Abstract
Introduction: Poor oral hygiene is linked to high risks of many systemic diseases, including cancers. Oral dysbiosis is closely associated with poor oral hygiene, causing tooth loss, gingivitis, and periodontitis. We provide a summary of studies and discuss the risk factors for oesophageal squamous cell carcinoma (ESCC) from a microbial perspective in this review.Methods: A literature search of studies published before December 31, 2022 from PubMed, Web of Science, and The Cochrane Library was performed. The search strategies included the following keywords: (1) oral care, oral health, oral hygiene, dental health, dental hygiene, tooth loss, teeth loss, tooth absence, missing teeth, edentulism, tooth brushing, mouthwash, and tooth cleaning; (2) esophageal, esophagus, oesophagus, and oesophageal; (3) cancer, carcinoma, tumor, and neoplasm.Discussion: Poor oral health, indicated by infrequent tooth brushing, chronic periodontitis, and tooth loss, has been associated with an increased risk of squamous dysplasia and ESCC. Oral microbial diversity and composition are profoundly dysregulated during oesophageal tumorigenesis. Similar to the oral microbiota, the oesophageal microbiota varies distinctly in multiple bacterial taxa in ESCC and gastric cardia adenocarcinoma, both of which have high co-occurrence rates in the “Oesophageal Cancer Belt”. In addition, the potential roles of oncogenic viruses in ESCC have also been discussed. We also briefly explore the potential mechanisms underlying the tumor-promoting role of dysregulated microbiota for the development of therapeutic targeting strategies.Conclusion: Poor oral health is an established risk indicator of ESCC. The dysbiosis of microbiota in upper gastrointestinal tract that highly resembles the oral microbial ecosystem but with distinct features at individual sites contributes to the development and progression of ESCC.
Keywords: Oesophageal squamous cell carcinoma, tooth loss, periodontitis, microbiota, porphyromonas gingivalis
Introduction
Oesophageal cancer ranks seventh and sixth in terms of incidence and death of cancer, respectively [1]. Two predominant histologic subtypes of oesophageal cancer, oesophageal squamous cell carcinoma (ESCC) and oesophageal adenocarcinoma (EAC), share few features in aetiology, regional distribution pattern, and time trend with the exception of poor prognosis [2]. Although EAC incidence has increased several folds over the past 40 years, ESCC still accounts for about 90% of oesophageal cancer globally at present, half of which occur in China [3]. The aetiology of ESCC varies by population. In China, Central Asia, and Sub-Saharan Africa, the major risk factors for ESCC comprise micronutrient deficiency, dietary carcinogen exposure, hot food and beverage ingestion, and low socioeconomic status [4–7]. In contrast, tobacco and alcohol consumption are important risk factors for ESCC in Western populations [8, 9]. Recently, chronic periodontitis and their related oral microorganisms have been linked to increased risks of various systemic diseases [10–13], including total cancer and certain site-specific cancers like ESCC [14–16].
Epidemiological studies suggest a causal link between infection-driven chronic inflammation and cancer [17, 18]. In general, approximately 20% of human tumours are associated with infectious agents, including virus, bacteria, and parasites [19]. Chronic inflammation is considered as the main risk factor for many cancers and contributes to acquisition of the majority of core cancer hallmarks [18]. The inflammatory milieu gives rise to large amounts of cytokines and growth factors, which foster the initiation and development of malignancies via accumulation of genetic instability, mutations, and epigenetic abnormalities [20–23]. Although one or several microbes can cause specific infection, the notion that the entire microbial community, termed microbiota, is reputed to be a pathogen, is becoming increasingly accepted [24, 25]. The human microbiota co-exists with its host in symbiosis and is beneficial for many physiological processes, such as nutrition absorption, metabolism, mucosal immune system development, and immune modulation [26–28]. Under conditions of dysbiosis, the dysregulated microbial community can promote the formation and development of many diseases including cancer, in synergism with other risk factors (e.g. smoking, alcohol intake, and dietary carcinogens).
Poor oral health, generally reflected by the degree of tooth loss, caries, and periodontitis, has been linked to a high risk of ESCC [15, 29–35]. Increasing evidence shows that health state of oral cavity is associated with oral microbiota, which has been implicated in the pathogenesis and development of various diseases [36]. The microbiota of the upper gastrointestinal tract has attracted less attention, particularly in relation to ESCC, compared with the microflora in the lower gastrointestinal tract. This review provides a summary of studies that have evaluated the effects of tooth loss, periodontitis, oral health, and practices of oral hygiene on the initiation and development of ESCC. In the light of culture-based and recent sequencing-based microbial profiling studies, we elaborate on latest data on microbiota in the upper gastrointestinal tract in relation to normal oesophageal mucosae and ESCC and assess the impact of microbial phylotypes on the carcinogenesis of ESCC. In addition, we summarize the associations between some extensively studied oncogenic viruses and ESCC. The potential tumorigenic mechanisms of microbiota dysbiosis in the context of ESCC are discussed.
Oral milieu
Several epidemiological studies support the association between an increased risk of ESCC and poor oral health, self-reported tooth loss, and chronic periodontitis [14–16, 29–35, 37,38] (Table 1).
Table 1.
Studies of oral health, tooth loss and periodontitis on esophageal squamous cell carcinoma.
| References | Oral health status | Study design | Participant details | Relative risk (95% CI) | Adjustment |
|---|---|---|---|---|---|
| Abnet et al. [30] | Tooth loss (Median splits) | Cohort | 28,868 person-cohort with 5.25 years follow-up, Linxian, China and 620 incident cases of ESCC | RR:1.3 (1.1–1.6) | Age, sex, tobacco use and alcohol use. |
| Wang et al. [35] | Tooth brushing (No vs. Yes) | Case–control | 116 cases and 189 controls, Linfen, China | OR:0.2 (0.1–0.5) | Age, sex, residence and occupation. |
| Chen et al. [29] | Tooth loss (6 teeth lost vs. none) | Case–control | 616 cases and 770 controls, Taixing, China | OR:1.48 (1.04–2.11) | Age, sex, education, marital status, tobacco use, alcohol consumption, family history, consumption of pickled vegetables and fresh fruits and wealth score. |
| Tooth brushing (less than once vs. twice per day) | OR:1.81 (1.37–2.38) | ||||
| Hiraki et al. [37] | Tooth loss (≥ 21 remaining teeth vs. 0) | Case–control | 5,240 cases and 10,480 controls, Aichi Cancer Centre, Japan. | OR:2.36 (1.17–4.75) | Age, sex, smoking and drinking status, vegetable and fruit intake, body mass index and regular exercise. |
| Dar et al. [31] | Teeth cleaning (≥ once vs. never per day) | Case–control | 703 cases and 1,664 controls, Kashmir, India. | OR:0.44 (0.25–0.77) | Age, sex, tobacco and alcohol use, and wealth score. |
| Abnet et al. [32] | Decayed, missing or filled teeth (32 vs. ≤ 15) | Case–control | 283 cases and 560 controls, Golestan, Iran. | OR:2.10 (1.19–3.70) | Age, sex, residence, tobacco and/or opium consumption, alcohol drinking, vegetable intake, socioeconomic status and ethnicity. |
| Tooth brushing (Never vs. daily) | OR:2.37 (1.42–3.97) | ||||
| Sato et al. [34] | Tooth brushing (≥ twice vs. once per day) | Case–control | 856 cases of upper aerodigestive tract cancer including 387 oesophageal cases and 2,696 controls, Aichi Cancer Centre, Japan. | OR:0.82 (0.68–0.99) | Age, sex, smoking and drinking status, vegetable and fruit intake, frequent intake of hot beverages, BMI, occupation and number of remaining teeth. |
| Sepehr et al. [40] | Good oral health vs. dental prosthesis | Case–control | 124 cases with oesophageal dysplasia and 50 controls, Turkoman plain, Iran. | OR:4.76 (1.48–15.31) | Age, sex, ethnic origin. |
| Guha et al. [33] | Tooth loss (≤5 vs. 6-15) | Case–control | 91 cases and 566 controls, central Europe. | OR: .84 (1.26–6.41) | Age, sex, education, country, tobacco pack-years, cumulative alcohol consumption. |
| 95 cases and 359 controls, Latin American | OR:2.18 (1.04–4.59) | Age, sex, education, centre, tobacco pack-years, cumulative alcohol consumption. | |||
| Abnet et al. [39] | Tooth loss (0 − 10 vs. 11-31) | Cohort | 29,124 person-cohort with 14–year follow-up and 49 incident cases of ESCC. | HR:0.92 (0.46–1.83) | Age, education and H. pylori. |
| Nwizu et al. [14] | Self-reported periodontal disease | Cohort | 65,869 women cohort with 8.32-year follow-up and 34 incident cases of oesophageal cancer | HR:3.28 (1.64–6.53) | Age, smoking status, BMI, history of diabetes and HT use. |
| Wen et al. [48] | Periodontitis vs. gingivitis | Cohort (Retrospective) | 15,3566 participants with 13-year follow-up and 82 incident cases of oesophageal cancer. | HR:1.50 (0.97–2.31) | Age, sex and comorbidities including diabetes, hypertension, hyperlipidaemia. |
| Chou et al. [49] | Periodontitis (Mild vs. severe) | Cohort | 25.485 individuals with mild chronic periodontitis and 25,485 individuals with severe chronic periodontitis using 1:1 propensity score matching. | HR:1.15 (0.62–2.15) | Age, sex, diabetes, cholecystectomy, Charlson comorbidity index score, medication use (aspirin and non-steroidal anti-inflammatory drugs, estimated monthly income and education level. |
| Michaud et al. [16] | Periodontitis (No vs. yes) | Cohort | 48,375 men with median of 17.7-year follow-up and 131 incident cases of oesophageal cancer. | HR:1.44 (0.98–2.11) | Ethnic origin, BMI, physical activity, smoking history, history of diabetes, geographical region, height, alcohol, vitamin D score, calcium intake, fruit and vegetable intake, red-meat intake, and total calorific intake. |
| Tooth loss (25-32 vs. 0-16 teeth remaining) | HR:1.34 (0.78-2.30) |
Tooth loss
The earliest evidence suggesting an etiological relationship between ESCC and poor oral health came from a general population-based prospective study conducted in 1985 in Linxian (renamed Linzhou in 1994), located in the Taihang Mountain range in northern China, with a 100-fold higher mortality rate of oesophageal cancer than that of Caucasian Americans. In this 28,868-person cohort with a prospective follow-up of 5.25 years, tooth loss was significantly associated with cancer risk increases in ESCC, GCA, and gastric non-cardia adenocarcinoma. Furthermore, the greatest cancer risk increase was strongly correlated with the loss of the first few teeth [30] (Table 1). In line with this, another population-based case-control study conducted in Taixing, another high-incidence area for ESCC in China, also revealed that the number of tooth loss was significantly associated with ESCC risk in a stepwise manner, and that the increased risks were stronger in patients ≥ 70 years of age, women, non-smokers, and non-drinkers in the stratification analyses [29]. In Japan, one large-scale, case-control study recruiting 5,240 cancer cases and 10,480 non-cancer controls reported that tooth loss significantly increased the risk of cancers from the head and neck, oesophagus, and lung [37] (Table 1). Furthermore, a case-control study performed in Golestan Province in Iran, a high-risk region for ESCC, demonstrated that poor oral hygiene and tooth loss were significantly associated with a progressively increased risk of ESCC, with the highest risk for ESCC in subjects who lost teeth earlier [32] (Table 1). In addition, positive associations of ESCC risk with tooth loss and poor oral health have been documented in other areas as well, such as India [31], Latin America and Eastern Europe [33] (Table 1). However, inconsistent with these results, tooth loss had no effect on the risks of ESCC or oesophageal/gastric cardia adenocarcinoma, but significantly increased the risk of gastric non-cardia cancer in a prospective Finnish cohort of male smokers [39] (Table 1). The reasons for this discrepancy may be threefold: first, the contributable risk factors for these three types of cancer arising from oesophagus and stomach are distinct in these two populations, that is, Linzhou, northern China versus Finland; second, the small number of incident ESCC cases (49 cases in Finland vs. 620 cases in Linzhou) and oesophageal/gastric cardia adenocarcinoma cases (66 cases in Finland vs. 431 cases in Linzhou) compared with those of gastric non-cardia cancer cases (179 cases in Finland vs. 102 cases in Linzhou) may limit the power of statistical analysis; third, no adjustments were performed for other potential confounders linking to tooth loss or periodontitis during the statistical analysis, such as smoking, dental caries, diabetes mellitus, and socioeconomic level. To further identify the potential role of oral pathogens in ESCC tumorigenesis, a population-based screening trial was initiated to examine oral health and hygiene status in high- and low-incidence regions for ESCC in China. In agreement with the findings of previous studies in China, our group observed that tooth loss and poor oral hygiene were more common in high-incidence areas than in low-incidence areas for ESCC in China. The pooled meta-analysis also revealed that more lost teeth was significantly associated with increased OR in ESCC (OR, 1.48; 95% confidence interval (CI), 1.21 − 1.82; Figure 1). As tooth loss is mainly caused by periodontitis due to dysbiosis in the oral cavity, it is tempting to speculate that tooth loss status may reflect oral health status and is closely correlated with microbial balance in the oral cavity. In addition, an alternative potential mechanism is that tooth loss may cause incomplete chewing and rapid swallowing of hot and large pieces of food.
