Abstract
Background
Cold atmospheric plasma (CAP), is a technology based on non‐thermal ionized gas that is used for cancer therapy in research. We evaluated the effect of CAP on malignant melanoma cancer cell line (B16) in comparison with normal cells (L929).
Methods
The effect of CAP on the cytotoxicity of B16 and L929 cell lines was assayed by the MTT method and inverted microscopy. The induction of apoptosis in cells was evaluated using a fluorescence microscope. FTIR monitored the CAP effect in biomacromolecules changes in these cell lines. QPCR assayed gene expression of BAX, BCL‐2, and Caspase‐3 (CASP‐3).
Results
The results of the MTT test showed CAP has a cytotoxic effect on the B16 cancer cell line more than L929 normal cells (p < 0.0001). The results of invert and fluorescence microscopy showed CAP‐induced apoptotic morphology on cancerous cells. FTIR spectroscopy indicated CAP changes biomacromolecules structure. Evaluation of gene expression showed CAP increased BAX and CASP‐3 gene expression. Also, it decreased BCL‐2 gene expression.
Conclusions
Taken together, CAP may change biomacromolecule structures involved in apoptosis pathways, decrease proliferation and induce apoptosis in cancer cells.
Keywords: cold atmospheric plasma, cytotoxicity, FTIR spectroscopy, melanoma cancer
1. INTRODUCTION
Cutaneous melanoma represents one of the most lethal forms of skin cancer and shows a highly metastatic phenotype. According to epidemiological studies, Melanoma is the 19th most common cancer worldwide, with estimated age‐standardized incidence rates of 2.8−3.1 per 100,000. 1
Despite diagnosing melanoma in early‐stage, often progression of melanoma could be increased up to stage IV with no prognostic assignment. 2 As long as surgery, immunotherapy, chemotherapy, and radiation therapy have been used in melanoma treatment. These treatments have rarely been successful, because melanoma is tolerated against many trial approaches. On the other view, the extensive cost, many side effects and not being selectiveness these trial options, it has been tried to find novel efficient methods with faster improvement potential than conventional clinical approaches. 2
Cold atmospheric plasma (CAP) is a non‐thermal ionized gas type such as argon, nitrogen, and helium, which is created from flowing the electric charge with high voltage through the gas medium tube resulting in components production including electrons, ions, reactive oxygen species (ROS), hydroxyl radicals (HO), reactive nitrogen radicals (RNS), and hydrogen peroxide. 3 , 4 CAP has been applied as a useful device in many various biomedicine aspects such as deactivating bacterial species, sterilization of surface, wound healing, skin regeneration, blood coagulation, and tooth caring. Also, many investigations have indicated that CAP could be successfully effective for cancer treatment. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 Noticeably, in these observations, normal cells or tissues are rarely implicated in exposure to CAP‐influent components. Following these investigations, more attempt has been made to survey the mechanisms that differentiate normal cell function in comparison to cancer cells. Various biochemical assays, molecular techniques, and apoptotic assays have been applied to probe pathways signaling to contribute cellular processes including cell cycle, proliferation, differentiation, invasion, and migration. But often these methods are time‐consuming and expensive. Despite various methods, Fourier transform infrared (FTIR) is a powerful, sensitive, non‐invasive, immediate, and chief technique that proves the distinction of macromolecules; phospholipids, proteins, nucleic acids, and carbohydrates through main functional groups markers in the fingerprint area of inferared (IR) wavelength in biological organisms. 5 Currently, biomedical scientists utilize FTIR for monitoring biochemical changes in cells and tissue during apoptosis, differentiation, and cell cycle. 6 Also, many studies have indicated that FTIR is a sensitive and useful tool for distinguishing normal cells from cancerous cells. 5 , 6 , 7 , 8 , 9 , 10 In our work, FTIR was used to investigate the CAP effect in biomacromolecule changes of B16 murine‐derived melanoma cell line and L929 mouse normal fibroblast cells.
