction; yellow dots mark the position of a row of cells at different times during development. The cells move slowly at first (S1–S5), but the speed picks up during S6–S8. During their migration, the follicle cells secrete ECM proteins into the basement membrane (far right egg chamber, light green) in a graded fashion such that the polar regions exert less tension on the egg chamber than the middle. During S9–S10A, pulsed basal contractions in the middle of the egg chamber squeeze the tissue outward. During the last stages of oogenesis (represented by S12), basal cell surfaces maintain contact with the ECM and exert tension on the tissue. The aspect ratio (length to width) of each stage is shown at the far left.
The first step in egg chamber elongation involves the establishment of a gradient of JAK/STAT signaling radiating from the 2 poles of the egg chamber (see Notch and JAK/STAT signaling define the termini of the egg chamber; reviewed by Horne-Badovinac and Bilder 2005). During S3–S7, this gradient induces pulses of apical constriction that pull on the 2 ends of the egg chamber and, due to the nonelastic nature of the tissue, slowly cause the sphere to elongate. Although myosin II is localized apically and is required for pulsatile contractions, the JAK/STAT pathway does not regulate myosin-II activity directly. Furthermore, cells intercalate but do not exhibit a clear planar cell polarity (Alégot et al. 2018). At the same time, JAK/STAT signaling induces expression of ADAM metallopeptidase with thrombospondin type 1 motif A (AdamTS-A); loss of function of the encoded matrix metalloprotease yields round egg chambers, likely by altering the relative stiffness of the basement membrane (Wittes and Schüpbach 2018).
A second process, egg chamber rotation, overlaps temporally with these apical contractions and also relies on information from the poles. Beginning at S1, egg chambers build a basal actin network oriented across the plane of the entire epithelium (Gutzeit 1990, 1991). By creating this unconventional form of planar polarity (see Unconventional PCP facilitates tissue rotation and egg chamber elongation), the follicle cells can coordinate their movement to rotate the entire egg chamber within the ovariole. Each egg chamber moves independently of its neighbor (and sometimes in opposite directions) by modulating adhesion with the interconnecting stalk cells, which are stationary and remain connected to the outside of the basement membrane of each neighboring egg chamber (Haigo and Bilder 2011; Cetera et al. 2014). As the follicle cells crawl along the basement membrane, they secrete additional collagen, laminin A, and other ECM components to build up the mesh such that it is also oriented within the plane (Gutzeit et al. 1991; Schneider et al. 2006; Haigo and Bilder 2011; Cetera et al. 2014; reviewed by Isabella and Horne-Badovinac 2015a, 2016; Loza et al. 2017). At first (S1–S5) the rotation is slow, but the tempo increases during S6–S8, and the movement of the egg chamber within this structured ECM framework elongates the egg chamber like a baker rolling out a ball of dough. Unlike the baker's bread dough, however, the egg chamber is continually growing, and this growth fuels expansion, particularly at the poles where resistance from the ECM is low (Gates 2012; reviewed by Bilder and Haigo 2012; Cetera Horne-Badovinac 2015). Surprisingly, overall egg chamber polarity (i.e. the oocyte at the posterior) depends on the correct regulation of these follicle cell–ECM interactions (Loza et al. 2017).
A third process begins in early S9, after egg chamber rotation ceases, and continues through S10. In this case, the basal surfaces of the follicle cells exhibit coordinated actomyosin contractions, pulsatile in nature and oriented in rings around the circumference of the egg chamber (Gutzeit 1991; He et al. 2010). This behavior squeezes the growing egg chamber like a corset, restricting expansion of the diameter while allowing an increase in length. Although myosin activity is regulated cell autonomously through the Rho kinase pathway (He et al. 2010), the follicle cells employ nonautonomous interactions between integrins and the ECM to regulate Rho activity within the epithelial plane (Qin et al. 2017). Anchored and activated by this integrin–matrix connection, the follicle cells extend filopodia across neighboring cells and form supracellular basal stress fibers, thereby coordinating the production of mechanical tension across the entire egg chamber (Mateos et al. 2020; Popkova et al. 2020).
Finally, at S11, nurse cell dumping enlarges the oocyte, and the egg chamber continues to lengthen during subsequent stages (see Nurse cell dumping and degradation; King 1970). Loss-of-function mutations in Dystrophin and Dystroglycan, which form a complex that links the actin cytoskeleton to the ECM, disrupt basal actin organization and the ECM and lead to a reduction in egg chamber length during S12–S14 (Cerqueira Campos et al. 2020). Similarly, RNAi studies suggest that core septate junction components contribute to egg chamber elongation and also impact morphogenesis of cells synthesizing specialized eggshell structures (Alhadyian et al. 2021).
In these ways, follicle cell–matrix interactions create a “molecular corset” that restrains the growing egg chamber and forces its elongation in an anterior-posterior direction.
Unconventional PCP facilitates tissue rotation and egg chamber elongation
The transformation of the round egg chamber to an ellipse involves an ∼2.5-fold change in aspect ratio that occurs mainly during S6–S9 (Haigo and Bilder 2011). To understand this change, scientists have focused on the collective cell migration that predominates during this period. Interestingly, mammary gland cell lines form cysts in 3D matrigel and rotate using a similar mechanism (Squarr et al. 2016).
During egg chamber rotation, the follicle cells must decide on a direction so that they can coordinate their movement, but unlike many other cell migrations, this tissue has no obvious leading edge (reviewed by Uechi and Kuranaga 2018). The cue for direction begins with a symmetry-breaking event in the germarium when the protocadherin Fat2 (encoded by kugelei) helps to orient the growth of MTs within the follicle cells. This process provides chirality to the epithelium (Viktorinová and Dahmann 2013; Chen et al. 2016).
Subsequently, at S1, Lar receptor tyrosine phosphatase (encoded by bola) works with integrins and Fat2 to initiate formation of the basal actin network across the epithelium (Bateman et al. 2001; Frydman and Spradling 2001; Viktorinová et al. 2009; Cha et al. 2017). Once egg chambers exit the germarium, Fat2 also coordinates the movement of cells to initiate rotation of the epithelium; this rotation occurs perpendicular to the A/P axis (Aurich and Dahmann 2016; Viktorinová et al. 2017) and in the opposite direction of the earlier plus-end-directed MT growth (Viktorinová and Dahmann 2013; Chen et al. 2016).
As migration begins, basal protrusions move cells forward along the ECM. Gain-of-function and loss-of-function studies show that while cells are moving, the basal actin stress fibers assemble new material at the leading edge and disassemble actin at the trailing edge; this treadmilling is mediated in part by the formin encoded by disheveled associated activator of morphogenesis (DAAM; Sherrard et al. 2021). In addition, clonal analyses demonstrate that Lar acts on the leading side of cells to induce retraction of membrane in the cells ahead, while the STE20-kinase Misshapen is needed on the trailing side to release follicle cell–matrix connections in the cells that follow (Lewellyn et al. 2013; Barlan et al. 2017). Signaling by Fat2 from the trailing side to the leading side recruits the WAVE complex to basal membranes, where it interacts with Lar to link the cytoskeleton to the membrane. Based on its role in other tissues, Lar likely also links the cytoskeleton, via integrins, to the ECM (Tootle et al. 2011; Cetera et al. 2014; Squarr et al. 2016; Barlan et al. 2017; Williams et al. 2022).
Fat2 and Lar also interact with other signaling complexes that are localized in a planar polarized fashion. Mosaic analyses, immunostaining, and RNAi studies suggest that the transmembrane protein Semaphorin5c (Sema5c) and its receptor PlexinA (PlexA), well known for their roles in axon guidance and other migratory processes (Jongbloets and Pasterkamp 2014), localize to leading and trailing edges of cells, respectively; there they interact with Fat2 or Lar to coordinate cell movement (Stedden et al. 2019; Williams and Horne-Badovinac 2023).
During migration, the follicle cells secrete basement membrane proteins oriented in the plane of their movement. Polarized secretion mediated by Stratum, Rab8, Rab10, and Crag (calmodulin-binding protein related to a Rab3 GDP/GTP exchange protein) ensures basal placement of molecules (Denef et al. 2008; Lerner et al. 2013; Devergne et al. 2017). Directionality depends on high levels of phosphatidylinositol 4,5-bisphosphate (PIP2) in apical and lateral membranes, which acts in part by affecting the localization of Crag (Devergne et al. 2014). Similarly, kinesin-based transport of Rab10-containing secretory vesicles along polarized MTs facilitates basal deposition of Col4a1 (Zajac and Horne-Badovinac 2022). In contrast, SPARC (Secreted protein, acidic, cysteine-rich) and other endoplasmic reticulum (ER)/Golgi-associated proteins act as chaperones that control ECM protein content by negatively regulating incorporation into the matrix (Isabella and Horne-Badovinac 2015b).
Through tight spatiotemporal control of ECM protein deposition (Fig. 7), the follicle cells produce a corset that is stiff in the middle and more relaxed at the ends (Isabella and Horne-Badovinac 2016; Crest et al. 2017). Ultimately, this gradient of basement membrane stiffness facilitates egg chamber elongation, not by controlling the plane of cell divisions but by driving expansion of tissue toward the poles and by optimizing cell packing for circumferential growth (Haigo and Bilder 2011; Loza et al. 2017; Chen, Crest et al. 2019). The degree of egg elongation impacts oviposition rates and is monitored by the anterior polar cells, which secrete matrix metalloproteinase (MMP) 1 to modulate basement membrane stiffness (Ku et al. 2023).
Live imaging approaches have driven many key observations that form the basis for our understanding of this process (Haigo and Bilder 2011; Shah and Devergne 2022). For example, the M-TRAIL method generates clones of GFP-tagged ECM proteins, allowing visualization of basement membrane deposition. Use of this technique clarified the activity of partial loss-of-function kugelei (Fat2) alleles and demonstrated a causal link between egg chamber rotation and egg chamber elongation (Chen et al. 2017). Atomic force microscopy of live egg chambers showed that differential stiffness across regions of the basement membrane directs egg chamber elongation (Chlasta et al. 2017; Crest et al. 2017; Chen, Crest et al. 2019). Near total internal reflection fluorescence microscopy allowed characterization of actin stress fibers and showed that their treadmilling involves formation of distinct types of adhesions at the leading and trailing edges of cells (Sherrard et al. 2021). A simple but ingenious method for mounting egg chambers allows easy exchange of culture medium, facilitating drug studies and use of lipid dyes (Zajac et al. 2023). Complementing these tools are computational methods such as imaging surface analysis environment (ImSAnE) that facilitate quantitation by virtually unrolling the epithelium into a flat sheet for morphometric analyses (Chen et al. 2016). Thus, egg chamber elongation has become a powerful system for investigating tissue morphogenesis.
Ovulation, egg activation, and successful fertilization
Once egg chambers reach the posterior end of the ovariole and become mature S14 egg chambers, encapsulated oocytes are released into the oviduct through a process called ovulation. Traveling down through the lateral and common oviduct (Fig. 1), mature oocytes are activated by hardening the eggshell, resuming meiosis, and modification of maternal mRNA and proteins for proper fertilization and embryogenesis, a process likely influenced by the microenvironment of the reproductive tract. Recent advances in this area have uncovered multiple similarities between mammalian and Drosophila ovulation and egg activation. Thus, Drosophila is an attractive genetic model system to discover and characterize novel molecules involved in ovulation, egg activation, and fertilization.
Ovulation
Cellular process of ovulation
Oocytes of S14 egg chambers are encapsulated by a thin layer of somatic follicle cells, which are essential for eggshell formation, oocyte development, and early embryogenesis (see Eggshell production). During ovulation, the oocyte is released into the lateral oviduct, while the follicle cells remain at the end of the ovariole (Deady et al. 2015; Mahowald 1972). Ovulation consists of 2 steps: (1) follicle trimming, in which posterior follicle cells of the S14 egg chamber reach the lateral oviduct and are broken down and (2) follicle rupture, in which the oocyte is actively squeezed out of the follicle cell capsule and into the lateral oviduct (Fig. 8; Supplementary Movie 1; Deady et al. 2015).
Fig. 8.
Process of ovulation and intrinsic factors in ovulation. a) A model shows the key steps in ovulation (adapted from Deady et al. 2017). Ovulation begins with activation of Matrix metalloprotease 2 (MMP2) in posterior follicle cells. Activation is followed by follicle cell trimming and follicle rupture. Once the oocyte is ovulated, the follicle cell sheath is maintained as a corpus luteum whose function is completely unknown. b) Describes the intrinsic roles for ecdysone signaling, calcium signaling, reactive oxygen species, and octopamine in main body or posterior follicle cells to control ovulation.
Once the oocyte is released into the lateral oviduct, the residual follicle cells stay at the end of the ovariole attached to the next younger egg chamber, presumably through the stalk cells (Spradling 1993). These residual follicle cells maintain anterior and posterior orientation and gene expression as they were in the S14 egg chamber and accumulate yellow pigmentation (Deady et al. 2015). Thus, these residual follicle cells are named Drosophila corpus luteum, analogous to the corpus luteum in mammals. Such pigmented materials are found in ovaries throughout the class Insecta, revealing shared biological processes (Büning 1994). Drosophila corpus luteum stays at the end of the ovariole for up to 12 hours, and its degradation may involve the oviduct epithelial cells (Nezis et al. 2002). Thus, ovulation in Drosophila not only consists of a follicle rupture but also results in corpus luteum formation, as occurs in mammalian ovulation (Conti et al. 2012; Fan et al. 2012). However, the function and the degradation mechanisms of the Drosophila corpus luteum are completely unknown.
Follicular intrinsic factors in ovulation
Follicle trimming during ovulation is driven by the activity of MMPs, a family of proteolytic enzymes that regulate ECM homeostasis (Page-McCaw et al. 2007). The Drosophila genome contains 2 MMP coding genes: Mmp1 and Mmp2. These enzymes have nonoverlapping roles in tissue remodeling and can both be inhibited by endogenous Tissue inhibitor of matrix metalloproteinase (Timp; Page-McCaw et al. 2003). MMP2 is specifically expressed in posterior follicle cells in S14 egg chambers (Deady et al. 2015). Functional studies show that MMP2, but not MMP1, has a central role in follicle trimming and rupture (Deady et al. 2015). MMP2 is implicated in the dissociation of larval fat body cells during pupal development through modulating basement membrane and cell–cell junctions (Jia et al. 2014). Presumably, MMP2 dissociates posterior follicle cells through a similar mechanism, but the exact targets of MMP2 during follicle trimming are still unknown.
MMP2 activity is tightly regulated to ensure only one oocyte ovulates at a time. At any given time, only a single S14 egg chamber (out of more than a dozen) exhibits prominent posterior MMP activity in vivo (Deady et al. 2015). Two signaling pathways regulate MMP2 activity during the process of ovulation: ecdysone signaling and octopaminergic signaling. The monooxygenase Shade (Shd), which converts ecdysone (E) to 20E, is not detected in S13 follicle cells but is significantly upregulated in S14 follicle cells. This upregulation of Shd is essential for MMP2 activity and ovulation (Knapp and Sun 2017). In addition, EcR is required in mature follicle cells to induce MMP2 activation and ovulation. EcR is encoded by a single gene, that, through alternative splicing, produces 3 isoforms (EcR.A, EcR.B1, and EcR.B2) with identical DNA/ligand binding domains but different N-terminal regions (Talbot et al. 1993). Expression analysis and genetic rescue experiments suggest that EcR.B2, the shortest isoform, is the receptor that mediates ecdysone signaling in mature follicle cells (Knapp and Sun 2017). Thus, mature follicle cells regulate both ecdysone production and its receptor expression to fine-tune the ecdysone signaling for MMP2 activation and ovulation. It is currently unknown how EcR.B2 mediates differential ecdysone signaling from EcR.A and EcR.B1 and what downstream targets affect MMP2 activity. Although we do not know the exact mechanism for how ecdysone signaling regulates MMP2 activation, it is clear that ecdysone does not affect MMP2 expression in posterior follicle cells (Knapp and Sun 2017) nor Oamb (Octopamine receptor in mushroom body) expression in all follicle cells; this latter process mediates follicular octopaminergic signaling for ovulation (see below).
MMP2 activity is also regulated by octopaminergic signaling. Octopamine signaling is thought to function as an equivalent to adrenergic signaling in mammals (Roeder 2005). Octopamine (OA) is produced from tyrosine by Tyrosine decarboxylase (Tdc) and Tyramine β-hydroxylase (TβH). The Drosophila genome contains 2 genes encoding Tdc (Tdc1 and Tdc2) and one gene encoding TβH (Tbh). There are 4 genes (Oamb, Octβ1R, Octβ2R, and Octβ3R) encoding 5 OA receptors (Oamb produces 2 different splicing isoforms Oamb.K3 and Oamb.AS) (Han et al. 1998; Maqueira et al. 2005).
Several lines of evidence demonstrate that octopaminergic signaling regulates Drosophila ovulation. Mutation of Tdc2 or Tbh leads to egg retention in the ovary and female sterility (Monastirioti et al. 1995, 1996; Cole et al. 2005). The latter can be rescued by feeding mutant flies with OA or by overexpression of TβH in a subset of abdominal ganglion neurons located at the ventral nerve cord (Monastirioti et al. 1996; Monastirioti 2003). These TβH-expressing neurons (also named octopaminergic neurons) project to the periphery where they innervate ovaries and the oviduct to regulate ovulation (Monastirioti 2003; Rodríguez-Valentin et al. 2006).
On the receiving end, Oamb is one of the OA receptors involved in ovulation, as Oamb mutant females are very weakly fertile, lay few eggs, and show strong egg retention in the ovary (Lee et al. 2003). Oamb is strongly expressed in oviduct epithelial cells, as well as in mature follicle cells (Lee et al. 2003, 2009), where it activates MMP2 for subsequent follicle rupture (see Follicular extrinsic factors in ovulation for the role of oviduct Oamb; Deady and Sun 2015). In addition, stimulation of isolated mature follicles with exogenous OA is sufficient to induce follicle trimming and rupture in an ex vivo culture system that lacks ovariole and oviduct muscle (Deady and Sun 2015; Knapp et al. 2018).
Ex vivo studies show that OA functions through the Oamb receptor in S14 follicle cells to induce the rise of intracellular Ca2+ and to activate MMP2 (Deady and Sun 2015). The mechanism linking the Ca2+ rise with MMP2 activation is still unclear, but it is not through regulating MMP2 expression (Deady and Sun 2015). One hypothesis is that the increase of intracellular Ca2+ leads to the secretion of MMP2, thus allowing MMP2 to degrade the ECM. MMP2 is unlikely to be the only target of the follicular adrenergic signaling because OA-induced Ca2+ rise spreads across the entire follicle cell layer (Deady et al. 2015; Deady and Sun 2015).
Consistent with this idea, genetic screens identified NADPH oxidase (Nox), a member of the Nox/Duox family for superoxide production (Ritsick et al. 2007), as another target of adrenergic signaling in S14 follicle cells that is required for follicle rupture (Li et al. 2018). Nox mRNA is enriched in S14 follicle cells (Eichhorn et al. 2016; Li et al. 2018; Oramas et al. 2023). When OA/Oamb-induced Ca2+ rise activates Nox in all follicle cells of S14 egg chambers, these cells produce superoxide. Superoxide is then converted by an extracellular superoxide dismutase 3 to hydrogen peroxide, which serves as a signaling molecule for follicle rupture (Li et al. 2018). Hydrogen peroxide does not seem to affect OA-induced MMP2 activation, but it is still unclear what molecules are targeted by hydrogen peroxide during follicle rupture.
These studies and others highlight that S14 follicle cells acquire a unique molecular signature (Jevitt et al. 2020) that allows them to respond to ovulatory stimuli. Interestingly, the zinc finger transcription factor Hindsight (Hnt, AKA Pebbled) is re-upregulated in S14 follicle cells after being suppressed in main body follicle cells during S10B–S13 (Deady et al. 2015). In contrast, multiple transcription factors, including Cut, Ttk69, and Br, are downregulated in S14 follicle cells (Knapp et al. 2019). The downregulation of these transcription factors ensures proper upregulation of Hnt, which in turn upregulates Oamb in all follicle cells and MMP2 in posterior follicle cells (Deady et al. 2017).
Hnt is not required for the upregulation of Nox expression in mature follicle cells. Instead, a basic helix-loop-helix/Per-ARNT-SIM (bHLH/PAS) domain transcription factor, Single-minded (Sim), drives Nox expression, as well as Hnt, Oamb, and MMP2 expression (Oramas et al. 2023). Sim is upregulated in follicle cells transiently during S10–S12 and then again in S14. At S10, ecdysone signaling induces expression of Ttk69 and Ftz-f1; the latter one is an NR5A family nuclear receptor that directly controls Sim expression at S10–S12 to promote follicle cell differentiation (Sun et al. 2008; Knapp et al. 2020). It is unclear, however, what signals upregulate Sim expression at early S14 to promote proper transition into S14. More work will be needed to understand the final maturation of S14 egg chambers and the regulatory mechanisms of follicular intrinsic factors in ovulation.
Finally, the intrinsic molecular mechanisms controlling ovulation in Drosophila are highly conserved in other species, including mammals. For example, MMPs, steroid signaling, reactive oxygen species (ROS), and adrenergic signaling also play key roles in vertebrate ovulation (Curry and Smith 2006; Shkolnik et al. 2011; Richards et al. 2015; Tokmakov et al. 2020). Compounds inhibiting Drosophila follicle rupture can also inhibit ovulation in mouse (Jiang et al. 2021). Therefore, Drosophila becomes a useful model for screening nonhormonal contraceptive compounds targeting the ovulation process.
Follicular extrinsic factors in ovulation
In addition to follicular intrinsic factors, multiple extrinsic factors regulate ovulation. These factors include muscle contraction, mating, and secretions from the reproductive tract and fat body.
Muscle contractions are coordinated by 2 types of neurons. Octopaminergic neurons from the abdominal ganglion innervate the peritoneal sheath and bilateral and common oviduct muscle to form type-II neuromuscular junctions (NMJs), while glutaminergic motor neurons innervate common oviduct and uterus muscle to form type-I NMJs (Middleton et al. 2006; Rodríguez-Valentin et al. 2006; Castellanos et al. 2013). Octopamine enhances muscle contraction in the peritoneal sheath but inhibits it in the oviduct (Middleton et al. 2006; Rodríguez-Valentin et al. 2006; Rubinstein and Wolfner 2013). In contrast, glutamate acts as an excitatory neurotransmitter that stimulates oviduct muscle contraction (Rodríguez-Valentin et al. 2006). Inactivating both types of neurons leads to egg retention in the ovary and defective ovulation, while ablating only the glutaminergic neurons results in egg jams in bilateral oviducts (Castellanos et al. 2013). Consistent with this observation, mutations in transformer (tra) and dissatisfaction (dsf), which control differentiation of the glutamatergic neurons that innervate the oviduct, result in similar egg jam phenotypes (Finley et al. 1997, 1998; Castellanos et al. 2013; Evans and Cline 2013; Gou et al. 2014). These studies indicate that glutamate-stimulated contraction of oviduct muscle plays a key role in moving eggs through the oviduct. It is therefore thought that the coordinated contraction of ovariole sheath and relaxation of oviduct muscle by octopamine likely facilitates the ovulation process (Middleton et al. 2006; Rodríguez-Valentin et al. 2006; Rubinstein and Wolfner 2013). In addition, recent work showed that JH promotes the assembly of ovarian muscle ECM and ovarian muscle contraction, and disruption of JH signaling reduces ovulation (Luo et al. 2021). Despite these recent advances, many questions regarding the role of muscle contraction in ovulation still remain. In particular, it is unclear how octopamine differentially regulates peritoneal and oviduct muscle contraction.
Ovulation is greatly induced upon mating, although virgin females can ovulate at a slower rate. Mating induces a profound change in female reproductive behaviors for several days, including increased ovulation, egg production, and sperm storage, and reduced sexual receptivity and lifespan, as well as a change in feeding behavior (Kubli 2010; Kubli and Bopp 2012). In addition, mating enhances neurotransmitter release in the female reproductive tract, stimulates the maturation of oviduct epithelium that lines the lumen, and induces the relaxation of oviduct musculature; these changes are likely responsible for the increased rate of ovulation and egg laying (Heifetz and Wolfner 2004; Kapelnikov, Rivlin et al. 2008; Kapelnikov, Zelinger et al. 2008; Rubinstein and Wolfner 2013; Heifetz et al. 2014).
Products of the male's accessory gland are necessary for all or most of these postmating responses. In particular, 2 male accessory gland proteins, Acp26Aa (Ovulin) and Acp 70A (sex peptide, SP), are responsible for the induction of ovulation and egg laying (Chen et al. 1988; Aigaki et al. 1991; Herndon and Wolfner 1995; Heifetz et al. 2000; Peng et al. 2005). Upon entering the female reproductive tract, Ovulin stimulates egg laying in females for the first day by increasing the ovulation rate immediately after mating (Herndon and Wolfner 1995; Heifetz et al. 2000, 2005). Ovulin's effect on ovulation is partly through enhancing octopaminergic signaling and relaxing oviduct musculature (Rubinstein and Wolfner 2013); however, it is still unknown what receptor mediates Ovulin's effect.
In contrast to Ovulin, SP is the primary seminal peptide that mediates both short- and long-term postmating responses (Chen et al. 1988; Kubli 2003). Females mated with SP-deficient males behave like virgin females except they exhibit a very weak and transient increase in egg laying and a decrease of sexual receptivity in the first 24 h after mating (Aigaki et al. 1991; Chapman et al. 2003; Liu and Kubli 2003). The residual stimulatory activity of SP-deficient males is likely due to the activity of Ovulin and Dup99B (Ductus ejaculatorius peptide 99B), an ejaculatory ductal peptide able to bind to the SP receptor (SPR; Saudan et al. 2002; Yapici et al. 2008). With the help of other male seminal proteins (Ram and Wolfner 2009), SP binds to sperm via its N-terminal end; there, its C-terminal end, known to be essential for postmating responses, is gradually released from stored sperm by cleavage at a trypsin cleavage site, thus prolonging the postmating responses (Peng et al. 2005).
SP induces postmating behavior changes through SPR, a G protein-coupled receptor highly expressed in the common oviduct, the SPT, and the sensory neurons innervating the reproductive tract (Yapici et al. 2008). Genetic studies identify the SP-sensing neurons as 2 bilateral clusters of 3 SPR+ neurons coexpressing pickpocket (ppk), dsx, and fruitless (fru) (Yang et al. 2009; Hasemeyer et al. 2009; Rezával et al. 2012). These SP-sensing neurons reside on the anterior uterus and project to a subset of dsx+ interneurons at the ventral nerve cord. The interneurons relay the signal to a higher order brain center for signal integration and ultimately to a subset of 9 dsx+ octopaminergic neurons that innervate the female reproductive system (Rezával et al. 2012; Rezával et al. 2014; Feng et al. 2014; Wang, Wang et al. 2020). Thus, mating ultimately leads to increased octopamine release (Heifetz et al. 2014), which is responsible for increased ovulation and egg laying.
Furthermore, secretions from oviduct epithelia, fat body, and reproductive-tract glands (SPT and parovaria) may also regulate ovulation. The entire oviduct is lined by a monolayer of epithelial cells that are joined by septate junctions along their lateral membranes to seal the oviduct lumen (Kapelnikov, Rivlin et al. 2008). Oamb and Octβ2R are highly expressed in oviduct epithelial cells and are required in these cells for normal ovulation (Lee et al. 2003, 2009; Lim et al. 2014; Li et al. 2015). This adrenergic signaling in the oviduct epithelium activates both cAMP-PKA and Ca2+-CaMKII signaling pathways, which are then thought to induce secretion of fluids needed for ovulation (Lim et al. 2014). In addition to the oviduct, a fat body-derived neuropeptide, CNMamide, also promotes ovulation, likely by acting on the brain center that ultimately regulates octopaminergic neurons innervating the female reproductive tract (Grmai et al. 2023). Furthermore, the SPT and parovaria also play important roles in ovulation, likely through the secretion of key factors (see Female reproductive tract secretions and reproductive success for details).
In summary, octopamine is the key signal that induces ovulation. It is released from octopaminergic neurons innervating the ovary and the oviduct and activates its receptors in mature follicle cells, oviduct epithelium, and ovarian and oviduct muscle. Mating increases ovulation rate by enhancing octopamine release. Other environmental factors, e.g. circadian rhythm, and female physiological status can also influence ovulation rate and likely function by influencing the octopamine signaling pathway (Manjunatha et al. 2008). This hypothesis is supported by the recent discovery that bacterial infection can acutely decrease ovulation rate through a NF-κB-dependent mechanism that dampens octopamine release from octopaminergic neurons (Kurz et al. 2017). Despite recent progress, many questions still remain in the ovulation field. For example, how do octopaminergic neurons precisely activate MMP2 in only one mature follicle and how are mature follicles selected for ovulation among multiple ovarioles in the same ovary and between 2 ovaries?
Egg activation
The end result of ovulation is the release of the mature oocyte into the oviduct. The passage of the mature oocyte through the oviduct into the uterus accompanies egg activation, a process transforming the mature oocyte into the haploid egg that is able to sustain embryogenesis upon fertilization. In contrast to egg activation in mammals, where sperm entry is the trigger, egg activation in Drosophila is triggered by osmotic/mechanical pressure when passing through the narrow lumen of the oviduct (Horner and Wolfner 2008a). Consistent with this idea, eggs in the uterus are better activated than those in the oviduct (Heifetz et al. 2001), and laid unfertilized eggs are fully activated (Doane 1960). Mature oocytes are dehydrated at the end of oogenesis (Drummond-Barbosa and Spradling 2004) and can be artificially activated in vitro with hypotonic buffer and/or hydrostatic pressure (Mahowald et al. 1983; Page and Orr-Weaver 1997; Horner and Wolfner 2008b).