Figure 1.
Forest Plot for the associations between oesophageal squamous cell carcinoma risk and tooth loss, and poor oral hygiene. The diamonds indicate best estimate of the true (pooled) risk with width indicating 95% confidence intervals (CI). for details, see text.
Tooth brushing
As tooth brushing is closely related to oral health and hygiene, several studies have shown that tooth brushing frequency is inversely associated with ESCC risk [29–32, 34,35] (Table 1). A case-control study conducted in Linfen, Shanxi, China, revealed that brushing teeth, an indicator of oral hygiene, was strongly associated with a lower risk of EC incidence [35] (Table 1). Furthermore, a population-based case–control study performed in Taixing, China, showed that infrequent tooth brushing contributed to an 80% excess risk of ESCC [29] (Table 1). In Iran, poor oral health was associated with a higher risk of precancerous lesions and squamous dysplasia in a dose-response fashion [40] (Table 1). Similar to tooth loss, never brushing teeth or brushing teeth less than once daily was significantly associated with an increased OR (OR, 2.28; 95% CI, 1.37 − 3.79). Furthermore, our meta-analysis also reveals a significant association between ESCC risk and the combination of tooth loss and poor oral hygiene indicated by tooth brushing (OR, 1.81; 95% CI, 1.39 − 2.35; Figure 1; Supplementary file 1).
Periodontitis
As dental caries also accounts for a proportion of tooth loss, particularly in younger individuals [41], it seems that tooth loss is not an optimal surrogate marker for periodontitis. Chronic periodontitis, characterized by chronic oral biofilm-mediated inflammation, are the major cause of tooth loss in the world and contribute to progressive destruction of the periodontium and alveolar bone loss [42, 43]. The development of periodontitis is strongly associated with oral bacteria, including Porphyromonas gingivalis, Actinobacillus actinomycetemcomitans, Tannerella forsythensis, and Treponema denticola [13, 44]. Thus, periodontitis is directly related to the microbial environment in the oral cavity. Several studies have linked periodontitis to many systemic diseases, including cancer [14, 16, 45–49]. One prospective cohort study, recruiting 65,869 postmenopausal women with a mean follow-up of 8.32 years in the USA, found that periodontal disease increased the risk of total cancer by 15% (hazard ratio (HR), 1.14; 95% CI, 1.08 − 1.20), with a stronger increased risk in the upper gastrointestinal tract, including the oesophagus and stomach (HR, 2.04; 95% CI, 1.35 − 3.09), among which oesophageal cancer risk was highest (HR, 3.28; 95% CI, 1.64 − 6.53) [14] (Table 1). A population-based retrospective cohort study in Taiwan reported that patients with periodontitis but not gingivitis exhibited a 1.14 times higher risk for total cancer, a 1.79 times higher risk for oral cancer, and a 1.5 times higher risk for oesophageal cancer compared with the control cohort [48] (Table 1). Relative to mild chronic periodontitis, the severity of chronic periodontitis did not increase the risk of total gastrointestinal cancers or individual gastrointestinal cancers, including esophageal cancer in Taiwan [49] (Table 1). However, in 48,375 older male health professionals with a median follow-up of 17.7 years in the USA, periodontitis was not associated with oesophageal cancer after adjusting for smoking status, in contrast to a 14% increased risk for overall total cancers in this male cohort [16] (Table 1). The inconsistency between males and females in the USA may stem from less smoking exposure in females, and the high prevalence of smoking in males contributes more to oesophageal cancer risk than other risk factors. Altogether, these findings indicate that a dysregulated microbiome leading to poor oral hygiene and periodontitis may play a causative role in the development of ESCC, which warrants further in-depth delineation of the composition of oral microflora and specific pathogens in relation to ESCC.
Oral microbiota
The oral microbiome comprises over 700 different bacterial species, representing greater than 11 bacterial phyla and 70 genera [50]. The majority of oral microbiome are commensal bacteria and play an important role in maintaining oral health, but a few species are real pathogenic factors in the development of certain oral diseases. The complex multispecies bacterial community maintains an exquisite equilibrium in the mouth ecosystem, which plays critical roles in many aspects of human health, such as immune response, carcinogen metabolism, and nutrient digestion [51–53]. Disruption of the equilibrium by Porphyromonas gingivalis, a key-stone oral pathogen, results in microbial dysbiosis, causing a dysregulated immune response and ultimately disease outcomes [53]. Periodontal pathogens have been identified in a number of organ systems, including lymph nodes [54], lung aspirates [55], arteries [56, 57], precancerous lesions of stomach [58] and colon [59], and oesophageal [60–63] and colorectal cancers [64–66]. These studies point to the potential implications of the oral dysbiosis in many systemic diseases, and identification of key oral pathogens would be instrumental in the development of novel preventive, diagnostic, and therapeutic strategies.
As the human oral microbiome defines all microorganisms in the oral cavity as well as its contiguous extensions up to the distal oesophagus, it is tempting to argue that oral pathogens may play crucial roles in the initiation and development of ESCC via local spread and dissemination stemming from the oral cavity or systemic mediators induced by a dysregulated oral microbiome. A pioneering culture-based study using oesophageal or oropharynx lavage samples isolated a common microorganism, Streptococcus viridans, from both the oropharynx and oesophagus. Using brush and biopsy samples from oral, upper, and lower oesophageal mucosae for both aerobic and anaerobic microbial cultivation and identification, Grusell et al. observed similar culture patterns in terms of bacterial number and diversity across samples from the oral cavity and oesophagus. In these three locations, the common bacteria were Streptococcus viridans, Fusobacterium spp., Neisseria spp., Haemophilus spp., and non-pigmented Prevotella spp., suggesting that the resident bacteria in oesophagus were of oral origin [67]. Using saliva from healthy subjects, a 16S rDNA-based sequencing study revealed that 43% of the clones were Streptococcus mitis, 13% were S. sanguinis, 10% were S. parasanguis, 7% were S. infantis, S. australis, and S. constellatus, 3% were S. cristatus, and 10% were unknown Streptococcus species, indicating that Streptococcus species are the most frequent oral commensal microbiota [60] (Table 2). In contrast, a sequencing-based study using swab samples from the uvula and biopsy samples from the proximal, middle, and distal oesophagus identified markedly different patterns of microbiome, indicating that the uvula was associated with a distinct and specific microflora as compared with all three levels of oesophagus. These data demonstrate that uvula is a distinct niche in the oral cavity and does not seem to be a surrogate of the oral cavity [68]. In a case–control study conducted in Taixing, China, which interrogated saliva microbiomes of 87 patients with ESCC, 63 cases with dysplasia, and 85 healthy subjects based on 16S rRNA gene sequencing of the V3–V4 region. The overall microbial diversity of the ESCC patients was significantly lower than that of dysplasia cases and control subjects. The most frequent phyla in the saliva included Bacteroidetes, Firmicutes, Proteobacteria, Fusobacteria and Actinobacteria. In patients with ESCC, the decreased genera included Lautropia, Bulleidia, Catonella, Corynebacterium, Moryella, Peptococcus, and Cardiobacterium, in contrast to the overabundant genera Prevotella, Streptococcus and Porphyromonas, suggesting an association between ESCC risk and aberrant oral microbiota [69] (Table 2). Chen and colleagues revealed that alpha diversity and beta diversity were distinct between the microbiota profiles of oral biofilms from healthy volunteers and ESCC patients. The abundance of Streptococcus species, Prevotella and P. gingivalis was significantly higher in the oral biofilms of ESCC patients than that from healthy controls. Moreover, P. gingivalis abundance in oral biofilm was associated with an increased ESCC risk [70]. A prospective study nested in two U.S. cohorts, that is, the NCI prostate, lung, colorectal, and ovarian (PLCO) Cancer Screening Trial Cohort and the American Cancer Society (ACS) Cancer Prevention Study II (CPS-II) Nutrition cohort, investigated the oral microbiota relationship between EAC and ESCC using 16S rRNA gene sequencing in mouthwash samples from 81/160 EAC cases, 25/50 ESCC cases, and matched controls. There were no significant associations between overall microbiota diversity or composition and risk for EAC or ESCC. Remarkably, an increased abundance of Prevotella nanceiensis, Bergeyella oral taxon 322, Neisseria weaveri, and Treponema vincentii was associated with a significantly higher risk, whereas reduced amount of Prevotella oral taxon 306 and Aggregatibacter paraphrophilus was associated with a decreased risk of ESCC. The authors also found that P. gingivalis, a key periodontal pathogen, was marginally associated with a higher risk of ESCC. In contrast to ESCC-related microorganisms, Tannerella forsythia was associated with a higher EAC risk whereas decreased abundance of the commensal genus Neisseria and the species Streptococcus pneumoniae was associated with a lower risk of EAC [71] (Table 2). It is plausible to suppose that distinct oral bacterial pathogens were linked to different subtypes of oesophageal cancer, that is, EAC and ESCC, suggesting that specific microbiota may underlie the histological origins, development, and mechanisms of EAC and ESCC.