2. MATERIALS AND METHODS
2.1. Cell culture
B16 and L929 cell lines were purchased from the cell bank of Pasteur Institute of Iran. The cell lines were grown separately in T25 flask in complete media containing Dulbecco's modified Eagle's medium (DMEM, Gibco, 12800116) with 10% fetal bovine serum (FBS, Gibco, 10270) and penicillin/streptomycin (Gibco, 15140) and kept at 37°C in 5% carbon dioxide. 11
2.2. Cold atmospheric plasma treatment
The CAP device was created in the school of physics at the University of Mazandaran which was previously reported. Briefly, it contains copper wire electrodes, a grounded copper ring electrode, and a Pyrex tube dielectric. There is a distance of 7 mm between the two electrodes. The used gas was Argon with purity of 99.9999%. Cold plasma treatments were carried out at 0–25 kV, and 9 kHz, using an Argon flow of 2.5 L/min, with a distance of 3 cm from the plasma source to the treatments. 12 , 13 , 14 , 15 The temperature of gas was 35–40 degree Celsius (°C). The hydroxyl radical (OH−) is the most abundant of reactive oxygen species (ROS) that is produced by this device. Other abundant ROS includes O, O2 +, O4 +, O2 −, O−, O3, NO, O2, and H2O2. Treatment was performed by two strategies direct and indirect approaches. In the direct method CAP device directly treated the cells seeded in a multi‐wells plate at variable times. In another approach, the CAP device stimulated the cell culture medium for three minutes of exposure to inhibit the growth of cancer cells during the cell culture process. In both approaches, the distance of CAP from treatment was 3 cm.
2.3. 3‐(4,5)‐dimethyl thiahiazo‐(‐z‐y1)−3, 5‐diphenytetrazoliumromide (MTT) assay
After being reached to 70% confluency condition, the medium was discarded and cells were separated with trypsin. 8×103 cells from the B16 cell line and 1×104 cells of L929 cells were seeded per well into 96 well plates and incubated in the standard condition. Then, cells in each well were treated with CAP by the direct treatment in variable times of 20, 30, 40, and 50 s of plasma radiation and maintained for 24 h in the standard condition. Afterward, a colorimetric MTT assay was used to examine cell viability based on the reduction of MTT to formazan in the mitochondrial membrane of live cells. Briefly, treated media was removed and 20 μL of MTT solution (5 mg/mL) was added to each well of 96 well‐plate and incubated for 4 h. For releasing formazan, 200 μL dimethyl sulfoxide (DMSO) was added to solve the formazan crystal. Finally, media absorbance was read with a microplate Elisa reader (Synergy HT, BioTek) at 570 nm. This test was repeated in triplicate. 11
2.4. Acridine orange and ethidium bromide staining
Evaluation of morphological change during cell death was performed with dual acridine orange (AO) and ethidium bromide (EB) staining. AO can be uptake with alive cells as shown with a green nucleus and early apoptotic cells as determined with a bright green nucleus and condensed pyknotic chromatin. EB labels late apoptotic cells which show bright orange nuclei with condensed pyknotic chromatin bodies and necrotic cells with a uniformly orange nucleus. For this work, 8×103 cells from the B16 cell line and 1×104 cells of L929 cells were seeded per well into 96 well plates and then incubated for 24 h. The cells were exposed directly to CAP for 40 seconds. After 24 h, the cells were washed twice with PBS. Either untreated or treated cells in both cell lines were stained with AO/EB solution and kept at room temperature for 5 min. Then, the staining solution was removed and washing was repeated. Next, the cells were monitored under the fluorescence microscope equipped with a digital camera. 11
2.5. FTIR
FTIR was performed to survey intra‐cellularmacromolecules following CAP treatment. B16 and L929 cells were seeded at the concentration of 18×104 cells of the B16 cell line and 2×10 5 cells of the L929 cell line per well in 6 well plates. After 24 h of culture, cells were exposed to indirect CAP treatment and kept two times for 2 and 4 h in the incubator. Then, cells were collected using centrifugation at 1800 RPM, washed with 0.9% NaCl solution 3 times, and finally re‐suspended in 0.9% NaCl solution. About 5 mL cell suspension was deposited on an IR transparent BaF2 window, then dried under vacuum at room temperature. Measurements were performed with WQF‐510 (Rayleigh Optics, China) spectrometer, equipped with a KBr beam splitter, a DLaTGS (deuterated, l‐alanine doped triglycine sulfate) detector, and N2 (99.999%) for refining the system. For each spectrum, 100 scans were co‐added with a 4 cm −1 resolution in the region of 400 and 4000 by Beijing software (second optical instrument factory main FTOS system V 1.0, China). The spectrum was normalized with baseline‐corrected using Beijing's second optical software. Resulted spectra were then scattering corrected using software developed in Shirazi's lab (Tehran, Iran). The average of all the spectra in the test and control groups were compared using domestic software and evaluated.