Despite the different triggers for egg activation between Drosophila and vertebrates, the downstream events for egg activation are highly conserved including (1) a rise of intracellular calcium (calcium wave), (2) physical and chemical changes to the oocyte's outer coverings (eggshell hardening), (3) resumption and completion of meiosis to form a haploid female pronucleus (meiotic resumption), (4) dynamic changes in maternal mRNA and proteins (maternal mRNA/protein processing), and (5) cytoskeletal rearrangement. Multiple excellent reviews have been published in the past 10 years (Pesin and Orr-Weaver 2008; Horner and Wolfner 2008a; Tadros and Lipshitz 2009; Stetina and Orr-Weaver 2011; Krauchunas and Wolfner 2013; Sartain and Wolfner 2013; Laver et al. 2015; Avilés-Pagán and Orr-Weaver 2018). Here, we briefly summarize the current understanding of egg activation.
Calcium wave
Earlier work shows that Ca2+ in hypotonic buffer is essential for egg activation in vitro (Horner and Wolfner 2008b). A genetically encoded Ca2+ sensor reveals a single, rapid calcium wave during egg activation in vitro and in vivo, a wave that starts at either the posterior pole or both poles of the oocyte and propagates across the entire oocyte cytoplasm within several minutes (Kaneuchi et al. 2015; York-Andersen et al. 2015). The Ca2+ ions are likely derived from perivitelline space. The rehydration of the oocyte due to osmotic pressure, as opposed to the mechanical pressure from the passage through the narrow oviduct, likely initiates the Ca2+ wave (York-Andersen et al. 2021). Both pharmacological and genetic experiments showed that mechanosensitive Transient receptor potential cation channel, subfamily M (TrpM) is the calcium channel for external calcium influx into oocytes during egg activation in vitro (Kaneuchi et al. 2015; Hu and Wolfner 2019; York-Andersen et al. 2021). Unexpectedly, eggs laid by TrpM germline KO females are still activated, albeit embryo hatch rates are reduced (Hu and Wolfner 2019). One potential explanation for this result is that additional channels are involved in calcium influx into oocytes in vivo.
The propagation of the calcium wave requires both release of internal ER calcium stores and actin cytoskeleton remodeling. Disruption of IP3R (inositol 1,4,5,-triphosphate receptor) or PLC21c (phospholipase C at 21C), 2 important components in ER calcium release, compromises the calcium wave propagation but does not affect the wave initiation (Kaneuchi et al. 2015; Hu et al. 2020). Treating oocytes with cytochalasin D, an actin polymerization inhibitor, also leads to a stuttered calcium wave, which retracts prematurely and never encompasses the whole oocyte (York-Andersen et al. 2015). It is still unclear how the TrpM-mediated external calcium leads to activation of PLC21c and actin cytoskeleton remodeling and how these events help the propagation of the calcium wave.
Eggshell hardening (egg swelling/eggshell crosslinking)
Eggshells from laid eggs are completely insoluble and water impermeable. The process of eggshell hardening is another area with large gaps in our knowledge. The eggshell matrix becomes recalcitrant to denaturing solutions at the end of oogenesis through the activity of peroxidases that crosslink the chorion on tyrosine residues (Petri et al. 1976). Crosslinking initiates at the poles and moves toward the central part of the eggshell. Recent studies identified candidate enzymes (Konstandi et al. 2005; Fakhouri et al. 2006; Tootle et al. 2011), including Pxd (Peroxidase), whose transcripts are enriched at anterior and posterior poles, potentially explaining the wave of hardening that initiates at these sites. Peroxidases reside in the inner chorion layer and endochorion as early as S11, but they become active only after secretion of hydrogen peroxide by the follicle cells at S14 (Margaritis 1985).
Vitelline membrane proteins also remain soluble until S14, but at S10B, several events occur to initiate maturation of the vitelline envelope. Genetic, biochemical, and cell biological studies demonstrate that the microvilli that protrude from the oocyte and follicle cells and that separate the vitelline membrane-containing droplets retract, allowing the droplets to coalesce (D’Alterio et al. 2005; Schlichting et al. 2006; Romani et al. 2016); some vitelline membrane proteins are processed (Pascucci et al. 1996), and some form disulfide bridges (Wu et al. 2010). A key regulator of these changes is Palisade (encoded by psd), which exhibits unique structural features compared to other vitelline membrane proteins (Popodi et al. 1988; Elalayli et al. 2008).
As a result of these changes at the end of S10B, large macromolecules can no longer pass into the oocyte, preventing the use of traditional in situ hybridization methods to analyze transcripts. Clever alternatives help circumvent this impermeability, including live image analysis (Forrest and Gavis 2003) and modifications of treatments used to analyze transcripts in fixed embryos (Ali-Murthy and Kornberg 2016). Note that the oocyte can still take up molecules that had been stored in the vitelline membrane (Noguerón et al. 2000).
The timing of this first phase of vitelline membrane maturation coincides with the termination of patency, the remodeling of tricellular junctions in the follicular epithelium that allows passage of molecules from the hemolymph into the oocyte (see Lipid accumulation and storage). These transitions might be regulated by Ttk69, since partial loss of ttk69 function disrupts both vitelline membrane integrity (French et al. 2003) and the cessation of patency (Row et al. 2021).
Biochemical analyses of deletion and amino acid substitution mutants demonstrate that at S13 and S14, extensive crosslinking generates covalent bonds between precisely spaced sulfhydryl groups of the major vitelline membrane proteins (Andrenacci et al. 2001; Wu et al. 2010). Similar types of structure—function studies suggest that a hydrophobic region at the amino terminus aligns the VM domains to facilitate crosslinking (Manogaran and Waring 2004). These disulfide bonds maintain the integrity of the eggshell during passage of the egg through the oviduct, where peroxidase-dependent crosslinking makes the vitelline membrane irreversibly insoluble and bleach resistant (Petri et al. 1976; Heifetz et al. 2001). Proper vitelline membrane maturation requires the activity of 3 other secreted proteins, Alpha methyl dopa-resistant (Amd), Yellow-g, and by inference, Yellow-g2; in these cases, drug inhibitor studies, mutant phenotypes, or comparisons to known functions of other family members suggest that these proteins might catalyze one or more of these late crosslinking steps (Konrad et al. 1993; Claycomb et al. 2004).
Meiosis resumption and completion
Early in oogenesis, oocytes enter meiosis and are arrested in meiotic prophase I. They resume first meiotic division with nuclear envelope breakdown (also referred to as germinal vesicle breakdown) during oocyte maturation from stages 12 to 14 (Fig. 2). As described extensively in several recent reviews (Stetina and Orr-Weaver 2011; Avilés-Pagán and Orr-Weaver 2018; Hughes et al. 2018), oocyte maturation is controlled by factors such as Polo kinase, Endosulfine, Cyclin A, Cyclin B3, and Cyclin-dependent kinase 1 (Cdk1). At the end of oocyte maturation in S14 egg chambers, oocyte centrosomes are depleted through reduction of Polo kinase and pericentriolar matrix; a specialized acentrosomal meiotic spindle is assembled with the active involvement of chromosomes and kinesin motor proteins (Pimenta-Marques et al. 2016; Radford et al. 2017). Ultimately, the oocyte arrests in metaphase I at the end of oogenesis (Fig. 2).
As part of egg activation, ovulated oocytes resume meiotic division while moving from the oviduct into the uterus, and they proceed to finish 2 meiotic divisions in the uterus (Heifetz et al. 2001). One of the key regulators that transduce the egg activation signal to resume meiosis is Sarah, the Drosophila homolog of regulator of Calcineurin (calcipressin). Eggs laid by sarah mutant females are arrested at anaphase I (Horner et al. 2006; Takeo et al. 2006). Upon egg activation, Sarah is phosphorylated at Ser215 by glycogen synthase kinase 3 (GSK3; encoded by shaggy; Takeo et al. 2010, 2012). It is currently unknown how GSK3 is activated upon egg activation and whether it requires the rise of intracellular Ca2+.
The phosphorylation of Sarah at Ser215 leads to activation of Calcineurin, a conserved Ca2+/Calmodulin-dependent serine/threonine phosphatase. Calcineurin acts as a heterodimer of catalytic A (CnA) and regulatory B (CnB) subunits (Rusnak and Mertz 2000). Although the Drosophila genome contains 3 genes (CanA1, Pp2B-14D, and CanA-14F) encoding CnA subunits and 2 genes (CanB and CanB2) encoding CnB subunits, expression studies (Takeo et al. 2006) and genetic knockouts (Takeo et al. 2010, 2012) demonstrate that only Pp2B-14D, CanA-14F, and CanB2 are required for eggs to proceed past anaphase I. The 2 catalytic subunits act redundantly in this process. Both Sarah and Calmodulin interact with CnA subunits and form stable complexes with Calcineurin in both oocytes and activated eggs (Takeo et al. 2012). It is unknown how phosphorylation of Sarah leads to activation of Calcineurin, presumably through inducing conformational changes of the complex. The activation of Calcineurin will lead to dephosphorylation of its downstream targets, some of which may be important for meiosis progression (Zhang et al. 2019).
The successful progression into anaphase I and completion of meiosis II requires activation of anaphase promoting complex/cyclosome (APC/C), an E3-ubiquitin ligase (Pesin and Orr-Weaver 2008). APC/C ubiquitinates target proteins such as Cyclin B/B3 and Securin for proteasome degradation and leads to anaphase progression (Swan and Schüpbach 2007). APC/C activity in activated eggs depends on cortex (cort), which encodes a member of the Cdc20 family of APC/C adaptors that is female germline specific (Swan and Schüpbach 2007; Pesin and Orr-Weaver 2007). Cort plays partially redundant roles in meiosis I with another member of the Cdc20 family, Fizzy (Fzy), but nonredundant roles in meiosis II (Swan and Schüpbach 2007). The evidence suggests that Cort mediates destruction of Cyclin B in the spindle mid zone at metaphase II, while Fzy mediates destruction of Cylin B along the spindle at anaphase II (Swan and Schüpbach 2007). In addition, Cort itself is a target of APC/C for degradation at the end of meiosis and is likely recognized by Fzy (Pesin and Orr-Weaver 2007).
Activation of Cort and Fzy likely depends on the Sarah/Calcineurin-mediated phosphorylation changes. Proteomic analysis showed significant changes of Fzy phosphorylation upon egg activation in a Calcineurin-dependent manner, similar to activation of Xenopus Cdc20 during egg activation (Mochida and Hunt 2007; Zhang et al. 2019). In addition, Cort from mature oocytes appears as a doublet on western blots but as a single, weak, low-mobility band from early embryos (Krauchunas et al. 2013). This change of Cort protein pattern depends on Sarah/Calcineurin. Further investigation will be required to establish the connection between Sarah, Calcineurin, and Cort.
Maternal mRNA/protein modification
The oocyte genome is transcriptionally quiescent during egg activation, and all cellular changes for egg activation rely on posttranscriptional and posttranslational modifications. Posttranslational modification of maternal proteins, such as phosphorylation/dephosphorylation, is not limited to meiotic regulators as mentioned above. A recent phosphoproteomic analysis estimated that hundreds of proteins change their phosphorylation state upon egg activation (Krauchunas et al. 2012; Zhang et al. 2019). Interestingly, egg activation is accompanied by more dephorsphorylation events than phosphorylation events, consistent with the major role of Sarah and Calcineurin in egg activation. Indeed, Sarah/Calcineurin signaling is required for the dephosphorylation of multiple proteins including Giant nuclei (Gnu), a regulatory protein for the serine/threonine kinase Pan Gu (Png) (see below; Krauchunas et al. 2013; Zhang et al. 2019). It is unlikely that Calcineurin is the only phosphatase responsible for dephosphorylation of maternal proteins, as dephosphorylation of MAPKs is not regulated by Sarah (Sackton et al. 2007).
Extensive changes in maternal mRNA translation occur during egg activation; the majority of these changes are controlled by the Png kinase complex, which is composed of the catalytic subunit Png and 2 activating subunits Gnu and Plutonium (Plu) (Kronja et al. 2014). The Png complex is inactive in mature oocytes due to the phosphorylation of Gnu by CycB/Cdk1. Upon egg activation, degradation of CycB leads to the dephosphorylation of Gnu in a Calcineurin-dependent manner and thus the assembly and activation of Png complex. The Png complex in turn promotes translation of CycB, thus facilitating entry into the first embryonic mitosis, and the translation of smaug, thereby initiating the degradation of many maternal mRNAs (Tadros et al. 2007; Vardy and Orr-Weaver 2007; Krauchunas et al. 2013; Hara et al. 2017). The Png complex also promotes the degradation of Gnu and thus restricts Png complex activity to a narrow time window used for massive translational changes via direct phosphorylation and inactivation of multiple translational repressors, such as Trailer hitch (Tral) and Pumilio (Pum) (Hara et al. 2018). The influence of CycB/Cdk1 on Png complex activation also coordinates these changes with the meiotic completion.
In addition to Png-mediated phosphorylation and inactivation of translational repressors, egg activation also leads to Ca2+-dependent dispersion of the processing body (P body), which is the site of translational repression (Horner et al. 2006; Weil et al. 2008, 2012; York-Andersen et al. 2015). It is unknown whether P-body dispersion is linked to phosphorylation of translation repressors by the Png complex. Furthermore, polyadenylation of multiple maternal mRNAs occurs during egg activation in a Wispy-dependent manner; however, selective poly(A)-tail shortening is the primary cause of translation changes during egg activation (Cui et al. 2013; Lim et al. 2016; Eichhorn et al. 2016). Due to the lack of genetic tools, it is much harder to decipher the exact downstream events in terms of protein and mRNA processing after egg activation.
Female reproductive gland secretions and reproductive success
Once the egg is activated in the oviduct and lodged in the uterus, it is fertilized by a single sperm stored in 1 of 2 distinct sperm storage organs: SR and SPT (Figs. 1 and 9). The SR is considered as the primary, short-term sperm-storage organ and holds 3 quarters of stored sperm, while the SPT is considered as the long-term storage organ (Pitnick et al. 1999; Bloch Qazi et al. 2003; Manier et al. 2010).
Fig. 9.
Anatomical structure of seminal receptacle, spermathecae, and parovaria. The top panel shows the anatomy of the proximal and distal seminal receptacle. The bottom panel shows the anatomy of the spermathecae and parovarian structures of the reproductive tract. The secretory unit consists of a secretory cell (SC), acellular end apparatus (EA), secretory cavity (space between the apical surface of SC and EA), and a canal that connects the secretory cell to the lumen. The cuticular intima lining the lumen and canal is highlighted in red.
The female reproductive tract must provide the appropriate microenvironment for proper storage and utilization of sperm to maximize fertilization success. In addition to the epithelium that lines the reproductive tract, the Drosophila female reproductive tract is equipped with 2 types of polyploid secretory cells surrounding the head capsules of spermathecae and parovaria (Fig. 9), and their secretions are essential for ovulation and sperm storage, thus influencing reproductive success (Allen and Spradling 2008; Schnakenberg et al. 2011; Sun and Spradling 2013). In this section, we will briefly discuss the role of glandular secretions in sperm storage and ovulation. We direct readers to the following papers for a comprehensive review of sperm storage and the fertilization process (Schnakenberg et al. 2012; Loppin et al. 2015).
Anatomical and cellular structure of sperm storage organs and their development
The SR is a compactly coiled tube localized to the anterior ventral end of the uterus and below the common oviduct (Fig. 1). It is a dead-end tubule that branches off the oviduct wall near its junction to the uterus and measures ∼2 mm in length, slightly longer than sperm, which are about 1.9 mm (Fig. 1; Pitnick et al. 1999). The SR is a heterogeneous structure with a variable lumen size across its length and distinct features in its proximal and distal halves (Fig. 9; Pattarini et al. 2006). The proximal half of the tube consists of a columnar epithelium with long and densely packed microvilli, and the distal portion consists of a cuboidal epithelium with brush border microvilli (Heifetz and Rivlin 2010). These structural characteristics suggest that the proximal and distal SR epithelia are functionally different from each other. Although expression sequence tag, microarray, RNAseq, and mass spectrometry analyses identified many SR-enriched genes (Prokupek et al. 2009, 2010; McDonough-Goldstein, Borziak et al. 2021; McDonough-Goldstein, Whittington et al. 2021), none of these genes has been assigned any function in the reproductive process. Thus, the roles of each region of the SR epithelium remain enigmatic.
Spermathecae are a pair of mushroom-shaped organs, each of which has a brown head capsule connected to the uterus by a thin epithelial duct. The 2 spermathecal ducts, surrounded by a layer of longitudinal muscle fibers, enter the dorsal uterus wall together on a low papillate elevation right behind the oviduct uterus junction (Miller 1950). The head capsule consists of a rigid lumen with a thick cuticular intima, lined by a layer of squamous epithelial cells and large cuboidal secretory cells (Fig. 9; Filosi and Perotti 1975). Slightly posterior to the spermathecae lies a pair of accessory glands named parovaria, which also have thin epithelial ducts open to the uterus wall behind spermathecal duct openings (Miller 1950). The parovarian head capsule, which is not pigmented, consists of a nonrigid lumen with a thin layer of cuticular intima surrounded by a layer of epithelial cells and fewer secretory cells.
Each secretory cell in both the spermathecae and parovaria is a separate secretory unit that discharges its secretions directly into the central lumen through its own ductule system (Fig. 9; Filosi and Perotti 1975; Allen and Spradling 2008). Each secretory unit consists of a secretory cell, an end-apparatus, and a canal (Fig. 9). The canal is a tubular invagination of the lumen cuticular intima. The terminal end of the canal is embraced by and open to the end apparatus, which consists of a network of highly branched lamellae and filamentous materials (Filosi and Perotti 1975; Mayhew and Merritt 2013). Secretory products are released into the apical space called the secretory cavity, which is defined by the microvilli of the secretory cell. They then penetrate through the end apparatus and are transported into the central lumen through the canal.
Both spermathecae and parovaria are derived from different segments of the genital disc (Keisman and Baker 2001). Early work reported that mutation of lozenge (lz), encoding a Runt domain transcription factor, causes loss of spermathecae and parovaria (Oliver and Green 1944; Anderson 1945). Lz is induced in precursors of both spermathecae and parovaria through a DsxF-dependent mechanism at the onset of pupation (Chatterjee et al. 2011; Wagamitsu et al. 2017). In addition, the zinc-finger transcription factor Glial cells missing (Gcm) acts with the NR5A family nuclear receptor Hr39 to promote precursor proliferation and bud off from the genital disc epithelium; this process forms rudimentary spermathecae and parovaria in early pupae (Allen and Spradling 2008; Sun and Spradling 2012; Cattenoz et al. 2016). These precursor cells further proliferate and utilize a defined cell lineage to build a 3-cell cluster in which asymmetric Notch signaling specifies each cell fate in this lineage (Sun and Spradling 2012; Shen and Sun 2017, 2020). The 3-cell cluster forms a concentric ring and ultimately builds the adult secretory unit (Sun and Spradling 2012; Mayhew and Merritt 2013).
The sophisticated structural arrangement of the secretory unit in spermathecae and parovaria predicts their specialized mechanisms in product secretion. Consistent with this idea, EM studies do not find traditional secretory vesicles in spermathecae and parovaria (Filosi and Perotti 1975); however, mounting evidence suggests that these cells do secrete products into the lumen. Variable amounts of electron-dense whorl-like laminae are first present in the secretory cavity and then in the spermathecal lumen (Filosi and Perotti 1975). These laminae are lipoproteins that are resistant to pronase digestion, and they are unique to spermathecae, suggesting that spermathecae and parovaria produce different secretory products and fulfill different biological functions. In addition, secretory cavity size and materials in the lumen change dynamically in females of different ages and genetic backgrounds (Filosi and Perotti 1975; Allen and Spradling 2008). Furthermore, 2 types of secretory cells are present in spermathecae: electron-dense dark cells with a well-developed rough ER and a large number of free ribosomes, and electron-clear light cells with poorly developed rough ER and cytoplasmic organelles (Filosi and Perotti 1975). Interestingly, Hr39 transcripts are also detected in a mosaic pattern in secretory cells of spermathecae and parovaria (Allen and Spradling 2008). It is unclear whether Hr39-positive secretory cells correspond to dark cells or light cells as described in Filosi and Perotti (1975). Finally, a recent study suggests that a heterogenous population of extracellular vesicles is utilized by spermathecal secretory cells to release their contents to the lumen (Sanchez-Lopez et al. 2022). Despite these advances, we still do not know much about the signaling pathways controlling gland secretions.
Secretions of spermathecae and parovaria regulate fertilization success
While studying lz mutant females, Anderson (1945) found that spermathecae and parovaria play important roles in fertilization success, as the loss of spermathecae and parovaria in lz mutant female correlated with the loss of female fertility. This key role is also manifested in Hr39 or gcm mutant females (Allen and Spradling 2008; Cattenoz et al. 2016). Recent studies further demonstrate that secretions from spermathecae and parovaria are not only required for efficient ovulation but also for proper sperm storage, both of which are essential for successful fertilization (Schnakenberg et al. 2011; Sun and Spradling 2013; Sanchez-Lopez et al. 2022).
Mutation of lz or Hr39 disrupts the formation of spermathecae and parovaria and reduces egg laying and ovulation rate, suggesting a role of spermathecae and parovaria in ovulation (Anderson 1945; Allen and Spradling 2008; Sun and Spradling 2013). Perturbing the developmental signals for secretory cell formation demonstrates that the number of secretory cells in spermathecae and parovaria is positively correlated with the ovulation rate (Sun and Spradling 2013). In addition, Hr39 functions in adult secretory cells to regulate ovulation (Sun and Spradling 2013). These studies suggest the hypothesis that Hr39-regulated secretions from spermathecae and parovaria influence the ovulation rate. More work will be required to identify the secreted products involved in ovulation and to decipher their precise mechanisms.
In addition to an ovulation defect, females lacking spermathecal secretory cells cannot store sperm in the spermathecal lumen, nor can they maintain sperm motility in the SR (Schnakenberg et al. 2011; Sun and Spradling 2013). These observations suggest that secretions from spermathecae function locally to attract sperm to the spermathecae and likely have a long-term effect to maintain sperm function in the SR. In addition, spermathecal secretory cells are also required for efficient sperm release from the SR, and this process depends on SPR in spermathecal secretory cells (Avila, Mattei et al. 2015). Therefore, secretions of spermathecae and parovaria have a plethora of roles in regulating sperm attraction, maintenance, and release.
The process of sperm storage is well studied in Drosophila, and multiple male factors have been identified (reviewed by Bloch Qazi et al. 2003; Avila et al. 2011; Schnakenberg et al. 2012). Upon copulation, ∼4000 sperm are transferred to the female reproductive tract along with seminal fluid. Upon entering the uterus, sperm exhibit activated flagellar beating, move in circular foci via arc line waves, and enter the SR in a parallel, tail-leading orientation (Yang and Lu 2011; Köttgen et al. 2011). Disruption of proper flagellar beating, such as in mutant sperm of Polycystic kidney disease 2 (Pkd2; Gao et al. 2003; Watnick et al. 2003), lost boys (lobo; Yang et al. 2011), and sheepish (shps; Tomaru et al. 2018), leads to fewer sperm stored in female sperm storage organs. Multiple male seminal proteins facilitate sperm entry into the sperm storage organs including Acp36DE (Accessory gland protein 36DE), which is known to regulate uterus contraction to push sperm to the sperm storage site (Neubaum and Wolfner 1999; Avila and Wolfner 2009; Avila et al. 2011). In addition, coagulation of seminal proteins at the posterior end of the semen mass forms an auto-fluorescent mating plug, which also facilitates sperm storage and whose removal is regulated by female neuron endocrine hormone Dh44 (Diuretic hormone 44) (Avila, Wong et al. 2015; Avila, Cohen et al. 2015; Lee et al. 2015; Arthur et al. 1998). Ultimately, ∼25% of transferred sperm will be stored in either SR or spermathecae and will be used to fertilize ovulated eggs. These sperm are constantly beating and can be stored for up to 2 weeks (Manier et al. 2010). A few factors regulate sperm maintenance and release, such as Wasted (Wst; Ohsako and Yamamoto 2011), Accessory gland protein 29AB (Acp29AB; Wong et al. 2008), SP (Avila et al. 2010), and tyramine/octopamine (Avila et al. 2012).
Despite these studies, many questions still remain. How do secretions of spermathecae and parovaria attract sperm? Do they function as chemo attractants to activate sperm calcium channel Pkd2 and flagella beating? Do they regulate sperm storage through activating/deactivating seminal fluid proteins? Do they provide an energy source for maintaining sperm activity in the storage organs? On the other hand, the identity of the secreted products from spermathecae and parovaria is still unknown; however, it is clear that these products are secreted through the canonical protein secretory pathway, as disruption of this pathway in the secretory cells produces the same phenotype as secretory cell-ablated females (Sun and Spradling 2013). Several genomic analyses suggest that spermathecae are abundant in transcripts encoding serine proteinases, antimicrobial peptides, antioxidants, and serpins (Arbeitman et al. 2004; Allen and Spradling 2008; Prokupek et al. 2009). Future work will be required for identifying these secreted factors and clarifying the underlying mechanism for sperm attraction and function.
Conclusions and future perspectives
In the last several decades, we have made significant progress in understanding the mechanisms of building a fertilizable egg for successful reproduction in Drosophila. Many conserved signaling pathways have been uncovered that regulate nutrient storage, oocyte maturation, pattern formation, cell cycle transitions, follicle cell differentiation, eggshell synthesis, egg shape, ovulation, egg activation, and fertilization. Drosophila oogenesis is and will continue to be an excellent model system not only for the study of reproductive biology but also for many cell biological questions in general. The breadth of knowledge of Drosophila oogenesis, the powerful and sophisticated genetic tools available, the ease of live imaging, and the short generation time provide Drosophila researchers with many advantages to tackle the multiple remaining questions we highlighted at the end of each section. A comprehensive knowledge of Drosophila oogenesis will undoubtedly advance our understanding of many biological processes and improve human health in the near future. We hope this chapter serves as a summary of previous findings and a foundation to launch new discoveries in this field.
Supplementary Material
Acknowledgments
We are grateful to our colleagues for many stimulating discussions, particularly Drs. Joe Campanale, Sally Horne-Badovinac, Lauren Penfield, Laurel Raftery, and Trudi Schüpbach. We especially appreciate the anonymous reviewers for reading this manuscript and giving us constructive criticisms. We also thank Virge Kask, Mark Kot, Rebecca Oramas, Sarah Shattuck, Anne Sustar, and Songdou Zhang for assisting with the drawing of the illustrations and Dr. Miriam Osterfield, UT Southwestern Medical Center, for providing a scanning electron microscope image of a laid egg.
Contributor Information
Celeste Berg, Department of Genome Sciences, University of Washington, Seattle, WA 98195-5065 USA.
Matthew Sieber, Department of Physiology, UT Southwestern Medical Center, Dallas, TX 75390 USA.
Jianjun Sun, Department of Physiology and Neurobiology, University of Connecticut, Storrs, CT 06269 USA.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Supplemental material available at GENETICS online.
Funding
This work is supported by a grant from the National Science Foundation Division of Integrative Organismal Systems (1755015) to CB; National Institutes of Health grants to CB (R01-GM079433), MS (R01-AG067604), and JS (R01-HD086175 and R01-HD097206); and Welch Foundation (I-2015-20190330), W. W. Caruth jr. Foundation, and UTSW Endowed Scholar Program awards to MS.