Table 2.
Studies of oral microbiota on oesophageal squamous cell carcinoma.
| References | Study design | Participants | Sample type | Method | Results |
|---|---|---|---|---|---|
| Chen et al. [69] | Case–control | 87 cases with ESCC, 63 individuals with dysplasia and 85 healthy controls from Taixing of Jiangsu, China | Saliva | (V3 to V4 regions of) 16S rRNA gene sequencing | The most common phyla in saliva included Bacteroidetes, Firmicutes, Proteobacteria, Fusobacteria and Actinobacteria; saliva microbiota in ESCC patients harboured the decreased genera including Lautropia, Bulleidia, Catonella, Corynebacterium, Moryella, Peptococcus and Cardiobacterium and the overabundant genera including Prevotella, Streptococcus and Porphyromonas. |
| Peters et al. [71] | Nested case–control | 81 EAC cases and 160 matched controls | Mouthwash | V4 regions 16S rRNA gene sequencing | Saliva microbiota contained overabundance of Tannerella forsythia, and decreased abundances of the commensal genus Neisseria and the species Streptococcus pneumoniae. |
| 25 ESCC cases and 50 matched controls | Saliva microbiota contained overabundances of Prevotella nanceiensis, Bergeyella oral taxon 322, Neisseria weaveri and Treponema vincentii, and decreased abundances of Prevotella oral taxon 306 and Aggregatibacter paraphrophilus; P. gingivalis was marginally associated with a higher ESCC risk. | ||||
| Narikiyo et al. [60] | Case–control | 20 healthy volunteers and 58 patients with oesophageal cancer from Tokyo, Japan. | Saliva mix | 16S rRNA gene amplification and clone sequencing | Streptococcus (S.) mitis (43%), S. sanguinis (13%), S. parasanguis (10%), S. infantis, S. australis, S. constellatus (7%), S. ctirtatus (3%) and unknown Streptococcus species (10%). |
In the light of the anatomical site adjacency and high consistency in microbiota between the oral cavity and oesophagus, the oral cavity may represent a microbial window mirroring alterations in the composition and structure of the microbial ecosystem colonizing the oesophagus. Thus, the identification of key oral pathogens associated with ESCC, particularly in high-risk regions of ESCC, is a potential microbial biomarker for screening and surveillance in high-risk population, early detection, and potential targets for preventive intervention.
Oesophageal microbiota
The oesophagus, a luminal organ that links the oral cavity with the stomach, allows the transient passage of food into the stomach, in contrast to mouth and other parts of the gastrointestinal tract, which retain and digest food to various degrees. The luminal surface of the oesophagus is lined with ample mucosa for bacterial colonization, and an oesophageal biofilm has been described by several research groups [67, 72–75]. Under normal physiological conditions, microorganisms are introduced into the oesophagus from the mouth by swallowing or from the stomach by reflux [72]. This may support the causal relationship between esophageal diseases and pathogenic agents from the oral cavity or stomach.
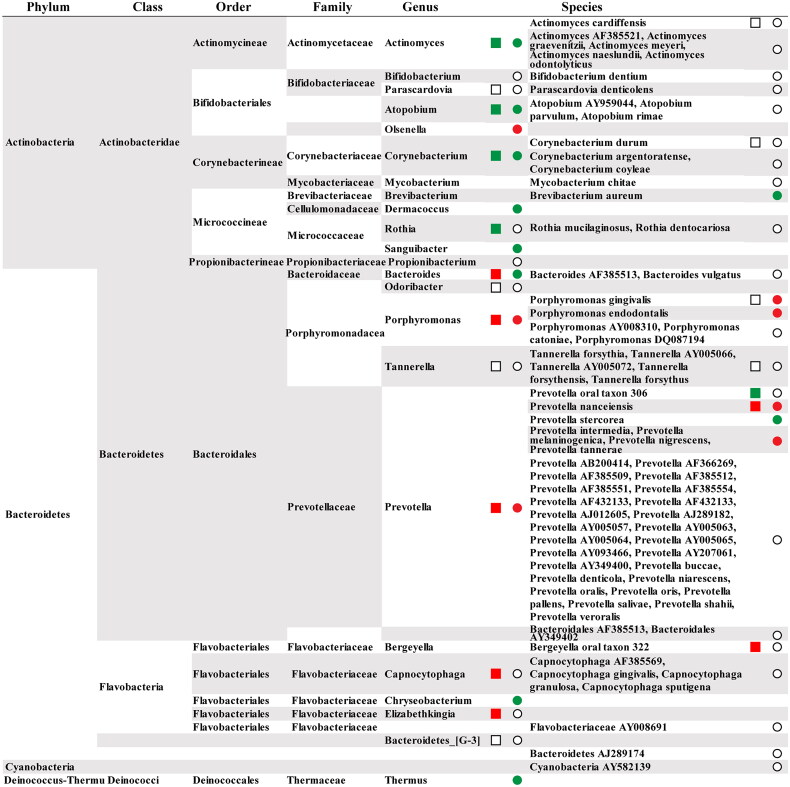
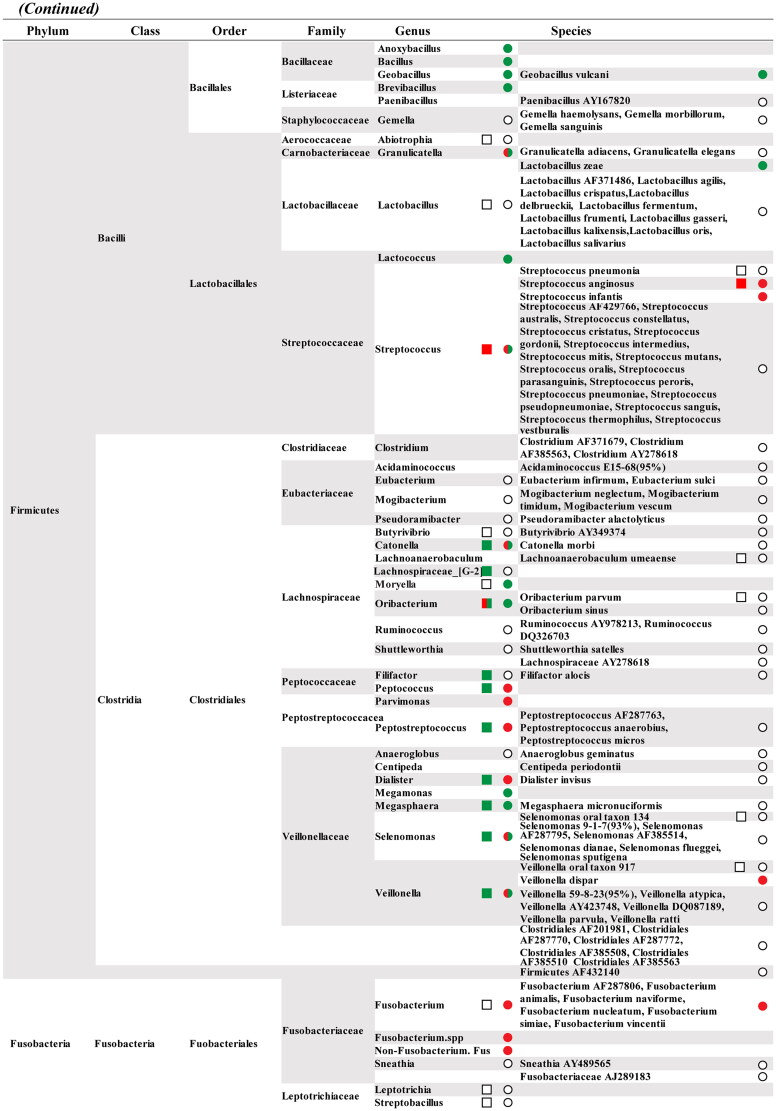
Using biopsy specimens from a healthy distal oesophagus, 900 16S rDNA PCR clones were identified, indicating abundant microbiota in the normal distal oesophagus. Six phyla, including Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and TM7, were found in the oesophageal microbiota. Among the 41 genus-level taxonomic units, Streptococcus (39%), Prevotella (17%), and Veilonella (14%) were the most common genera. Most species-level operational taxonomic units (SLOU) of the microbiota in the distal oesophagus were similar to the up-stream oral flora [73] (Table 3). Consistent with these findings, a culture-based study found streptococcal species are the most common organisms in oesophagus [67]. Most of oesophageal bacteria (82%) are cultivable and derived from known families [73], while 70% of oral bacteria, including 57% officially named and 13% unnamed, are cultivable [76]. Accumulating studies reveal that the core microbiota in healthy oesophageal mucosa comprises Streptococcus, Fusobacterium, Veillonella and Prevotella genera, whose composition changes under pathological conditions [50, 76]. A total of 10 phyla have been identified in oral cavity and oesophagus, among which the most common phyla include Firmicutes, Proteobacteria, Bacteroidetes and Actinobacteria (Figure 2). Certainly, the normal distal oesophagus contains a complicated but conserved bacterial community that is closely related to the oral microbiota.
Table 3.
Studies of oesophageal microbiota on oesophageal squamous cell carcinoma.