2.6. Evaluation of apoptosis gene expression upon CAP treatment
Total RNA was extracted from treated and untreated cells by Favorgen column kit, Taiwan. The cDNA was prepared by Addbio cDNA Synthesis Kit, Korea. Real‐time quantitative polymerase chain reaction was done by stem‐loop TaqMan real‐time PCR assay, unique sequence index (USI) barcodes and probe. GAPDH (housekeeping gene) expression level was used for normalization. Finally, the average of Double Ct values was assayed to determine relative expression levels of BAX, BCL‐2, and Caspase‐3 (CASP‐3) apoptosis genes that were calculated using the comparative Ct method. 16
2.7. Statistical analysis
Results were expressed as mean ± SD. Data analysis was performed by analysis of variance (ANOVA) and Tukey's post hoc test, using SPSS V.16. p < 0.05 was considered for significant statistical differences.
3. RESULTS
3.1. CAP reduced B16 cell proliferation
The effect of CAP on cell proliferation of cancer and normal cell lines was detected using observation in the light microscope and MTT test. Microscopic images revealed CAP had significant damage to the morphology of the B16 cancer cell line compared to the untreated control group, while this treatment didn't affect morphological changes of L929 normal cells (Figure 1A). These findings might show the selective toxicity of CAP on tumor cells but not on normal cells. MTT assay results showed that CAP strongly inhibited the viability of the B16 cell line, while it has not a significant effect on L929 cells (Figure 1B). Taken together, our results showed that CAP was capable of inhibiting B16 cell proliferation, suggesting its role in modulating melanoma cancer cells, without any anti‐proliferative activities on normal cells.
FIGURE 1.
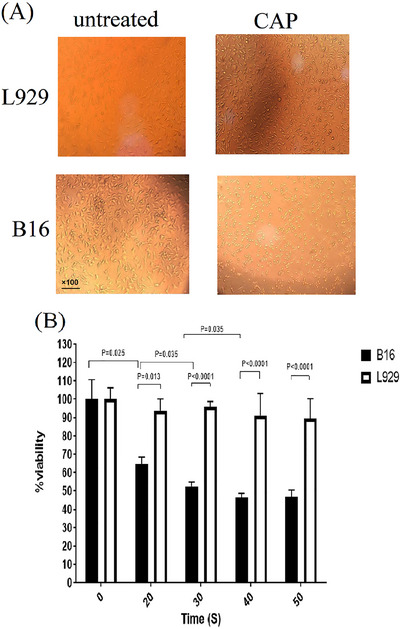
(A) Morphological analysis of the cells using an inverted microscope (×100). (B) MTT assay results in 24 h after CAP application. CAP selectively diminished the viability of B16 melanoma cancer cells in a dose‐response manner, while it not affected on the viability of normal L929 cells. The tests were performed three times.
3.2. CAP can selectively increase apoptotic activity in the B16 cancer cell line
Cancer and normal cells were labeled by AO/EB after 24 h of CAP treatment. Dual staining was examined under a fluorescent microscope. Apoptotic cells in the early stage, are marked by the crescent‐shaped or granular yellow‐green nuclear staining. Apoptotic cells in late stage were also detected with concentrated and asymmetrically localized orange nuclear staining. Necrotic cells were detected with uniform orange staining. As Figure 2 Shows AO/EB staining revealed the high occurrence of apoptosis in CAP‐treated B16 cells, while no significant apoptotic cells were detected in the L929 cell line after treatment of CAP.
FIGURE 2.

Fluorescence images of B16 and L929 cell lines that are stained with acridine orange/ethidium bromide (AO/EB) after being exposed to CAP for 24 h. The test was performed three times.
3.3. Cellular biochemical alteration detected using the FTIR microspectroscopy
Biomolecular changes in control and treated cells were investigated using FTIR. The scatter‐corrected spectra of treated and untreated cells are shown in the range of 900 to 2000 cm−1 in Figure 3. The Results of absorbance changes corresponding to the cellular biomolecules were summarized as follows:
FIGURE 3.