Literature cited
- Aggarwal BD, Calvi BR. 2004. Chromatin regulates origin activity in Drosophila follicle cells. Nature. 430(6997):372–376. doi: 10.1038/nature02694. [DOI] [PubMed] [Google Scholar]
- Aigaki T, Fleischmann I, Chen P-S, Kubli E. 1991. Ectopic expression of sex peptide alters reproductive behavior of female D. melanogaster. Neuron. 7(4):557–563. doi: 10.1016/0896-6273(91)90368-A. [DOI] [PubMed] [Google Scholar]
- Airoldi SJ, McLean PF, Shimada Y, Cooley L. 2011. Intercellular protein movement in syncytial Drosophila follicle cells. J Cell Sci. 124(23):4077–4086. doi: 10.1242/jcs.090456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akey CW, Edelstein SJ. 1987. The innermost chorionic layer of Drosophila. I. The role of chorin octamers in the formation of a family of interdigitating crystalline plates. J Mol Biol. 193(4):673–683. doi: 10.1016/0022-2836(87)90350-0. [DOI] [PubMed] [Google Scholar]
- Alégot H, Pouchin P, Bardot O, Mirouse V. 2018. Jak-Stat pathway induces Drosophila follicle elongation by a gradient of apical contractility. Elife. 7:773. doi: 10.7554/eLife.32943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JL, Beagan K, Orr-Weaver TL, McVey M. 2016. Multiple mechanisms contribute to double-strand break repair at rereplication forks in Drosophila follicle cells. Proc Natl Acad Sci U S A. 113(48):13809–13814. doi: 10.1073/pnas.1617110113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadyian H, Shoaib D, Ward RE. 2021. Septate junction proteins are required for egg elongation and border cell migration during oogenesis in Drosophila. G3 (Bethesda). 11(7):jkab127. doi: 10.1093/g3journal/jkab127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali-Murthy Z, Kornberg TB. 2016. Bicoid gradient formation and function in the Drosophila pre-syncytial blastoderm. Elife. 5:e13222. doi: 10.7554/eLife.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AK, Spradling AC. 2008. The Sf1-related nuclear hormone receptor Hr39 regulates Drosophila female reproductive tract development and function. Development. 135(2):311–21. doi:dev.015156. [DOI] [PubMed] [Google Scholar]
- Amarnath S, Stevens LM, Stein DS. 2017. Reconstitution of Torso signaling in cultured cells suggests a role for both Trunk and Torso-like in receptor activation. Development. 144:677–686. doi: 10.1242/dev.146076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameku T, Niwa R. 2016. Mating-induced increase in germline stem cells via the neuroendocrine system in female Drosophila. PLoS Genet. 12(6):e1006123. doi: 10.1371/journal.pgen.1006123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RC. 1945. A study of the factors affecting fertility of lozenge females of Drosophila melanogaster. Genetics. 30(3):280–96. doi: 10.1093/genetics/30.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrenacci D, Cernilogar FM, Taddel C, Rotoli D, Cavaliere V, Graziani F, Gargiulo G. 2001. Specific domains drive VM32E protein distribution and integration in Drosophila eggshell layers. J Cell Sci. 114(15):2819–2829. doi: 10.1242/jcs.114.15.2819. [DOI] [PubMed] [Google Scholar]
- Andrew DJ, Ewald AJ. 2010. Morphogenesis of epithelial tubes: insights into tube formation, elongation, and elaboration. Dev Biol. 341(1):34–55. doi: 10.1016/j.ydbio.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anllo L, Schüpbach T. 2016. Signaling through the G-protein-coupled receptor rickets is important for polarity, detachment, and migration of the border cells in Drosophila. Dev Biol. 414(2):193–206. doi: 10.1016/j.ydbio.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranjuez G, Burtscher A, Sawant K, Majumder P, McDonald JA. 2016. Dynamic myosin activation promotes collective morphology and migration by locally balancing oppositional forces from surrounding tissue. Mol Biol Cell. 27(12):1898–1910. doi: 10.1091/mbc.e15-10-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeitman MN, Fleming AA, Siegal ML, Null BH, Baker BS. 2004. A genomic analysis of Drosophila somatic sexual differentiation and its regulation. Development. 131(9):2007–21. doi: 10.1242/dev.01077. [DOI] [PubMed] [Google Scholar]
- Arthur BI, Hauschteck-Jungen E, thiger RN, Ward PI. 1998. A female nervous system is necessary for normal sperm storage in Drosophila melanogaster: a masculinized nervous system is as good as none. Proc R Soc B Biol Sci. 265(1407):1749–1753. doi: 10.1098/rspb.1998.0498. [DOI] [Google Scholar]
- Auman T, Chipman AD. 2017. The evolution of gene regulatory networks that define arthropod body plans. Integr Comp Biol. 57(3):523–532. doi: 10.1093/icb/icx035. [DOI] [PubMed] [Google Scholar]
- Aurich F, Dahmann C. 2016. A mutation in fat2 uncouples tissue elongation from global tissue rotation. Cell Rep. 14(11):2503–2510. doi: 10.1016/j.celrep.2016.02.044. [DOI] [PubMed] [Google Scholar]
- Avila FW, Cohen AB, Ameerudeen FS, Duneau D, Suresh S, Mattei AL, Wolfner MF. 2015. Retention of ejaculate by Drosophila melanogaster females requires the male-derived mating plug protein PEBme. Genetics. 200(4):1171–1179. doi: 10.1534/genetics.115.176669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila FW, Mattei AL, Wolfner MF. 2015. Sex peptide receptor is required for the release of stored sperm by mated Drosophila melanogaster females. J Insect Physiol. 76:1–6. doi: 10.1016/j.jinsphys.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila FW, Qazi MCB, Rubinstein CD, Wolfner MF. 2012. A requirement for the neuromodulators octopamine and tyramine in Drosophila melanogaster female sperm storage. Proc Natl Acad Sci U S A. 109(12):4562–4567. doi: 10.1073/pnas.1117689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila FW, Ravi Ram K, Bloch Qazi MC, Wolfner MF. 2010. Sex peptide is required for the efficient release of stored sperm in mated Drosophila females. Genetics. 186(2):595–600. doi:genetics.110.119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. 2011. Insect seminal fluid proteins: identification and function. Annu Rev Entomol. 56(1):21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila FW, Wolfner MF. 2009. Acp36DE is required for uterine conformational changes in mated Drosophila females. Proc Natl Acad Sci U S A. 106(37):15796–800. doi:0904029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila FW, Wong A, Sitnik JL, Wolfner MF. 2015. Don’t pull the plug! the Drosophila mating plug preserves fertility. Fly (Austin). 9(2):62–67. doi: 10.1080/19336934.2015.1120931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avilés-Pagán EE, Orr-Weaver TL. 2018. Activating embryonic development in Drosophila. Semin Cell Dev Biol. 84:100–110. doi: 10.1016/j.semcdb.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badmos H, Cobbe N, Campbell A, Jackson R, Bennett D. 2021. Drosophila Usp22/nonstop polarizes the actin cytoskeleton during collective border cell migration. J Cell Biol. 220(7):e202007005. doi: 10.1083/jcb.202007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J, Uehara Y, Montell DJ. 2000. Regulation of invasive cell behavior by taiman, a Drosophila protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. Cell. 103(7):1047–1058. doi: 10.1016/s0092-8674(00)00208-7. [DOI] [PubMed] [Google Scholar]
- Barlan K, Cetera M, Horne-Badovinac S. 2017. Fat2 and Lar define a basally localized planar signaling system controlling collective cell migration. Dev Cell. 40(5):467–477.e5. doi: 10.1016/j.devcel.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass BP, Tanner EA, Mateos San Martín D, Blute T, Kinser RD, Dolph PJ, McCall K. 2009. Cell-autonomous requirement for DNaseII in nonapoptotic cell death. Cell Death Differ. 16(10):1362–71. doi: 10.1038/cdd.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastock R, Strutt D. 2007. The planar polarity pathway promotes coordinated cell migration during Drosophila oogenesis. Development. 134:3055–3064. doi: 10.1242/dev.010447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J, Reddy RS, Saito H, Vactor DV. 2001. The receptor tyrosine phosphatase Dlar and integrins organize actin filaments in the Drosophila follicular epithelium. Curr Biol. 11(17):1317–1327. doi: 10.1016/s0960-9822(01)00420-1. [DOI] [PubMed] [Google Scholar]
- Bauer BJ, Waring GL. 1987. 7C female sterile mutants fail to accumulate early eggshell proteins necessary for later chorion morphogenesis in Drosophila. Dev Biol. 121(2):349–358. doi: 10.1016/0012-1606(87)90171-0. [DOI] [PubMed] [Google Scholar]
- Baum JS, Arama E, Steller H, McCall K. 2007. The Drosophila caspases Strica and Dronc function redundantly in programmed cell death during oogenesis. Cell Death Differ. 14(8):1508–17. doi: 10.1038/sj.cdd.4402155. [DOI] [PubMed] [Google Scholar]
- Beall EL, Manak JR, Zhou S, Bell M, Lipsick JS, Botchan MR. 2002. Role for a Drosophila Myb-containing protein complex in site-specific DNA replication. Nature. 420(6917):833–837. doi: 10.1038/nature01228. [DOI] [PubMed] [Google Scholar]
- Bell WJ, Barth RH. 1971. Initiation of yolk deposition by juvenile hormone. Nature New Biol. 230(15):220–222. doi: 10.1038/newbio230220a0. [DOI] [PubMed] [Google Scholar]
- Bellés X, Piulachs M-D. 2015. Ecdysone signalling and ovarian development in insects: from stem cells to ovarian follicle formation. Biochim Biophys Acta. 1849(2):181–186. doi: 10.1016/j.bbagrm.2014.05.025. [DOI] [PubMed] [Google Scholar]
- Berg CA. 2005. The Drosophila shell game: patterning genes and morphological change. Trends Genet. 21(6):346–355. doi: 10.1016/j.tig.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Bernard F, Lepesant J-A, Guichet A. 2017. Nucleus positioning within Drosophila egg chamber. Semin Cell Dev Biol. 82:25–33. doi: 10.1016/j.semcdb.2017.10.013. [DOI] [PubMed] [Google Scholar]
- Bernardi F, Cavaliere V, Andrenacci D, Gargiulo G. 2006. Dpp signaling down-regulates the expression of VM32E eggshell gene during Drosophila oogenesis. Dev Dyn. 235(3):768–775. doi: 10.1002/dvdy.20660. [DOI] [PubMed] [Google Scholar]
- Bernardi F, Duchi S, Cavaliere V, Donati A, Andrenacci D, Gargiulo G. 2007. Egfr signaling modulates VM32E gene expression during Drosophila oogenesis. Dev Genes Evol. 217(7):529–540. doi: 10.1007/s00427-007-0164-1. [DOI] [PubMed] [Google Scholar]
- Bernardi F, Romani P, Tzertzinis G, Gargiulo G, Cavaliere V. 2009. EcR-B1 and Usp nuclear hormone receptors regulate expression of the VM32E eggshell gene during Drosophila oogenesis. Dev Biol. 328(2):541–551. doi: 10.1016/j.ydbio.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Bianco A, Poukkula M, Cliffe A, Mathieu J, Luque CM, Fulga TA, Rørth P. 2007. Two distinct modes of guidance signalling during collective migration of border cells. Nature. 448(7151):362–365. doi: 10.1038/nature05965. [DOI] [PubMed] [Google Scholar]
- Bielinsky AK, Blitzblau H, Beall EL, Ezrokhi M, Smith HS, Botchan MR, Gerbi SA. 2001. Origin recognition complex binding to a metazoan replication origin. Curr Biol. 11(18):1427–1431. doi: 10.1016/s0960-9822(01)00444-4. [DOI] [PubMed] [Google Scholar]
- Bilder D, Haigo SL. 2012. Expanding the morphogenetic repertoire: perspectives from the Drosophila egg. Dev Cell. 22(1):12–23. doi: 10.1016/j.devcel.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch Qazi MC, Heifetz Y, Wolfner MF. 2003. The developments between gametogenesis and fertilization: ovulation and female sperm storage in Drosophila melanogaster. Dev Biol. 256(2):195–211. doi: 10.1016/S0012-1606(02)00125-2. [DOI] [PubMed] [Google Scholar]
- Bor VVD, Hartswood E, Jones C, Finnegan D, Davis I. 2005. Gurken and the I factor retrotransposon RNAs share common localization signals and machinery. Dev Cell. 9(1):51–62. doi: 10.1016/j.devcel.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Borreguero-Muñoz N, Fletcher GC, Aguilar-Aragon M, Elbediwy A, Vincent-Mistiaen ZI, Thompson BJ. 2019. The Hippo pathway integrates PI3K–Akt signals with mechanical and polarity cues to control tissue growth. PLoS Biol. 17(10):e3000509. doi: 10.1371/journal.pbio.3000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bownes M. 1982. Hormonal and genetic regulation of vitellogenesis in Drosophila. Q Rev Biol. 57(3):247–274. doi: 10.1086/412802. [DOI] [PubMed] [Google Scholar]
- Bownes M, Dempster M, Blair M. 1983. Expression of the yolk-protein genes in the mutant doublesex dominant (dsxD) of Drosophila melanogaster. J Embryol Exp Morphol. 75:241–57. [PubMed] [Google Scholar]
- Bownes M, Hobson B. 1980. Mutant fs(2)1163 of Drosophila melanogaster alters yolk protein secretion from the fat body. Mol Gen Genet. 180(2):411–418. doi: 10.1007/BF00425856. [DOI] [Google Scholar]
- Bownes M, Ronaldson E, Mauchline D. 1996. 20-Hydroxyecdysone, But not juvenile hormone, regulation of yolk protein gene expression can be mapped to cis-acting DNA sequences. Dev Biol. 173(2):475–89. doi: 10.1006/dbio.1996.0041. [DOI] [PubMed] [Google Scholar]
- Boyle MJ, Berg CA. 2009. Control in time and space: Tramtrack69 cooperates with Notch and Ecdysone to repress ectopic fate and shape changes during Drosophila egg chamber maturation. Development. 136:4187–4197. doi: 10.1242/dev.042770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle MJ, French RL, Cosand KA, Dorman JB, Kiehart DP, Berg CA. 2010. Division of labor: subsets of dorsal-appendage-forming cells control the shape of the entire tube. Dev Biol. 346(1):68–79. doi: 10.1016/j.ydbio.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan D, Araujo HM. 2021. Evolution of the dorsoventral axis in insects: the changing role of bone morphogenetic proteins. Curr Opin Insect Sci. 49:1–7. doi: 10.1016/j.cois.2021.09.004. [DOI] [PubMed] [Google Scholar]
- Brigaud I, Duteyrat JL, Chlasta J, Le Bail S, Couderc JL, Grammont M. 2015. Transforming growth factor β/activin signalling induces epithelial cell flattening during Drosophila oogenesis. Biol Open. 4(3):345–354. doi: 10.1242/bio.201410785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JS, Lockwood WK, Li L, Cohen SM, Edgar BA. 2002. Drosophila's insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev Cell. 2(2):239–49. doi: 10.1016/S1534-5807(02)00117-X. [DOI] [PubMed] [Google Scholar]
- Brookes VJ. 1969. The induction of yolk protein synthesis in the fat body of an insect, Leucophaea maderae, by an analog of the juvenile hormone. Dev Biol. 20(5):459–471. doi: 10.1016/0012-1606(69)90026-8. [DOI] [PubMed] [Google Scholar]
- Büning J. 1994. The Insect Ovary: Ultrastructure, Previtellogenic Growth and Evolution. 1st ed. London; New York: Chapman & Hall. [Google Scholar]
- Burn KM, Shimada Y, Ayers K, Vemuganti S, Lu F, Hudson AM, Cooley L. 2015. Somatic insulin signaling regulates a germline starvation response in Drosophila egg chambers. Dev Biol. 398(2):206–217. doi: 10.1016/j.ydbio.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtis KC, Coschigano KT, Baker BS, Wensink PC. 1991. The doublesex proteins of Drosophila melanogaster bind directly to a sex-specific yolk protein gene enhancer. EMBO J. 10(9):2577–82. doi: 10.1002/j.1460-2075.1991.tb07798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buszczak M, Cooley L. 2000. Eggs to die for: cell death during Drosophila oogenesis. Cell Death Differ. 7(11):1071–1074. doi: 10.1038/sj.cdd.4400755. [DOI] [PubMed] [Google Scholar]
- Buszczak M, Freeman MR, Carlson JR, Bender M, Cooley L, Segraves WA. 1999. Ecdysone response genes govern egg chamber development during mid-oogenesis in Drosophila. Development. 126(20):4581–9. doi: 10.1242/dev.126.20.4581. [DOI] [PubMed] [Google Scholar]
- Buszczak M, Lu X, Segraves WA, Chang TY, Cooley L. 2002. Mutations in the midway gene disrupt a Drosophila acyl coenzyme A: diacylglycerol acyltransferase. Genetics. 160(4):1511–8. doi: 10.1093/genetics/160.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth FM. 1999. A yolk protein mutant leads to defects in the secretion machinery of Drosophila melanogaster. Tissue Cell. 31(2):212–222. doi: 10.1054/tice.1999.0022. [DOI] [PubMed] [Google Scholar]
- Butterworth FM, Burde VS, Bownes M. 1992. Mutant yolk proteins lead to female sterility in Drosophila. Dev Biol. 154(1):182–194. doi: 10.1016/0012-1606(92)90058-O. [DOI] [PubMed] [Google Scholar]
- Cáceres L, Nilson LA. 2005. Production of gurken in the nurse cells is sufficient for axis determination in the Drosophila oocyte. Development. 132:2345–2353. doi: 10.1242/dev.01820. [DOI] [PubMed] [Google Scholar]
- Cáceres L, Nilson LA. 2009. Translational repression of gurken mRNA in the Drosophila oocyte requires the hnRNP Squid in the nurse cells. Dev Biol. 326(2):327–334. doi: 10.1016/j.ydbio.2008.11.030. [DOI] [PubMed] [Google Scholar]
- Cai D, Chen SC, Prasad M, He L, Wang X, Choesmel-Cadamuro V, Sawyer JK, Danuser G, Montell DJ. 2014. Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell. 157(5):1146–1159. doi: 10.1016/j.cell.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Montell DJ. 2014. Diverse and dynamic sources and sinks in gradient formation and directed migration. Curr Opin Cell Biol. 30:91–98. doi: 10.1016/j.ceb.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi BR, Byrnes BA, Kolpakas AJ. 2007. Conservation of epigenetic regulation, ORC binding and developmental timing of DNA replication origins in the genus Drosophila. Genetics. 177(3):1291–1301. doi: 10.1534/genetics.107.070862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi BR, Lilly MA, Spradling AC. 1998. Cell cycle control of chorion gene amplification. Genes Dev. 12(5):734–744. doi: 10.1101/gad.12.5.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi BR, Spradling AC. 1999. Chorion gene amplification in Drosophila: a model for metazoan origins of DNA replication and S-phase control. Methods. 18(3):407–417. doi: 10.1006/meth.1999.0799. [DOI] [PubMed] [Google Scholar]
- Campanale JP, Mondo JA, Montell DJ. 2022. A Scribble/Cdep/Rac pathway controls follower-cell crawling and cluster cohesion during collective border-cell migration. Dev Cell. 57(21):2483–2496.e4. doi: 10.1016/j.devcel.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant K, Knowles BA, Mooseker MS, Cooley L. 1994. Drosophila singed, a fascin homolog, is required for actin bundle formation during oogenesis and bristle extension. J Cell Biol. 125(2):369–80. doi: 10.1083/jcb.125.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali A, Casanova J. 2001. The spatial control of Torso RTK activation: a C-terminal fragment of the Trunk protein acts as a signal for Torso receptor in the Drosophila embryo. Development. 128:1709–1715. [DOI] [PubMed] [Google Scholar]
- Casanova J, Furriols M, McCormick CA, Struhl G. 1995. Similarities between trunk and spätzle, putative extracellular ligands specifying body pattern in Drosophila. Genes Dev. 9(20):2539–2544. doi: 10.1101/gad.9.20.2539. [DOI] [PubMed] [Google Scholar]
- Castellanos MC, Tang JCY, Allan DW. 2013. Female-biased dimorphism underlies a female-specific role for post-embryonic Ilp7 neurons in Drosophila fertility. Development. 140(18):3915–3926. doi: 10.1242/dev.094714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattenoz PB, Delaporte C, Bazzi W, Giangrande A. 2016. An evolutionary conserved interaction between the Gcm transcription factor and the SF1 nuclear receptor in the female reproductive system. Sci Rep. 6(1):37792. doi: 10.1038/srep37792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaliere V, Bernardi F, Romani P, Duchi S, Gargiulo G. 2008. Building up the Drosophila eggshell: first of all the eggshell genes must be transcribed. Dev Dyn. 237(8):2061–2072. doi: 10.1002/dvdy.21625. [DOI] [PubMed] [Google Scholar]
- Cavaliere V, Spanò S, Andrenacci D, Cortesi L, Gargiulo G. 1997. Regulatory elements in the promoter of the vitelline membrane gene VM32E of Drosophila melanogaster direct gene expression in distinct domains of the follicular epithelium. Mol Gen Genet. 254(3):231–237. doi: 10.1007/s004380050411. [DOI] [PubMed] [Google Scholar]
- Cavaliere V, Taddei C, Gargiulo G. 1998. Apoptosis of nurse cells at the late stages of oogenesis of Drosophila melanogaster. Dev Genes Evol. 208(2):106–112.. doi: 10.1007/s004270050160. [DOI] [PubMed] [Google Scholar]
- Cernilogar FM, Fabbri F, Andrenacci D, Taddei C, Gargiulo G. 2001. Drosophila vitelline membrane cross-linking requires the fs(1)Nasrat, fs(1)polehole and chorion genes activities. Dev Genes Evol. 211(12):573–580. doi: 10.1007/s00427-001-0192-1. [DOI] [PubMed] [Google Scholar]
- Cerqueira Campos F, Dennis C, Alégot H, Fritsch C, Isabella A, Pouchin P, Bardot O, Horne-Badovinac S, Mirouse V. 2020. Oriented basement membrane fibrils provide a memory for F-actin planar polarization via the Dystrophin-Dystroglycan complex during tissue elongation. Development. 147:dev186957. doi: 10.1242/dev.186957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetera M, Horne-Badovinac S. 2015. Round and round gets you somewhere: collective cell migration and planar polarity in elongating Drosophila egg chambers. Curr Opin Genet Dev. 32:10–15. doi: 10.1016/j.gde.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetera M, Ramirez-San Juan GR, Oakes PW, Lewellyn L, Fairchild MJ, Tanentzapf G, Gardel ML, Horne-Badovinac S. 2014. Epithelial rotation promotes the global alignment of contractile actin bundles during Drosophila egg chamber elongation. Nat Commun. 5(1):5511. doi: 10.1038/ncomms6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha IJ, Lee JH, Cho KS, Lee S-B. 2017. Drosophila Tensin plays an essential role in cell migration and planar polarity formation during oogenesis by mediating integrin-dependent extracellular signals to actin organization. Biochem Biophys Res Commun. 484(3):702–709. doi: 10.1016/j.bbrc.2017.01.183. [DOI] [PubMed] [Google Scholar]
- Chang WL, Liou W, Pen HC, Chou HY, Chang YW, Li WH, Chiang W, Pai LM. 2008. The gradient of Gurken, a long-range morphogen, is directly regulated by Cbl-mediated endocytosis. Development. 135(11):1923–1933. doi: 10.1242/dev.017103. [DOI] [PubMed] [Google Scholar]
- Chang YC, Wu JW, Hsieh YC, Huang TH, Liao ZM, Huang YS, Mondo JA, Montell D, Jang AC. 2018. Rap1 negatively regulates the hippo pathway to polarize directional protrusions in collective cell migration. Cell Rep. 22(8):2160–2175. doi: 10.1016/j.celrep.2018.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T, Bangham J, Vinti G, Seifried B, Lung O, Wolfner MF, Smith HK, Partridge L. 2003. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc Natl Acad Sci U S A. 100(17):9923–9928. doi: 10.1073/pnas.1631635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RF, Simpson SJ, Douglas AE. 2013. The Insects: Structure and Function. New York (NY): Cambridge University Press. [Google Scholar]
- Charbonnier E, Fuchs A, Cheung LS, Chayengia M, Veikkolainen V, Seyfferth J, Shvartsman SY, Pyrowolakis G. 2015. BMP-dependent gene repression cascade in Drosophila eggshell patterning. Dev Biol. 400(2):258–265. doi: 10.1016/j.ydbio.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee SS, Uppendahl LD, Chowdhury MA, Ip PL, Siegal ML. 2011. The female-specific Doublesex isoform regulates pleiotropic transcription factors to pattern genital development in Drosophila. Development. 138(6):1099–109. doi:138/6/1099. [DOI] [PubMed] [Google Scholar]
- Chen D-Y, Crest J, Bilder D. 2017. A cell migration tracking tool supports coupling of tissue rotation to elongation. Cell Rep. 21(3):559–569. doi: 10.1016/j.celrep.2017.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D-Y, Crest J, Streichan SJ, Bilder D. 2019. Extracellular matrix stiffness cues junctional remodeling for 3D tissue elongation. Nat Commun. 10(1):3339–15. doi: 10.1038/s41467-019-10874-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Kotian N, Aranjuez G, Chen L, Messer CL, Burtscher A, Sawant K, Ramel D, Wang X, McDonald JA. 2019. Protein phosphatase 1 activity controls a balance between collective and single cell modes of migration. Elife. 9:e52979. doi: 10.7554/elife.52979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D-Y, Lipari KR, Dehghan Y, Streichan SJ, Bilder D. 2016. Symmetry breaking in an edgeless epithelium by Fat2-regulated microtubule polarity. Cell Rep. 15(6):1125–1133. doi: 10.1016/j.celrep.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Nohata J, Guo H, Li S, Liu J, Guo Y, Yamamoto K, Kadono-Okuda K, Liu C, Arunkumar KP, et al. 2015. A comprehensive analysis of the chorion locus in silkmoth. Sci Rep. 5(1):16424. doi: 10.1038/srep16424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Schüpbach T. 2006. The role of brinker in eggshell patterning. Mech Dev. 123(5):395–406. doi: 10.1016/j.mod.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Chen PS, Stumm-Zollinger E, Aigaki T, Balmer J, Bienz M, Böhlen P. 1988. A male accessory gland peptide that regulates reproductive behavior of female D. melanogaster. Cell. 54(3):291–298. doi: 10.1016/0092-8674(88)90192-4. [DOI] [PubMed] [Google Scholar]
- Cherbas L, Hu X, Zhimulev I, Belyaeva E, Cherbas P. 2003. Ecr isoforms in Drosophila: testing tissue-specific requirements by targeted blockade and rescue. Development. 130:271–284. doi: 10.1242/dev.00205. [DOI] [PubMed] [Google Scholar]
- Cheung KJ, Ewald AJ. 2016. A collective route to metastasis: seeding by tumor cell clusters. Science. 352(6282):167–169. doi: 10.1126/science.aaf6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung LS, Schüpbach T, Shvartsman SY. 2011. Pattern formation by receptor tyrosine kinases: analysis of the Gurken gradient in Drosophila oogenesis. Curr Opin Genet Dev. 21(6):719–725. doi: 10.1016/j.gde.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlasta J, Milani P, Runel G, Duteyrat JL, Arias L, Lamiré LA, Boudaoud A, Grammont M. 2017. Variations in basement membrane mechanics are linked to epithelial morphogenesis. Development. 144:4350–4362. doi: 10.1242/dev.152652. [DOI] [PubMed] [Google Scholar]
- Choi SH, Park J-H, Nguyen TTN, Shim HJ, Song Y-H. 2017. Initiation of Drosophila chorion gene amplification requires Claspin and mus101, whereas Claspin, but not mus101, plays a major role during elongation. Dev Dyn. 246(6):466–474. doi: 10.1002/dvdy.24499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson AMK, Kafatos FC. 1993. Binding affinity of the Drosophila melanogaster CF1/USP protein to the chorion s15 promoter. Biochem Biophys Res Commun. 193(3):1318–1323. doi: 10.1006/bbrc.1993.1769. [DOI] [PubMed] [Google Scholar]
- Claycomb JM, Benasutti M, Bosco G, Fenger DD, Orr-Weaver TL. 2004. Gene amplification as a developmental strategy: isolation of two developmental amplicons in Drosophila. Dev Cell. 6(1):145–155. doi: 10.1016/s1534-5807(03)00398-8. [DOI] [PubMed] [Google Scholar]
- Claycomb JM, MacAlpine DM, Evans JG, Bell SP, Orr-Weaver TL. 2002. Visualization of replication initiation and elongation in Drosophila. J Cell Biol. 159(2):225–236. doi: 10.1083/jcb.200207046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claycomb JM, Orr-Weaver TL. 2005. Developmental gene amplification: insights into DNA replication and gene expression. Trends Genet. 21(3):149–162. doi: 10.1016/j.tig.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Cliffe A, Doupé DP, Sung H, Lim IK, Ong KH, Cheng L, Yu W. 2017. Quantitative 3D analysis of complex single border cell behaviors in coordinated collective cell migration. Nat Commun. 8(1):14905. doi: 10.1038/ncomms14905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SH, Carney GE, McClung CA, Willard SS, Taylor BJ, Hirsh J. 2005. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J Biol Chem. 280(15):14948–55. doi:M414197200. [DOI] [PubMed] [Google Scholar]
- Colonnetta MM, Lym LR, Wilkins L, Kappes G, Castro EA, Ryder PV, Schedl P, Lerit DA, Deshpande G. 2021. Antagonism between germ cell-less and Torso receptor regulates transcriptional quiescence underlying germline/soma distinction. Elife. 10:e54346. doi: 10.7554/elife.54346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M, Hsieh M, Zamah AM, Oh JS. 2012. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol Cell Endocrinol. 356(1–2):65–73. doi:S0303-7207(11)00655-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley L, Verheyen E, Ayers K. 1992. Chickadee encodes a profilin required for intercellular cytoplasm transport during Drosophila oogenesis. Cell. 69(1):173–84. doi: 10.1016/0092-8674(92)90128-y. [DOI] [PubMed] [Google Scholar]