| References | Participants | Sample type | Method | Results |
|---|---|---|---|---|
| Pei et al. [73] | Four persons with gastrointestinal symptoms | Four distal oesophageal biopsies with normal oesophageal histology | 16S rDNA PCR | Six phyla, 41 genera including 13 common genera among 4 persons, and 95 SLOTU including 14 common SLOTU among 4 persons. |
| Yang et al. [77] | Twelve normal oesophagus, 12 oesophagitis, 10 Barrett’s oesophagus | 34 biopsy samples of distal oesophagus. | 16S rRNA gene amplification and clone sequencing | Nine phyla, 70 genera and 166 SLOTU; Type I microbiome dominated by Streptococcus in normal oesophagus; Type II microbiome represented by Gram-negative anaerobes in esophagitis and Barrett’s oesophagus |
| Yu et al. [78] | 333 subjects including 142 with ESD and 191 without ESD from Linxian (Linzhou), Henan, China | Upper digestive tract cell samples collected by inflatable rubber balloon covered with cotton mesh or Cytomesh Oesophageal Cytology Device | Human Oral Microbe Identification Microarray for detection of 272 bacterial species. | An inverse association between microbial richness and ESD, and a positive association between microbial richness and PGI/II ratio. |
| Yang et al.[79] | 18 patients with ESCC and 11 volunteers with physiological normal oesophagus from Guangzhou, China | 18 ESCC samples and 11 biopsy samples with normal oesophagus | PCR amplification of V4 region of 16S rDNA and sequencing | ESCC tissues had significantly decreased microbial diversity and high abundances of Bacteroidetes, Fusobacteria and Spirochaetes, which were associated with reduced nitrate reductase and nitrite reductase function |
| Shao et al. [81] | 67 patients with ESCC and from Linzhou, Henan, China | Paired ESCC tissue and non-tumour tissue samples | PCR amplification of V4region of 16S rDNA and sequencing | Microbial composition of ESCC comprised of Firmicutes, Bacteroidetes and Proteobacteria phyla; ESCC contained higher abundance of Fusobacteria phylum including Fusobacterium genus, and less abundance of Firmicutes phylum including Streptococcus genus in comparison with non-tumour tissues; ESCC harboured greater abundances of Fusobacteria and Tenericutes phyla, greater abundances of Mycoplasma and Fusobacterium genera in comparison with GCA tumour tissues. |
| Narikiyo et al. [60] | 58 patients with oesophageal cancer from Tokyo, Japan, 4 from China, 2 from France, 5 from Italy. | Oesophageal cancer tissues with or without paired normal tissues. | 16S rRNA gene amplification and clone sequencing | Frequent infection of the oral periodontopathic spirochete Treponema denticola, Streptococcus mitis, Streptococcus anginosus in ESCC from different regions of the world. |
| Yamamura, et al. [62] | 325 patients with oesophageal cancer from Kumamoto, Japan | Formalin-fixed, paraffin-embedded (FFPE) oesophageal cancer tissues including 300 ESCCs, 12 adenocarcinomas and 13 of others | qPCR quantification of Fusobacterium nucleatum | F. nucleatum positivity in tumour is 23% (74/325) and is positively correlated with tumour stage. F. nucleatum-positive patients showed poor prognosis with multivariate HR: 1.98 (1.14-3.37) |
| Yamamura, et al. [63] | 551 cases with ESCC | A training cohort of 207 cases with fresh frozen tissues and a validation cohort of 344 cases with FFPE tissues from Nagoya, Kumamoto, Japan, respectively. | qPCR quantification of Fusobacterium nucleatum | High levels of F. nucleatum in ESCC associate with an invasion depth, poor recurrence-free survival, poor chemotherapeutic response |
| Gao et al. [61] | 100 patients with ESCC | 100 ESCCs with paired nontumor tissues, and 30 biopsy samples or normal oesophagus from Henan, | Immunohistochemistry and qPCR of 16S rDNA | Porphyromonas gingivalis positivity was 61% in ESCC, 12% in adjacent tissues, and negative in normal oesophagus. High burden of P. gingivalis in ESCC was |
| China | positively correlated with poor differentiation, lymph node metastasis, advanced TNM stage, and poor overall survival. | |||
| Gao et al. [90] | Patients with ESCC or esophagitis, and healthy controls. | 96 cases with ESCC, 50 cases with esophagitis, and 80 healthy controls. | ELISA to measure the serum IgG and IgA antibodies against P. gingivalis. | Serum levels of IgG and IgA against P. gingivalis were higher in ESCC patients than those in patients with esophagitis and healthy controls. Higher levels of IgG and IgA were associated with poor prognosis. |
Note: SLOTU denotes species-level operational taxonomic units.
Figure 2.
The distribution of previously published bacterial taxa derived from oral cavity and oesophagus that represented by the square and circle, respectively. The red/green indicates a detrimental and preventive bacterial taxa. For details, see text.
In the human distal oesophagus, the microbiota in histologically defined normal, oesophagitis, and Barrett’s oesophagus were classified as type I or II. The type I microbiome was well represented by the Streptococcus genus in the phenotypically normal oesophagus, whereas the type II microbiome was dominated by Gram-negative anaerobes/microaerophiles in oesophagitis and Barrett’s oesophagus. This study identified a total of nine phyla represented by 166 species, which were associated with histological phenotypes of the distal oesophagus [77] (Table 3, Figure 2). Notably, an inverse association between microbial richness and oesophageal squamous dysplasia was observed, in contrast to the opposite in normal upper digestive tract [78] (Table 3). These results suggest that lower microbial richness in the upper digestive tract in subjects from Linxian, China, could increase the risk of cancer-predisposing states in the oesophagus and stomach. Furthermore, the ESCC tissues had significantly decreased microbial diversity and high abundances of Bacteroidetes, Fusobacteria and Spirochaetes, which were associated with reduced nitrate reductase and nitrite reductase function [79]. Interestingly, a recent study from the United Kingdom also reported that the abundance of genera decreased in oesophageal adenocarcinoma comprised of Gram-negative (Veillonella, Megasphaera, and Campylobacter) and Gram-positive taxa (Granulicatella, Atopobium, Actinomyces, and Solobacterium) [75]. As the prevalence of GCA concurs with that of ESCC in the Asian Oesophageal Cancer Belt [80], Dantong and colleagues characterized the bacterial microbiota of ESCC and GCA with paired nontumor tissues from Linzhou, China. The microbial composition of ESCC and GCA tissues was primarily composed of Firmicutes, Bacteroidetes and Proteobacteria at the phylum level. ESCC harboured a greater abundance of Fusobacterium (3.2% vs. 1.3%) and a lower abundance of multiple taxa such as Streptococcus (12.0% vs. 30.2%) than nontumor tissues, whereas GCA contained a higher abundance of multiple taxa, including Firmicutes, Bacteroidetes, Fusobacteria, Actinobacteria and Proteobacteria phyla, and Helicobacter genus (60.5% vs. 11.8%) than nontumor tissues. Significant clustering between paired ESCC/GCA and nontumor tissues was found based on β-diversity, reflecting the microbial community composition by the weighted UniFrac distance [81] (Table 3). These results demonstrate that the microbial ecosystems of ESCC and GCA are distinct despite anatomical site adjacency, which warrants further validation. The abundance of P. gingivalis and T. forsythus was significantly enriched in ESCC compared with adjacent nontumor and healthy control tissues using 16S rDNA sequencing of the oesophageal mucosa biopsy of healthy controls, ESCC, and matched adjacent nontumor tissues. Notably, we also found that P. gingivalis-associated risk for ESCC was predominantly observed in ESCC patients with early clinical stage, and enrichment of Ruminococcus genus was positively correlated with poor prognosis in Anyang, a high-incidence region for ESCC. In contrast, using cloning and Sanger sequencing of 100 clones, Michihiro and colleagues reported frequent infection of the oral periodontopathic spirochete Treponema denticola, Streptococcus mitis, Streptococcus anginosus in ESCC, all of which are of oral origin and present in nearby non-tumour tissues. The oesophageal cancer samples used in this study were from Japan, China, France, and Italy, without histologic subtype information [60] (Table 3). The reasons for the discrepancy between these two studies may include different detection methods and tumour histology.
Fusobacterium nucleatum, a proportion of the normal flora inhabiting the human oral cavity, vagina, and gut, is an opportunistic pathogen implicated in a variety of inflammatory diseases and contributes to the development of many human cancers [59, 62, 65, 66, 82, 83]. The abundance of F. nucleatum was significantly increased in ESCC than in matched normal oesophageal mucosa and was positively associated with advanced tumour stage [62] (Table 3). Additionally, ESCC patients with higher abundance of F. nucleatum had significantly shorter cancer-specific and overall survival than those with lower F. nucleatum abundance. Furthermore, a higher abundance of intratumoral F. nucleatum in ESCC patients was correlated with a worse response to neoadjuvant chemotherapy and higher tumour recurrence [63] (Table 3). As a keystone pathogen in chronic periodontitis [84, 85], P. gingivalis was present in many extraoral infection-related diseases, such as cardiovascular diseases, diabetes, rheumatoid arthritis, Alzheimer’s disease, and various cancers [86–88]. A meta-analysis revealed that the prevalence of P. gingivalis in cancer patients was significantly greater than that in healthy controls, and P. gingivalis carriers have a 1.36-fold increase in risk for cancer and periodontitis [89]. Colonization of P. gingivalis in oesophageal mucosa was detected and the prevalence of P. gingivalis in ESCC was 61% compared with 12% in matched adjacent nontumor tissues. A high abundance of P. gingivalis in ESCC was positively associated with dismal survival and aggressive clinical features, including poor differentiation, lymph node metastasis, and advanced clinical stage [61] (Table 3). Furthermore, the median serum levels of IgG and IgA antibodies against P. gingivalis increased remarkably in patients with ESCC compared to those in non-ESCC controls. The combination of IgG and IgA against P. gingivalis produced a sensitivity and specificity of 68.75% and 68.46%, respectively, for the diagnosis of ESCC and even performed well in early ESCC. Furthermore, higher levels of IgG and IgA antibodies against P. gingivalis predicted worse prognosis in ESCC patients [90] (Table 3). These results indicate that F. nucleatum and P. gingivalis may play causative and tumorigenic roles in the onset and development of ESCC, which requires further investigation. Furthermore, tumor enrichment of F. nucleatum and P. gingivalis represents potential prognostic and predictive biomarkers for the clinical management of ESCC and potential targets of antibiotic intervention for improving the therapeutic response in ESCC patients.
Gastric microbiota
Despite being hostile for bacterial survival in the normal human stomach, 16S rRNA gene sequencing identified a very complex bacterial flora inhabiting the acidic niche of the stomach (Table 4), which refutes the dogma that the stomach is a sterile organ [24, 92]. Helicobacter pylori infects half of the world population [91] and is the most abundant bacterium in the gastric microbiota of H. pylori-positive subjects. The presence of H. pylori, however, did not affect the diversity and composition of gastric bacterial community [93]. Apart from H. pylori, the gastric microbial community is distinct from the oral, oesophageal and respiratory microflora despite the majority of bacteria reminiscent of upstream components of alimentary and respiratory tracts [94]. The core gastric microbiota comprises five major phyla, that is, Proteobacteria, Firmicutes, Actinobacteria, Bacteroidetes and Fusobacteria [93], common to those of oesophageal microbiota.
Table 4.
Studies of gastric microbiota on esophageal squamous cell carcinoma.
