Abstract
Eagle syndrome is defined as a collection of symptoms affecting the cervical and cranial regions, resulting from an elongated styloid process or ossified stylohyoid ligament encroaching on surrounding structures and causing a variety of symptoms. Classically, Eagle syndrome presents as neck, throat, or ear pain. Carotid artery dissection is a rare complication of Eagle syndrome. We report the case of a 40-year-old man who presented with bilateral internal carotid artery dissection secondary to pathological elongation of the styloid processes.
Keywords: Carotid dissection, Eagle syndrome, Elongated styloid process
Introduction
Eagle syndrome was first described in 1937 by otolaryngologist Watt W. Eagle as a collection of symptoms resulting from the compression of adjacent local structures by an elongated styloid process or a calcified stylohyoid ligament. Stylocarotid syndrome, which is a less common vascular variant of Eagle syndrome, occurs when the styloid process compresses the internal or external carotid artery. Although it is a rare complication of stylocarotid syndrome, carotid artery dissection has been reported. We present the case of a 40-year-old man who had bilateral internal carotid artery dissection secondary to the pathological elongation of the styloid processes.
Case report
We report the case of a 40-year-old man followed for type 2 diabetes and hypertension under treatment. He presented to the emergency department with a sudden onset of motor deficit.
The initial clinical examination revealed a patient who was well-oriented in time and space, with stable hemodynamic and respiratory status. Neurological examination showed a Glasgow Coma Scale score of 15 associated with facial paralysis and left sensorimotor deficit, consistent with left hemiplegia.
An initial noncontrast brain CT scan was performed with no specific abnormalities. Due to the worsening of the facial paralysis, left hemiplegia, and the emergence of right-sided weakness, a second brain CT scan was conducted, revealing a subacute right frontoparietal hypodense area (Fig. 1).
Fig. 1.
Axial sections of the brain scan performed after 6 hour showing a deep junctional stroke at the level of the corona radiata (A), at the level of the superficial posterior right (B) and posterior left (C) junctional territories.
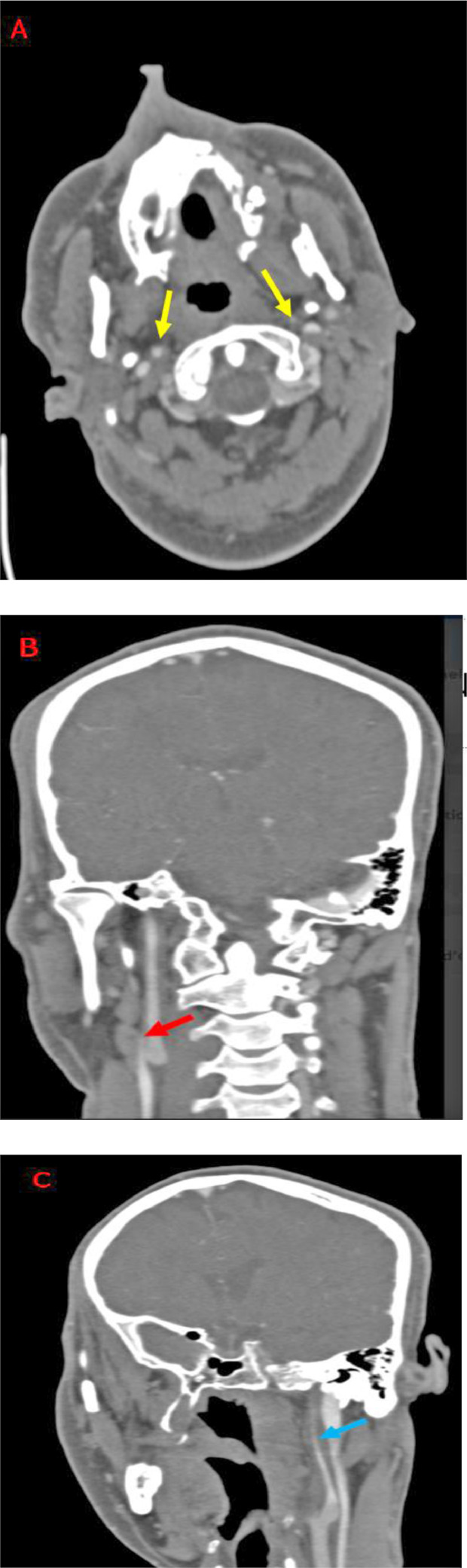
An angio-CT scan was performed, demonstrating reduced opacification of both internal carotid arteries, along with a slender appearance of the Willis polygon and elongation of the stylohyoid processes measuring 5 mm on the right and 4 mm on the left Fig. 2 and 3.
Fig. 2.
Following the context of bilateral ischemic stroke in a young adult, initially a CT angiography of the supra-aortic trunks in search of a carotid dissection was performed showing reduced opacification of both extra-cranial internal carotid arteries more marked to the left on the axial sections (A: yellow arrows) and coronal reconstructions (B: red arrow and C: blue arrow).
Fig. 3.
CT scan bone reconstructions were essential for the search for the elongated styloid process measuring 4 cm on the right (A–C, yellow arrow) and 3.8 cm on the left (B, blue arrow).