- Crest J, Diz-Muñoz A, Chen D-Y, Fletcher DA, Bilder D. 2017. Organ sculpting by patterned extracellular matrix stiffness. eLife. 6:773. doi: 10.7554/eLife.24958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau DL, Popuri V, Opresko PL, Bohr VA. 2014. Human RecQ helicases in DNA repair, recombination, and replication. Annu Rev Biochem. 83(1):519–552. doi: 10.1146/annurev-biochem-060713-035428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Sartain CV, Pleiss JA, Wolfner MF. 2013. Cytoplasmic polyadenylation is a major mRNA regulator during oogenesis and egg activation in Drosophila. Dev Biol. 383(1):121–131. doi: 10.1016/j.ydbio.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry T, Smith M. 2006. Impact of extracellular matrix remodeling on ovulation and the folliculo-luteal transition. Semin Reprod Med. 24(4):228–241. doi: 10.1055/s-2006-948552. [DOI] [PubMed] [Google Scholar]
- Dahlgaard K, Raposo AASF, Niccoli T, St Johnston D. 2007. Capu and Spire assemble a cytoplasmic actin mesh that maintains microtubule organization in the Drosophila oocyte. Dev Cell. 13(4):539–553. doi: 10.1016/j.devcel.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Guo X, Cao Y, Mondo JA, Campanale JP, Montell BJ, Burrous H, Streichan S, Gov N, Rappel WJ, et al. 2020. Tissue topography steers migrating Drosophila border cells. Science. 370(6519):987–990. doi: 10.1126/science.aaz4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alterio C, Tran DD, Yeung MW, Hwang MS, Li MA, Arana CJ, Mulligan VK, Kubesh M, Sharma P, Chase M, et al. 2005. Drosophila melanogaster Cad99C, the orthologue of human Usher cadherin PCDH15, regulates the length of microvilli. J Cell Biol. 171(3):549–558. doi: 10.1083/jcb.200507072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A, Parton RM, Rabouille C, Weil TT, Davis I. 2016. Localized translation of gurken/TGF-α mRNA during axis specification is controlled by access to Orb/CPEB on processing bodies. Cell Rep. 14(10):2451–2462. doi: 10.1016/j.celrep.2016.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deady LD, Li W, Sun J. 2017. The zinc-finger transcription factor hindsight regulates ovulation competency of Drosophila follicles. Elife. 6:e29887. doi: 10.7554/eLife.29887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deady LD, Shen W, Mosure SA, Spradling AC, Sun J. 2015. Matrix metalloproteinase 2 is required for ovulation and corpus luteum formation in Drosophila. PLoS Genet. 11(2):e1004989. doi: 10.1371/journal.pgen.1004989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deady LD, Sun J. 2015. A follicle rupture assay reveals an essential role for follicular adrenergic signaling in Drosophila ovulation. PLoS Genet. 11(10):e1005604. doi: 10.1371/journal.pgen.1005604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degelmann A, Hardy PA, Mahowald AP. 1990. Genetic analysis of two female-sterile loci affecting eggshell integrity and embryonic pattern formation in Drosophila melanogaster. Genetics. 126(2):427–434. doi: 10.1093/genetics/126.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graeve FM, Van de Bor V, Ghiglione C, Cerezo D, Jouandin P, Ueda R, Shashidhara LS, Noselli S. 2012. Drosophila Apc regulates delamination of invasive epithelial clusters. Dev Biol. 368(1):76–85. doi: 10.1016/j.ydbio.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Delanoue R, Herpers B, Soetaert J, Davis I, Rabouille C. 2007. Drosophila Squid/hnRNP helps Dynein switch from a gurken mRNA transport motor to an ultrastructural static anchor in sponge bodies. Dev Cell. 13(4):523–538. doi: 10.1016/j.devcel.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Denef N, Chen Y, Weeks SD, Barcelo G, Schüpbach T. 2008. Crag regulates epithelial architecture and polarized deposition of basement membrane proteins in Drosophila. Dev Cell. 14(3):354–364. doi: 10.1016/j.devcel.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng WM, Bownes M. 1997. Two signalling pathways specify localised expression of the broad-complex in Drosophila eggshell patterning and morphogenesis. Development. 124(22):4639–4647. doi: 10.1242/dev.124.22.4639. [DOI] [PubMed] [Google Scholar]
- Deng WM, Ruohola-Baker H. 2000. Laminin A is required for follicle cell-oocyte signaling that leads to establishment of the anterior-posterior axis in Drosophila. Curr Biol. 10(11):683–686. doi: 10.1016/S0960-9822(00)00514-5. [DOI] [PubMed] [Google Scholar]
- Deng WM, Schneider M, Frock R, Castillejo-Lopez C, Gaman EA, Baumgartner S, Ruohola-Baker H. 2003. Dystroglycan is required for polarizing the epithelial cells and the oocyte in Drosophila. Development. 130(1):173–184. doi: 10.1242/dev.00199. [DOI] [PubMed] [Google Scholar]
- Derrick CJ, Weil TT. 2017. Translational control of gurken mRNA in Drosophila development. Cell Cycle. 16(1):23–32. doi: 10.1080/15384101.2016.1250048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devergne O, Sun GH, Schüpbach T. 2017. Stratum, a homolog of the human GEF Mss4, partnered with Rab8, controls the basal restriction of basement membrane proteins in epithelial cells. Cell Rep. 18(8):1831–1839. doi: 10.1016/j.celrep.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devergne O, Tsung K, Barcelo G, Schüpbach T. 2014. Polarized deposition of basement membrane proteins depends on phosphatidylinositol synthase and the levels of phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci U S A. 111(21):7689–7694. doi: 10.1073/pnas.1407351111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz de la Loza MC, Díaz-Torres A, Zurita F, Rosales-Nieves AE, Moeendarbary E, Franze K, Martín-Bermudo MD, González-Reyes A. 2017. Laminin levels regulate tissue migration and anterior-posterior polarity during egg morphogenesis in Drosophila. Cell Rep. 20(1):211–223. doi: 10.1016/j.celrep.2017.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digan ME, Spradling AC, Waring GL, Mahowald AP. 1979. The genetic analysis of chorion morphogenesis in Drosophila melanogaster. In: Axel R, Maniatis T, Fox CF, editors. Eukaryotic Gene Regulation. New York (NY): Academic Press. p. 171–181. [Google Scholar]
- DiMario PJ, Mahowald AP. 1987. Female sterile (1) yolkless: a recessive female sterile mutation in Drosophila melanogaster with depressed numbers of coated pits and coated vesicles within the developing oocytes. J Cell Biol. 105(1):199–206. doi: 10.1083/jcb.105.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doane WW. 1960. Completion of meiosis in uninseminated eggs of Drosophila melanogaster. Science. 132(3428):677–678. doi: 10.1126/science.132.3428.677. [DOI] [PubMed] [Google Scholar]
- Dobens L, Jaeger A, Peterson JS, Raftery LA. 2005. Bunched sets a boundary for Notch signaling to pattern anterior eggshell structures during Drosophila oogenesis. Dev Biol. 287(2):425–437. doi: 10.1016/j.ydbio.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Dobens LL, Peterson JS, Treisman J, Raftery LA. 2000. Drosophila bunched integrates opposing DPP and EGF signals to set the operculum boundary. Development. 127:745–754. doi: 10.1242/dev.127.4.745. [DOI] [PubMed] [Google Scholar]
- Dobens LL, Raftery LA. 2000. Integration of epithelial patterning and morphogenesis in Drosophila ovarian follicle cells. Dev Dyn. 218(1):80–93. doi:10.1002/(SICI)1097-0177(200005)218:1< 80::AID-DVDY7>3.0.CO; 2-8. [DOI] [PubMed] [Google Scholar]
- Dobrosotskaya IY, Seegmiller AC, Brown MS, Goldstein JL, Rawson RB. 2002. Regulation of SREBP processing and membrane lipid production by phospholipids in Drosophila. Science. 296(5569):879–83. doi: 10.1126/science.1071124. [DOI] [PubMed] [Google Scholar]
- Domanitskaya E, Anllo L, Schupbach T. 2014. Phantom, a cytochrome P450 enzyme essential for ecdysone biosynthesis, plays a critical role in the control of border cell migration in Drosophila. Dev Biol. 386(2):408–18. doi: 10.1016/j.ydbio.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman JB, James KE, Fraser SE, Kiehart DP, Berg CA. 2004. Bullwinkle is required for epithelial morphogenesis during Drosophila oogenesis. Dev Biol. 267(2):320–341. doi: 10.1016/j.ydbio.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Drummond-Barbosa D, Spradling AC. 2001. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol. 231(1):265–78. doi: 10.1006/dbio.2000.0135. [DOI] [PubMed] [Google Scholar]
- Drummond-Barbosa D, Spradling AC. 2004. α-Endosulfine, a potential regulator of insulin secretion, is required for adult tissue growth control in Drosophila. Dev Biol. 266(2):310–321. doi: 10.1016/j.ydbio.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Duchek P, Rørth P. 2001. Guidance of cell migration by EGF receptor signaling during Drosophila oogenesis. Science. 291(5501):131–133. doi: 10.1126/science.291.5501.131. [DOI] [PubMed] [Google Scholar]
- Duchek P, Somogyi K, Jékely G, Beccari S, Rørth P. 2001. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell. 107(1):17–26. doi: 10.1016/S0092-8674(01)00502-5. [DOI] [PubMed] [Google Scholar]
- Duhart JC, Parsons TT, Raftery LA. 2017. The repertoire of epithelial morphogenesis on display: progressive elaboration of Drosophila egg structure. Mech Dev. 148:18–39. doi: 10.1016/j.mod.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Duncan EJ, Benton MA, Dearden PK. 2013. Canonical terminal patterning is an evolutionary novelty. Dev Biol. doi: 10.1016/j.ydbio.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Duncan JE, Warrior R. 2002. The cytoplasmic dynein and kinesin motors have interdependent roles in patterning the Drosophila oocyte. Curr Biol. 12(23):1982–1991. doi: 10.1016/S0960-9822(02)01303-9. [DOI] [PubMed] [Google Scholar]
- Easwaran S, Van Ligten M, Kui M, Montell DJ. 2022. Enhanced germline stem cell longevity in Drosophila diapause. Nat Commun. 13(1):711. doi: 10.1038/s41467-022-28347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards KA, Demsky M, Montague RA, Weymouth N, Kiehart DP. 1997. GFP-moesin illuminates actin cytoskeleton dynamics in living tissue and demonstrates cell shape changes during morphogenesis in Drosophila. Dev Biol. 191(1):103–117. doi: 10.1006/dbio.1997.8707. [DOI] [PubMed] [Google Scholar]
- Edwards KA, Kiehart DP. 1996. Drosophila Nonmuscle myosin II has multiple essential roles in imaginal disc and egg chamber morphogenesis. Development. 122(5):1499–511. doi: 10.1242/dev.122.5.1499. [DOI] [PubMed] [Google Scholar]
- Eichhorn SW, Subtelny AO, Kronja I, Kwasnieski JC, Orr-Weaver TL, Bartel DP. 2016. Mrna poly(A)-tail changes specified by deadenylation broadly reshape translation in Drosophila oocytes and early embryos. Elife. 5:e16955. doi: 10.7554/eLife.16955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elalayli M, Hall JD, Fakhouri M, Neiswender H, Ellison TT, Han Z, Roon P, LeMosy EK. 2008. Palisade is required in the Drosophila ovary for assembly and function of the protective vitelline membrane. Dev Biol. 319(2):359–369. doi: 10.1016/j.ydbio.2008.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espenshade PJ. 2006. Srebps: sterol-regulated transcription factors. J Cell Sci. 119(6):973–6. doi: 10.1242/jcs02866. [DOI] [PubMed] [Google Scholar]
- Espinoza CY, Berg CA. 2020. Detecting new allies: modifier screen identifies a genetic interaction between imaginal disc growth factor 3 and combover, a Rho-kinase substrate, during dorsal appendage tube formation in Drosophila. G3 (Bethesda). 10(10):3585–3599. doi: 10.1534/g3.120.401476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DS, Cline TW. 2013. Drosophila Switch gene sex-lethal can bypass its switch-gene target transformer to regulate aspects of female behavior. Proc Natl Acad Sci U S A. 110(47):E4474–81. doi:1319063110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhouri M, Elalayli M, Sherling D, Hall JD, Miller E, Sun X, Wells L, LeMosy EK. 2006. Minor proteins and enzymes of the Drosophila eggshell matrix. Dev Biol. 293(1):127–141. doi: 10.1016/j.ydbio.2006.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H-Y, Liu Z, Mullany LK, Richards JS. 2012. Consequences of RAS and MAPK activation in the ovary: the good, the bad and the ugly. Mol Cell Endocrinol. 356(1–2):74–79. doi: 10.1016/j.mce.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargnoli J, Waring GL. 1982. Identification of vitelline membrane proteins in Drosophila melanogaster. Dev Biol. 92(2):306–314. doi: 10.1016/0012-1606(82)90177-4. [DOI] [PubMed] [Google Scholar]
- Fauré A, Vreede BMI, Sucena E, Chaouiya C. 2014. A discrete model of Drosophila eggshell patterning reveals cell-autonomous and juxtacrine effects. PLoS Comput Biol. 10(3):e1003527. doi: 10.1371/journal.pcbi.1003527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng K, Palfreyman MT, Häsemeyer M, Talsma A, Dickson BJ. 2014. Ascending SAG neurons control sexual receptivity of Drosophila females. Neuron. 83(1):135–148. doi: 10.1016/j.neuron.2014.05.017. [DOI] [PubMed] [Google Scholar]
- Ferguson SB, Blundon MA, Klovstad MS, Schüpbach T. 2012. Modulation of gurken translation by insulin and TOR signaling in Drosophila. J Cell Sci. 125(Pt\ 6):1407–1419. doi: 10.1242/jcs.090381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Espartero CH, Ramel D, Farago M, Malartre M, Luque CM, Limanovich S, Katzav S, Emery G, Martín-Bermudo MD. 2013. Gtp exchange factor Vav regulates guided cell migration by coupling guidance receptor signalling to local Rac activation. J Cell Sci. 126(Pt\ 10):2285–2293. doi: 10.1242/jcs.124438. [DOI] [PubMed] [Google Scholar]
- Ferreira T, Prudencio P, Martinho RG. 2014. Drosophila Protein kinase N (Pkn) is a negative regulator of actin-myosin activity during oogenesis. Dev Biol. 394(2):277–91. doi: 10.1016/j.ydbio.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Filosi M, Perotti ME. 1975. Fine structure of the spermatheca of Drosophila melanogaster Meig. J Submicr Cytol. 7:259–270. [Google Scholar]
- Finley KD, Edeen PT, Foss M, Gross E, Ghbeish N, Palmer RH, Taylor BJ, McKeown M. 1998. Dissatisfaction encodes a tailless-like nuclear receptor expressed in a subset of CNS neurons controlling Drosophila sexual behavior. Neuron. 21(6):1363–1374. doi: 10.1016/S0896-6273(00)80655-8. [DOI] [PubMed] [Google Scholar]
- Finley KD, Taylor BJ, Milstein M, McKeown M. 1997. Dissatisfaction, a gene involved in sex-specific behavior and neural development of Drosophila melanogaster. Proc Natl Acad Sci U S A. 94(3):913–918. doi: 10.1073/pnas.94.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher GC, Diaz-de-la-Loza MD, Borreguero-Muñoz N, Holder M, Aguilar-Aragon M, Thompson BJ. 2018. Mechanical strain regulates the Hippo pathway in Drosophila. Development. 145:dev159467. doi: 10.1242/dev.159467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley K, Cooley L. 1998. Apoptosis in late stage Drosophila nurse cells does not require genes within the H99 deficiency. Development. 125(6):1075–1082. doi: 10.1242/dev.125.6.1075. [DOI] [PubMed] [Google Scholar]
- Forrest KM, Gavis ER. 2003. Live imaging of endogenous RNA reveals a diffusion and entrapment mechanism for nanos mRNA localization in Drosophila. Curr Biol. 13(14):1159–1168. doi: 10.1016/S0960-9822(03)00451-2. [DOI] [PubMed] [Google Scholar]
- Fox EF, Mellentine SQ, Tootle TL. 2020. Prostaglandins regulate invasive, collective border cell migration. Mol Biol Cell. 31(15):1584–1594. doi: 10.1091/mbc.e19-10-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French RL, Cosand KA, Berg CA. 2003. The Drosophila female sterile mutation twin peaks is a novel allele of tramtrack and reveals a requirement for Ttk69 in epithelial morphogenesis. Dev Biol. 253(1):18–35. doi: 10.1006/dbio.2002.0856. [DOI] [PubMed] [Google Scholar]
- Frydman HM, Spradling AC. 2001. The receptor-like tyrosine phosphatase lar is required for epithelial planar polarity and for axis determination within Drosophila ovarian follicles. Development. 128:3209–3220. doi: 10.1242/dev.128.16.3209. [DOI] [PubMed] [Google Scholar]
- Gans M, Audit C, Masson M. 1975. Isolation and characterization of sex-linked female-sterile mutants in Drosophila melanogaster. Genetics. 81(4):683–704. doi: 10.1093/genetics/81.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Ruden DM, Lu X. 2003. Pkd2 cation channel is required for directional sperm movement and male fertility. Curr Biol. 13(24):2175–2178. doi: 10.1016/j.cub.2003.11.053. [DOI] [PubMed] [Google Scholar]
- Garbiec A, Kubrakiewicz J. 2012. Differentiation of follicular cells in polytrophic ovaries of Neuroptera (Insecta: Holometabola). Arthropod Struct Dev. 41(2):165–176. doi: 10.1016/j.asd.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Garbiec A, Kubrakiewicz J, Mazurkiewicz-Kania M, zena Simiczyjew B, Jędrzejowska I. 2016. Asymmetry in structure of the eggshell in Osmylus fulvicephalus (Neuroptera: Osmylidae): an exceptional case of breaking symmetry during neuropteran oogenesis. Protoplasma. 253(4):1033–1042. doi: 10.1007/s00709-015-0860-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates J. 2012. Drosophila Egg chamber elongation: insights into how tissues and organs are shaped. Fly (Austin). 6(4):213–227. doi: 10.4161/fly.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaziova I, Bonnette PC, Henrich VC, Jindra M. 2004. Cell-autonomous roles of the ecdysoneless gene in Drosophila development and oogenesis. Development. 131(11):2715–2725. doi: 10.1242/dev.01143. [DOI] [PubMed] [Google Scholar]
- Ge W, Deng Q, Guo T, Hong X, Kugler JM, Yang X, Cohen SM. 2015. Regulation of pattern formation and gene amplification during Drosophila oogenesis by the miR-318 microRNA. Genetics. 200(1):255–265. doi: 10.1534/genetics.115.174748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelti-Douka H, Gingeras TR, Kambysellis MP. 1974. Yolk proteins in Drosophila: identification and site of synthesis. J Exp Zool. 187(1):167–72. doi: 10.1002/jez.1401870120. [DOI] [PubMed] [Google Scholar]
- Gilbert LI, Rybczynski R, Warren JT. 2002. Control and biochemical nature of the ecdysteroidogenic pathway. Annu Rev Entomol. 47(1):883–916. doi: 10.1146/annurev.ento.47.091201.145302. [DOI] [PubMed] [Google Scholar]
- Gillespie DE, Berg CA. 1995. Homeless is required for RNA localization in Drosophila oogenesis and encodes a new member of the DE-H family of RNA-dependent ATPases. Genes Dev. 9(20):2495–2508. doi: 10.1101/gad.9.20.2495. [DOI] [PubMed] [Google Scholar]
- Giorgi F. 1978. Intercellular bridges in ovarian follicle cells of Drosophila melanogaster. Cell Tissue Res. 186(3):413–422. doi: 10.1007/BF00224931. [DOI] [PubMed] [Google Scholar]
- Giorgi F. 1979. In vitro induced pinocytotic activity by a juvenile hormone analogue in oocytes of Drosophila melanogaster. Cell Tissue Res. 203(2):241–247. doi: 10.1007/BF00237238. [DOI] [PubMed] [Google Scholar]
- Giorgi F, Deri P. 1976. Cytochemistry of late ovarian chambers of Drosophila melanogaster. Histochemistry. 48(4):325–34. doi: 10.1007/BF00499249. [DOI] [PubMed] [Google Scholar]
- Giorgi F, Jacob J. 1977. Recent findings on oogenesis of Drosophila melanogaster. I. Ultrastructural observations on the developing ooplasm. J Embryol Exp Morphol. 38:115–24. [PubMed] [Google Scholar]
- Giorgi F, Lucchesi P, Morelli A, Bownes M. 1993. Ultrastructural analysis of Drosophila ovarian follicles differing in yolk polypeptide (yps) composition. Development. 117(1):319–28. doi: 10.1242/dev.117.1.319. [DOI] [PubMed] [Google Scholar]
- Goentoro LA, Reeves GT, Kowal CP, Martinelli L, Schüpbach T, Shvartsman SY. 2006. Quantifying the Gurken morphogen gradient in Drosophila oogenesis. Dev Cell. 11(2):263–272. doi: 10.1016/j.devcel.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogos JA, Hsu T, Bolton J, Kafatos FC. 1992. Sequence discrimination by alternatively spliced isoforms of a DNA binding zinc finger domain. Science. 257(5078):1951–1955. doi: 10.1126/science.1290524. [DOI] [PubMed] [Google Scholar]
- González-Reyes A, Elliott H, Johnston DS. 1995. Polarization of both major body axes in Drosophila by gurken-torpedo signalling. Nature. 375(6533):654–658. doi: 10.1038/375654a0. [DOI] [PubMed] [Google Scholar]
- González-Reyes A, Johnston DS. 1998. Patterning of the follicle cell epithelium along the anterior-posterior axis during Drosophila oogenesis. Development. 125(15):2837–2846. doi: 10.1242/dev.125.15.2837. [DOI] [PubMed] [Google Scholar]
- Gou B, Liu Y, Guntur AR, Stern U, Yang C-H. 2014. Mechanosensitive neurons on the internal reproductive tract contribute to egg-laying-induced acetic acid attraction in Drosophila. Cell Rep. 9(2):522–530. doi: 10.1016/j.celrep.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal Y, Schüpbach T, Shvartsman SY. 2018. A quantitative model of developmental RTK signaling. Dev Biol. doi: 10.1016/j.ydbio.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammont M, Irvine KD. 2002. Organizer activity of the polar cells during Drosophila oogenesis. Development. 129(22):5131–5140. doi: 10.1242/dev.129.22.5131. [DOI] [PubMed] [Google Scholar]
- Griffin-Shea R, Thireos G, Kafatos FC. 1982. Organization of a cluster of four chorion genes in Drosophila and its relationship to developmental expression and amplification. Dev Biol. 91(2):325–336. doi: 10.1016/0012-1606(82)90039-2. [DOI] [PubMed] [Google Scholar]
- Grmai L, Michaca M, Lackner E, Nampoothiri VP, Vasudevan D. Integrated stress response signaling acts as a metabolic sensor in fat tissues to regulate oocyte maturation and ovulation. BioRxiv 530289. doi: 10.1101/2023.02.27.530289, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed]
- Groen CM, Spracklen AJ, Fagan TN, Tootle TL. 2012. Drosophila Fascin is a novel downstream target of prostaglandin signaling during actin remodeling. Mol Biol Cell. 23(23):4567–78. doi: 10.1091/mbc.E12-05-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guild GM, Connelly PS, Shaw MK, Tilney LG. 1997. Actin filament cables in Drosophila nurse cells are composed of modules that slide passively past one another during dumping. J Cell Biol. 138(4):783–97. doi: 10.1083/jcb.138.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzeit HO. 1990. The microfilament pattern in the somatic follicle cells of mid-vitellogenic ovarian follicles of Drosophila. Eur J Cell Biol. 53:349–356. [PubMed] [Google Scholar]
- Gutzeit HO. 1991. Organization and in vitro activity of microfilament bundles associated with the basement membrane of Drosophila follicles. Acta Histochem Suppl. 41:201–210. [PubMed] [Google Scholar]
- Gutzeit HO, Eberhardt W, Gratwohl E. 1991. Laminin and basement membrane-associated microfilaments in wild-type and mutant Drosophila ovarian follicles. J Cell Sci. 100(4):781–788. doi: 10.1242/jcs.100.4.781. [DOI] [PubMed] [Google Scholar]
- Hackney JF, Pucci C, Naes E, Dobens L. 2007. Ras signaling modulates activity of the ecdysone receptor EcR during cell migration in the Drosophila ovary. Dev Dyn. 236(5):1213–1226. doi: 10.1002/dvdy.21140. [DOI] [PubMed] [Google Scholar]
- Haigo SL, Bilder D. 2011. Global tissue revolutions in a morphogenetic movement controlling elongation. Science. 331(6020):1071–4. doi: 10.1126/science.1199424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RS, Hartswood E, Vendra G, Jones C, Van De Bor V, Finnegan D, Davis I. 2009. A bioinformatics search pipeline, RNA2DSearch, identifies RNA localization elements in Drosophila retrotransposons. RNA. 15(2):200–207. doi: 10.1261/rna.1264109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K-A, Millar NS, Davis RL. 1998. A novel octopamine receptor with preferential expression in Drosophila mushroom bodies. J Neurosci. 18(10):3650–3658. doi: 10.1523/JNEUROSCI.18-10-03650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M, Lourido S, Petrova B, Lou HJ, Von Stetina JR, Kashevsky H, Turk BE, Orr-Weaver TL. 2018. Identification of PNG kinase substrates uncovers interactions with the translational repressor TRAL in the oocyte-to-embryo transition. Elife. 7:e33150. doi: 10.7554/eLife.33150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M, Petrova B, Orr-Weaver TL. 2017. Control of PNG kinase, a key regulator of mRNA translation, is coupled to meiosis completion at egg activation. Elife. 6:e22219. doi: 10.7554/eLife.22219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshman LG, Loeb AM, Johnson BA. 1999. Ecdysteroid titers in mated and unmated Drosophila melanogaster females. J Insect Physiol. 45(6):571–577. doi: 10.1016/S0022-1910(99)00038-4. [DOI] [PubMed] [Google Scholar]
- Hartl T, Boswell C, Orr-Weaver TL, Bosco G. 2007. Developmentally regulated histone modifications in Drosophila follicle cells: initiation of gene amplification is associated with histone H3 and H4 hyperacetylation and H1 phosphorylation. Chromosoma. 116(2):197–214. doi: 10.1007/s00412-006-0092-2. [DOI] [PubMed] [Google Scholar]
- Hasemeyer M, Yapici N, Heberlein U, Dickson BJ. 2009. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron. 61(4):511–8. doi:S0896-6273(09)00076-2. [DOI] [PubMed] [Google Scholar]
- Hawley RJ, Waring GL. 1988. Cloning and analysis of the dec-1 female-sterile locus, a gene required for proper assembly of the Drosophila eggshell. Genes Dev. 2(3):341–349. doi: 10.1101/gad.2.3.341. [DOI] [PubMed] [Google Scholar]
- He L, Wang X, Tang HL, Montell DJ. 2010. Tissue elongation requires oscillating contractions of a basal actomyosin network. Nat Cell Biol. 12(12):1133–1142. doi: 10.1038/ncb2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifetz Y, Lindner M, Garini Y, Wolfner MF. 2014. Mating regulates neuromodulator ensembles at nerve termini innervating the Drosophila reproductive tract. Curr Biol. 24(7):731–737. doi: 10.1016/j.cub.2014.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifetz Y, Lung O, Frongillo EA Jr, Wolfner MF. 2000. The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary. Curr Biol. 10(2):99–102. doi: 10.1016/S0960-9822(00)00288-8. [DOI] [PubMed] [Google Scholar]
- Heifetz Y, Rivlin PK. 2010. Beyond the mouse model: using Drosophila as a model for sperm interaction with the female reproductive tract. Theriogenology. 73(6):723–739. doi: 10.1016/j.theriogenology.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Heifetz Y, Vandenberg LN, Cohn HI, Wolfner MF. 2005. Two cleavage products of the Drosophila accessory gland protein ovulin can independently induce ovulation. Proc Natl Acad Sci U S A. 102(3):743–8. doi:0407692102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifetz Y, Wolfner MF. 2004. Mating, seminal fluid components, and sperm cause changes in vesicle release in the Drosophila female reproductive tract. Proc Natl Acad Sci U S A. 101(16):6261–6. doi: 10.1073/pnas.0401337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifetz Y, Yu J, Wolfner MF. 2001. Ovulation triggers activation of Drosophila oocytes. Dev Biol. 234(2):416–424. doi: 10.1006/dbio.2001.0246. [DOI] [PubMed] [Google Scholar]
- Henstridge MA, Johnson TK, Warr CG, Whisstock JC. 2014. Trunk cleavage is essential for Drosophila terminal patterning and can occur independently of Torso-like. Nat Commun. 5(1):3419. doi: 10.1038/ncomms4419. [DOI] [PubMed] [Google Scholar]
- Herndon LA, Wolfner MF. 1995. A Drosophila seminal fluid protein, Acp26Aa, stimulates egg laying in females for 1 day after mating. Proc Natl Acad Sci U S A. 92(22):10114–10118. doi: 10.1073/pnas.92.22.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins MJ, Walker VK, Holden JA, White BN. 1984. Isolation of two Drosophila melanogaster genes abundantly expressed in the ovary during vitelline membrane synthesis. Dev Biol. 105(1):155–165. doi: 10.1016/0012-1606(84)90271-9. [DOI] [PubMed] [Google Scholar]
- Hinton HE. 1960. The structure and function of the respiratory horns of the eggs of some flies. Philos Trans R Soc Lond B Biol Sci. 243(699):45–73. doi: 10.1098/rstb.1960.0004. [DOI] [Google Scholar]
- Hinton HE. 1981. Biology of Insect Eggs. Volume I–III. 1st ed. Oxford England; New York: Pergamon Press. [Google Scholar]
- Hogan BLM, Kolodziej PA. 2002. Organogenesis: molecular mechanisms of tubulogenesis. Nat Rev Genet. 3(7):513–523. doi: 10.1038/nrg840. [DOI] [PubMed] [Google Scholar]
- Hong CC, Hashimoto C. 1996. The maternal nudel protein of Drosophila has two distinct roles important for embryogenesis. Genetics. 143(4):1653–1661. doi: 10.1093/genetics/143.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne-Badovinac S. 2020. The Drosophila micropyle as a system to study how epithelia build complex extracellular structures. Philos Trans R Soc B. 375(1809):20190561. doi: 10.1098/rstb.2019.0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne-Badovinac S, Bilder D. 2005. Mass transit: epithelial morphogenesis in the Drosophila egg chamber. Dev Dyn. 232:559–574. doi: 10.1002/dvdy.20286. [DOI] [PubMed] [Google Scholar]
- Horner VL, Czank A, Jang JK, Singh N, Williams BC, Puro J, Kubli E, Hanes SD, McKim KS, Wolfner MF, Goldberg ML. 2006. The Drosophila calcipressin Sarah is required for several aspects of egg activation. Curr Biol. 16(14):1441–1446. doi: 10.1016/j.cub.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Horner VL, Wolfner MF. 2008a. Transitioning from egg to embryo: triggers and mechanisms of egg activation. Dev Dyn. 237(3):527–544. doi: 10.1002/dvdy.21454. [DOI] [PubMed] [Google Scholar]