| References | Participants | Sample type | Method | Results |
|---|---|---|---|---|
| Bik et al. [93] | 23 adult subjects comprising 12 H. pylori-positive and 11 H. pylori-negative subjects | 9 gastric corpus biopsy samples and 14 gastric antrum biopsy samples | 16S rRNA gene amplification and clone sequencing | A total of 128 phylotypes were assigned to five major phyla, i.e. Proteobacteria, Firmicutes, Actinobacteria, Bacteroidetes and Fusobacteria; no significant association between gastric microbial composition and H. pylori status, gastric anatomical location, and gastric pH value; gastric microbiota is distinct from oral and oesophageal microbiotas. |
| Nasrollahzadeh et al. [95] | Case cohort comprising 19 patients with stage I to II ESCC and 18 patients with oesophageal squamous dysplasia (ESD), 17 subjects with esophagitis as disease cohort, and 37 normal oesophagus as healthy control cohort from Golestan, Iran | Snap frozen gastric corpus tissue samples | PCR amplification of V3-V4 region of 16S rDNA and sequencing | Five major core phyla across ESCC, ESD, esophagitis and normal oesophagus; higher abundances of Clostridiales and Erysipelotrichales orders in patients with ESCC or ESD; no microbial composition difference between esophagitis and normal oesophagus. |
| Shao et al. [81] | 36 patients with GCA from Linzhou, Henan, China. | Paired GCA tissue and nontumor tissue samples | PCR amplification of V4 region of 16S rDNA and sequencing | Microbial composition of GCA comprised of Firmicutes, Bacteroidetes and Proteobacteria phyla; GCA contained higher levels of Firmicutes, Bacteroidetes, Fusobacteria, Actinobacteria phyla; higher levels of Prevotella, Streptococcus, Veillonella, Haemophilus and Neisseria genera, and lower levels of Proteobacteria phylum and |
| Helicobacter genus in comparison with nontumor tissues; GCA had higher abundance of Proteobacteria phylum and Helicobacter genus in comparison with ESCC. | ||||
| Yu et al. [96] | 80 gastric cardia cancer patients from Taiyuan, Shanxi, China | Paired GCA tissue and nontumor tissue samples | PCR amplification of V3-V4 region of 16S rDNA and sequencing | GCA had higher amounts of Bacteriodetes, Firmicutes, Fusobacteria and Spirochaetes phyla, lower amounts of Proteobacteria phylum, Helicobacter genus and H. pylori compared to non-malignant tissues; either H. pylori status or gastric anatomic sites had no effects on gastric microbiota composition; |
| Yu et al. [97] | 80 gastric cardia cancer patients from Taiyuan, Shanxi, China | Paired GCA tissue and non-tumour tissue samples | PCR amplification of V3-V4 region of 16S rDNA and sequencing | In non-malignant tissue, higher level of H. pylori was positively associated with the family history of upper gastrointestinal cancer and the tumour grade; higher abundance of Lactobacillales in patients without metastasis; higher abundance of Bacteroidetes in patients with lower tumour grade. |
Chronic atrophic gastritis caused by chronic H. pylori infection is associated with an increased risk of ESCC as well as its precursor, oesophageal squamous dysplasia [38, 78]. Nevertheless, the tumorigenic effect of H. pylori infection may be more potent for gastric cardia mucosa than for oesophageal squamous mucosa. In a study conducted in Golestan, Northern Iran within the ‘oesophageal cancer belt’, gastric corpus microbiota harboured higher relative abundance of orders Clostridiales and Erysipelotrichales in cases with early ESCC and ESD compared with those of diseased and healthy controls. Although accounting for nearly 43% of the total reads, Helicobacteriacea failed to cluster the microbial communities in terms of the pathological status of the oesophagus. Based on the Unifrac distance and weighted Unifrac distance, the pattern of the gastric microbiota was significantly altered between cases and controls [95] (Table 4). Although co-occurrence of ESCC and GCA in the Asian ‘oesophageal cancer belt’ including Linzhou, China, the microbial compositions in these two types of cancer with distinct histology were different. The relative amounts of genera Helicobacter and Proteobacteria were higher in GCA, whereas ESCC was enriched with higher levels of genera Mycoplasma and Fusobacterium, and phyla Fusobacteria and Tenericutes, and less Streptococcus. In comparison with matched non-tumour tissue, GCA showed a decreased relative abundance of helicobacter genus [81] (Table 4). In Taiyuan, Shanxi, which has a high incidence of GCA, 16S rRNA gene sequencing analysis of GCA revealed that the microbiota of GCA had lower amounts of Proteobacteria and greater amounts of Bacteriodetes, Firmicutes, Fusobacteria and Spirochaetes than non-malignant tissues. The relative abundances of the genera Helicobacter and H. pylori in GCA were lower than those in matched non-malignant gastric tissues. Although H. pylori colonization was present in 94% of the gastric tissue of patients with GCA, the gastric microbiota composition did not differ according to H. pylori infection or sub-anatomic sites in the stomach [96] (Table 4). Nevertheless, no associations between the features of the GCA microbiota and clinical variables were uncovered. In contrast, the microbiota features in matched nontumor tissues were correlated with a family history of upper gastrointestinal tract cancer, tumour grade, and metastasis. A higher relative abundance of H. pylori was detected in patients with a positive family history and an advanced tumour grade. Overabundance of Bacteroidetes was associated with lower tumour grade, and higher abundance of Lactobacillales was negatively associated with metastases, suggesting a protective role for these two microoranisms [97] (Table 4). These results demonstrate that distinct microbial communities exist in non-malignant and tumor tissues in ESCC and GCA, which underlies the potential for the identification of distinguishable preventive, diagnostic, and therapeutic microbial targets between ESCC and GCA.
Virus
The complex microbial community of the microbiota includes bacteria, archaea, fungi, protozoa, and viruses. Apart from certain cancers related to bacterial infection, viruses cause approximately 10% of all human cancers worldwide [98]. Several studies have demonstrated that infection with oncogenic viruses may play potential direct pathogenic roles in the development of ESCC.
Human papilloma virus (HPV)
The role of HPV in ESCC development and progression remains controversial. The pioneering study reporting the close relationship between HPV and ESCC dates back to 1982 by Kari Syrjänen, who subsequently published several updated literature reviews summarizing this topic in 2002, 2006, 2010, and 2013 [99, 100]. The prevalence of HPV DNA in ESCC tissues is characterized by great variation with a range of 0% to 70% [101]. To date, the latest systematic review involving the largest number of ESCC cases included 14 788 cases from 187 studies performed in 32 countries from six continents (Africa, Asia, Australia, Europe, North America, and Latin America) published between 1982 and 2017 [102]. In agreement with previously published studies [100, 101, 103–108], the overall prevalence of HPV among these 14 788 cases was 30.9% (95% CI, 30.1–31.6%) with remarkable geographical differences. The highest prevalence of HPV was from China (40.0%; 95% CI, 39.0–41.1%), followed by South Africa (29.7%; 95% CI, 26.0–33.6%), and several other Asian countries, with the exception of China, which is among the highest incidence area of ESCC. In addition, forty-two reports from 12 countries between 2012 and 2017 revealed that Alpha-PV prevalence of alpha-PV was 31.1% (95% CI, 29.8-32.4%), of which HPV16/18 prevalence was 73.8% (95% CI, 71.5–76.0%). The most common HPV type detected in ESCC is HPV 16 followed by HPV 18. Integration of HPV16 into the host genome contributes to malignant conversion and cancer in the oesophageal epithelia [109].
In sharp contrast, only 1.1% of HPV infections were detected in 272 cases of ESCC from China, including Linxian, contradicting the etiological association between HPV and ESCC [110]. A prospective nested case-control study reported that the prevalence of alpha, beta, and gamma HPV in the oral cavity did not increase significantly in incident oesophageal cancer cases compared to controls matched for age, sex, and race/ethnicity. Moreover, none of 28 ESCC cases showed positive oral HPV 16 [111]. Using centralized multiplex serological assays to detect antibodies against L1 and E6/E7, only four cases and two controls were positive for HPV E6 and E7 among 1561 case subjects and 2502 controls subjects, respectively, from six case–control studies [112]. In 86 patients with early ESCC undergoing endoscopic resection or esophagectomy in France, RNAscope HPV-HR18 Probe was performed to detect 18 high-risk HPV genotypes and no cases were positive for the HPV types tested [113]. Furthermore, there was no evidence of integrated HPV DNA in ESCC samples from Western and Eastern countries by genome sequencing analysis [114, 115]. These findings indicate that HPV plays a negligible role in the pathogenesis of ESCC.
Epstein–Barr virus (EBV)
EBV is a member of the gamma-herpes virus family and preferentially infects B-lymphocytes as well as epithelial cells. It is a widespread oncovirus that affects more than 95% of all adults as a lifelong asymptomatic latent infection [116, 117]. Several studies have indicated associations between EBV and a variety of malignancies, such as multiple types of lymphoma, nasopharyngeal carcinoma, and gastric adenocarcinoma. EBV infection contributes to approximately 2% of all cancer-related mortality per year [118, 119]. Since the first report revealed the presence of EBV in microdissected ESCC samples in 1994, the frequency of EBV positivity ranged from 0% to 35.5% in ESCC tissues [104, 120, 121]. The whole-exome sequencing of 90 ESCC samples by The Cancer Genome Atlas (TCGA) failed to find evidence of EBV infection [114]. In line with this, none of the esophageal cancer samples in a large cohort of 988 esophageal cancer samples were EBV-encoded RNA (EBER) positive by EBER in situ hybridization [121]. The conflicting results on the association between EBV and ESCC can be attributed to different detection techniques and geographic factors. However, the very rare ESCC variant of lymphoepithelioma-like carcinoma is frequently EBV-positive and results in an improved outcome compared with conventional ESCC, probably due to hypermutation induced by EBV infection in parallel with heavy lymphocyte infiltration [118]. Nevertheless, despite the general null relationship between EBV and ESCC, at least some ESCC subtypes exhibit evidence of EBV infection in ESCC, to some extent, in certain races and countries.
Other viruses
In addition to the extensively studied viruses such as HPV and EBV, other viruses may play an etiological role in the development of ESCC in a similar fashion. These viruses include herpes simplex virus (HSV), cytomegalovirus (CMV) [122], Merkel cell polyomavirus (MCPyV) [104, 123] and John Cunningham virus (JCV) [124], which could serve as cofactors for HPV-related carcinogenesis.
In the oesophagus, HSV causes esophagitis as a distinct clinical entity. To date, three studies have been performed to determine the prevalence of HSV in ESCC. Among these three studies, two studies used ESCC samples from the high-incidence population of Shantou China and the HSV prevalence was 31.7% and 30.0%, respectively [125, 126]. In sharp contrast, another study investigated HSV infection by immunohistochemistry in 103 ESCC samples from Anyang, northern China, and none of the tested samples were HSV-positive [122]. The discrepancy of HSV infection in ESCC may be associated with geographic variation.
Although CMV, MCPyV, and JCV are well-recognized causative agents of other malignancies, few studies have reported their prevalence in ESCC tissues with variation. Thus, conclusive evidence of the etiological role of these viruses in ESCC is still lacking, and more studies are warranted.
Potential mechanisms of microbiota-associated tumorigenesis in ESCC
Accumulating data have demonstrated that the cancer microbiome plays a key role in the aetiology and development of some types of cancer including ESCC [127]. Elucidating the cancer-promoting mechanisms holds the potential for rational development of microbiome-based detection, prevention and treatment. Nowadays, multiple clinical and experimental studies have revealed the mechanisms which enhance malignant progression through microbial entity, cancer cell, tumour microenvironment, and immune escape.