FTIR spectral analysis. (A) The scatter‐corrected FTIR bio spectra of B16 melanoma cancer cells. (B) The scatter‐corrected FTIR bio spectra of L929 normal cell line. The average of all spectra in test (in red) and all spectra in control (in blue) group. The test was performed two times.
In the B16 cell line after 2 h of CAP treatment, the absorbance changes in peaks of 1816, 1841, 3010, 1914, and 1914 cm−1 were to the higher frequencies. These changes may represent the connection of CAP to Succinimidyl ester anhydrides and Pyrolidinone suximide (peak index of 1816 cm−1), Biomolecules containing repeated CH2 groups such as cellular fats (peak index of 1841 cm−1), CH groups of fatty acids and Ring structures (peak index of 3010 cm−1), Carbonyl structures (peak index of 1914 cm−1), and carbon with a double bond (peak index of 1934 cm−1). On other hand, after 2 h of CAP, the absorbance changes in peaks of 1816, 1841, 3010, 1914, and 1914 cm‐1 were to the lower frequencies. These changes may represent the connection of CAP to the next structures to the following groups:
C‐N (peak index of 1346 cm−1), unusual hydrocarbon structures, cellular NH (peak index of 1413 cm−1), and carbonyl in polysaccharide structures (peak index of 1936 cm−1).
Increasing the time of CAP treatment to 4 h increases absorbance peaks of 1263, 1517, 1816, 1914, and 1934 cm−1. These changes are indicating the connection of CAP to the asymmetric structure of phosphate I (peak index of 1263 cm−1), Amide II in collagen biomolecules (peak index of 1517 cm−1), anidrides in Succinimidyl ester anhydrides and Pyrrolidinone suximide (peak index of 1816 cm−1), Carbonyl structures (peak index of 1914 cm−1), and carbon with double bonds (peak index of 1934 cm‐1). On the other hand, increasing the time of CAP treatment to 4 h decreases absorbance peaks of 1450, 1550, and 1893 cm−1. These changes are indicating the connection of CAP to the molecules associated with these structures:
CH3 in Valine and vibrations of the ring in tyrosine and phenylalanine amino acids (peak index of 1934 cm−1), Amide II in the cellular proteins (peak index of 1550 cm−1), and Abnormal carbonyl and aldehyde structures (peak index of 1893 cm−1).
In the L929 normal cell line after 2 h of CAP treatment, all the absorbance changes in peak index were to the higher frequencies. Changes in the peak index maybe include the connection of CAP to carbon with double bonds (peak index of 1936 cm−1), carbonyl (peak index of 1968 cm−1), changed the structure of NH3 in amino acid of lysine, the tensile form of carbon with double bonds, and C = N in nucleic acids (peak index of 1535 cm−1), and C = C = N (peak index of 2009 cm−1).
3.4. CAP can selectively increase BAX, and CASP‐3 and decrease BCL‐2 gene expression in the B16 cancer cell line
QPCR test was for evaluation of BAX, BCL‐2, and CASP‐3 gene expression levels in the effect of CAP on B16 and L929 cells. As Figure 4 showed, the expression of BAX (p < 0.0001), and CASP‐3 (p < 0.0001) was significantly increased after treatment of CAP in B16 cancer cells but not L929 cells. On the other hand, CAP treatment decreased BCL‐2 expression in both of B16 (p < 0.0001) and L929 (p = 0.004) cells in comparison with untreated cells. But CAP has more effect on B16 cancer cells in comparison with L929 normal cells (p = 0.034).
FIGURE 4.
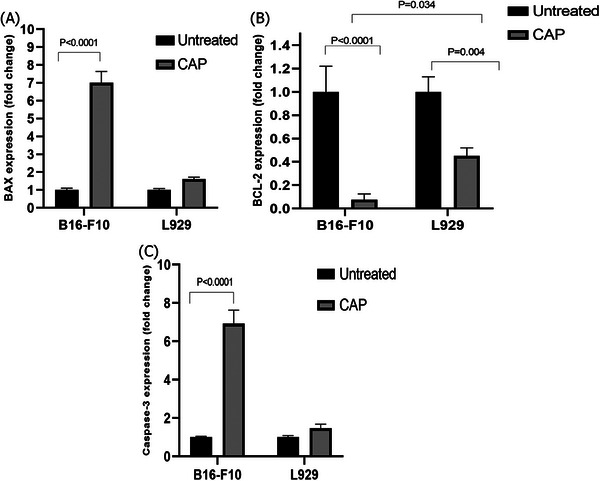
Expression of apoptosis related genes of BAX, BCL‐2, and Caspase‐3 in B16 and L929 cells after 24 h of CAP treatment. The tests were performed three times.