- Horner VL, Wolfner MF. 2008b. Mechanical stimulation by osmotic and hydrostatic pressure activates Drosophila oocytes in vitro in a calcium-dependent manner. Dev Biol. 316(1):100–9. doi:S0012-1606(08)00027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q-L, Chen E-H, Xie Y-F, Dou W, Wang J-J. 2021. Ovary-Specific transcriptome and essential role of nanos in ovary development in the oriental fruit fly (Diptera: Tephritidae). J Econ Entomol. 114(2):947–958. doi: 10.1093/jee/toab004. [DOI] [PubMed] [Google Scholar]
- Hsu T, Bagni C, Sutherland JD, Kafatos FC. 1996. The transcriptional factor CF2 is a mediator of EGF-R-activated dorsoventral patterning in Drosophila oogenesis. Genes Dev. 10(11):1411–1421. doi: 10.1101/gad.10.11.1411. [DOI] [PubMed] [Google Scholar]
- Hsu HJ, Drummond-Barbosa D. 2011. Insulin signals control the competence of the Drosophila female germline stem cell niche to respond to Notch ligands. Dev Biol. 350(2):290–300. doi: 10.1016/j.ydbio.2010.11.032. [DOI] [PubMed] [Google Scholar]
- Hsu T, Gogos JA, Kirsh SA, Kafatos FC. 1992. Multiple zinc finger forms resulting from developmentally regulated alternative splicing of a transcription factor gene. Science. 257(5078):1946–1950. doi: 10.1126/science.1411512. [DOI] [PubMed] [Google Scholar]
- Hsu SJ, Stow EC, Simmons JR, Wallace HA, Lopez AM, Stroud S, Labrador M. 2020. Mutations in the insulator protein suppressor of hairy wing induce genome instability. Chromosoma. 129(3–4):255–274. doi: 10.1007/s00412-020-00743-8. [DOI] [PubMed] [Google Scholar]
- Hua BL, Orr-Weaver TL. 2017. Dna replication control during Drosophila development: insights into the onset of S phase, replication initiation, and fork progression. Genetics. 207(1):29–47. doi: 10.1534/genetics.115.186627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Vélez-Avilés AN, Wolfner MF. 2020. Drosophila Plc21c is involved in calcium wave propagation during egg activation. MicroPublication Biol. 2020: 10.17912/micropub.biology.000235. doi: 10.17912/micropub.biology.000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Wolfner MF. 2019. The Drosophila Trpm channel mediates calcium influx during egg activation. Proc Natl Acad Sci U S A. 116(38):18994–19000. doi: 10.1073/pnas.1906967116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Yokoyama C, Wu J, Briggs MR, Brown MS, Goldstein JL, Wang X. 1993. SREBP-2, a second basic-helix-loop-helix-leucine zipper protein that stimulates transcription by binding to a sterol regulatory element. Proc Natl Acad Sci U S A. 90(24):11603–7. doi: 10.1073/pnas.90.24.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y-C, Smith L, Poulton J, Deng W-M. 2013. The microRNA miR-7 regulates Tramtrack69 in a developmental switch in Drosophila follicle cells. Development. 140(4):897–905. doi: 10.1242/dev.080192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AM, Cooley L. 2002. Understanding the function of actin-binding proteins through genetic analysis of Drosophila oogenesis. Annu Rev Genet. 36(1):455–488. doi: 10.1146/annurev.genet.36.052802.114101. [DOI] [PubMed] [Google Scholar]
- Hudson AM, Petrella LN, Tanaka AJ, Cooley L. 2008. Mononuclear muscle cells in Drosophila ovaries revealed by GFP protein traps. Dev Biol. 314(2):329–340. doi: 10.1016/j.ydbio.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SE, Miller DE, Miller AL, Hawley RS. 2018. Female meiosis: synapsis, recombination, and segregation in Drosophila melanogaster. Genetics. 208(3):875–908. doi: 10.1534/genetics.117.300081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh J-R, Johnston DS. 2004. The origin of asymmetry: early polarisation of the Drosophila germline cyst and oocyte. Curr Biol CB. 14(11):R438–49. doi: 10.1016/j.cub.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Imran Alsous J, Romeo N, Jackson JA, Mason FM, Dunkel J, Martin AC. 2021. Dynamics of hydraulic and contractile wave-mediated fluid transport during Drosophila oogenesis. Proc Natl Acad Sci U S A. 118(10):e2019749118. doi: 10.1073/pnas.2019749118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac PG, Bownes M. 1982. Ovarian and fat-body vitellogenin synthesis in Drosophila melanogaster. Eur J Biochem. 123(3):527–34. doi: 10.1111/j.1432-1033.1982.tb06563.x. [DOI] [PubMed] [Google Scholar]
- Isabella AJ, Horne-Badovinac S. 2015a. Building from the ground up: basement membranes in Drosophila development. Curr Top Membr. 76:305–336. doi: 10.1016/bs.ctm.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isabella AJ, Horne-Badovinac S. 2015b. Dynamic regulation of basement membrane protein levels promotes egg chamber elongation in Drosophila. Dev Biol. 406(2):212–221. doi: 10.1016/j.ydbio.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isabella AJ, Horne-Badovinac S. 2016. Rab10-Mediated secretion synergizes with tissue movement to build a polarized basement membrane architecture for organ morphogenesis. Dev Cell. 38(1):47–60. doi: 10.1016/j.devcel.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isasti-Sanchez J, Münz-Zeise F, Lancino M, Luschnig S. 2021. Transient opening of tricellular vertices controls paracellular transport through the follicle epithelium during Drosophila oogenesis. Dev Cell. 56(8):1083–1099.e5. doi: 10.1016/j.devcel.2021.03.021. [DOI] [PubMed] [Google Scholar]
- Jackson RJ, Hellen CUT, Pestova TV. 2010. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol. 11(2):113–127. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglarz MK, Krzeminski W, Bilinski SM. 2008. Structure of the ovaries and follicular epithelium morphogenesis in Drosophila and its kin. Dev Genes Evol. 218(8):399–411. doi: 10.1007/s00427-008-0233-0. [DOI] [PubMed] [Google Scholar]
- Jaglarz MK, Kubrakiewicz J, Bilinski SM. 2010. A novel pattern of follicular epithelium morphogenesis in higher dipterans. Zoology (Jena). 113:91–99. doi: 10.1016/j.zool.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Jambor H, Surendranath V, Kalinka AT, Mejstrik P, Saalfeld S, Tomancak P. 2015. Systematic imaging reveals features and changing localization of mRNAs in Drosophila development. Elife. 4:e05003. doi: 10.7554/eLife.05003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang AC-C, Chang Y-C, Bai J, Montell D. 2009. Border-cell migration requires integration of spatial and temporal signals by the BTB protein Abrupt. Nat Cell Biol. 11(5):569–579. doi: 10.1038/ncb1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jékely G, Rørth P. 2003. Hrs mediates downregulation of multiple signalling receptors in Drosophila. EMBO Rep. 4(12):1163–1168. doi: 10.1038/sj.embor.7400019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellusova J, Cato MH, Apgar JR, Ramezani-Rad P, Leung CR, Chen C, Richardson AD, Conner EM, Benschop RJ, Woodgett JR, et al. 2017. Gsk3 is a metabolic checkpoint regulator in B cells. Nat Immunol. 18(3):303–312. doi: 10.1038/ni.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins VK, Timmons AK, McCall K. 2013. Diversity of cell death pathways: insight from the fly ovary. Trends Cell Biol. 23(11):567–574. doi: 10.1016/j.tcb.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevitt A, Chatterjee D, Xie G, Wang XF, Otwell T, Huang YC, Deng WM. 2020. A single-cell atlas of adult Drosophila ovary identifies transcriptional programs and somatic cell lineage regulating oogenesis. PLoS Biol. 18(4):e3000538. doi: 10.1371/journal.pbio.3000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia D, Huang Y-C, Deng W-M. 2015. Analysis of cell cycle switches in Drosophila oogenesis. Methods Mol Biol. 1328:207–216. doi: 10.1007/978-1-4939-2851-4_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia D, Jevitt A, Huang Y-C, Ramos B, Deng W-M. 2022. Developmental regulation of epithelial cell cuboidal-to-squamous transition in Drosophila follicle cells. Dev Biol. 491:113–125. doi: 10.1016/j.ydbio.2022.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Q, Liu Y, Liu H, Li S. 2014. Mmp1 and Mmp2 cooperatively induce Drosophila fat body cell dissociation with distinct roles. Sci Rep. 4(1):7535. doi: 10.1038/srep07535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K, Zhang J, Huang Y, Wang Y, Xiao S, Hadden MK, Woodruff TK, Sun J. 2021. A platform utilizing Drosophila ovulation for nonhormonal contraceptive screening. Proc Natl Acad Sci U S A. 118(28):e2026403118. doi: 10.1073/pnas.2026403118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez G, González-Reyes A, Casanova J. 2002. Cell surface proteins nasrat and polehole stabilize the torso-like extracellular determinant in Drosophila oogenesis. Genes Dev. 16(8):913–918. doi: 10.1101/gad.223902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns AR, Henstridge MA, Saligari MJ, Moore KA, Whisstock JC, Warr CG, Johnson TK. 2018. Genome-Wide screen for new components of the Drosophila melanogaster torso receptor tyrosine kinase pathway. G3 (Bethesda). 8(3):761–769. doi: 10.1534/g3.117.300491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TK, Henstridge MA, Herr A, Moore KA, Whisstock JC, Warr CG. 2015. Torso-like mediates extracellular accumulation of furin-cleaved trunk to pattern the Drosophila embryo termini. Nat Commun. 6(1):8759. doi: 10.1038/ncomms9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TK, Henstridge MA, Warr CG. 2017. Macpf/CDC proteins in development: insights from Drosophila torso-like. Semin Cell Dev Biol. 72:163–170. doi: 10.1016/j.semcdb.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Johnson TK, Moore KA, Whisstock JC, Warr CG. 2017. Maternal Torso-like coordinates tissue folding during Drosophila gastrulation. Genetics. 206(3):1459–1468. doi: 10.1534/genetics.117.200576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone O, Lasko P. 2001. Translational regulation and RNA localization in Drosophila oocytes and embryos. Annu Rev Genet. 35(1):365–406. doi: 10.1146/annurev.genet.35.102401.090756. [DOI] [PubMed] [Google Scholar]
- Jongbloets BC, Pasterkamp RJ. 2014. Semaphorin signalling during development. Development. 141(17):3292–3297. doi: 10.1242/dev.105544. [DOI] [PubMed] [Google Scholar]
- Kaneuchi T, Sartain CV, Takeo S, Horner VL, Buehner NA, Aigaki T, Wolfner MF. 2015. Calcium waves occur as Drosophila oocytes activate. Proc Natl Acad Sci U S A. 112(3):791–6. doi: 10.1073/pnas.1420589112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D, Wang D, Xu J, Quan C, Guo X, Wang H, Luo J, Yang Z, Chen S, Chen J. 2018. The InR/akt/TORC1 growth-promoting signaling negatively regulates JAK/STAT activity and migratory cell fate during morphogenesis. Dev Cell. 44(4):524–531.e5. doi: 10.1016/j.devcel.2018.01.017. [DOI] [PubMed] [Google Scholar]
- Kapelnikov A, Rivlin PK, Hoy RR, Heifetz Y. 2008. Tissue remodeling: a mating-induced differentiation program for the Drosophila oviduct. BMC Dev Biol. 8(1):114. doi:1471-213X-8-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapelnikov A, Zelinger E, Gottlieb Y, Rhrissorrakrai K, Gunsalus KC, Heifetz Y. 2008. Mating induces an immune response and developmental switch in the Drosophila oviduct. Proc Natl Acad Sci U S A. 105(37):13912–13917. doi: 10.1073/pnas.0710997105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keisman EL, Baker BS. 2001. The Drosophila sex determination hierarchy modulates wingless and decapentaplegic signaling to deploy dachshund sex-specifically in the genital imaginal disc. Development. 128(9):1643–56. doi: 10.1242/dev.128.9.1643. [DOI] [PubMed] [Google Scholar]
- Kelpsch DJ, Groen CM, Fagan TN, Sudhir S, Tootle TL. 2016. Fascin regulates nuclear actin during Drosophila oogenesis. Mol Biol Cell. 27(19):2965–79. doi: 10.1091/mbc.E15-09-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keramaris KE, Konstantopoulos K, Margaritis LH, Velentzas AD, Papassideri IS, Stravopodis DJ. 2020. Exploitation of Drosophila choriogenesis process as a model cellular system for assessment of compound toxicity: the phloroglucinol paradigm. Sci Rep. 10(1):242. doi: 10.1038/s41598-019-57113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes LN, Spradling AC. 1997. The Drosophila gene fs(2)cup interacts with otu to define a cytoplasmic pathway required for the structure and function of germ-line chromosomes. Development. 124(7):1419–1431. doi: 10.1242/dev.124.7.1419. [DOI] [PubMed] [Google Scholar]
- Khokhar A, Chen N, Yuan JP, Li Y, Landis GN, Beaulieu G, Kaur H, Tower J. 2008. Conditional switches for extracellular matrix patterning in Drosophila melanogaster. Genetics. 178(3):1283–1293. doi: 10.1534/genetics.106.065912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehart DP, Crawford JM, Aristotelous A, Venakides S, Edwards GS. 2017. Cell sheet morphogenesis: dorsal closure in Drosophila melanogaster as a model system. Annu Rev Cell Dev Biol. 33(1):169–202. doi: 10.1146/annurev-cellbio-111315-125357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JC, Nordman J, Xie F, Kashevsky H, Eng T, Li S, MacAlpine DM, Orr-Weaver TL. 2011. Integrative analysis of gene amplification in Drosophila follicle cells: parameters of origin activation and repression. Genes Dev. 25(13):1384–1398. doi: 10.1101/gad.2043111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JC, Orr-Weaver TL. 2011. Analysis of a Drosophila amplicon in follicle cells highlights the diversity of metazoan replication origins. Proc Natl Acad Sci U S A. 108(40):16681–6. doi: 10.1073/pnas.1114209108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrell DA, Beutler B. 2001. The evolution and genetics of innate immunity. Nat Rev Genet. 2(4):256–267. doi: 10.1038/35066006. [DOI] [PubMed] [Google Scholar]
- King-Jones K, Thummel CS. 2005. Nuclear receptors—a perspective from Drosophila. Nat Rev Genet. 6(4):311–323. doi: 10.1038/nrg1581. [DOI] [PubMed] [Google Scholar]
- King RC. 1970. Ovarian Development in Drosophila Melanogaster. New York, New York, USA: Academic Press. [Google Scholar]
- Kleve CD, Siler DA, Syed SK, Eldon ED. 2006. Expression of 18-wheeler in the follicle cell epithelium affects cell migration and egg morphology in Drosophila. Dev Dyn. 235(7):1953–1961. doi: 10.1002/dvdy.20820. [DOI] [PubMed] [Google Scholar]
- Klusza S, Deng W-M. 2011. At the crossroads of differentiation and proliferation: precise control of cell-cycle changes by multiple signaling pathways in Drosophila follicle cells. BioEssays. 33(2):124–134. doi: 10.1002/bies.201000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp E, Deady L, Sun J. 2018. Ex vivo follicle rupture and in situ zymography in Drosophila. BIO-Protoc. 8(10):e2846. doi: 10.21769/BioProtoc.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp EM, Li W, Singh V, Sun J. 2020. Nuclear receptor Ftz-f1 promotes follicle maturation and ovulation partly via bHLH/PAS transcription factor Sim. Elife. 9:e54568. doi: 10.7554/eLife.54568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp EM, Li W, Sun J. 2019. Downregulation of homeodomain protein Cut is essential for Drosophila follicle maturation and ovulation. Development. 146(18):dev179002. doi: 10.1242/dev.179002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp E, Sun J. 2017. Steroid signaling in mature follicles is important for Drosophila ovulation. Proc Natl Acad Sci U S A. 114(4):699–704. doi: 10.1073/pnas.1614383114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles BA, Cooley L. 1994. The specialized cytoskeleton of the Drosophila egg chamber. Trends Genet. 10(7):235–41. doi: 10.1016/0168-9525(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Kohzaki H, Asano M, Murakami Y, Mazo A. 2020. Epigenetic regulation affects gene amplification in Drosophila development. Front Biosci (Landmark Ed). 25(4):632–645. doi: 10.2741/4825. [DOI] [PubMed] [Google Scholar]
- Kolahi KS, White PF, Shreter DM, Classen AK, Bilder D, Mofrad MR. 2009. Quantitative analysis of epithelial morphogenesis in Drosophila oogenesis: new insights based on morphometric analysis and mechanical modeling. Dev Biol. 331(2):129–139. doi: 10.1016/j.ydbio.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komitopoulou K, Margaritis LH, Kafatos FC. 1988. Structural and biochemical studies on four sex-linked chorion mutants of Drosophila melanogaster. Dev Genet. 9(1):37–48. doi: 10.1002/dvg.1020090105. [DOI] [PubMed] [Google Scholar]
- Konrad KD, Wang D, Marsh JL. 1993. Vitelline membrane biogenesis in Drosophila requires the activity of the alpha-methyl dopa hypersensitive gene (I(2)amd) in both the germline and follicle cells. Insect Mol Biol. 1(4):179–187. doi: 10.1111/j.1365-2583.1993.tb00090.x. [DOI] [PubMed] [Google Scholar]
- Konstandi OA, Papassideri IS, Stravopodis DJ, Kenoutis CA, Hasan Z, Katsorchis T, Wever R, Margaritis LH. 2005. The enzymatic component of Drosophila melanogaster chorion is the Pxd peroxidase. Insect Biochem Mol Biol. 35(9):1043–1057. doi: 10.1016/j.ibmb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Kotian N, Troike KM, Curran KN, Lathia JD, McDonald JA. 2021. A Drosophila RNAi screen reveals conserved glioblastoma-related adhesion genes that regulate collective cell migration. G3 (Bethesda). 12(1):jkab356. doi: 10.1093/g3journal/jkab356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köttgen M, Hofherr A, Li W, Chu K, Cook S, Montell C, Watnick T. 2011. Drosophila sperm swim backwards in the female reproductive tract and are activated via TRPP2 Ion channels. PLoS One. 6(5):e20031. doi: 10.1371/journal.pone.0020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauchunas AR, Horner VL, Wolfner MF. 2012. Protein phosphorylation changes reveal new candidates in the regulation of egg activation and early embryogenesis in D. melanogaster. Dev Biol. 370(1):125–134. doi: 10.1016/j.ydbio.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauchunas AR, Sackton KL, Wolfner MF. 2013. Phospho-regulation pathways during egg activation in Drosophila melanogaster. Genetics. 195(1):171–80. doi:genetics.113.150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauchunas AR, Wolfner MF, Wassarman PM. 2013. Chapter Ten—molecular changes during egg activation. In: Wassarman PM, editor. Current Topics in Developmental Biology, Gametogenesis. Oxford: Academic Press. p. 267–292. [DOI] [PMC free article] [PubMed]
- Kronja I, Yuan B, Eichhorn SW, Dzeyk K, Krijgsveld J, Bartel DP, Orr-Weaver TL. 2014. Widespread changes in the posttranscriptional landscape at the Drosophila oocyte-to-embryo transition. Cell Rep. 7(5):1495–1508. doi: 10.1016/j.celrep.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku H-Y, Harris LK, Bilder D. 2023. Specialized cells that sense tissue mechanics to regulate Drosophila morphogenesis. Dev Cell. 58(3):211–223.e5. doi: 10.1016/j.devcel.2023.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubli E. 2003. Sex-peptides: seminal peptides of the Drosophila male. Cell Mol Life Sci. 60(8):1689–1704. doi: 10.1007/s00018-003-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubli E. 2010. Sexual behavior: dietary food switch induced by sex. Curr Biol. 20(11):R474–6. doi:S0960-9822(10)00516-6. [DOI] [PubMed] [Google Scholar]
- Kubli E, Bopp D. 2012. Sexual behavior: how sex peptide flips the postmating switch of female flies. Curr Biol. 22(13):R520–R522. doi: 10.1016/j.cub.2012.04.058. [DOI] [PubMed] [Google Scholar]
- Kugler J-M, Lasko P. 2009. Localization, anchoring and translational control of oskar, gurken, bicoid and nanos mRNA during Drosophila oogenesis. Fly (Austin). 3(1):15–28. doi: 10.4161/fly.3.1.7751. [DOI] [PubMed] [Google Scholar]
- Kurogi Y, Imura E, Mizuno Y, Hoshino R, Nouzova M, Matsuyama S, Mizoguchi A, Kondo S, Tanimoto H, Noriega FG, et al. 2023. Female reproductive dormancy in Drosophila is regulated by DH31-producing neurons projecting into the corpus allatum. Development. 150(10):dev201186. doi: 10.1242/dev.201186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz CL, Charroux B, Chaduli D, Viallat-Lieutaud A, Royet J. 2017. Peptidoglycan sensing by octopaminergic neurons modulates Drosophila oviposition. Elife. 6:e21937. doi: 10.7554/eLife.21937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachance J-FB, Lomas MF, Eleiche A, Kerr PB, Nilson LA. 2009. Graded Egfr activity patterns the Drosophila eggshell independently of autocrine feedback. Development. 136(17):2893–2902. doi: 10.1242/dev.036103. [DOI] [PubMed] [Google Scholar]
- LaFever L, Drummond-Barbosa D. 2005. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science. 309(5737):1071–3. doi: 10.1126/science.1111410. [DOI] [PubMed] [Google Scholar]
- LaFever L, Feoktistov A, Hsu HJ, Drummond-Barbosa D. 2010. Specific roles of target of rapamycin in the control of stem cells and their progeny in the Drosophila ovary. Development. 137(13):2117–26. doi: 10.1242/dev.050351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake CM, Hawley RS. 2012. The molecular control of meiotic chromosomal behavior: events in early meiotic prophase in Drosophila oocytes. Annu Rev Physiol. 74(1):425–451. doi: 10.1146/annurev-physiol-020911-153342. [DOI] [PubMed] [Google Scholar]
- Lamb MC, Kaluarachchi CP, Lansakara TI, Mellentine SQ, Lan Y, Tivanski AV, Tootle TL. 2021. Fascin limits Myosin activity within Drosophila border cells to control substrate stiffness and promote migration. Elife. 10:e69836. doi: 10.7554/elife.69836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan L, Lin S, Zhang S, Cohen RS. 2010. Evidence for a transport-trap mode of Drosophila melanogaster gurken mRNA localization. PLoS One. 5(11):e15448. doi: 10.1371/journal.pone.0015448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis G, Tower J. 1999. The Drosophila chiffon gene is required for chorion gene amplification, and is related to the yeast Dbf4 regulator of DNA replication and cell cycle. Development. 126(19):4281–4293. doi: 10.1242/dev.126.19.4281. [DOI] [PubMed] [Google Scholar]
- Lasko P. 2012. Mrna localization and translational control in Drosophila oogenesis. Cold Spring Harb Perspect Biol. 4(10):a012294. doi: 10.1101/cshperspect.a012294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver JD, Marsolais AJ, Smibert CA, Lipshitz HD. 2015. Chapter Two—regulation and function of maternal gene products during the maternal-to-zygotic transition in Drosophila. In: Lipshitz HD, editor. Current Topics in Developmental Biology, the Maternal-to-Zygotic Transition. Oxford: Academic Press. p. 43–84. [DOI] [PubMed]
- Laws KM, Sampson LL, Drummond-Barbosa D. 2015. Insulin-independent role of adiponectin receptor signaling in Drosophila germline stem cell maintenance. Dev Biol. 399(2):226–36. doi: 10.1016/j.ydbio.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebo DPV, McCall K. 2021. Murder on the ovarian express: a tale of non-autonomous cell death in the Drosophila ovary. Cells. 10(6):1454. doi: 10.3390/cells10061454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Daubnerová I, Isaac RE, Zhang C, Choi S, Chung J, Kim YJ. 2015. A neuronal pathway that controls sperm ejection and storage in female Drosophila. Curr Biol. 25(6):790–797. doi: 10.1016/j.cub.2015.01.050. [DOI] [PubMed] [Google Scholar]
- Lee HG, Rohila S, Han KA. 2009. The octopamine receptor OAMB mediates ovulation via ca2+/calmodulin-dependent protein kinase II in the Drosophila oviduct epithelium. PLoS One. 4(3):e4716. doi: 10.1371/journal.pone.0004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-G, Seong C-S, Kim Y-C, Davis RL, Han K-A. 2003. Octopamine receptor OAMB is required for ovulation in Drosophila melanogaster. Dev Biol. 264(1):179–190. doi: 10.1016/j.ydbio.2003.07.018. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. 1996. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 86(6):973–983. doi: 10.1016/S0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- Lembong J, Yakoby N, Shvartsman SY. 2008. Spatial regulation of BMP signaling by patterned receptor expression. Tissue Eng Part A. 14(9):1469–1477. doi: 10.1089/ten.tea.2008.0098. [DOI] [PubMed] [Google Scholar]
- Lembong J, Yakoby N, Shvartsman SY. 2009. Pattern formation by dynamically interacting network motifs. Proc Natl Acad Sci U S A. 106(9):3213–3218. doi: 10.1073/pnas.0810728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMosy EK, Hashimoto C. 2000. The nudel protease of Drosophila is required for eggshell biogenesis in addition to embryonic patterning. Dev Biol. 217(2):352–361. doi: 10.1006/dbio.1999.9562. [DOI] [PubMed] [Google Scholar]
- Lerner DW, McCoy D, Isabella AJ, Mahowald AP, Gerlach GF, Chaudhry TA, Horne-Badovinac S. 2013. A Rab10-dependent mechanism for polarized basement membrane secretion during organ morphogenesis. Dev Cell. 24(2):159–168. doi: 10.1016/j.devcel.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Hackney JF, Bergen A, Truesdale A, Dobens L. 2010. Opposing interactions between Drosophila cut and the C/EBP encoded by slow border cells direct apical constriction and epithelial invagination. Dev Biol. 344(1):196–209. doi: 10.1016/j.ydbio.2010.04.030. [DOI] [PubMed] [Google Scholar]
- Levine B, Jean-Francois M, Bernardi F, Gargiulo G, Dobens L. 2007. Notch signaling links interactions between the C/EBP homolog slow border cells and the GILZ homolog bunched during cell migration. Dev Biol. 305(1):217–231. doi: 10.1016/j.ydbio.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Lewellyn L, Cetera M, Horne-Badovinac S. 2013. Misshapen decreases integrin levels to promote epithelial motility and planar polarity in Drosophila. J Cell Biol. 200(6):721–729. doi: 10.1083/jcb.201209129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WX. 2005. Functions and mechanisms of receptor tyrosine kinase Torso signaling: lessons from Drosophila embryonic terminal development. Dev Dyn. 232(3):656–672. doi: 10.1002/dvdy.20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fink C, El-Kholy S, Roeder T. 2015. The octopamine receptor octß2R is essential for ovulation and fertilization in the fruit fly Drosophila melanogaster. Arch Insect Biochem Physiol. 88(3):168–178. doi: 10.1002/arch.21211. [DOI] [PubMed] [Google Scholar]
- Li Z, Thiel K, Thul PJ, Beller M, Kühnlein RP, Welte MA. 2012. Lipid droplets control the maternal histone supply of Drosophila embryos. Curr Biol. 22(22):2104–13. doi: 10.1016/j.cub.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Young JF, Sun J. 2018. Nadph oxidase-generated reactive oxygen species in mature follicles are essential for Drosophila ovulation. Proc Natl Acad Sci U S A. 115(30):7765–7770. doi: 10.1073/pnas.1800115115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber T, Jeedigunta SP, Palozzi JM, Lehmann R, Hurd TR. 2019. Mitochondrial fragmentation drives selective removal of deleterious mtDNA in the germline. Nature. 570(7761):380–384. doi: 10.1038/s41586-019-1213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew GM, Foulk MS, Gerbi SA. 2013. The ecdysone receptor (ScEcR-A) binds DNA puffs at the start of DNA amplification in Sciara coprophila. Chromosome Res. 21(4):345–360. doi: 10.1007/s10577-013-9360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly MA, Spradling AC. 1996. The Drosophila endocycle is controlled by Cyclin E and lacks a checkpoint ensuring S-phase completion. Genes Dev. 10(19):2514–2526. doi: 10.1101/gad.10.19.2514. [DOI] [PubMed] [Google Scholar]
- Lim J, Lee M, Son A, Chang H, Kim VN. 2016. mTAIL-seq reveals dynamic poly(A) tail regulation in oocyte-to-embryo development. Genes Dev. 30(14):1671–1682. doi: 10.1101/gad.284802.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Sabandal PR, Fernandez A, Sabandal JM, Lee HG, Evans P, Han KA. 2014. The octopamine receptor Octβ2R regulates ovulation in Drosophila melanogaster. PLoS One. 9(8):e104441. doi: 10.1371/journal.pone.0104441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T-H, Yeh T-H, Wang T-W, Yu J-Y. 2014. The Hippo pathway controls border cell migration through distinct mechanisms in outer border cells and polar cells of the Drosophila ovary. Genetics. 198(3):1087–1099. doi: 10.1534/genetics.114.167346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Abreu-Blanco MT, Barry KC, Linardopoulou EV, Osborn GE, Parkhurst SM. 2009. Wash functions downstream of Rho and links linear and branched actin nucleation factors. Development. 136(16):2849–2860. doi: 10.1242/dev.035246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kubli E. 2003. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc Natl Acad Sci U S A. 100(17):9929–9933. doi: 10.1073/pnas.1631700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llense F, Martín-Blanco E. 2008. Jnk signaling controls border cell cluster integrity and collective cell migration. Curr Biol. 18(7):538–544. doi: 10.1016/j.cub.2008.03.029. [DOI] [PubMed] [Google Scholar]