The first potential mechanism is inflammation which has been recognized as a hallmark and cause of cancer [128]. P. gingivalis and F. nucleatum, two potential pathogens with oral origin, can induce chronic inflammatory response and establish a dysbiotic milieu which disrupts the local immunoinflammatory equilibrium [129, 130]. P. gingivalis infection led to upregulation of IL-6 and TGFβ1 in cultured ESCC cells and in ESCC specimens [131, 132]. In F. nucleatum-infected ESCC tissues, cytokine-cytokine receptor interaction was the significant enriched pathway and CCL20 was the most upregulated chemokine [62]. Second, the inflammatory cells in tumour environment can promote cancer cell proliferation as well as suppress antitumor immunity. Accumulation of CD11b+ myeloid cells in inflammatory microenvironment was observed in orthotopic MC38 rectal cancer tissues [133]. P. gingivalis can induce upregulation of B7-H1, B7-DE, and B7- H4, which inhibit effector T cells and cause immune evasion [130, 134]. Third, growing evidence suggests that intratumor microbiome and gut microbiome cancer can significantly influence response to cancer therapy [135, 136]. Microbiome ablation by oral antibiotic administration prevents oncogenic progression. Both P. gingivalis and F. nucleatum in ESCC are associated with worse chemotherapeutic efficacy and have the potential to predict response to neoadjuvant chemotherapy in patients with ESCC [63, 137]. Fourth, microbial metabolites from dietary and environmental sources contributes to the initiation and development of ESCC [2]. Dysbiosis of esophageal microbiota led to dysregulation of nitrate reductase activity [79]. Nitrosamine was increased in subjects with poor oral hygiene and the increase of nitrosamine was significantly reduced by antiseptic mouthwashes [30]. Acetaldehyde, another carcinogen in upper gastrointestinal tract, is produced from ethanol by oral bacteria, such as P. gingivalis [2]. Lastly, microbial entity itself produce a variety of virulence factors, such as LPS, gingipains, and FimA, Fap2 and FadA adhesins, which play a role in initiation and development of malignancies [61, 128, 130]. Through interaction with cancer cells, pathogenic bacteria in upper gastrointestinal tract promotes survival and suppresses death via subversion of multiple signaling pathways, including PI3K/Akt, ERK, TGFβ, Jak1/Stat3, and miR194/GRHL3/PTEN pathways [128, 130, 132, 138].
Conclusions and future challenges
Lifestyle factors, including dietary habits, oral healthcare, tobacco smoking, and alcohol consumption, play critical roles in the aetiology of ESCC. Many epidemiological studies have provided supportive evidence for poor oral hygiene status, such as tooth loss and chronic periodontitis, as risk indicators for ESCC. With the growing appreciation of microbiota in the pathophysiology of multiple diseases, including ESCC, beneficial and detrimental bacterial candidates implicated in aggressive progression and impact on therapeutic response and recurrence of ESCC have been increasingly identified. However, the causality of these detrimental bacteria, such as P. gingivalis and F. nucleatum, in the initiation and development of ESCC warrants experimental evidence and follow-up of high-risk subjects with precancerous lesions. Additionally, the etiological role of oncogenic viruses in the initiation and development of ESCC remains unclear. Further in-depth studies are needed to elucidate the biological mechanisms underlying the suppressive or promoting roles of these distinct microbial patterns in ESCC pathogenesis. The next challenge is to translate dysbiotic microbiota and related signaling effectors and metabolites into clinical utility as potential biomarkers for diagnosis, prognostication, and therapeutic targets [127].[AQ]
Supplementary Material
Funding Statement
This work was supported in part by grants from the National Natural Science Foundation of China (81872037 and U1604191).
Author contributions
SG and YJQ conceived and designed the project. ZZ and KS performed the literature collection. MXL performed the meta-analysis. YJQ wrote the manuscript and SG revised the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interests was reported by the author(s).
Data availability
All data generated or analysed during this study are included in this published article.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):1–20. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Abnet CC, Arnold M, Wei WQ.. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154(2):360–373. doi: 10.1053/j.gastro.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qi YJ, Chao WX, Chiu JF.. An overview of esophageal squamous cell carcinoma proteomics. J Proteomics. 2012;75(11):3129–3137. doi: 10.1016/j.jprot.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 4.Yang CX, Wang HY, Wang ZM, et al. Risk factors for esophageal cancer: a case-control study in South-Western China. Asian Pacific J Cancer Prevent: APJCP. 2005;6(1):48–53. [PubMed] [Google Scholar]
- 5.Siassi F, Ghadirian P.. Riboflavin deficiency and esophageal cancer: a case control-household study in the caspian Littoral of Iran. Cancer Detect Prev. 2005;29(5):464–469. doi: 10.1016/j.cdp.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Sun G, Wang S, Hu X, et al. Fumonisin B1 contamination of home-grown corn in high-risk areas for esophageal and liver cancer in China. Food Addit Contam. 2007;24(2):181–185. doi: 10.1080/02652030601013471. [DOI] [PubMed] [Google Scholar]
- 7.Wang LD, Zhou FY, Li XM, et al. Genome-wide association study of esophageal squamous cell carcinoma in Chinese subjects identifies susceptibility loci at PLCE1 and C20orf54. Nat Genet. 2010;42(9):759–763. doi: 10.1038/ng.648. [DOI] [PubMed] [Google Scholar]
- 8.Morita M, Kumashiro R, Kubo N, et al. Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophagus: epidemiology, clinical findings, and prevention. Int J Clin Oncol. 2010;15(2):126–134. doi: 10.1007/s10147-010-0056-7. [DOI] [PubMed] [Google Scholar]
- 9.Engel LS, Chow WH, Vaughan TL, et al. Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst. 2003;95(18):1404–1413. doi: 10.1093/jnci/djg047. [DOI] [PubMed] [Google Scholar]
- 10.Southerland JH, Taylor GW, Moss K, et al. Commonality in chronic inflammatory diseases: periodontitis, diabetes, and coronary artery disease. Periodontol 2000. 2006;40(1):130–143. doi: 10.1111/j.1600-0757.2005.00138.x. [DOI] [PubMed] [Google Scholar]
- 11.Teng YT, Taylor GW, Scannapieco F, et al. Periodontal health and systemic disorders. J Canadian Dental Assoc. 2002;68(3):188–192. [PubMed] [Google Scholar]
- 12.Beck JD, Offenbacher S.. Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. J Periodontol. 2005;76(11 Suppl):2089–2100. doi: 10.1902/jop.2005.76.11-S.2089. [DOI] [PubMed] [Google Scholar]
- 13.Pihlstrom BL, Michalowicz BS, Johnson NW.. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 14.Nwizu NN, Marshall JR, Moysich K, et al. Periodontal disease and incident cancer risk among postmenopausal women: results from the women’s health initiative observational cohort. Cancer Epidemiol Biomarkers Prev. 2017;26(8):1255–1265. doi: 10.1158/1055-9965.EPI-17-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer MS, Joshipura K, Giovannucci E, et al. A review of the relationship between tooth loss, periodontal disease, and cancer. Cancer Causes Control. 2008;19(9):895–907. doi: 10.1007/s10552-008-9163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michaud DS, Liu Y, Meyer M, et al. Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. Lancet Oncol. 2008;9(6):550–558. doi: 10.1016/S1470-2045(08)70106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coussens LM, Werb Z.. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balkwill F, Mantovani A.. Inflammation and cancer: back to virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 19.Allavena P, Garlanda C, Borrello MG, et al. Pathways connecting inflammation and cancer. Curr Opin Genet Dev. 2008;18(1):3–10. doi: 10.1016/j.gde.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu Rev Immunol. 2012;30(1):677–706. doi: 10.1146/annurev-immunol-020711-075008. [DOI] [PubMed] [Google Scholar]
- 21.Schetter AJ, Heegaard NH, Harris CC.. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31(1):37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin WW, Karin M.. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117(5):1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hattori N, Ushijima T.. Epigenetic impact of infection on carcinogenesis: mechanisms and applications. Genome Med. 2016;8(1):10. doi: 10.1186/s13073-016-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nardone G, Compare D, Rocco A.. A microbiota-centric view of diseases of the upper gastrointestinal tract. Lancet Gastroenterol Hepatol. 2017;2(4):298–312. doi: 10.1016/S2468-1253(16)30108-X. [DOI] [PubMed] [Google Scholar]
- 25.Ley RE, Turnbaugh PJ, Klein S, et al. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 26.Hooper LV, Gordon JI.. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 27.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134(2):577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 28.Tözün N, Vardareli E, Proceedings from the 8th Probiotics, Prebiotics & New Foods for Microbiota and Human Health meeting held in Rome, Italy on September 13-15, 2015 . Gut microbiome and gastrointestinal cancer: les liaisons dangereuses. J Clin Gastroenterol. 2016;50 Suppl 2, Proceedings from the 8th Probiotics, Prebiotics & New Foods for Microbiota and Human Health meeting held in Rome, Italy on September 13-15, 2015Suppl(Supplement 2):S191–s196. doi: 10.1097/MCG.0000000000000714. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Yuan Z, Lu M, et al. Poor oral health is associated with an increased risk of esophageal squamous cell carcinoma - a population-based case-control study in China. Int J Cancer. 2017;140(3):626–635. doi: 10.1002/ijc.30484. [DOI] [PubMed] [Google Scholar]
- 30.Abnet CC, Qiao YL, Mark SD, et al. Prospective study of tooth loss and incident esophageal and gastric cancers in China. Cancer Causes Control. 2001;12(9):847–854. doi: 10.1023/a:1012290009545. [DOI] [PubMed] [Google Scholar]
- 31.Dar NA, Islami F, Bhat GA, et al. Poor oral hygiene and risk of esophageal squamous cell carcinoma in Kashmir. Br J Cancer. 2013;109(5):1367–1372. doi: 10.1038/bjc.2013.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abnet CC, Kamangar F, Islami F, et al. Tooth loss and lack of regular oral hygiene are associated with higher risk of esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17(11):3062–3068. doi: 10.1158/1055-9965.EPI-08-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guha N, Boffetta P, Wünsch Filho V, et al. Oral health and risk of squamous cell carcinoma of the head and neck and esophagus: results of two multicentric case-control studies. Am J Epidemiol. 2007;166(10):1159–1173. doi: 10.1093/aje/kwm193. [DOI] [PubMed] [Google Scholar]
- 34.Sato F, Oze I, Kawakita D, et al. Inverse association between toothbrushing and upper aerodigestive tract cancer risk in a Japanese population. Head Neck. 2011;33(11):1628–1637. doi: 10.1002/hed.21649. [DOI] [PubMed] [Google Scholar]
- 35.Wang YP, Han XY, Su W, et al. Esophageal cancer in Shanxi province, people’s republic of China: a case-control study in high and moderate risk areas. Cancer Causes Control. 1992;3(2):107–113. doi: 10.1007/BF00051650. [DOI] [PubMed] [Google Scholar]