4. DISCUSSION
This study evaluated the inhibition effect of CAP in the B16 melanoma cancer cell line in comparison with normal cells and the mechanisms underlying this effect.
The results showed CAP can kill cancer cells and does not have cytotoxicity on normal cells (p < 0.05). These results are in line with other studies in tumor cell lines of melanoma, 17 , 18 , 19 , 20 , 21 , 22 , 23 breast, 24 , 25 colorectal, 19 leukemia, 26 glioblastoma, 27 liver, 28 , 29 head and neck, 30 and prostate 31 cancer cell lines.
Melanoma cells are resistant to a variety of anticancer drugs using their intrinsic resistance to apoptosis. 32 So, induction of apoptosis is very important for the treatment of this disease. Several studies have suggested CAP induces apoptosis in cancer cells. 23 , 25 , 31 , 33 , 34 , 35 This study showed CAP induced significant apoptotic activity in B16 melanoma cells, while this treatment did not affect on apoptosis of normal cells. It can increase BAX, and CASP‐3 and decreases of BCL‐2 gene expression. These results showed CAP induces apoptosis selectivity in B16 cancer cells but not in L929 normal cells. The selective response of tumor cells to CAP may also be due to cellular biochemical alternation. In this study for the first time, we evaluated the effects of CAP on biomolecular structures that have roles in the induction of apoptosis of melanoma cancer and normal cell lines. Analysis of FTIR bio spectroscopy revealed that CAP changed the conformation and configuration of cellular DNA, proteins, and membrane lipid bilayer matrix in B16 cancer cell lines more normal cells. In another word, treatment with CAP changed smoothly structure of nucleic acids, groups—characterizing carbohydrate and secondary structure of proteins in L929 normal cell line (carbon with double bonds, carbonyl, changed the structure of NH3 in amino acid of lysine, the tensile form of carbon with double bonds, C = N and C = C = N). But the CAP treatment had a stronger effect on the alteration of cellular biochemistry of the B16 cancer cell line. Treatment of CAP in 2 h changed cellular fat and groups—characterizing carbohydrates, nucleic acids, cyclic nucleotides, and proteins (e.g., those occurring during the cell cycle and/or apoptosis). 4 h of CAP treatment, changed the structure of cellular proteins strongly, in particular Amid II bands in cellular proteins and Collagen, and changes in the amino acids of Valine, Tyrosine, and phenylalanine. Also, this treatment changed the structures of nucleic acids, cyclic nucleotides, carbohydrates, and lipids. The changes in the biomolecule's structure may change the expression of genes and proteins that regulate apoptosis. These can change inactivate enzymes involved in cell proliferation, destroy cytoskeleton, cleavage of DNA, and finally induce cell death suggestive of apoptosis and cytotoxic selectivity effect in cancer cells. Finally, this study had some limitations. This study assayed effect of CAP on changing of biomolecular structures involved in apoptosis pathways of melanoma cancer and expression of apoptosis genes. But it did not assay apoptosis directly. Further studies are needed to show inducing of apoptosis such as measurement of cleaved caspase or TUNEL assay.
5. CONCLUSION
In conclusion, this study suggests that CAP may change biomacromolecule structures involved in apoptosis pathways in cancer cells. It can inactivate proteins and enzymes involved in cell proliferation, destroying cytoskeleton, and cleavage of DNA. Finally, it can induce cell death suggestive of apoptosis in cancer cells.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The protocol of this study was approved by the Research Ethics committee of Mazandaran University of Medical Sciences.
ACKNOWLEDGEMENTS
This research was funded by the Research and Technology Deputy of Mazandaran University of Medical Sciences [grant number: 853].