- Lomas MF, Hails F, Lachance JF, Nilson LA. 2013. Response to the dorsal anterior gradient of EGFR signaling in Drosophila oogenesis is prepatterned by earlier posterior EGFR activation. Cell Rep. 4(4):791–802. doi: 10.1016/j.celrep.2013.07.038. [DOI] [PubMed] [Google Scholar]
- Lomas MF, Vito SD, Lachance J-FB, Houde J, Nilson LA. 2016. Determination of EGFR signaling output by opposing gradients of BMP and JAK/STAT activity. Curr Biol. 26(19):2572–2582. doi: 10.1016/j.cub.2016.07.073. [DOI] [PubMed] [Google Scholar]
- Loppin B, Dubruille R, Horard B. 2015. The intimate genetics of Drosophila fertilization. Open Biol. 5(8):150076. doi: 10.1098/rsob.150076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossky M, Wensink PC. 1995. Regulation of Drosophila yolk protein genes by an ovary-specific GATA factor. Mol Cell Biol. 15(12):6943–52. doi: 10.1128/mcb.15.12.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett JA, Goldstein ES. 1977. The cytoplasmic distribution and characterization of poly(A)+RNA in oocytes and embryos of Drosophilia. Dev Biol. 61(1):70–8.. doi: 10.1016/0012-1606(77)90342-6. [DOI] [PubMed] [Google Scholar]
- Lu L, Jin W, Liu H, Wang LL. 2014. Recq DNA helicases and osteosarcoma. Adv Exp Med Biol. 804:129–145. doi: 10.1007/978-3-319-04843-7_7. [DOI] [PubMed] [Google Scholar]
- Lu W, Lakonishok M, Serpinskaya AS, Gelfand VI. 2022. A novel mechanism of bulk cytoplasmic transport by cortical dynein in Drosophila ovary. Elife. 11:e75538. doi: 10.7554/eLife.75538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubarsky B, Krasnow MA. 2003. Tube morphogenesis: making and shaping biological tubes. Cell. 112(1):19–28. doi: 10.1016/S0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- Lunyak VV, Ezrokhi M, Smith HS, Gerbi SA. 2002. Developmental changes in the Sciara II/9A initiation zone for DNA replication. Mol Cell Biol. 22(24):8426–8437. doi: 10.1128/MCB.22.24.8426-8437.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Liu S, Zhang W, Yang L, Huang J, Zhou S, Feng Q, Palli SR, Wang J, Roth S, Li S. 2021. Juvenile hormone signaling promotes ovulation and maintains egg shape by inducing expression of extracellular matrix genes. Proc Natl Acad Sci U S A. 118(39):e2104461118. doi: 10.1073/pnas.2104461118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JA, Roth S. 2011. The evolution of dorsal-ventral patterning mechanisms in insects. Genes Dev. 25(2):107–118. doi: 10.1101/gad.2010711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall N, Clark A, MacDougall E, Davis I. 2003. Drosophila Gurken (TGFalpha) mRNA localizes as particles that move within the oocyte in two dynein-dependent steps. Dev Cell. 4(3):307–319. doi: 10.1016/s1534-5807(03)00058-3. [DOI] [PubMed] [Google Scholar]
- MacDougall N, Lad Y, Wilkie GS, Francis-Lang H, Sullivan W, Davis I. 2001. Merlin, the Drosophila homologue of neurofibromatosis-2, is specifically required in posterior follicle cells for axis formation in the oocyte. Development. 128(5):665–673. doi: 10.1242/dev.128.5.665. [DOI] [PubMed] [Google Scholar]
- Mahajan-Miklos S, Cooley L. 1994a. The villin-like protein encoded by the Drosophila quail gene is required for actin bundle assembly during oogenesis. Cell. 78(2):291–301. doi: 10.1016/0092-8674(94)90298-4. [DOI] [PubMed] [Google Scholar]
- Mahajan-Miklos S, Cooley L. 1994b. Intercellular cytoplasm transport during Drosophila oogenesis. Dev Biol. 165(2):336–351. doi: 10.1006/dbio.1994.1257. [DOI] [PubMed] [Google Scholar]
- Mahone M, Saffman EE, Lasko PF. 1995. Localized Bicaudal-C RNA encodes a protein containing a KH domain, the RNA binding motif of FMR1. EMBO J. 14(9):2043–2055. doi: 10.1002/j.1460-2075.1995.tb07196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahowald AP. 1972. Ultrastructural observations on oogenesis in Drosophila. J Morphol. 137(1):29–48. doi: 10.1002/jmor.1051370103. [DOI] [PubMed] [Google Scholar]
- Mahowald AP, Goralski TJ, Caulton JH. 1983. In vitro activation of Drosophila eggs. Dev Biol. 98(2):437–445. doi: 10.1016/0012-1606(83)90373-1. [DOI] [PubMed] [Google Scholar]
- Mahowald AP, Tiefert M. 1970. Fine structural changes in the Drosophila oocyte nucleus during a short period of RNA synthesis. Wilhelm Roux Arch Für Entwicklungsmechanik Org. 165(1):8–25. doi: 10.1007/BF00576994. [DOI] [PubMed] [Google Scholar]
- Manier MK, Belote JM, Berben KS, Novikov D, Stuart WT, Pitnick S. 2010. Resolving mechanisms of competitive fertilization success in Drosophila melanogaster. Science. 328(5976):354–357. doi: 10.1126/science.1187096. [DOI] [PubMed] [Google Scholar]
- Manjunatha T, Hari Dass S, Sharma VK. 2008. Egg-laying rhythm in Drosophila melanogaster. J Genet. 87(5):495–504. doi: 10.1007/s12041-008-0072-9. [DOI] [PubMed] [Google Scholar]
- Manning L, Sheth J, Bridges S, Saadin A, Odinammadu K, Andrew D, Spencer S, Montell D, Starz-Gaiano M. 2017. A hormonal cue promotes timely follicle cell migration by modulating transcription profiles. Mech Dev. 148:56–68. doi: 10.1016/j.mod.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manogaran A, Waring GL. 2004. The N-terminal prodomain of sV23 is essential for the assembly of a functional vitelline membrane network in Drosophila. Dev Biol. 270(1):261–271. doi: 10.1016/j.ydbio.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Manseau LJ, Schüpbach T. 1989. Cappuccino and spire: two unique maternal-effect loci required for both the anteroposterior and dorsoventral patterns of the Drosophila embryo. Genes Dev. 3(9):1437–1452. doi: 10.1101/gad.3.9.1437. [DOI] [PubMed] [Google Scholar]
- Mantrova EY, Hsu T. 1998. Down-regulation of transcription factor CF2 by Drosophila Ras/MAP kinase signaling in oogenesis: cytoplasmic retention and degradation. Genes Dev. 12(8):1166–1175. doi: 10.1101/gad.12.8.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqueira B, Chatwin H, Evans PD. 2005. Identification and characterization of a novel family of Drosophilaβ-adrenergic-like octopamine G-protein coupled receptors. J Neurochem. 94(2):547–560. doi: 10.1111/j.1471-4159.2005.03251.x. [DOI] [PubMed] [Google Scholar]
- Margaritis LH. 1985. The egg-shell of Drosophila melanogaster III. Covalent crosslinking of the chorion proteins involves endogenous hydrogen peroxide. Tissue Cell. 17(4):553–559. doi: 10.1016/0040-8166(85)90031-X. [DOI] [PubMed] [Google Scholar]
- Margaritis LH. 1986. The eggshell of Drosophila melanogaster. II New staging characteristics and fine structural analysis of choriogenesis. Can J Zool. 64(10):2152–2175. doi: 10.1139/z86-330. [DOI] [Google Scholar]
- Margaritis LH, Kafatos FC, Petri WH. 1980. The eggshell of Drosophila melanogaster. I fine structure of the layers and regions of the wild-type eggshell. J Cell Sci. 43(1):1–35. doi: 10.1242/jcs.43.1.1. [DOI] [PubMed] [Google Scholar]
- Margolis J, Spradling A. 1995. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development. 121:3797–3807. doi: 10.1242/dev.121.11.3797. [DOI] [PubMed] [Google Scholar]
- Marín I, Baker BS. 1998. The evolutionary dynamics of sex determination. Science. 281(5385):1990–1994. doi: 10.1126/science.281.5385.1990. [DOI] [PubMed] [Google Scholar]
- Martin JR, Raibaud A, Ollo R. 1994. Terminal pattern elements in Drosophila embryo induced by the torso-like protein. Nature. 367(6465):741–745. doi: 10.1038/367741a0. [DOI] [PubMed] [Google Scholar]
- Mateos CS-C, Valencia-Expósito A, Palacios IM, Martín-Bermudo MD. 2020. Integrins regulate epithelial cell shape by controlling the architecture and mechanical properties of basal actomyosin networks. PLoS Genet. 16(6):e1008717. doi: 10.1371/journal.pgen.1008717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, Korn BS, Hammer RE, Moon YA, Komuro R, Horton JD, Goldstein JL, Brown MS, Shimomura I. 2001. Srebp cleavage-activating protein (SCAP) is required for increased lipid synthesis in liver induced by cholesterol deprivation and insulin elevation. Genes Dev. 15(10):1206–16. doi: 10.1101/gad.891301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui A, Ihara T, Suda H, Mikami H, Semba K. 2013. Gene amplification: mechanisms and involvement in cancer. Biomol Concepts. 4(6):567–582. doi: 10.1515/bmc-2013-0026. [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Armstrong AR, Sampson LL, Laws KM, Drummond-Barbosa D. 2017. Adipocyte metabolic pathways regulated by diet control the female germline stem cell lineage in Drosophila melanogaster. Genetics. 206(2):953–971. doi: 10.1534/genetics.117.201921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauzy-Melitz D, Waring GL. 2003. Fc177, a minor dec-1 proprotein, is necessary to prevent ectopic aggregation of the endochorion during eggshell assembly in Drosophila. Dev Biol. 255(2):193–205. doi: 10.1016/s0012-1606(02)00084-2. [DOI] [PubMed] [Google Scholar]
- Mayhew ML, Merritt DJ. 2013. The morphogenesis of spermathecae and spermathecal glands in Drosophila melanogaster. Arthropod Struct Dev. 42(5):385–393. doi: 10.1016/j.asd.2013.07.002. [DOI] [PubMed] [Google Scholar]
- McDonald JA, Pinheiro EM, Kadlec L, Schüpbach T, Montell DJ. 2006. Multiple EGFR ligands participate in guiding migrating border cells. Dev Biol. 296(1):94–103. doi: 10.1016/j.ydbio.2006.04.438. [DOI] [PubMed] [Google Scholar]
- McDonald JA, Pinheiro EM, Montell DJ. 2003. Pvf1, a PDGF/VEGF homolog, is sufficient to guide border cells and interacts genetically with Taiman. Development. 130(15):3469–3478. doi: 10.1242/dev.00574. [DOI] [PubMed] [Google Scholar]
- McDonough-Goldstein CE, Borziak K, Pitnick S, Dorus S. 2021. Drosophila female reproductive tract gene expression reveals coordinated mating responses and rapidly evolving tissue-specific genes. G3 (Bethesda). 11(3):jkab020. doi: 10.1093/g3journal/jkab020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough-Goldstein CE, Whittington E, McCullough EL, Buel SM, Erdman S, Pitnick S, Dorus S. 2021. Pronounced postmating response in the Drosophila female reproductive tract fluid proteome. Mol Cell Proteomics. 20:100156. doi: 10.1016/j.mcpro.2021.100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor JR, Xi R, Harrison DA. 2002. Jak signaling is somatically required for follicle cell differentiation in Drosophila. Development. 129(3):705–717. doi: 10.1242/dev.129.3.705. [DOI] [PubMed] [Google Scholar]
- McLean PF, Cooley L. 2013. Protein equilibration through somatic ring canals in Drosophila. Science. doi: 10.1126/science.1234887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan EA, Longo SM, Smith MD, Broskin S, Lin B, Singh NK, Strochlic TI. 2018. The protein kinase CK2 substrate Jabba modulates lipid metabolism during Drosophila oogenesis. J Biol Chem. 293(8):2990–3002. doi: 10.1074/jbc.M117.814657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meignin C, Alvarez-Garcia I, Davis I, Palacios IM. 2007. The salvador-warts-hippo pathway is required for epithelial proliferation and axis specification in Drosophila. Curr Biol. 17(21):1871–1878. doi: 10.1016/j.cub.2007.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekus Z, Cooley J, George A, Sabo V, Strzegowski M, Starz-Gaiano M, Peercy B. 2018. Effects of cell packing on chemoattractant distribution within a tissue. AIMS Biophys. 5(1):1–21. doi: 10.3934/biophy.2018.1.1. [DOI] [Google Scholar]
- Mensah LB, Goberdhan DCI, Wilson C. 2017. Mtorc1 signalling mediates PI3K-dependent large lipid droplet accumulation in Drosophila ovarian nurse cells. Biol Open. 6(5):563–570. doi: 10.1242/bio.022210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle JA, Wittes J, Schüpbach T. 2020. Chapter Three—signaling between somatic follicle cells and the germline patterns the egg and embryo of Drosophila. In: Marlow FL, editor. Current Topics in Developmental Biology, Maternal Effect Genes in Development. Oxford: Academic Press. p. 55–86. [DOI] [PubMed] [Google Scholar]
- Mermod JJ, Jacobs-Lorena M, Crippa M. 1977. Changes in rate of RNA synthesis and ribosomal gene number during oogenesis of Drosophila melanogaster. Dev Biol. 57(2):393–402. doi: 10.1016/0012-1606(77)90224-X. [DOI] [PubMed] [Google Scholar]
- Miao G, Godt D, Montell DJ. 2020. Integration of migratory cells into a new site in vivo requires channel-independent functions of innexins on microtubules. Dev Cell. 54(4):501–515. doi: 10.1016/j.devcel.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton CA, Nongthomba U, Parry K, Sweeney ST, Sparrow JC, Elliott CJ. 2006. Neuromuscular organization and aminergic modulation of contractions in the Drosophila ovary. BMC Biol. 4(1):17. doi:1741-7007-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milas A, de-Carvalho J, Telley IA. 2022. Follicle cell contact maintains main body axis polarity in the Drosophila melanogaster oocyte. J Cell Biol. 222(2):e202209052. doi: 10.1083/jcb.202209052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. 1950. The internal anatomy and histology of the imago of Drosophila melanogaster. Biol Drosoph. 421–534. [Google Scholar]
- Mindrinos MN, Scherer LJ, Garcini FJ, Kwan H, Jacobs KA, Petri WH. 1985. Isolation and chromosomal location of putative vitelline membrane genes in Drosophila melanogaster. EMBO J. 4(1):147–153. doi: 10.1002/j.1460-2075.1985.tb02329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo A, Fuentes E, Furriols M, Casanova J. 2018. Holes in the plasma membrane mimic Torso-like perforin in torso tyrosine kinase receptor activation in the Drosophila embryo. Genetics. doi: 10.1534/genetics.118.301397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo A, Furriols M, Casanova J. 2015. Accumulation of the Drosophila torso-like protein at the blastoderm plasma membrane suggests that it translocates from the eggshell. Development. 142(7):1299–1304. doi: 10.1242/dev.117630. [DOI] [PubMed] [Google Scholar]
- Mineo A, Furriols M, Casanova J. 2017. Transfer of dorsoventral and terminal information from the ovary to the embryo by a common group of eggshell proteins in Drosophila. Genetics. 205(4):1529–1536. doi: 10.1534/genetics.116.197574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo A, Furriols M, Casanova J. 2018. The trigger (and the restriction) of Torso RTK activation. Open Biol. 8(12):180180. doi: 10.1098/rsob.180180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra AK, Mondo JA, Campanale JP, Montell DJ. 2019. Coordination of protrusion dynamics within and between collectively migrating border cells by myosin II. Mol Biol Cell. 30(19):2490–2502. doi: 10.1091/mbc.E19-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S, Hunt T. 2007. Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature. 449(7160):336–340. doi: 10.1038/nature06121. [DOI] [PubMed] [Google Scholar]
- Monastirioti M. 2003. Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster. Dev Biol. 264(1):38–49. doi: 10.1016/j.ydbio.2003.07.019. [DOI] [PubMed] [Google Scholar]
- Monastirioti M, Gorczyca M, Rapus J, Eckert M, White K, Budnik V. 1995. Octopamine immunoreactivity in the fruit fly Drosophila melanogaster. J Comp Neurol. 356(2):275–287. doi: 10.1002/cne.903560210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastirioti M, Linn CE Jr, White K. 1996. Characterization of Drosophila tyramine β-hydroxylase gene and isolation of mutant flies lacking octopamine. J Neurosci. 16(12):3900–3911. doi: 10.1523/JNEUROSCI.16-12-03900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondragon AA, Yalonetskaya A, Ortega AJ, Zhang Y, Naranjo O, Elguero J, Chung WS, McCall K. 2019. Lysosomal machinery drives extracellular acidification to direct non-apoptotic cell death. Cell Rep. 27(1):11–19.e3. doi: 10.1016/j.celrep.2019.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell DJ, Rørth P, Spradling AC. 1992. Slow border cells, a locus required for a developmentally regulated cell migration during oogenesis, encodes Drosophila C/EBP. Cell. 71(1):51–62. doi: 10.1016/0092-8674(92)90265-E. [DOI] [PubMed] [Google Scholar]
- Montell DJ, Yoon WH, Starz-Gaiano M. 2012. Group choreography: mechanisms orchestrating the collective movement of border cells. Nat Rev Mol Cell Biol. 13(10):631–645. doi: 10.1038/nrm3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisato D, Anderson KV. 1994. The spätzle gene encodes a component of the extracellular signaling pathway establishing the dorsal-ventral pattern of the Drosophila embryo. Cell. 76(4):677–688. doi: 10.1016/0092-8674(94)90507-X. [DOI] [PubMed] [Google Scholar]
- Nallamothu G, Woolworth JA, Dammai V, Hsu T. 2008. Awd, the homolog of metastasis suppressor gene Nm23, regulates Drosophila epithelial cell invasion. Mol Cell Biol. 28(6):1964–1973. doi: 10.1128/MCB.01743-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubaum DM, Wolfner MF. 1999. Mated Drosophila melanogaster females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics. 153(2):845–857. doi: 10.1093/genetics/153.2.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman-Silberberg FS, Schüpbach T. 1993. The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGF alpha-like protein. Cell. 75(1):165–174. doi: 10.1016/S0092-8674(05)80093-5. [DOI] [PubMed] [Google Scholar]
- Nezis IP, Shravage BV, Sagona AP, Johansen T, Baehrecke EH, Stenmark H. 2010. Autophagy as a trigger for cell death: autophagic degradation of inhibitor of apoptosis dBruce controls DNA fragmentation during late oogenesis in Drosophila. Autophagy. 6(8):1214–1215. doi: 10.4161/auto.6.8.13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezis IP, Shravage BV, Sagona AP, Lamark T, Bjørkøy G, Johansen T, Rusten TE, Brech A, Baehrecke EH, Stenmark H. 2010. Autophagic degradation of dBruce controls DNA fragmentation in nurse cells during late Drosophila melanogaster oogenesis. J Cell Biol. 190(4):523–31. doi: 10.1083/jcb.201002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezis IP, Stravopodis DJ, Papassideri I, Robert-Nicoud M, Margaritis LH. 2002. Dynamics of apoptosis in the ovarian follicle cells during the late stages of Drosophila oogenesis. Cell Tissue Res. 307(3):401–409. doi: 10.1007/s00441-001-0498-3. [DOI] [PubMed] [Google Scholar]
- Niepielko MG, Yakoby N. 2014. Evolutionary changes in TGFα distribution underlie morphological diversity in eggshells from Drosophila species. Development. 141(24):4710–4715. doi: 10.1242/dev.111898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewiadomska P, Godt D, Tepass U. 1999. DE-Cadherin is required for intercellular motility during Drosophila oogenesis. J Cell Biol. 144(3):533–547. doi: 10.1083/jcb.144.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilson LA, Schüpbach T. 1998. Localized requirements for windbeutel and pipe reveal a dorsoventral prepattern within the follicular epithelium of the Drosophila ovary. Cell. 93(2):253–262. doi: 10.1016/S0092-8674(00)81576-7. [DOI] [PubMed] [Google Scholar]
- Niu W, Spradling AC. 2022. Mouse oocytes develop in cysts with the help of nurse cells. Cell. 185(14):2576–2590.e12. doi: 10.1016/j.cell.2022.05.001. [DOI] [PubMed] [Google Scholar]
- Noguerón MI, Mauzy-Melitz D, Waring GL. 2000. Drosophila dec-1 eggshell proteins are differentially distributed via a multistep extracellular processing and localization pathway. Dev Biol. 225(2):459–470. doi: 10.1006/dbio.2000.9805. [DOI] [PubMed] [Google Scholar]
- Noguerón MI, Waring GL. 1995. Regulated processing of dec-1 eggshell proteins in Drosophila. Dev Biol. 172(1):272–279. doi: 10.1006/dbio.1995.0022. [DOI] [PubMed] [Google Scholar]
- Nomura T, Horikawa M, Shimamura S, Hashimoto T, Sakamoto K. 2010. Fat accumulation in Caenorhabditis elegans is mediated by SREBP homolog SBP-1. Genes Nutr. 5(1):17–27. doi: 10.1007/s12263-009-0157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordman JT, Kozhevnikova EN, Verrijzer CP, Pindyurin AV, Andreyeva EN, Shloma VV, Zhimulev IF, Orr-Weaver TL. 2014. Dna copy-number control through inhibition of replication fork progression. Cell Rep. 9(3):841–849. doi: 10.1016/j.celrep.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordman J, Orr-Weaver TL. 2012. Regulation of DNA replication during development. Development. 139:455–464. doi: 10.1242/dev.061838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norvell A, Wong J, Randolph K, Thompson L. 2015. Wispy and Orb cooperate in the cytoplasmic polyadenylation of localized gurken mRNA. Dev Dyn. 244(10):1276–1285. doi: 10.1002/dvdy.24311. [DOI] [PubMed] [Google Scholar]
- Nystul T, Spradling A. 2010. Regulation of epithelial stem cell replacement and follicle formation in the Drosophila ovary. Genetics. 184(2):503–515. doi: 10.1534/genetics.109.109538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogienko AA, Karagodin DA, Lashina VV, Baiborodin SI, Omelina ES, Baricheva EM. 2013. Capping protein beta is required for actin cytoskeleton organisation and cell migration during Drosophila oogenesis. Cell Biol Int. 37(2):149–159. doi: 10.1002/cbin.10025. [DOI] [PubMed] [Google Scholar]
- Ogienko AA, Yarinich LA, Fedorova EV, Dorogova NV, Bayborodin SI, Baricheva EM, Pindyurin AV. 2020. Gaga regulates border cell migration in Drosophila. Int J Mol Sci. 21(20):7468. doi: 10.3390/ijms21207468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hanlon KN, Dam RA, Archambeault SL, Berg CA. 2018. Two Drosophilids exhibit distinct EGF pathway patterns in oogenesis. Dev Genes Evol. 228(1):31–48. doi: 10.1007/s00427-017-0601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsako T, Yamamoto M-T. 2011. Sperm of the wasted mutant are wasted when females utilize the stored sperm in Drosophila melanogaster. Genes Genet Syst. 86(2):97–108. doi: 10.1266/ggs.86.97. [DOI] [PubMed] [Google Scholar]
- Okamoto N, Fujinaga D, Yamanaka N. 2023. Steroid hormone signaling: What we can learn from insect models. In: Litwack G, editor. Vitamins and Hormones. Oxford: Academic Press. p. 525–554. [DOI] [PubMed] [Google Scholar]
- Oliver CP, Green MM. 1944. Heterosis in compounds of lozenge alleles in Drosophila melanogaster. Genetics. 29(4):331–347. doi: 10.1093/genetics/29.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oramas R, Knapp EM, Zeng B, Sun J. 2023. The bHLH-PAS transcriptional complex sim:Tgo plays active roles in late oogenesis to promote follicle maturation and ovulation. Development. 150(12):dev201566. doi: 10.1242/dev.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oro AE, McKeown M, Evans RM. 1990. Relationship between the product of the Drosophila ultraspiracle locus and the vertebrate retinoid X receptor. Nature. 347(6290):298–301. doi: 10.1038/347298a0. [DOI] [PubMed] [Google Scholar]
- Orr W, Komitopoulou K, Kafatos FC. 1984. Mutants suppressing in trans chorion gene amplification in Drosophila. Proc Natl Acad Sci U S A. 81(12):3773–3777. doi: 10.1073/pnas.81.12.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osheim YN, Miller OL. 1983. Novel amplification and transcriptional activity of chorion genes in Drosophila melanogaster follicle cells. Cell. 33(2):543–553. doi: 10.1016/0092-8674(83)90435-X. [DOI] [PubMed] [Google Scholar]
- Osheim YN, Miller OL, Beyer AL. 1988. Visualization of Drosophila melanogaster chorion genes undergoing amplification. Mol Cell Biol. 8(7):2811–2821. doi: 10.1128/mcb.8.7.2811-2821.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterfield M, Berg CA, Shvartsman SY. 2017. Epithelial patterning, morphogenesis, and evolution: Drosophila eggshell as a model. Dev Cell. 41(4):337–348. doi: 10.1016/j.devcel.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterfield M, Du X, Schüpbach T, Wieschaus E, Shvartsman SY. 2013. Three-dimensional epithelial morphogenesis in the developing Drosophila egg. Dev Cell. 24(4):400–410. doi: 10.1016/j.devcel.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterfield M, Schüpbach T, Wieschaus E, Shvartsman SY. 2015. Diversity of epithelial morphogenesis during eggshell formation in drosophilids. Development. 142:1971–1977. doi: 10.1242/dev.119404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen JL, Zhang Y, Bae SH, Farooqi MS, Liang G, Hammer RE, Goldstein JL, Brown MS. 2012. Insulin stimulation of SREBP-1c processing in transgenic rat hepatocytes requires p70 S6-kinase. Proc Natl Acad Sci U S A. 109(40):16184–9. doi: 10.1073/pnas.1213343109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pae J, Cinalli RM, Marzio A, Pagano M, Lehmann R. 2017. Gcl and CUL3 control the switch between cell lineages by mediating localized degradation of an RTK. Dev Cell. 42(2):130–142.e7. doi: 10.1016/j.devcel.2017.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. 2007. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 8(3):221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-McCaw A, Serano J, Sante JM, Rubin GM. 2003. Drosophila matrix metalloproteinases are required for tissue remodeling, but not embryonic development. Dev Cell. 4(1):95–106. doi:S1534580702004008. [DOI] [PubMed] [Google Scholar]
- Page AW, Orr-Weaver TL. 1997. Activation of the meiotic divisions inDrosophilaOocytes. Dev Biol. 183(2):195–207. doi: 10.1006/dbio.1997.8506. [DOI] [PubMed] [Google Scholar]
- Palm W, Sampaio JL, Brankatschk M, Carvalho M, Mahmoud A, Shevchenko A, Eaton S. 2012. Lipoproteins in Drosophila melanogaster–assembly, function, and influence on tissue lipid composition. PLoS Genet. 8(7):e1002828. doi: 10.1371/journal.pgen.1002828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Connacher RP, O’Connor MB. 2021. Control of the insect metamorphic transition by ecdysteroid production and secretion. Curr Opin Insect Sci. 43:11–20. doi: 10.1016/j.cois.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papantonis A, Swevers L, Iatrou K. 2015. Chorion genes: a landscape of their evolution, structure, and regulation. Annu Rev Entomol. 60(1):177–194. doi: 10.1146/annurev-ento-010814-020810. [DOI] [PubMed] [Google Scholar]
- Papassideri IS, Margaritis LH. 1996. The eggshell of Drosophila melanogaster: iX. Synthesis and morphogenesis of the innermost chorionic layer. Tissue Cell. 28(4):401–409. doi: 10.1016/S0040-8166(96)80026-7. [DOI] [PubMed] [Google Scholar]
- Papassideri IS, Margaritis LH, Gulik-Krzywicki T. 1993. The eggshell of Drosophila melanogaster. VIII Morphogenesis of the wax layer during oogenesis. Tissue Cell. 25(6):929–936. doi: 10.1016/0040-8166(93)90041-I. [DOI] [PubMed] [Google Scholar]
- Papassideri IS, Trougakos IP, Leonard KR, Margaritis LH. 2007. Crystalline yolk spheroids in Drosophila melanogaster oocyte: freeze fracture and two-dimensional reconstruction analysis. J Insect Physiol. 53(4):370–6.. doi: 10.1016/j.jinsphys.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Park EA, MacAlpine DM, Orr-Weaver TL. 2007. Drosophila Follicle cell amplicons as models for metazoan DNA replication: a cyclinE mutant exhibits increased replication fork elongation. Proc Natl Acad Sci U S A. 104(43):16739–16746. doi: 10.1073/pnas.0707804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks S, Spradling A. 1987. Spatially regulated expression of chorion genes during Drosophila oogenesis. Genes Dev. 1(5):497–509. doi: 10.1101/gad.1.5.497. [DOI] [Google Scholar]
- Parra-Peralbo E, Culi J. 2011. Drosophila Lipophorin receptors mediate the uptake of neutral lipids in oocytes and imaginal disc cells by an endocytosis-independent mechanism. PLoS Genet. 7(2):e1001297. doi: 10.1371/journal.pgen.1001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons TT, Mosallaei S, Raftery LA. 2023. Two phases for centripetal migration of Drosophila melanogaster follicle cells: initial ingression followed by epithelial migration. Development. 150(6):dev200492. doi: 10.1242/dev.200492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascucci T, Perrino J, Mahowald AP, Waring GL. 1996. Eggshell assembly in Drosophila: processing and localization of vitelline membrane and chorion proteins. Dev Biol. 177(2):590–598. doi: 10.1006/dbio.1996.0188. [DOI] [PubMed] [Google Scholar]
- Pastor-Pareja JC, Xu T. 2011. Shaping cells and organs in Drosophila by opposing roles of fat body-secreted Collagen IV and perlecan. Dev Cell. 21(2):245–56. doi: 10.1016/j.devcel.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattarini JM, Starmer WT, Bjork A, Pitnick S. 2006. Mechanisms underlying the sperm quality advantage in Drosophila melanogaster. Evolution. 60:2064–2080. [PubMed] [Google Scholar]
- Peercy BE, Starz-Gaiano M. 2020. Clustered cell migration: modeling the model system of Drosophila border cells. Semin Cell Dev Biol. 100:167–176. doi: 10.1016/j.semcdb.2019.11.010. [DOI] [PubMed] [Google Scholar]
- Peng J, Chen S, Büsser S, Liu H, Honegger T, Kubli E. 2005. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr Biol. 15(3):207–13. doi:S0960982205000424. [DOI] [PubMed] [Google Scholar]