- 36.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8(7):481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 37.Hiraki A, Matsuo K, Suzuki T, et al. Teeth loss and risk of cancer at 14 common sites in Japanese. Cancer Epidemiol Biomarkers Prev. 2008;17(5):1222–1227. doi: 10.1158/1055-9965.EPI-07-2761. [DOI] [PubMed] [Google Scholar]
- 38.Nasrollahzadeh D, Malekzadeh R, Aghcheli K, et al. Gastric atrophy and oesophageal squamous cell carcinoma: possible interaction with dental health and oral hygiene habit. Br J Cancer. 2012;107(5):888–894. doi: 10.1038/bjc.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abnet CC, Kamangar F, Dawsey SM, et al. Tooth loss is associated with increased risk of gastric non-cardia adenocarcinoma in a cohort of Finnish smokers. Scand J Gastroenterol. 2005;40(6):681–687. doi: 10.1080/00365520510015430. [DOI] [PubMed] [Google Scholar]
- 40.Sepehr A, Kamangar F, Fahimi S, et al. Poor oral health as a risk factor for esophageal squamous dysplasia in northeastern Iran. Anticancer Res. 2005;25(1b):543–546. [PubMed] [Google Scholar]
- 41.Papapanou PN. Periodontal diseases: epidemiology. Ann Periodontol. 1996;1(1):1–36. doi: 10.1902/annals.1996.1.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Irfan UM, Dawson DV, Bissada NF.. Epidemiology of periodontal disease: a review and clinical perspectives. J Int Acad Periodontol. 2001;3(1):14–21. [PubMed] [Google Scholar]
- 43.Tonetti MS, Van Dyke TE, working group 1 of the joint EFP/AAP workshop . Periodontitis and atherosclerotic cardiovascular disease: consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases. J Periodontol. 2013;84(4 Suppl):S24–S29. doi: 10.1902/jop.2013.1340019. [DOI] [PubMed] [Google Scholar]
- 44.Oringer RJ. Modulation of the host response in periodontal therapy. J Periodontol. 2002;73(4):460–470. [DOI] [PubMed] [Google Scholar]
- 45.Baima G, Muwalla M, Testa G, et al. Periodontitis prevalence and severity in inflammatory bowel disease: a case-control study. J Periodontol. 2023;94(3):313–322. doi: 10.1002/JPER.22-0322. [DOI] [PubMed] [Google Scholar]
- 46.Sung CE, Lin FG, Huang RY, et al. Periodontitis, Helicobacter pylori infection, and gastrointestinal tract cancer mortality. J Clin Periodontol. 2022;49(3):210–220. doi: 10.1111/jcpe.13590. [DOI] [PubMed] [Google Scholar]
- 47.Fan X, Alekseyenko AV, Wu J, et al. Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut. 2018;67(1):120–127. doi: 10.1136/gutjnl-2016-312580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wen BW, Tsai CS, Lin CL, et al. Cancer risk among gingivitis and periodontitis patients: a nationwide cohort study. QJM. 2014;107(4):283–290. doi: 10.1093/qjmed/hct248. [DOI] [PubMed] [Google Scholar]
- 49.Chou SH, Tung YC, Wu LS, et al. Severity of chronic periodontitis and risk of gastrointestinal cancers: a population-based follow-up study from Taiwan. Medicine (Baltimore). 2018;97(27):e11386. doi: 10.1097/MD.0000000000011386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dewhirst FE, Chen T, Izard J, et al. The human oral microbiome. J Bacteriol. 2010;192(19):5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kudo Y, Tada H, Fujiwara N, et al. Oral environment and cancer. Genes Environ. 2016;38(1):13. doi: 10.1186/s41021-016-0042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Niu Q, Fan W, et al. Oral microbiota and gastrointestinal cancer. Onco Targets Ther. 2019;12:4721–4728. doi: 10.2147/OTT.S194153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hajishengallis G, Lamont RJ.. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27(6):409–419. doi: 10.1111/j.2041-1014.2012.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amodini Rajakaruna G, Umeda M, Uchida K, et al. Possible translocation of periodontal pathogens into the lymph nodes draining the oral cavity. J Microbiol. 2012;50(5):827–836. doi: 10.1007/s12275-012-2030-8. [DOI] [PubMed] [Google Scholar]
- 55.El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167(12):1650–1654. doi: 10.1164/rccm.200212-1543OC. [DOI] [PubMed] [Google Scholar]
- 56.Gaetti-Jardim E, Jr., Marcelino SL, Feitosa AC, et al. Quantitative detection of periodontopathic bacteria in atherosclerotic plaques from coronary arteries. J Med Microbiol. 2009;58(Pt 12):1568–1575. doi: 10.1099/jmm.0.013383-0. [DOI] [PubMed] [Google Scholar]
- 57.Ford PJ, Gemmell E, Chan A, et al. Inflammation, heat shock proteins and periodontal pathogens in atherosclerosis: an immunohistologic study. Oral Microbiol Immunol. 2006;21(4):206–211. doi: 10.1111/j.1399-302X.2006.00276.x. [DOI] [PubMed] [Google Scholar]
- 58.Salazar CR, Sun J, Li Y, et al. Association between selected oral pathogens and gastric precancerous lesions. PLoS One. 2013;8(1):e51604. doi: 10.1371/journal.pone.0051604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14(2):207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Narikiyo M, Tanabe C, Yamada Y, et al. Frequent and preferential infection of Treponema denticola, Streptococcus mitis, and Streptococcus anginosus in esophageal cancers. Cancer Sci. 2004;95(7):569–574. doi: 10.1111/j.1349-7006.2004.tb02488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao S, Li S, Ma Z, et al. Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer. Infect Agent Cancer. 2016;11(1):3. doi: 10.1186/s13027-016-0049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamamura K, Baba Y, Nakagawa S, et al. Human microbiome Fusobacterium Nucleatum in esophageal cancer tissue is associated with prognosis. Clin Cancer Res. 2016;22(22):5574–5581. doi: 10.1158/1078-0432.CCR-16-1786. [DOI] [PubMed] [Google Scholar]
- 63.Yamamura K, Izumi D, Kandimalla R, et al. Intratumoral Fusobacterium Nucleatum levels predict therapeutic response to neoadjuvant chemotherapy in esophageal squamous cell carcinoma. Clin Cancer Res. 2019;25(20):6170–6179. doi: 10.1158/1078-0432.CCR-19-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of fusobacterium with colorectal carcinoma. Genome Res. 2012;22(2):292–298. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22(2):299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu T, Guo F, Yu Y, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170(3):548–563.e516. doi: 10.1016/j.cell.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Norder Grusell E, Dahlén G, Ruth M, et al. Bacterial flora of the human oral cavity, and the upper and lower esophagus. Dis Esophagus. 2013;26(1):84–90. doi: 10.1111/j.1442-2050.2012.01328.x. [DOI] [PubMed] [Google Scholar]
- 68.Okereke I, Hamilton C, Reep G, et al. Microflora composition in the gastrointestinal tract in patients with Barrett’s esophagus. J Thorac Dis. 2019;11(Suppl 12):S1581–s1587. doi: 10.21037/jtd.2019.06.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen X, Winckler B, Lu M, et al. Oral microbiota and risk for esophageal squamous cell carcinoma in a high-risk area of China. PLoS One. 2015;10(12):e0143603. doi: 10.1371/journal.pone.0143603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wen L, Mu W, Lu H, et al. Porphyromonas gingivalis promotes oral squamous cell carcinoma progression in an immune microenvironment. J Dent Res. 2020;99(6):666–675. doi: 10.1177/0022034520909312. [DOI] [PubMed] [Google Scholar]
- 71.Peters BA, Wu J, Pei Z, et al. Oral microbiome composition reflects prospective risk for esophageal cancers. Cancer Res. 2017;77(23):6777–6787. doi: 10.1158/0008-5472.CAN-17-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gagliardi D, Makihara S, Corsi PR, et al. Microbial flora of the normal esophagus. Dis Esophagus. 1998;11(4):248–250. doi: 10.1093/dote/11.4.248. [DOI] [PubMed] [Google Scholar]
- 73.Pei Z, Bini EJ, Yang L, et al. Bacterial biota in the human distal esophagus. Proc Natl Acad Sci U S A. 2004;101(12):4250–4255. doi: 10.1073/pnas.0306398101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blackett KL, Siddhi SS, Cleary S, et al. Oesophageal bacterial biofilm changes in gastro-oesophageal reflux disease, barrett’s and oesophageal carcinoma: association or causality? Aliment Pharmacol Ther. 2013;37(11):1084–1092. doi: 10.1111/apt.12317. [DOI] [PubMed] [Google Scholar]
- 75.Elliott DRF, Walker AW, O’Donovan M, et al. A non-endoscopic device to sample the oesophageal microbiota: a case-control study. Lancet Gastroenterol Hepatol. 2017;2(1):32–42. doi: 10.1016/S2468-1253(16)30086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Verma D, Garg PK, Dubey AK.. Insights into the human oral microbiome. Arch Microbiol. 2018;200(4):525–540. doi: 10.1007/s00203-018-1505-3. [DOI] [PubMed] [Google Scholar]
- 77.Yang L, Lu X, Nossa CW, et al. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology. 2009;137(2):588–597. doi: 10.1053/j.gastro.2009.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu G, Gail MH, Shi J, et al. Association between upper digestive tract microbiota and cancer-predisposing states in the esophagus and stomach. Cancer Epidemiol Biomarkers Prev. 2014;23(5):735–741. doi: 10.1158/1055-9965.EPI-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang W, Chen CH, Jia M, et al. Tumor-Associated microbiota in esophageal squamous cell carcinoma. Front Cell Dev Biol. 2021;9:641270. doi: 10.3389/fcell.2021.641270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Glenn TF, Esophageal C. Facts, figures, and screening. Gastroenterol Nurs. 2001;24(6):271–273. quiz 274-275. doi: 10.1097/00001610-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 81.Shao D, Vogtmann E, Liu A, et al. Microbial characterization of esophageal squamous cell carcinoma and gastric cardia adenocarcinoma from a high-risk region of China. Cancer. 2019;125(22):3993–4002. doi: 10.1002/cncr.32403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cho I, Blaser MJ.. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13(4):260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamamura K, Baba Y, Miyake K, et al. Fusobacterium nucleatum in gastroenterological cancer: evaluation of measurement methods using quantitative polymerase chain reaction and a literature review. Oncol Lett. 2017;14(6):6373–6378. doi: 10.3892/ol.2017.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hajishengallis G, Darveau RP, Curtis MA.. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10(10):717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hajishengallis G, Lamont RJ.. Dancing with the stars: how choreographed bacterial interactions dictate nososymbiocity and give rise to keystone pathogens, accessory pathogens, and pathobionts. Trends Microbiol. 2016;24(6):477–489. doi: 10.1016/j.tim.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Olsen I. From the acta prize lecture 2014: the periodontal-systemic connection seen from a microbiological standpoint. Acta Odontol Scand. 2015;73(8):563–568. doi: 10.3109/00016357.2015.1007480. [DOI] [PubMed] [Google Scholar]
- 87.Atanasova KR, Yilmaz O.. Prelude to oral microbes and chronic diseases: past, present and future. Microbes Infect. 2015;17(7):473–483. doi: 10.1016/j.micinf.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dominy SS, Lynch C, Ermini F, et al. Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019;5(1):eaau3333. doi: 10.1126/sciadv.aau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sayehmiri F, Sayehmiri K, Asadollahi K, et al. The prevalence rate of Porphyromonas gingivalis and its association with cancer: a systematic review and meta-analysis. Int J Immunopathol Pharmacol. 2015;28(2):160–167. doi: 10.1177/0394632015586144. [DOI] [PubMed] [Google Scholar]