Yazdani Z, Pasandi MS, Golpour M, Eslami M, Rafiei A. Effect of cold atmospheric plasma on changing of biomolecular structures involved in apoptosis pathways of melanoma cancer. Skin Res Technol. 2024;30:e13544. 10.1111/srt.13544
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Ali Z, Yousaf N, Larkin J. Melanoma epidemiology, biology and prognosis. EJC. Suppl 2013;11(2):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davis LE, Shalin SC, Tackett AJ. Current state of melanoma diagnosis and treatment. Cancer Biol Ther 2019;20(11):1366‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Keidar M, Shashurin A, Volotskova O, et al. Cold atmospheric plasma in cancer therapy. Phys Plasmas. 2013;20(5):057101. [Google Scholar]
- 4. Yan D, Sherman JH, Keidar M. Cold atmospheric plasma, a novel promising anti‐cancer treatment modality. Oncotarget. 2017;8(9):15977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Derenne A, Van Hemelryck V, Lamoral‐Theys D, Kiss R, Goormaghtigh E. FTIR spectroscopy: a new valuable tool to classify the effects of polyphenolic compounds on cancer cells. Biochim Biophys Acta Mol Basis Dis. 2013;1832(1):46‐56. [DOI] [PubMed] [Google Scholar]
- 6. Movasaghi Z, Rehman S, ur Rehman DI. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl Spectrosc Rev 2008;43(2):134‐179. [Google Scholar]
- 7. Sitnikova VE, Kotkova MA, Nosenko TN, Kotkova TN, Martynova DM, Uspenskaya MV. Breast cancer detection by ATR‐FTIR spectroscopy of blood serum and multivariate data‐analysis. Talanta. 2020;214:120857. [DOI] [PubMed] [Google Scholar]
- 8. Yousif ES, Abdulkareem DT, Mohammad EJ. Detection of urinary bladder cancer by (ATR‐FTIR) spectroscopy. Syst Rev Pharm. 2020;11(12):1932. [Google Scholar]
- 9. Tiernan H, Byrne B, Kazarian SG. ATR‐FTIR spectroscopy and spectroscopic imaging for the analysis of biopharmaceuticals. Spectrochim Acta A Mol Biomol Spectrosc. 2020;241:118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang X, Ou Q, Yang W, Shi Y, Liu G. Diagnosis of liver cancer by FTIR spectra of serum. Spectrochim Acta A Mol Biomol Spectrosc. 2021;263:120181. [DOI] [PubMed] [Google Scholar]
- 11. Kardan M, Yazdani Z, Morsaljahan Z, Ebrahimzadeh MA, Rafiei A. Cytotoxic effect of methanolic extracts of Fritillaria imperialis bulbs and Eryngium caucasicum leaves on hepatoma and colon cancer cells. Asian Pac J Trop Biomed. 2019;9(8):353. [Google Scholar]
- 12. Rafiei A, Sohbatzadeh F, Hadavi S, Bekeschus S, Alimohammadi M, Valadan R. Inhibition of murine melanoma tumor growth in vitro and in vivo using an argon‐based plasma jet. Clin Plasma Med. 2020;19:100102. [Google Scholar]
- 13. Alimohammadi M, Golpur M, Sohbatzadeh F, et al. Cold atmospheric plasma is a potent tool to improve chemotherapy in melanoma in vitro and in vivo. Biomolecules. 2020;10(7):1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yazdani Z, Mehrabanjoubani P, Biparva P, Rafiei A. Cytotoxicity Effect of Cold Atmospheric Plasma on Melanoma (B16‐F10), Breast (MCF‐7) and Lung (A549) Cancer Cell Lines Compared with Normal Cells. Majallahi Danishgahi Ulumi Pizishkii Mazandaran. 2020;30(187):38‐48. [Google Scholar]
- 15. Yazdani Z, Mehrabanjoubani P, Rafiei A, Biparva P, Kardan M. Combined effect of cold atmospheric plasma and curcumin in melanoma cancer. Biomed Res Int. 2021;2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sharifi E, Yazdani Z, Najafi M, Hosseini‐khah Z, Jafarpour A, Rafiei A. The combined effect of fish oil containing Omega‐3 fatty acids and Lactobacillus plantarum on colorectal cancer. Food Sci Nutr. 2022;10(12):4411‐4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zucker SN, Zirnheld J, Bagati A, et al. Preferential induction of apoptotic cell death in melanoma cells as compared with normal keratinocytes using a non‐thermal plasma torch. Cancer Biol Ther. 2012;13(13):1299‐1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zirnheld JL, Zucker SN, DiSanto TM, Berezney R, Etemadi K. Nonthermal plasma needle: development and targeting of melanoma cells. IEEE Trans Plasma Sci. 2010;38(4):948‐952. [Google Scholar]