- Peng Q, Chen J, Wang R, Zhu H, Han C, Ji X, Pan Y. 2022. The sex determination gene doublesex regulates expression and secretion of the basement membrane protein Collagen IV. J Genet Genomics. 49(7):636–644. doi: 10.1016/j.jgg.2021.12.010. [DOI] [PubMed] [Google Scholar]
- Peri F, Roth S. 2000. Combined activities of Gurken and decapentaplegic specify dorsal chorion structures of the Drosophila egg. Development. 127(4):841–850. doi: 10.1242/dev.127.4.841. [DOI] [PubMed] [Google Scholar]
- Pesin JA, Orr-Weaver TL. 2007. Developmental role and regulation of cortex, a meiosis-specific anaphase-promoting Complex/cyclosome activator. PLoS Genet. 3(11):e202. doi: 10.1371/journal.pgen.0030202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesin JA, Orr-Weaver TL. 2008. Regulation of APC/C activators in mitosis and meiosis. Annu Rev Cell Dev Biol. 24(1):475–499. doi: 10.1146/annurev.cellbio.041408.115949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters NC, Berg CA. 2016a. In vitro culturing and live imaging of Drosophila egg chambers: a history and adaptable method. Methods Mol Biol. 1457:35–68. doi: 10.1007/978-1-4939-3795-0_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters NC, Berg CA. 2016b. Dynamin-mediated endocytosis is required for tube closure, cell intercalation, and biased apical expansion during epithelial tubulogenesis in the Drosophila ovary. Dev Biol. 409(1):39–54. doi: 10.1016/j.ydbio.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters NC, Thayer NH, Kerr SA, Tompa M, Berg CA. 2013. Following the “tracks”: tramtrack69 regulates epithelial tube expansion in the Drosophila ovary through paxillin, dynamin, and the homeobox protein mirror. Dev Biol. 378(2):154–169. doi: 10.1016/j.ydbio.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JS, Barkett M, McCall K. 2003. Stage-specific regulation of caspase activity in Drosophila oogenesis. Dev Biol. 260(1):113–23. doi: 10.1016/s0012-1606(03)00240-9. [DOI] [PubMed] [Google Scholar]
- Peterson JS, Bass BP, Jue D, Rodriguez A, Abrams JM, McCall K. 2007. Noncanonical cell death pathways act during Drosophila oogenesis. Genesis. 45(6):396–404. doi: 10.1002/dvg.20306. [DOI] [PubMed] [Google Scholar]
- Peterson JS, McCall K. 2013. Combined inhibition of autophagy and caspases fails to prevent developmental nurse cell death in the Drosophila melanogaster ovary. PLoS One. 8(9):e76046. doi: 10.1371/journal.pone.0076046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JS, Timmons AK, Mondragon AA, McCall K. 2015. The End of the beginning: cell death in the germline. Curr Top Dev Biol. 114:93–119. doi: 10.1016/bs.ctdb.2015.07.025. [DOI] [PubMed] [Google Scholar]
- Petri WH, Wyman AR, Kafatos FC. 1976. Specific protein synthesis in cellular differentiation: III. The eggshell proteins of Drosophila melanogaster and their program of synthesis. Dev Biol. 49(1):185–199. doi: 10.1016/0012-1606(76)90266-9. [DOI] [PubMed] [Google Scholar]
- Pimenta-Marques A, Bento I, Lopes CA, Duarte P, Jana SC, Bettencourt-Dias M. 2016. A mechanism for the elimination of the female gamete centrosome in Drosophila melanogaster. Science 353:(6294)aaf.4866. doi: 10.1126/science.aaf4866. [DOI] [PubMed] [Google Scholar]
- Pitnick S, Markow T, Spicer GS. 1999. Evolution of multiple kinds of female sperm-storage organs in Drosophila. Evolution. 53(6):1804. doi: 10.2307/2640442. [DOI] [PubMed] [Google Scholar]
- Pizette S, Rabouille C, Cohen SM, Thérond P. 2009. Glycosphingolipids control the extracellular gradient of the Drosophila EGFR ligand Gurken. Development. 136(4):551–561. doi: 10.1242/dev.031104. [DOI] [PubMed] [Google Scholar]
- Plutoni C, Keil S, Zeledon C, Delsin LEA, Decelle B, Roux PP, Carréno S, Emery G. 2019. Misshapen coordinates protrusion restriction and actomyosin contractility during collective cell migration. Nat Commun. 10(1):3940. doi: 10.1038/s41467-019-11963-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokrywka NJ, Stephenson EC. 1995. Microtubules are a general component of mRNA localization systems in Drosophila oocytes. Dev Biol. 167(1):363–370. doi: 10.1006/dbio.1995.1030. [DOI] [PubMed] [Google Scholar]
- Polesello C, Tapon N. 2007. Salvador-warts-hippo signaling promotes Drosophila posterior follicle cell maturation downstream of notch. Curr Biol. 17(21):1864–1870. doi: 10.1016/j.cub.2007.09.049. [DOI] [PubMed] [Google Scholar]
- Pomiankowski A, Nöthiger R, Wilkins A. 2004. The evolution of the Drosophila sex-determination pathway. Genetics. 166(4):1761–1773. doi: 10.1093/genetics/166.4.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkova A, Stone OJ, Chen L, Qin X, Liu C, Liu J, Belguise K, Montell DJ, Hahn KM, Rauzi M, et al. 2020. A Cdc42-mediated supracellular network drives polarized forces and Drosophila egg chamber extension. Nat Commun. 11(1):1921–15. doi: 10.1038/s41467-020-15593-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popodi E, Minoo P, Burke T, Waring GL. 1988. Organization and expression of a second chromosome follicle cell gene cluster in Drosophila. Dev Biol. 127(2):248–256. doi: 10.1016/0012-1606(88)90312-0. [DOI] [PubMed] [Google Scholar]
- Poulton JS, Deng W-M. 2006. Dystroglycan down-regulation links EGF receptor signaling and anterior-posterior polarity formation in the Drosophila oocyte. Proc Natl Acad Sci U S A. 103(34):12775–12780. doi: 10.1073/pnas.0603817103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad M, Montell DJ. 2007. Cellular and molecular mechanisms of border cell migration analyzed using time-lapse live-cell imaging. Dev Cell. 12(6):997–1005. doi: 10.1016/j.devcel.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Price JV, Clifford RJ, Schüpbach T. 1989. The maternal ventralizing locus torpedo is allelic to faint little ball, an embryonic lethal, and encodes the Drosophila EGF receptor homolog. Cell. 56(6):1085–1092. doi: 10.1016/0092-8674(89)90641-7. [DOI] [PubMed] [Google Scholar]
- Pritchett T, Tanner E, McCall K. 2009. Cracking open cell death in the Drosophila ovary. Apoptosis. 14(8):969–979. doi: 10.1007/s10495-009-0369-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokupek AM, Eyun S-I, Ko L, Moriyama EN, Harshman LG. 2010. Molecular evolutionary analysis of seminal receptacle sperm storage organ genes of Drosophila melanogaster. J Evol Biol. 23(7):1386–1398. doi: 10.1111/j.1420-9101.2010.01998.x. [DOI] [PubMed] [Google Scholar]
- Prokupek AM, Kachman SD, Ladunga I, Harshman LG. 2009. Transcriptional profiling of the sperm storage organs of Drosophila melanogaster. Insect Mol Biol. 18(4):465–475. doi: 10.1111/j.1365-2583.2009.00887.x. [DOI] [PubMed] [Google Scholar]
- Qin X, Park BO, Liu J, Chen B, Choesmel-Cadamuro V, Belguise K, Heo WD, Wang X. 2017. Cell-matrix adhesion and cell-cell adhesion differentially control basal myosin oscillation and Drosophila egg chamber elongation. Nat Commun. 8(1):14708. doi: 10.1038/ncomms14708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan ME. 2016. Cytoplasmic streaming in the Drosophila oocyte. Annu Rev Cell Dev Biol. 32(1):173–195. doi: 10.1146/annurev-cellbio-111315-125416. [DOI] [PubMed] [Google Scholar]
- Radford SJ, Nguyen AL, Schindler K, McKim KS. 2017. The chromosomal basis of meiotic acentrosomal spindle assembly and function in oocytes. Chromosoma. 126(3):351–364. doi: 10.1007/s00412-016-0618-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram KR, Wolfner MF. 2009. A network of interactions among seminal proteins underlies the long-term postmating response in Drosophila. Proc Natl Acad Sci U S A. 106(36):15384–15389. doi: 10.1073/pnas.0902923106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel D, Wang X, Laflamme C, Montell DJ, Emery G. 2013. Rab11 regulates cell-cell communication during collective cell movements. Nat Cell Biol. 15(3):317–324. doi: 10.1038/ncb2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezával C, Nojima T, Neville MC, Lin AC, Goodwin SF. 2014. Sexually dimorphic octopaminergic neurons modulate female postmating behaviors in Drosophila. Curr Biol. 24(7):725–730. doi: 10.1016/j.cub.2013.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezával C, Pavlou HJ, Dornan AJ, Chan YB, Kravitz EA, Goodwin SF. 2012. Neural circuitry underlying Drosophila female postmating behavioral responses. Curr Biol. 22(13):1155–65. doi:S0960-9822(12)00518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard DS, Gilbert M, Crum B, Hollinshead DM, Schelble S, Scheswohl D. 2001. Yolk protein endocytosis by oocytes in Drosophila melanogaster: immunofluorescent localization of clathrin, adaptin and the yolk protein receptor. J Insect Physiol. 47(7):715–723. doi: 10.1016/S0022-1910(00)00165-7. [DOI] [PubMed] [Google Scholar]
- Richard DS, Rybczynski R, Wilson TG, Wang Y, Wayne ML, Zhou Y, Partridge L, Harshman LG. 2005. Insulin signaling is necessary for vitellogenesis in Drosophila melanogaster independent of the roles of juvenile hormone and ecdysteroids: female sterility of the chico1 insulin signaling mutation is autonomous to the ovary. J Insect Physiol. 51(4):455–64. doi: 10.1016/j.jinsphys.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Richards JS, Liu Z, Shimada M. 2015. Chapter 22—ovulation. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill's Physiology of Reproduction. 4th ed. San Diego: Elsevier Science & Technology. p. 997–1021. [Google Scholar]
- Richter JD, Sonenberg N. 2005. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 433(7025):477–480. doi: 10.1038/nature03205. [DOI] [PubMed] [Google Scholar]
- Riddiford LM, Cherbas P, Truman JW. 2000. Ecdysone receptors and their biological actions. Vitam Horm. 60:1–73. doi: 10.1016/S0083-6729(00)60016-X. [DOI] [PubMed] [Google Scholar]
- Riechmann V, Ephrussi A. 2001. Axis formation during Drosophila oogenesis. Curr Opin Genet Dev. 11(4):374–383. doi: 10.1016/s0959-437x(00)00207-0. [DOI] [PubMed] [Google Scholar]
- Ritsick DR, Edens WA, Finnerty V, Lambeth JD. 2007. Nox regulation of smooth muscle contraction. Free Radic Biol Med. 43(1):31–38. doi: 10.1016/j.freeradbiomed.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse KR, Berg CA. 1995. Mutations in the Drosophila gene bullwinkle cause the formation of abnormal eggshell structures and bicaudal embryos. Development. 121:3023–3033. doi: 10.1242/dev.121.9.3023. [DOI] [PubMed] [Google Scholar]
- Roberto GM, Emery G. 2021. Directing with restraint: mechanisms of protrusion restriction in collective cell migrations. Semin Cell Dev Biol. 129:75–81. doi: 10.1016/j.semcdb.2022.03.037. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Valentín R, López-González I, Jorquera R, Labarca P, Zurita M, Reynaud E. 2006. Oviduct contraction in Drosophila is modulated by a neural network that is both, octopaminergic and glutamatergic. J Cell Physiol. 209(1):183–98. doi: 10.1002/jcp.20722. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Vazquez M, Vaquero D, Parra-Peralbo E, Mejia-Morales JE, Culi J. 2015. Drosophila Lipophorin receptors recruit the lipoprotein LTP to the plasma membrane to mediate lipid uptake. PLoS Genet. 11(6):e1005356. doi: 10.1371/journal.pgen.1005356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder T. 2005. Tyramine AND OCTOPAMINE: ruling behavior and metabolism. Annu Rev Entomol. 50(1):447–477. doi: 10.1146/annurev.ento.50.071803.130404. [DOI] [PubMed] [Google Scholar]
- Rom I, Faicevici A, Almog O, Neuman-Silberberg FS. 2007. Drosophila Dynein light chain (DDLC1) binds to gurken mRNA and is required for its localization. Biochim Biophys Acta. 1773(10):1526–1533. doi: 10.1016/j.bbamcr.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Romani P, Gargiulo G, Cavaliere V. 2016. The ecdysone receptor signalling regulates microvilli formation in follicular epithelial cells. Cell Mol Life Sci. 73(2):409–425. doi: 10.1007/s00018-015-1999-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales-Nieves AE, González-Reyes A. 2014. Genetics and mechanisms of ovarian cancer: parallels between Drosophila and humans. Semin Cell Dev Biol. 28:104–109. doi: 10.1016/j.semcdb.2014.03.031. [DOI] [PubMed] [Google Scholar]
- Roth S. 2003. The origin of dorsoventral polarity in Drosophila. Philos Trans R Soc Lond B Biol Sci. 358(1436):1317–1329. doi: 10.1098/rstb.2003.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S, Neuman-Silberberg FS, Barcelo G, Schüpbach T. 1995. Cornichon and the EGF receptor signaling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell. 81(6):967–978. doi: 10.1016/0092-8674(95)90016-0. [DOI] [PubMed] [Google Scholar]
- Row S, Huang Y-C, Deng W-M. 2021. Developmental regulation of oocyte lipid intake through ‘patent’ follicular epithelium in Drosophila melanogaster. iScience. 24(4):102275. doi: 10.1016/j.isci.2021.102275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Saha TT, Zou Z, Raikhel AS. 2018. Regulatory pathways controlling female insect reproduction. Annu Rev Entomol. 63(1):489–511. doi: 10.1146/annurev-ento-020117-043258. [DOI] [PubMed] [Google Scholar]
- Rubinstein CD, Wolfner MF. 2013. Drosophila Seminal protein ovulin mediates ovulation through female octopamine neuronal signaling. Proc Natl Acad Sci U S A. 110(43):17420–17425. doi: 10.1073/pnas.1220018110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson EJ, Kim SK, Nusse R. 2002. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science. 296(5570):1118–20. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- Ruohola H, Bremer KA, Baker D, Swedlow JR, Jan LY, Jan YN. 1991. Role of neurogenic genes in establishment of follicle cell fate and oocyte polarity during oogenesis in Drosophila. Cell. 66(3):433–449. doi: 10.1016/0092-8674(81)90008-8. [DOI] [PubMed] [Google Scholar]
- Rusnak F, Mertz P. 2000. Calcineurin: form and function. Physiol Rev. 80(4):1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- Saadin A, Starz-Gaiano M. 2016. Circuitous genetic regulation governs a straightforward cell migration. Trends Genet. 32(10):660–673. doi: 10.1016/j.tig.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Sackton KL, Buehner NA, Wolfner MF. 2007. Modulation of MAPK activities during egg activation in Drosophila. Fly (Austin). 1(4):222–227. doi: 10.4161/fly.5200. [DOI] [PubMed] [Google Scholar]
- Saito K. 2013. The epigenetic regulation of transposable elements by PIWI-interacting RNAs in Drosophila. Genes Genet Syst. 88(1):9–17. doi: 10.1266/ggs.88.9. [DOI] [PubMed] [Google Scholar]
- Salz HK. 2011. Sex determination in insects: a binary decision based on alternative splicing. Curr Opin Genet Dev. 21(4):395–400. doi: 10.1016/j.gde.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Lopez JA, Twena S, Apel I, Kornhaeuser SC, Chasnitsky M, Miklosi AG, Vega-Dominguez PJ, Shephard A, Hefetz A, Heifetz Y. 2022. Male-female communication enhances release of extracellular vesicles leading to high fertility in Drosophila. Commun Biol. 5(1):815. doi: 10.1038/s42003-022-03770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, Ricardo S, Lehmann R. 2007. Tumbling, an interactive way to move forward. Sci STKE Signal Transduct Knowl Environ. 2007:pe63–pe63. doi: 10.1126/stke.4122007pe63. [DOI] [PubMed] [Google Scholar]
- Santos RCD, Garay C, Antonescu CN. 2015. Charming neighborhoods on the cell surface: plasma membrane microdomains regulate receptor tyrosine kinase signaling. Cell Signal. 27(10):1963–1976. doi: 10.1016/j.cellsig.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Santoso CS, Meehan TL, Peterson JS, Cedano TM, Turlo CV, McCall K. 2018. The ABC transporter Eato promotes cell clearance in the Drosophila melanogaster ovary. G3 (Bethesda). 8(3):833–843. doi: 10.1534/g3.117.300427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarov M, Barz C, Jambor H, Hein MY, Schmied C, Suchold D, Stender B, Janosch S, Vinay Vikas KJ, Krishnan RT, et al. 2016. A genome-wide resource for the analysis of protein localisation in Drosophila. Elife. 5:e12068. doi: 10.7554/elife.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartain CV, Wolfner MF. 2013. Calcium and egg activation in Drosophila. Cell Calcium. 53(1):10–15. doi: 10.1016/j.ceca.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudan P, Hauck K, Soller M, Choffat Y, Ottiger M, Spörri M, Ding Z, Hess D, Gehrig PM, Klauser S, et al. 2002. Ductus ejaculatorius peptide 99B (DUP99B), a novel Drosophila melanogaster sex-peptide pheromone. Eur J Biochem. 269(3):989–997. doi: 10.1046/j.0014-2956.2001.02733.x. [DOI] [PubMed] [Google Scholar]
- Saunders DS, Richard DS, Applebaum SW, Ma M, Gilbert LI. 1990. Photoperiodic diapause in Drosophila melanogaster involves a block to the juvenile hormone regulation of ovarian maturation. Gen Comp Endocrinol. 79(2):174–184. doi: 10.1016/0016-6480(90)90102-R. [DOI] [PubMed] [Google Scholar]
- Savant-Bhonsale S, Montell DJ. 1993. Torso-like encodes the localized determinant of Drosophila terminal pattern formation. Genes Dev. 7(12b):2548–2555. doi: 10.1101/gad.7.12b.2548. [DOI] [PubMed] [Google Scholar]
- Savant SS, Waring GL. 1989. Molecular analysis and rescue of a vitelline membrane mutant in Drosophila. Dev Biol. 135(1):43–52. doi: 10.1016/0012-1606(89)90156-5. [DOI] [PubMed] [Google Scholar]
- Schejter ED, Shilo BZ. 1989. The Drosophila EGF receptor homolog (DER) gene is allelic to faint little ball, a locus essential for embryonic development. Cell. 56(6):1093–104. doi: 10.1016/0092-8674(89)90642-9. [DOI] [PubMed] [Google Scholar]
- Schlichting K, Wilsch-Bräuninger M, Demontis F, Dahmann C. 2006. Cadherin Cad99C is required for normal microvilli morphology in Drosophila follicle cells. J Cell Sci. 119(6):1184–1195. doi: 10.1242/jcs.02831. [DOI] [PubMed] [Google Scholar]
- Schloop AE, Bandodkar PU, Reeves GT. 2020. Gradients and tissue patterning. Curr Top Dev Biol. 137:143–191. doi: 10.1016/bs.ctdb.2019.11.007. [DOI] [PubMed] [Google Scholar]
- Schnakenberg SL, Matias WR, Siegal ML. 2011. Sperm-storage defects and live birth in Drosophila females lacking spermathecal secretory cells. PLoS Biol. 9(11):e1001192. doi: 10.1371/journal.pbio.1001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnakenberg SL, Siegal ML, Bloch Qazi MC. 2012. Oh, the places they’ll go: female sperm storage and sperm precedence in Drosophila melanogaster. Spermatogenesis. 2(3):224–235. doi: 10.4161/spmg.21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Khalil AA, Poulton J, Castillejo-Lopez C, Egger-Adam D, Wodarz A, Deng WM, Baumgartner S. 2006. Perlecan and dystroglycan act at the basal side of the Drosophila follicular epithelium to maintain epithelial organization. Development. 133:3805–3815. doi: 10.1242/dev.02549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonbaum CP, Lee S, Mahowald AP. 1995. The Drosophila yolkless gene encodes a vitellogenin receptor belonging to the low density lipoprotein receptor superfamily. Proc Natl Acad Sci U S A. 92(5):1485–9. doi: 10.1073/pnas.92.5.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonbaum CP, Perrino JJ, Mahowald AP. 2000. Regulation of the vitellogenin receptor during Drosophila melanogaster oogenesis. Mol Biol Cell. 11(2):511–21. doi: 10.1091/mbc.11.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüpbach T. 1987. Germ line and soma cooperate during oogenesis to establish the dorsoventral pattern of egg shell and embryo in Drosophila melanogaster. Cell. 49(5):699–707. doi: 10.1016/0092-8674(87)90546-0. [DOI] [PubMed] [Google Scholar]
- Schüpbach T. 2016. The complexities and unexpected insights of developmental genetic analysis. Curr Top Dev Biol. 117:319–330. doi: 10.1016/bs.ctdb.2015.10.015. [DOI] [PubMed] [Google Scholar]
- Schüpbach T. 2019. Genetic screens to analyze pattern formation of egg and embryo in Drosophila: a personal history. Annu Rev Genet. 53(1):1–18. doi: 10.1146/annurev-genet-112618-043708. [DOI] [PubMed] [Google Scholar]
- Schüpbach T, Wieschaus E. 1991. Female sterile mutations on the second chromosome of Drosophila melanogaster. II mutations blocking oogenesis or altering egg morphology. Genetics. 129(4):1119–1136. doi: 10.1093/genetics/129.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwedes CC, Carney GE. 2012. Ecdysone signaling in adult Drosophila melanogaster. J Insect Physiol. 58(3):293–302. doi: 10.1016/j.jinsphys.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Sen J, Goltz JS, Stevens L, Stein D. 1998. Spatially restricted expression of pipe in the Drosophila egg chamber defines embryonic dorsal-ventral polarity. Cell. 95(4):471–481. doi: 10.1016/S0092-8674(00)81615-3. [DOI] [PubMed] [Google Scholar]
- Sergeev P, Streit A, Heller A, Steinmann-Zwicky M. 2001. The Drosophila dorsoventral determinant PIPE contains ten copies of a variable domain homologous to mammalian heparan sulfate 2-sulfotransferase. Dev Dyn. 220(2):122–132. doi:10.1002/1097-0177(2000)9999:9999< ::AID-DVDY1094>3.0.CO; 2-A. [DOI] [PubMed] [Google Scholar]
- Shah HP, Devergne O. 2022. Confocal and super-resolution imaging of polarized intracellular trafficking and secretion of basement membrane proteins during Drosophila oogenesis. J Vis Exp. doi: 10.3791/63778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Halder S, Felix M, Nisaa K, Deshpande G, Prasad M. 2018. Insulin signaling modulates border cell movement in Drosophila oogenesis. Development. 145:dev166165. doi: 10.1242/dev.166165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea MJ, King DL, Conboy MJ, Mariani BD, Kafatos FC. 1990. Proteins that bind to Drosophila chorion cis-regulatory elements: a new C2H2 zinc finger protein and a C2C2 steroid receptor-like component. Genes Dev. 4(7):1128–1140. doi: 10.1101/gad.4.7.1128. [DOI] [PubMed] [Google Scholar]
- Shen W, Sun J. 2017. Dynamic notch signaling specifies each cell fate in Drosophila spermathecal lineage. G3 (Bethesda). 7(5):1417–1427. doi: 10.1534/g3.117.040212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Sun J. 2020. Different modes of Notch activation and strength regulation in the spermathecal secretory lineage. Development. 147:dev184390. doi: 10.1242/dev.184390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrard KM, Cetera M, Horne-Badovinac S. 2021. Daam mediates the assembly of long-lived, treadmilling stress fibers in collectively migrating epithelial cells in Drosophila. Elife. 10:e72881. doi: 10.7554/eLife.72881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirk PD, Minoo P, Postlethwait JH. 1983. 20-Hydroxyecdysone stimulates the accumulation of translatable yolk polypeptide gene transcript in adult male Drosophila melanogaster. Proc Natl Acad Sci U S A. 80(1):186–90. doi: 10.1073/pnas.80.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shklover J, Levy-Adam F, Kurant E. 2015a. The role of Drosophila TNF Eiger in developmental and damage-induced neuronal apoptosis. FEBS Lett. 589(8):871–879. doi:doi: 10.1016/j.febslet.2015.02.032. [DOI] [PubMed] [Google Scholar]
- Shklover J, Levy-Adam F, Kurant E. 2015b. Chapter Eleven—apoptotic cell clearance in development. In: Steller H, editor. Current Topics in Developmental Biology, Apoptosis and Development. Oxford: Academic Press. p. 297–334. [Google Scholar]
- Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, Dekel N. 2011. Reactive oxygen species are indispensable in ovulation. Proc Natl Acad Sci U S A. 108(4):1462–1467. doi: 10.1073/pnas.1017213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shravage BV, Altmann G, Technau M, Roth S. 2007. The role of Dpp and its inhibitors during eggshell patterning in Drosophila. Development. 134(12):2261–2271. doi: 10.1242/dev.02856. [DOI] [PubMed] [Google Scholar]
- Sieber MH, Spradling AC. 2015. Steroid signaling establishes a female metabolic state and regulates SREBP to control oocyte lipid accumulation. Curr Biol. 25(8):993–1004. doi: 10.1016/j.cub.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber MH, Thomsen MB, Spradling AC. 2016. Electron transport chain remodeling by GSK3 during oogenesis connects nutrient state to reproduction. Cell. 164(3):420–432. doi: 10.1016/j.cell.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simakov DSA, Cheung LS, Pismen LM, Shvartsman SY. 2012. EGFR-dependent network interactions that pattern Drosophila eggshell appendages. Development. 139(15):2814–2820. doi: 10.1242/dev.077669. [DOI] [PubMed] [Google Scholar]
- Sinsimer KS, Jain RA, Chatterjee S, Gavis ER. 2011. A late phase of germ plasm accumulation during Drosophila oogenesis requires lost and rumpelstiltskin. Development. 138(16):3431–3440. doi: 10.1242/dev.065029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly J, Pushparajan C, Duncan EJ, Dearden PK. 2019. Evolution of the Torso activation cassette, a pathway required for terminal patterning and moulting. Insect Mol Biol. 28(3):392–408. doi: 10.1111/imb.12560. [DOI] [PubMed] [Google Scholar]
- Smits CM, Shvartsman SY. 2020. The design and logic of terminal patterning in Drosophila. Curr Top Dev Biol. 137:193–217. doi: 10.1016/bs.ctdb.2019.11.008. [DOI] [PubMed] [Google Scholar]
- Spangenberg DK, Waring GL. 2007. A mutant dec-1 transgene induces dominant female sterility in Drosophila melanogaster. Genetics. 177(3):1595–1608. doi: 10.1534/genetics.107.080168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spracklen AJ, Kelpsch DJ, Chen X, Spracklen CN, Tootle TL. 2014. Prostaglandins temporally regulate cytoplasmic actin bundle formation during Drosophila oogenesis. Mol Biol Cell. 25(3):397–411. doi: 10.1091/mbc.E13-07-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spracklen AJ, Lamb MC, Groen CM, Tootle TL. 2019. Pharmaco-genetic screen to uncover actin regulators targeted by prostaglandins during Drosophila oogenesis. G3 (Bethesda). 9(11):3555–3565. doi: 10.1534/g3.119.400704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling AC. 1981. The organization and amplification of two chromosomal domains containing Drosophila chorion genes. Cell. 27(1):193–201. doi: 10.1016/0092-8674(81)90373-1. [DOI] [PubMed] [Google Scholar]
- Spradling AC. 1993. Developmental genetics of oogenesis. In: Bate M, Martinez-Arias A, editors. The Development of Drosophila Melanogaster. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press. p. 1–70 . [Google Scholar]
- Spradling AC, Digan ME, Mahowald AP, Scott M. 1980. Two clusters of genes for major chorion proteins of Drosophila melanogaster. Cell. 19(4):905–914. doi: 10.1016/0092-8674(80)90082-3. [DOI] [PubMed] [Google Scholar]
- Spradling AC, Mahowald AP. 1980. Amplification of genes for chorion proteins during oogenesis in Drosophila melanogaster. Proc Natl Acad Sci U S A. 77(2):1096–1100. doi: 10.1073/pnas.77.2.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squarr AJ, Brinkmann K, Chen B, Steinbacher T, Ebnet K, Rosen MK, Bogdan S. 2016. Fat2 acts through the WAVE regulatory complex to drive collective cell migration during tissue rotation. J Cell Biol. 212(5):591–603. doi: 10.1083/jcb.201508081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedden CG, Menegas W, Zajac AL, Williams AM, Cheng S, Özkan E, Horne-Badovinac S. 2019. Planar-polarized Semaphorin-5c and Plexin A promote the collective migration of epithelial cells in Drosophila. Curr Biol. 29(6):908–920.e6. doi: 10.1016/j.cub.2019.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DS, Stevens LM. 2014. Maternal control of the Drosophila dorsal-ventral body axis. Wiley Interdiscip Rev Dev Biol. 3(5):301–330. doi: 10.1002/wdev.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson R, Hosler MR, Gavande NS, Ghosh AK, Weake VM. 2015. Characterization of a Drosophila ortholog of the Cdc7 kinase: a role for Cdc7 in endoreplication independent of chiffon. J Biol Chem. 290(3):1332–1347. doi: 10.1074/jbc.M114.597948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson RA, Thomalla JM, Chen L, Kolkhof P, White RP, Beller M, Welte MA. 2021. Sequestration to lipid droplets promotes histone availability by preventing turnover of excess histones. Development. 148(15):dev199381. doi: 10.1242/dev.199381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetina JRV, Orr-Weaver TL. 2011. Developmental control of oocyte maturation and egg activation in metazoan models. Cold Spring Harb Perspect Biol. 3(10):a005553. doi: 10.1101/cshperspect.a005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LM, Beuchle D, Jurcsak J, Tong X, Stein D. 2003. The Drosophila embryonic patterning determinant torsolike is a component of the eggshell. Curr Biol. 13(12):1058–1063. doi: 10.1016/S0960-9822(03)00379-8. [DOI] [PubMed] [Google Scholar]
- Stevens LM, Frohnhöfer HG, Klingler M, Nüsslein-Volhard C. 1990. Localized requirement for torso-like expression in follicle cells for development of terminal anlagen of the Drosophila embryo. Nature. 346(6285):660–663. doi: 10.1038/346660a0. [DOI] [PubMed] [Google Scholar]