- 90.Gao SG, Yang JQ, Ma ZK, et al. Preoperative serum immunoglobulin G and a antibodies to Porphyromonas gingivalis are potential serum biomarkers for the diagnosis and prognosis of esophageal squamous cell carcinoma. BMC Cancer. 2018;18(1):17. doi: 10.1186/s12885-017-3905-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Williams MP, Pounder RE.. Helicobacter pylori: from the benign to the malignant. Am J Gastroenterol. 1999;94(11 Suppl):S11–S16. doi: 10.1016/s0002-9270(99)00657-7. [DOI] [PubMed] [Google Scholar]
- 92.Sheh A, Fox JG.. The role of the gastrointestinal microbiome in Helicobacter pylori pathogenesis. Gut Microbes. 2013;4(6):505–531. doi: 10.4161/gmic.26205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bik EM, Eckburg PB, Gill SR, et al. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci USA. 2006;103(3):732–737. doi: 10.1073/pnas.0506655103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Delgado S, Cabrera-Rubio R, Mira A, et al. Microbiological survey of the human gastric ecosystem using culturing and pyrosequencing methods. Microb Ecol. 2013;65(3):763–772. doi: 10.1007/s00248-013-0192-5. [DOI] [PubMed] [Google Scholar]
- 95.Nasrollahzadeh D, Malekzadeh R, Ploner A, et al. Variations of gastric corpus microbiota are associated with early esophageal squamous cell carcinoma and squamous dysplasia. Sci Rep. 2015;5(1):8820. doi: 10.1038/srep08820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu G, Torres J, Hu N, et al. Molecular characterization of the human stomach microbiota in gastric cancer patients. Front Cell Infect Microbiol. 2017;7:302. doi: 10.3389/fcimb.2017.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu G, Hu N, Wang L, et al. Gastric microbiota features associated with cancer risk factors and clinical outcomes: a pilot study in gastric cardia cancer patients from shanxi, China. Int J Cancer. 2017;141(1):45–51. doi: 10.1002/ijc.30700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Plummer M, de Martel C, Vignat J, et al. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4(9):e609–616. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 99.Syrjänen KJ. HPV infections and oesophageal cancer. J Clin Pathol. 2002;55(10):721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Syrjänen K. Geographic origin is a significant determinant of human papillomavirus prevalence in oesophageal squamous cell carcinoma: systematic review and meta-analysis. Scand J Infect Dis. 2013;45(1):1–18. doi: 10.3109/00365548.2012.702281. [DOI] [PubMed] [Google Scholar]
- 101.Zhang SK, Guo LW, Chen Q, et al. Prevalence of human papillomavirus 16 in esophageal cancer among the chinese population: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2014;15(23):10143–10149. doi: 10.7314/apjcp.2014.15.23.10143. [DOI] [PubMed] [Google Scholar]
- 102.Hošnjak L, Poljak M.. A systematic literature review of studies reporting human papillomavirus (HPV) prevalence in esophageal carcinoma over 36 years (1982-2017). Acta Dermatovenerologica Alpina, Pannonica, et Adriatica. 2018;27(3):127–136. [PubMed] [Google Scholar]
- 103.Petrick JL, Wyss AB, Butler AM, et al. Prevalence of human papillomavirus among oesophageal squamous cell carcinoma cases: systematic review and meta-analysis. Br J Cancer. 2014;110(9):2369–2377. doi: 10.1038/bjc.2014.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yahyapour Y, Rahmani R, Alipour M, et al. Prevalence and association of human papillomavirus, Epstein-Barr virus and merkel cell polyomavirus with neoplastic esophageal lesions in Northern Iran. Caspian J Intern Med. 2018;9(4):353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yong F, Xudong N, Lijie T.. Human papillomavirus types 16 and 18 in esophagus squamous cell carcinoma: a meta-analysis. Ann Epidemiol. 2013;23(11):726–734. doi: 10.1016/j.annepidem.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 106.Guo LW, Zhang SK, Liu SZ, et al. Human papillomavirus type-18 prevalence in oesophageal cancer in the chinese population: a meta-analysis. Epidemiol Infect. 2016;144(3):469–477. doi: 10.1017/S0950268815001703. [DOI] [PubMed] [Google Scholar]
- 107.Guo F, Liu Y, Wang X, et al. Human papillomavirus infection and esophageal squamous cell carcinoma: a case-control study. Cancer Epidemiol Biomarkers Prev. 2012;21(5):780–785. doi: 10.1158/1055-9965.EPI-11-1206. [DOI] [PubMed] [Google Scholar]
- 108.Liyanage SS, Rahman B, Ridda I, et al. The aetiological role of human papillomavirus in oesophageal squamous cell carcinoma: a meta-analysis. PLoS One. 2013;8(7):e69238. doi: 10.1371/journal.pone.0069238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li S, Shen H, Li J, et al. Prevalence of the integration status for human papillomavirus 16 in esophageal carcinoma samples. Turk J Gastroenterol. 2018;29(2):157–163. doi: 10.5152/tjg.2018.17568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Koshiol J, Wei WQ, Kreimer AR, et al. No role for human papillomavirus in esophageal squamous cell carcinoma in China. Int J Cancer. 2010;127(1):93–100. doi: 10.1002/ijc.25023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Agalliu I, Chen Z, Wang T, et al. Oral alpha, beta, and gamma HPV types and risk of incident esophageal cancer. Cancer Epidemiol Biomarkers Prev. 2018;27(10):1168–1175. doi: 10.1158/1055-9965.EPI-18-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sitas F, Egger S, Urban MI, et al. InterSCOPE study: Associations between esophageal squamous cell carcinoma and human papillomavirus serological markers. J Natl Cancer Inst. 2012;104(2):147–158. doi: 10.1093/jnci/djr499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kanaan C, Lorenzo D, Barret M, et al. Early esophageal squamous cell carcinoma in a Western series is not associated with active HPV infection. Virchows Arch. 2020;477(5):697–704. doi: 10.1007/s00428-020-02860-2. [DOI] [PubMed] [Google Scholar]
- 114.Network. CGAR . Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541(7636):169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu W, Snell JM, Jeck WR, et al. Subtyping Sub-Saharan esophageal squamous cell carcinoma by comprehensive molecular analysis. JCI Insight. 2016;1(16):e88755. doi: 10.1172/jci.insight.88755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stanfield BA, Luftig MA.. Recent advances in understanding Epstein-Barr virus. F1000Res. 2017;6:386. doi: 10.12688/f1000research.10591.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Young LS, Rickinson AB.. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4(10):757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 118.El-Zimaity H, Di Pilato V, Novella Ringressi M, et al. Risk factors for esophageal cancer: emphasis on infectious agents. Ann N Y Acad Sci. 2018;1434(1):319–332. doi: 10.1111/nyas.13858. [DOI] [PubMed] [Google Scholar]
- 119.Xu W, Liu Z, Bao Q, et al. Viruses, other pathogenic microorganisms and esophageal cancer. Gastrointest Tumors. 2015;2(1):2–13. doi: 10.1159/000380897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jenkins TD, Nakagawa H, Rustgi AK.. The association of Epstein-Barr virus DNA with esophageal squamous cell carcinoma. Oncogene. 1996;13(8):1809–1813. [PubMed] [Google Scholar]
- 121.Hewitt LC, Inam IZ, Saito Y, et al. Epstein-Barr virus and mismatch repair deficiency status differ between oesophageal and gastric cancer: a large multi-Centre study. Eur J Cancer. 2018;94:104–114. doi: 10.1016/j.ejca.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chang F, Syrjänen S, Shen Q, et al. Evaluation of HPV, CMV, HSV and EBV in esophageal squamous cell carcinomas from a high-incidence area of China. Anticancer Research. 2000;20(5c):3935–3940. [PubMed] [Google Scholar]
- 123.Yahyapour Y, Sadeghi F, Alizadeh A, et al. Detection of merkel cell polyomavirus and human papillomavirus in esophageal squamous cell carcinomas and non-Cancerous esophageal samples in Northern Iran. Pathol Oncol Res. 2016;22(4):667–672. doi: 10.1007/s12253-016-0048-7. [DOI] [PubMed] [Google Scholar]
- 124.Del Valle L, White MK, Enam S, et al. Detection of JC virus DNA sequences and expression of viral T antigen and agnoprotein in esophageal carcinoma. Cancer. 2005;103(3):516–527. doi: 10.1002/cncr.20806. [DOI] [PubMed] [Google Scholar]
- 125.Wu MY, Wu XY, Zhuang CX.. Detection of HSV and EBV in esophageal carcinomas from a high-incidence area in Shantou China. Dis Esophagus. 2005;18(1):46–50. doi: 10.1111/j.1442-2050.2005.00423.x. [DOI] [PubMed] [Google Scholar]
- 126.Zhang DH, Zhang QY, Hong CQ, et al. Prevalence and association of human papillomavirus 16, Epstein-Barr virus, herpes simplex virus-1 and cytomegalovirus infection with human esophageal carcinoma: a case-control study. Oncology Reports. 2011;25(6):1731–1738. [DOI] [PubMed] [Google Scholar]
- 127.Poore GD, Kopylova E, Zhu Q, et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature. 2020;579(7800):567–574. doi: 10.1038/s41586-020-2095-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 128.El Tekle G, Garrett WS.. Bacteria in cancer initiation, promotion and progression. Nat Rev Cancer. 2023;23(9):600–618. doi: 10.1038/s41568-023-00594-2. [DOI] [PubMed] [Google Scholar]
- 129.Reyes L. Porphyromonas gingivalis. Trends Microbiol. 2021;29(4):376–377. doi: 10.1016/j.tim.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 130.Whitmore SE, Lamont RJ.. Oral bacteria and cancer. PLoS Pathog. 2014;10(3):e1003933. doi: 10.1371/journal.ppat.1003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen MF, Lu MS, Hsieh CC, et al. Porphyromonas gingivalis promotes tumor progression in esophageal squamous cell carcinoma. Cell Oncol (Dordr). 2021;44(2):373–384. doi: 10.1007/s13402-020-00573-x. [DOI] [PubMed] [Google Scholar]
- 132.Gao S, Liu K, Jiao Y, et al. Selective activation of TGFβ signaling by P. gingivalis-mediated upregulation of GARP aggravates esophageal squamous cell carcinoma. Am J Cancer Res. 2023;13(5):2013–2029. [PMC free article] [PubMed] [Google Scholar]
- 133.Wang X, Jia Y, Wen L, et al. Porphyromonas gingivalis promotes colorectal carcinoma by activating the hematopoietic NLRP3 inflammasome. Cancer Res. 2021;81(10):2745–2759. doi: 10.1158/0008-5472.CAN-20-3827. [DOI] [PubMed] [Google Scholar]
- 134.Yuan X, Liu Y, Li G, et al. Blockade of immune-Checkpoint B7-H4 and lysine demethylase 5B in esophageal squamous cell carcinoma confers protective immunity against P. gingivalis infection. Cancer Immunol Res. 2019;7(9):1440–1456. doi: 10.1158/2326-6066.CIR-18-0709. [DOI] [PubMed] [Google Scholar]
- 135.Geller LT, Barzily-Rokni M, Danino T, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357(6356):1156–1160. doi: 10.1126/science.aah5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 137.Gao S, Liu Y, Duan X, et al. Porphyromonas gingivalis infection exacerbates oesophageal cancer and promotes resistance to neoadjuvant chemotherapy. Br J Cancer. 2021;125(3):433–444. doi: 10.1038/s41416-021-01419-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liang G, Wang H, Shi H, et al. Porphyromonas gingivalis promotes the proliferation and migration of esophageal squamous cell carcinoma through the miR-194/GRHL3/PTEN/akt axis. ACS Infect Dis. 2020;6(5):871–881. doi: 10.1021/acsinfecdis.0c00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article.