- 19. Lupu A‐R, Georgescu N, Cãlugãru A, Cremer L, Szegli G, Kerek F. The effects of cold atmospheric plasma jets on B16 and COLO320 tumoral cells. Roum Arch Microbiol Immunol. 2009;68(3):136‐144. [PubMed] [Google Scholar]
- 20. Kim GC, Lee HJ, Shon CH. The effects of a micro plasma on melanoma (G361) cancer cells. J Korean Phys Soc. 2009;54(2):628‐632. [Google Scholar]
- 21. Arndt S, Wacker E, Li YF, et al. Cold atmospheric plasma, a new strategy to induce senescence in melanoma cells. Exp Dermatol. 2013;22(4):284‐289. [DOI] [PubMed] [Google Scholar]
- 22. Lee H, Shon C, Kim Y, Kim S, Kim G, Kong MG. Degradation of adhesion molecules of G361 melanoma cells by a non‐thermal atmospheric pressure microplasma. New J Phys. 2009;11(11):115026. [Google Scholar]
- 23. Ishaq M, Kumar S, Varinli H, et al. Atmospheric gas plasma–induced ROS production activates TNF‐ASK1 pathway for the induction of melanoma cancer cell apoptosis. Mol Biol Cell. 2014;25(9):1523‐1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adil Ban H, Al‐Shammri Ahmed M, Murbat Hamid H. Cold atmospheric plasma generated by FE‐DBD scheme cytotoxicity against breast cancer cells. Res J Biotech. 2019.14: 192‐195. [Google Scholar]
- 25. Kim SJ, Chung T, Bae S, Leem S. Induction of apoptosis in human breast cancer cells by a pulsed atmospheric pressure plasma jet. Appl Phys Lett. 2010;97(2):023702. [Google Scholar]
- 26. Barekzi N, Laroussi M. Dose‐dependent killing of leukemia cells by low‐temperature plasma. J Phys D. 2012;45(42):422002. [Google Scholar]
- 27. Chen Z, Simonyan H, Cheng X, et al. A novel micro cold atmospheric plasma device for glioblastoma both in vitro and in vivo. Cancers. 2017;9(6):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yan X, Xiong Z, Zou F, et al. Plasma‐induced death of HepG2 cancer cells: intracellular effects of reactive species. Plasma Process Polym. 2012;9(1):59‐66. [Google Scholar]
- 29. Zhang X, Li M, Zhou R, Feng K, Yang S. Ablation of liver cancer cells in vitro by a plasma needle. Appl Phys Lett. 2008;93(2):021502. [Google Scholar]
- 30. Guerrero‐Preston R, Ogawa T, Uemura M, et al. Cold atmospheric plasma treatment selectively targets head and neck squamous cell carcinoma cells. Int J Mol Med. 2014;34(4):941‐946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weiss M, Gümbel D, Hanschmann E‐M, et al. Cold atmospheric plasma treatment induces anti‐proliferative effects in prostate cancer cells by redox and apoptotic signaling pathways. PloS One. e0130350, 2015;10(7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Soengas MS, Lowe SW. Apoptosis and melanoma chemoresistance. Oncogene. 2003;22(20):3138‐3151. [DOI] [PubMed] [Google Scholar]
- 33. Irani S, Shahmirani Z, Atyabi SM, Mirpoor S. Induction of growth arrest in colorectal cancer cells by cold plasma and gold nanoparticles. Arch Med Sci. 2015;11(6):1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sensenig R, Kalghatgi S, Cerchar E, et al. Retracted article: non‐thermal plasma induces apoptosis in melanoma cells via production of intracellular reactive oxygen species. Ann Biomed Eng. 2011;39(2):674‐687. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35. Iseki S, Nakamura K, Hayashi M, et al. Selective killing of ovarian cancer cells through induction of apoptosis by nonequilibrium atmospheric pressure plasma. Appl Phys Lett. 2012;100(11):113702. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