- Streicher R, Kotzka J, Müller-Wieland D, Siemeister G, Munck M, Avci H, Krone W. 1996. SREBP-1 mediates activation of the low density lipoprotein receptor promoter by insulin and insulin-like growth factor-I. J Biol Chem. 271(12):7128–33. doi: 10.1074/jbc.271.12.7128. [DOI] [PubMed] [Google Scholar]
- Strisovsky K, Sharpe HJ, Freeman M. 2009. Sequence-specific intramembrane proteolysis: identification of a recognition motif in rhomboid substrates. Mol Cell. 36(6):1048–1059. doi: 10.1016/j.molcel.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Smith L, Armento A, Deng W-M. 2008. Regulation of the endocycle/gene amplification switch by notch and ecdysone signaling. J Cell Biol. 182(5):885–896. doi: 10.1083/jcb.200802084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Spradling AC. 2012. Nr5a nuclear receptor Hr39 controls three-cell secretory unit formation in Drosophila female reproductive glands. Curr Biol. 22(10):862–871. doi: 10.1016/j.cub.2012.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Spradling AC. 2013. Ovulation in Drosophila is controlled by secretory cells of the female reproductive tract. Elife. 2:e00415. doi: 10.7554/eLife.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Yan Y, Denef N, Schüpbach T. 2011. Regulation of somatic myosin activity by protein phosphatase 1 controls Drosophila oocyte polarization. Development. 138(10):1991–2001. doi: 10.1242/dev.062190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sustar AE, Strand LG, Zimmerman SG, Berg CA. 2023. Imaginal disk growth factors are Drosophila chitinase-like proteins with roles in morphogenesis and CO2 response. Genetics. 223(2):iyac185. doi: 10.1093/genetics/iyac185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan A, Schüpbach T. 2007. The Cdc20 (Fzy)/Cdh1-related protein, Cort, cooperates with Fzy in cyclin destruction and anaphase progression in meiosis I and II in Drosophila. Development. 134(5):891–899. doi: 10.1242/dev.02784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan A, Suter B. 1996. Role of Bicaudal-D in patterning the Drosophila egg chamber in mid-oogenesis. Development. 122:3577–3586. doi: 10.1242/dev.122.11.3577. [DOI] [PubMed] [Google Scholar]
- Swevers L. 2019. An update on ecdysone signaling during insect oogenesis. Curr Opin Insect Sci. 31:8–13. doi: 10.1016/j.cois.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Tadros W, Goldman AL, Babak T, Menzies F, Vardy L, Orr-Weaver T, Hughes TR, Westwood JT, Smibert CA, Lipshitz HD. 2007. Smaug is a Major regulator of maternal mRNA destabilization in Drosophila and its translation is activated by the PAN GU kinase. Dev Cell. 12(1):143–155. doi: 10.1016/j.devcel.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Tadros W, Lipshitz HD. 2009. The maternal-to-zygotic transition: a play in two acts. Development. 136(18):3033–3042. doi: 10.1242/dev.033183. [DOI] [PubMed] [Google Scholar]
- Takeo S, Hawley RS, Aigaki T. 2010. Calcineurin and its regulation by Sra/RCAN is required for completion of meiosis in Drosophila. Dev Biol. 344(2):957–967. doi: 10.1016/j.ydbio.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Takeo S, Swanson SK, Nandanan K, Nakai Y, Aigaki T, Washburn MP, Florens L, Hawley RS. 2012. Shaggy/glycogen synthase kinase 3beta and phosphorylation of Sarah/regulator of calcineurin are essential for completion of Drosophila female meiosis. Proc Natl Acad Sci U S A. 109(17):6382–9. doi: 10.1073/pnas.1120367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeo S, Tsuda M, Akahori S, Matsuo T, Aigaki T. 2006. The calcineurin regulator Sra plays an essential role in female meiosis in Drosophila. Curr Biol. 16(14):1435–1440. doi: 10.1016/j.cub.2006.05.058. [DOI] [PubMed] [Google Scholar]
- Talbot WS, Swyryd EA, Hogness DS. 1993. Drosophila Tissues with different metamorphic responses to ecdysone express different ecdysone receptor isoforms. Cell. 73(7):1323–1337.. doi: 10.1016/0092-8674(93)90359-X. [DOI] [PubMed] [Google Scholar]
- Tan L, Chang JS, Costa A, Schedl P. 2001. An autoregulatory feedback loop directs the localized expression of the Drosophila CPEB protein Orb in the developing oocyte. Development. 128(7):1159–1169. doi: 10.1242/dev.128.7.1159. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Tani N, Nakamura A. 2021. Receptor-mediated yolk uptake is required for oskar mRNA localization and cortical anchorage of germ plasm components in the Drosophila oocyte. PLoS Biol. 19(4):e3001183. doi: 10.1371/journal.pbio.3001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Tuffery J, Bakopoulos D, Lequeux S, Warr CG, Johnson TK, Dearden PK. 2019. The torso-like gene functions to maintain the structure of the vitelline membrane in Nasonia vitripennis, implying its co-option into Drosophila axis formation. Biol Open. 8(9):bio046284. doi: 10.1242/bio.046284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennessen JM, Bertagnolli NM, Evans J, Sieber MH, Cox J, Thummel CS. 2014. Coordinated metabolic transitions during Drosophila embryogenesis and the onset of aerobic glycolysis. G3 (Bethesda). 3(5):839–50. doi: 10.1534/g3.114.010652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra P, Ab G. 1988. Homology of Drosophila yolk proteins and the triacylglycerol lipase family. J Mol Biol. 202(3):663–5. doi: 10.1016/0022-2836(88)90294-X. [DOI] [PubMed] [Google Scholar]
- Theurkauf WE, Smiley S, Wong ML, Alberts BM. 1992. Reorganization of the cytoskeleton during Drosophila oogenesis: implications for axis specification and intercellular transport. Development. 115(4):923–936. doi: 10.1242/dev.115.4.923. [DOI] [PubMed] [Google Scholar]
- Thio GL, Ray RP, Barcelo G, Schüpbach T. 2000. Localization of gurken RNA in Drosophila oogenesis requires elements in the 5′ and 3′ regions of the transcript. Dev Biol. 221(2):435–446. doi: 10.1006/dbio.2000.9690. [DOI] [PubMed] [Google Scholar]
- Throckmorton LH. 1962. Throckmorton: the problem of phylogeny in the genus. Stud Genet II. 207–343. [Google Scholar]
- Tilney LG, Tilney MS, Guild GM. 1996. Formation of actin filament bundles in the ring canals of developing Drosophila follicles. J Cell Biol. 133(1):61–74. doi: 10.1083/jcb.133.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons AK, Mondragon AA, Meehan TL, McCall K. 2017. Control of non-apoptotic nurse cell death by engulfment genes in Drosophila. Fly (Austin). 11(2):104–111. doi: 10.1080/19336934.2016.1238993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons AK, Mondragon AA, Schenkel CE, Yalonetskaya A, Taylor JD, Moynihan KE, Etchegaray JI, Meehan TL, McCall K. 2016. Phagocytosis genes nonautonomously promote developmental cell death in the Drosophila ovary. Proc Natl Acad Sci U S A. 113(9):E1246-55. doi: 10.1073/pnas.1522830113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd BL, Stewart EV, Burg JS, Hughes AL, Espenshade PJ. 2006. Sterol regulatory element binding protein is a principal regulator of anaerobic gene expression in fission yeast. Mol Cell Biol. 26(7):2817–31. doi: 10.1128/MCB.26.7.2817-2831.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokmakov AA, Stefanov VE, Sato K-I. 2020. Dissection of the ovulatory process using ex vivo approaches. Front Cell Dev Biol. 8:605379. doi: 10.3389/fcell.2020.605379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolias PP, Kafatos FC. 1990. Functional dissection of an early Drosophila chorion gene promoter: expression throughout the follicular epithelium is under spatially composite regulation. EMBO J. 9(5):1457–1464. doi: 10.1002/j.1460-2075.1990.tb08262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolias PP, Konsolaki M, Halfon MS, Stroumbakis ND, Kafatos FC. 1993. Elements controlling follicular expression of the s36 chorion gene during Drosophila oogenesis. Mol Cell Biol. 13(9):5898–5906. doi: 10.1128/mcb.13.9.5898-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaru M, Ohsako T, Watanabe M, Juni N, Matsubayashi H, Sato H, Takahashi A, Yamamoto MT. 2018. Severe fertility effects of sheepish sperm caused by failure to enter female sperm storage organs in Drosophila melanogaster. G3 (Bethesda). 8(1):149–160. doi: 10.1534/g3.117.300171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootle TL, Spradling AC. 2008. Drosophila Pxt: a cyclooxygenase-like facilitator of follicle maturation. Development. 135(5):839–847. doi: 10.1242/dev.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootle TL, Williams D, Hubb A, Frederick R, Spradling A. 2011. Drosophila eggshell production: identification of new genes and coordination by Pxt. PLoS One. 6(5):e19943. doi: 10.1371/journal.pone.0019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tower J. 2004. Developmental gene amplification and origin regulation. Annu Rev Genet. 38(1):273–304. doi: 10.1146/annurev.genet.37.110801.143851. [DOI] [PubMed] [Google Scholar]
- Tracy K, Baehrecke EH. 2013. The role of autophagy in Drosophila metamorphosis. Curr Top Dev Biol. 103:101–25. doi: 10.1016/B978-0-12-385979-2.00004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran DH, Berg CA. 2003. Bullwinkle and shark regulate dorsal-appendage morphogenesis in Drosophila oogenesis. Development. 130:6273–6282. doi: 10.1242/dev.00854. [DOI] [PubMed] [Google Scholar]
- Trougakos IP, Margaritis LH. 1998a. Immunolocalization of the temporally “early” secreted major structural chorion proteins, Dvs38 and Dvs36, in the eggshell layers and regions of Drosophila virilis. J Struct Biol. 123(2):111–123. doi: 10.1006/jsbi.1998.4028. [DOI] [PubMed] [Google Scholar]
- Trougakos IP, Margaritis LH. 1998b. The formation of the functional chorion structure of Drosophila virilis involves inercalation of the “middle” and “late” major chorion proteins: a general model for chorion assembly in Drosophilidae. J Struct Biol. 123(2):97–110. doi: 10.1006/jsbi.1998.4999. [DOI] [PubMed] [Google Scholar]
- Truman JW, Riddiford LM. 2002. Endocrine insights into the evolution of metamorphosis in insects. Annu Rev Entomol. 47(1):467–500. doi: 10.1146/annurev.ento.47.091201.145230. [DOI] [PubMed] [Google Scholar]
- Turner FR, Mahowald AP. 1976. Scanning electron microscopy of Drosophila embryogenesis: 1. The structure of the egg envelopes and the formation of the cellular blastoderm. Dev Biol. 50(1):95–108. doi: 10.1016/0012-1606(76)90070-1. [DOI] [PubMed] [Google Scholar]
- Twombly V, Blackman RK, Jin H, Graff JM, Padgett RW, Gelbart WM. 1996. The TGF-beta signaling pathway is essential for Drosophila oogenesis. Development. 122(5):1555–1565. doi: 10.1242/dev.122.5.1555. [DOI] [PubMed] [Google Scholar]
- Uechi H, Kuranaga E. 2018. Mechanisms of unusual collective cell movement lacking a free front edge in Drosophila. Curr Opin Genet Dev. 51:46–51. doi: 10.1016/j.gde.2018.06.012. [DOI] [PubMed] [Google Scholar]
- Underwood EM, Briot AS, Doll KZ, Ludwiczak RL, Otteson DC, Tower J, Vessey KB, Yu K. 1990. Genetics of 51D-52A, a region containing several maternal-effect genes and two maternal-specific transcripts in Drosophila. Genetics. 126(3):639–650. doi: 10.1093/genetics/126.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urnov FD, Liang C, Blitzblau HG, Smith HS, Gerbi SA. 2002. A DNase I hypersensitive site flanks an origin of DNA replication and amplification in Sciara. Chromosoma. 111(5):291–303. doi: 10.1007/s00412-002-0194-4. [DOI] [PubMed] [Google Scholar]
- Vardy L, Orr-Weaver TL. 2007. The Drosophila PNG kinase complex regulates the translation of Cyclin B. Dev Cell. 12(1):157–166. doi: 10.1016/j.devcel.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Velentzas AD, Velentzas PD, Katarachia SA, Anagnostopoulos AK, Sagioglou NE, Thanou EV, Tsioka MM, Mpakou VE, Kollia Z, Gavriil VE, et al. 2018. The indispensable contribution of s38 protein to ovarian-eggshell morphogenesis in Drosophila melanogaster. Sci Rep. 8(1):16103–17. doi: 10.1038/s41598-018-34532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velentzas AD, Velentzas PD, Sagioglou NE, Konstantakou EG, Anagnostopoulos AK, Tsioka MM, Mpakou VE, Kollia Z, Consoulas C, Margaritis LH, et al. 2016. Targeted downregulation of s36 protein unearths its cardinal role in chorion biogenesis and architecture during Drosophila melanogaster oogenesis. Sci Rep. 6(1):35511. doi: 10.1038/srep35511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura G, Furriols M, Martín N, Barbosa V, Casanova J. 2010. Closca, a new gene required for both Torso RTK activation and vitelline membrane integrity. Germline proteins contribute to Drosophila eggshell composition. Dev Biol. 344(1):224–232. doi: 10.1016/j.ydbio.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Verdier V, Johndrow JE, Betson M, Chen GC, Hughes DA, Parkhurst SM, Settleman J. 2006. Drosophila Rho-kinase (DRok) is required for tissue morphogenesis in diverse compartments of the egg chamber during oogenesis. Dev Biol. 297(2):417–32. doi: 10.1016/j.ydbio.2006.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereshchagina N, Wilson C. 2006. Cytoplasmic activated protein kinase Akt regulates lipid-droplet accumulation in Drosophila nurse cells. Development. 133(23):4731–5. doi: 10.1242/dev.02659. [DOI] [PubMed] [Google Scholar]
- Viktorinová I, Dahmann C. 2013. Microtubule polarity predicts direction of egg chamber rotation in Drosophila. Curr Biol. 23(15):1472–1477. doi: 10.1016/j.cub.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Viktorinová I, Henry I, Tomancak P. 2017. Epithelial rotation is preceded by planar symmetry breaking of actomyosin and protects epithelial tissue from cell deformations. PLoS Genet. 13(11):e1007107. doi: 10.1371/journal.pgen.1007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viktorinová I, König T, Schlichting K, Dahmann C. 2009. The cadherin Fat2 is required for planar cell polarity in the Drosophila ovary. Development. 136(24):4123–4132. doi: 10.1242/dev.039099. [DOI] [PubMed] [Google Scholar]
- Vorobyeva NE, Erokhin M, Chetverina D, Krasnov AN, Mazina MY. 2021. Su(Hw) primes 66D and 7F Drosophila chorion genes loci for amplification through chromatin decondensation. Sci Rep. 11(1):16963. doi: 10.1038/s41598-021-96488-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreede BM, Lynch JA, Roth S, Sucena E. 2013. Co-option of a coordinate system defined by the EGFr and Dpp pathways in the evolution of a morphological novelty. EvoDevo. 4(1):7. doi: 10.1186/2041-9139-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagamitsu S, Takase D, Aoki F, Suzuki MG. 2017. Identification of the doublesex protein binding sites that activate expression of lozenge in the female genital disc in Drosophila melanogaster. Mech Dev. 143:26–31. doi: 10.1016/j.mod.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Wang X, Bo J, Bridges T, Dugan KD, Pan TC, Chodosh LA, Montell DJ. 2006. Analysis of cell migration using whole-genome expression profiling of migratory cells in the Drosophila ovary. Dev Cell. 10(4):483–495. doi: 10.1016/j.devcel.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Wang H, Guo X, Wang X, Wang X, Chen J. 2020. Supracellular actomyosin mediates cell-cell communication and shapes collective migratory morphology. iScience. 23(6):101204. doi: 10.1016/j.isci.2020.101204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, He L, Wu YI, Hahn KM, Montell DJ. 2010. Light-mediated activation reveals a key role for Rac in collective guidance of cell movement in vivo. Nat Cell Biol. 12(6):591–597. doi: 10.1038/ncb2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZH, Liu Y, Chaitankar V, Pirooznia M, Xu H. 2019. Electron transport chain biogenesis activated by a JNK-insulin-Myc relay primes mitochondrial inheritance in Drosophila. Elife. 8:e49309. doi: 10.7554/eLife.49309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P-Y, Pai L-M. 2011. D-Cbl binding to Drk leads to dose-dependent down-regulation of EGFR signaling and increases receptor-ligand endocytosis. PLoS One. 6(2):e17097. doi: 10.1371/journal.pone.0017097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Qiu Z, Xu Z, Chen SJ, Luo J, Wang X, Chen J. 2018. Apkc is a key polarity determinant in coordinating the function of three distinct cell polarities during collective migration. Development. 145:dev158444. doi: 10.1242/dev.158444. [DOI] [PubMed] [Google Scholar]
- Wang L, Sexton TR, Venard C, Giedt M, Guo Q, Chen Q, Harrison DA. 2014. Pleiotropy of the Drosophila JAK pathway cytokine unpaired 3 in development and aging. Dev Biol. 395(2):218–231. doi: 10.1016/j.ydbio.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Wang F, Wang K, Forknall N, Patrick C, Yang T, Parekh R, Bock D, Dickson BJ. 2020. Neural circuitry linking mating and egg laying in Drosophila females. Nature. 579(7797):101–105. doi: 10.1038/s41586-020-2055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang H, Liu L, Li S, Emery G, Chen J. 2020. Temporal coordination of collective migration and lumen formation by antagonism between two nuclear receptors. iScience. 23(7):101335. doi: 10.1016/j.isci.2020.101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward EJ, Berg CA. 2005. Juxtaposition between two cell types is necessary for dorsal appendage tube formation. Mech Dev. 122(2):241–255. doi: 10.1016/j.mod.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Waring GL. 2000. Morphogenesis of the eggshell in Drosophila. Int Rev Cytol. 198:67–108. doi: 10.1016/S0074-7696(00)98003-3. [DOI] [PubMed] [Google Scholar]
- Waring GL, Hawley RJ, Schoenfeld T. 1990. Multiple proteins are produced from the dec-1 eggshell gene in Drosophila by alternative RNA splicing and proteolytic cleavage events. Dev Biol. 142(1):1–12. doi: 10.1016/0012-1606(90)90146-A. [DOI] [PubMed] [Google Scholar]
- Waring GL, Mahowald AP. 1979. Identification and time of synthesis of chorion proteins in Drosophila melanogaster. Cell. 16(3):599–607. doi: 10.1016/0092-8674(79)90033-3. [DOI] [PubMed] [Google Scholar]
- Watnick TJ, Jin Y, Matunis E, Kernan MJ, Montell C. 2003. A flagellar polycystin-2 homolog required for male fertility in Drosophila. Curr Biol. 13(24):2179–2184. doi: 10.1016/j.cub.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Weaver LN, Drummond-Barbosa D. 2018. Maintenance of proper germline stem cell number requires adipocyte collagen in adult Drosophila females. Genetics. 209(4):1155–1166. doi: 10.1534/genetics.118.301137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Lilly MA. 2014. The TORC1 inhibitors Nprl2 and Nprl3 mediate an adaptive response to amino-acid starvation in Drosophila. Cell Death Differ. 21(9):1460–8. doi: 10.1038/cdd.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichselberger V, Dondl P, Classen A-K. 2022. Eya-controlled affinity between cell lineages drives tissue self-organization during Drosophila oogenesis. Nat Commun. 13(1):6377. doi: 10.1038/s41467-022-33845-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil TT, Forrest KM, Gavis ER. 2006. Localization of bicoid mRNA in late oocytes is maintained by continual active transport. Dev Cell. 11(2):251–262. doi: 10.1016/j.devcel.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Weil TT, Parton R, Davis I, Gavis ER. 2008. Changes in bicoid mRNA anchoring highlight conserved mechanisms during the Oocyte-to-Embryo transition. Curr Biol. 18(14):1055–1061. doi: 10.1016/j.cub.2008.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil TT, Parton RM, Herpers B, Soetaert J, Veenendaal T, Xanthakis D, Dobbie IM, Halstead JM, Hayashi R, Rabouille C, et al. 2012. Drosophila patterning is established by differential association of mRNAs with P bodies. Nat Cell Biol. 14(12):1305–1313. doi: 10.1038/ncb2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbrod A, Cohen M, Chipman AD. 2013. Evolution of the insect terminal patterning system–insights from the milkweed bug, Oncopeltus fasciatus. Dev Biol. 380(1):125–131. doi: 10.1016/j.ydbio.2013.04.030. [DOI] [PubMed] [Google Scholar]
- Wilfling F, Wang H, Haas JT, Krahmer N, Gould TJ, Uchida A, Cheng JX, Graham M, Christiano R, Fröhlich F, et al. 2013. Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev Cell. 24(4):384–99. doi: 10.1016/j.devcel.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AM, Donoughe S, Munro E, Horne-Badovinac S. 2022. Fat2 polarizes the WAVE complex in trans to align cell protrusions for collective migration. Elife. 11:e78343. doi: 10.7554/eLife.78343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AM, Horne-Badovinac S. 2023. Fat2 polarizes Lar and Sema5c to coordinate the motility of collectively migrating epithelial cells. J Cell Sci. 137(5):jcs261173. doi: 10.1242/jcs.261173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TG, Ashok M. 1998. Insecticide resistance resulting from an absence of target-site gene product. Proc Natl Acad Sci. 95(24):14040–14044. doi: 10.1073/pnas.95.24.14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittes J, Schüpbach T. 2018. A gene expression screen in Drosophila melanogaster identifies novel JAK/STAT and EGFR targets during oogenesis. G3 (Bethesda). 9(1):47–60. doi: 10.1534/g3.118.200786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A, Albright SN, Giebel JD, Ram KR, Ji S, Fiumera AC, Wolfner MF. 2008. A role for Acp29AB, a predicted seminal fluid lectin, in female sperm storage in Drosophila melanogaster. Genetics. 180(2):921–931. doi: 10.1534/genetics.108.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LC, Costa A, McLeod I, Sarkeshik A, Yates J 3rd, Kyin S, Perlman D, Schedl P. 2011. The functioning of the Drosophila CPEB protein Orb is regulated by phosphorylation and requires casein kinase 2 activity. PLoS One. 6(9):e24355. doi: 10.1371/journal.pone.0024355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Capp C, Feng L, Hsieh T. 2008. Drosophila homologue of the Rothmund-Thomson syndrome gene: essential function in DNA replication during development. Dev Biol. 323(1):130–142. doi: 10.1016/j.ydbio.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Manogaran AL, Beauchamp JM, Waring GL. 2010. Drosophila vitelline membrane assembly: a critical role for an evolutionarily conserved cysteine in the “VM domain” of sV23. Dev Biol. 347(2):360–368. doi: 10.1016/j.ydbio.2010.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi R, McGregor JR, Harrison DA. 2003. A gradient of JAK pathway activity patterns the anterior-posterior axis of the follicular epithelium. Dev Cell. 4(2):167–177. doi: 10.1016/S1534-5807(02)00412-4. [DOI] [PubMed] [Google Scholar]
- Xue F, Cooley L. 1993. Kelch encodes a component of intercellular bridges in Drosophila egg chambers. Cell. 72(5):681–93. doi: 10.1016/0092-8674(93)90397-9. [DOI] [PubMed] [Google Scholar]
- Yakoby N, Bristow CA, Gong D, Schafer X, Lembong J, Zartman JJ, Halfon MS, Schüpbach T, Shvartsman SY. 2008. A combinatorial code for pattern formation in Drosophila oogenesis. Dev Cell. 15(5):725–737. doi: 10.1016/j.devcel.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakoby N, Lembong J, Schüpbach T, Shvartsman SY. 2008. Drosophila eggshell is patterned by sequential action of feedforward and feedback loops. Development. 135(2):343–351. doi: 10.1242/dev.008920. [DOI] [PubMed] [Google Scholar]
- Yalonetskaya A, Mondragon AA, Hintze ZJ, Holmes S, McCall K. 2020. Nuclear degradation dynamics in a nonapoptotic programmed cell death. Cell Death Differ. 27(2):711–724. doi: 10.1038/s41418-019-0382-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Gustafson EA, Foulk MS, Smith HS, Gerbi SA. 2021. Anatomy and evolution of a DNA replication origin. Chromosoma. 130(2–3):199–214. doi: 10.1007/s00412-021-00756-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka N, Rewitz KF, O’Connor MB. 2013. Ecdysone control of developmental transitions: lessons from Drosophila research. Annu Rev Entomol. 58(1):497–516. doi: 10.1146/annurev-ento-120811-153608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Cochran DA, Gargano MD, King I, Samhat NK, Burger BP, Sabourin KR, Hou Y, Awata J, Parry DA, et al. 2011. Regulation of flagellar motility by the conserved flagellar protein CG34110/Ccdc135/FAP50. Mol Biol Cell. 22(7):976–987. doi: 10.1091/mbc.E10-04-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Lu X. 2011. Drosophila Sperm motility in the reproductive tract. Biol Reprod. 84(5):1005–1015. doi: 10.1095/biolreprod.110.088773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Rumpf S, Xiang Y, Gordon MD, Song W, Jan LY, Jan YN. 2009. Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron. 61(4):519–526. doi: 10.1016/j.neuron.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yapici N, Kim YJ, Ribeiro C, Dickson BJ. 2008. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 451(7174):33–7. doi:nature06483. [DOI] [PubMed] [Google Scholar]
- Yoo H, Roth-Johnson EA, Bor B, Quinlan ME. 2015. Drosophila Cappuccino alleles provide insight into formin mechanism and role in oogenesis. Mol Biol Cell. 26(10):1875–1886. doi: 10.1091/mbc.e14-11-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York-Andersen AH, Parton RM, Bi CJ, Bromley CL, Davis I, Weil TT. 2015. A single and rapid calcium wave at egg activation in Drosophila. Biol Open. 4(4):553–560. doi: 10.1242/bio.201411296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York-Andersen AH, Wood BW, Wilby EL, Berry AS, Weil TT. 2021. Osmolarity-regulated swelling initiates egg activation in Drosophila. Open Biol. 11(8):210067. doi: 10.1098/rsob.210067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Poulton J, Huang Y-C, Deng W-M. 2008. The hippo pathway promotes notch signaling in regulation of cell differentiation, proliferation, and oocyte polarity. PLoS One. 3(3):e1761. doi: 10.1371/journal.pone.0001761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue S, Wang L, DeMartino GN, Zhao F, Liu Y, Sieber MH. 2022. Highly conserved shifts in ubiquitin-proteasome system (UPS) activity drive mitochondrial remodeling during quiescence. Nat Commun. 13(1):4462. doi: 10.1038/s41467-022-32206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac AL, Horne-Badovinac S. 2022. Kinesin-directed secretion of basement membrane proteins to a subdomain of the basolateral surface in Drosophila epithelial cells. Curr Biol. 32(4):735–748.e10. doi: 10.1016/j.cub.2021.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac AL, Williams AM, Horne-Badovinac S. 2023. A low-tech flow chamber for live imaging of Drosophila egg chambers during drug treatments. In: Giedt MS, Tootle TL, editors. Drosophila Oogenesis: Methods and Protocols, Methods in Molecular Biology. New York (NY): Springer US. p. 277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarani FE, Margaritis LH. 1986. The eggshell of Drosophila melanogaster. V Structure and morphogenesis of the micropylar apparatus. Can J Zool. 64(11):2509–2519. doi: 10.1139/z86-372. [DOI] [Google Scholar]
- Zartman JJ, Cheung LS, Niepielko MG, Bonini C, Haley B, Yakoby N, Shvartsman SY. 2011. Pattern formation by a moving morphogen source. Phys Biol. 8(4):045003. doi: 10.1088/1478-3975/8/4/045003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zartman JJ, Kanodia JS, Cheung LS, Shvartsman SY. 2009. Feedback control of the EGFR signaling gradient: superposition of domain-splitting events in Drosophila oogenesis. Development. 136(17):2903–2911. doi: 10.1242/dev.039545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zartman JJ, Kanodia JS, Yakoby N, Schafer X, Watson C, Schlichting K, Dahmann C, Shvartsman SY. 2009. Sciencedirect—gene expression patterns: expression patterns of cadherin genes in Drosophila oogenesis. Gene Expr Patterns. 9(1):31–36. doi: 10.1016/j.gep.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Ahmed-Braimah YH, Goldberg ML, Wolfner MF. 2019. Calcineurin-dependent protein phosphorylation changes during egg activation in Drosophila melanogaster. Mol Cell Proteomics. 18:S145–S158. doi: 10.1074/mcp.RA118.001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Stevens LM, Stein D. 2009. Sulfation of eggshell components by pipe defines dorsal-ventral polarity in the Drosophila embryo. Curr Biol. 19(14):1200–1205. doi: 10.1016/j.cub.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhu X, Stevens LM, Stein D. 2009. Distinct functional specificities are associated with protein isoforms encoded by the Drosophila dorsal-ventral patterning gene pipe. Development. 136(16):2779–2789. doi: 10.1242/dev.034413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman SG, Merrihew GE, MacCoss MJ, Berg CA. 2017. Proteomics analysis identifies orthologs of human chitinase-like proteins as inducers of tube morphogenesis defects in Drosophila melanogaster. Genetics. 206(2):973–984. doi: 10.1534/genetics.116.199323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.
Supplemental material available at GENETICS online.