Regarding measurement process, only neuroradiologists measured styloid process for bias prevention. Further MRI was carried out, revealing bilateral dissection of the 2 internal carotid arteries in the postbulbar region (Fig. 4). The right carotid artery dissection exhibited greater severity with a lumen occlusion measured at 79% according to The North American Symptomatic Carotid Endarterectomy Trial (NASCET), in comparison to the left side with NACEST indicating 70%. This difference in severity helps explain the extent of the stroke on the right side.
Fig. 4.
A cerebral MRI confirmed junctional strokes on the diffusion sequence (A and B), with subacute parietal hematomas at the level of internal carotid arteries on the sequence of axial T1 fat sat (C and D) as well as the lack of opacification on the sequence of flow 2 TOF achieving the flaming appearance testifying to the subtotal occlusion.
Discussion
Eagle syndrome is defined as a collection of symptoms that affect the cervical and cranial regions. It results from an elongated styloid process or ossified stylohyoid ligament encroaching on the surrounding anatomical structures, leading to a variety of symptoms.
In 1937, the otolaryngologist Eagle described cervical pain [1] caused by elongation of the styloid process. Ten years later, he reported 254 cases, 44 of which were operated on [2]. However, its incidence remains low in the general population, ranging from 4% to 7%, with a slight male predominance [3].
An elongated styloid process is defined as being longer than 3 cm in length [4], slightly longer in men, and increasing with age to reach an average of 37 mm after 80 years [5]. Multiple anatomical variations based on length, angulation, and proximity to the carotid artery can affect the styloid process.
Anatomically, the styloid process is a bony prominence located on the outer surface of the temporal bone. This process measures 3 cm in length, extends anteromedially, and continues as the stylohyoid ligament. The styloid process serves as an attachment point for multiple anatomical structures, with significant relationships to branches of the external carotid artery, the internal carotid artery, cranial nerves IX to XII, and the internal jugular vein.
Clinically, when symptomatic, Eagle syndrome is characterized by a typically dull neck pain that can radiate towards the throat and ear, and it may worsen during swallowing.
Imaging is very crucial for diagnosis. A cervical CT scan, both before and after the injection of contrast material, centered on the axis of the styloid process, enables precise measurements of its length, thickness, and, most importantly, evaluation of its relationships with nearby vascular structures through 2D and 3D reconstructions. Conventional X-rays have no place in diagnosing an elongated styloid process due to their limitations in assessing vascular relationships [6].
Due to its close relationships with blood vessels and nerves, pathological elongation of the styloid process can lead to multiple vascular complications, including carotid artery dissection, pseudoaneurysm, and strokes [3].
Stylocarotid syndrome is a rare vascular variant of Eagle syndrome. Patients with this condition may experience symptoms ranging from headaches to a stroke due to the direct compression of the internal or external carotid arteries by an elongated styloid process [7].
Extrinsic compression of the extracranial internal jugular vein (due to an elongated styloid process has seldom been reported in the literature. In a study conducted by Jayaraman et al., 108 patients underwent CT angiography to assess the occurrence of internal jugular vein compression by extrinsic structures in the upper neck. They discovered compressions on the right side in 24.1% of cases and on the left side in 30.6% of cases. The study's conclusion was that jugular vein compression represents an anatomical variation and is unlikely to have a pathological nature [8].
Carotid artery dissection is a rare complication of stylocarotid syndrome and can result from direct compression by an elongated styloid process [9]. When patients have an elongated styloid process, they are four times more likely to develop carotid artery dissection [9]. The extracranial segments of the internal carotid artery, to 3 cm upon the bulb, has a significantly higher risk of undergoing dissection compared to their intracranial counterparts. This difference can be attributed to their increased mobility and the potential for injury due to contact with the styloid process [10]. Only a few cases of bilateral carotid artery dissection secondary to Eagle syndrome have been reported in the literature [3].
In a study on a total of 145 affected vessels from 118 patients with characteristics of cervical-cranial dissection, severe stenosis/occlusion(>70%) on high-resolution magnetic resonance imaging can be associated with hypoperfusion and a greater risk of thrombogenesis resulting in ischemic [11].
The reference measurements for determining styloid process elongation can indeed vary between males and females. Various studies have established different cut-off values for diagnosing Eagle's syndrome. Eagle originally proposed a cut-off value of 25 mm, while Basekim et al. suggested 40 mm, Jung et al. determined a limit of 45 mm, and Ramadan et al. recommended any measurement above 30 mm as indicative of an elongated styloid process [[12], [13], [14]]. In a study conducted by Ekici et al., it was found that the mean length of the styloid process was longer in males than in females (33.2 ± 13.2 vs 29.6 ± 10.5 mm, P < 0.001). Typically, the standard measurements are as follows: for males, a styloid process length exceeding 30-35 mm is often considered elongated, and for females, a length over 25-30 mm is typically considered elongated [15].
The measurement standards were established using CT scans of patients in the supine position. Styloid process measurements were conducted on multiplanar reconstructed images (MPR) in the coronal plane. The length of the styloid process, from its junction with the temporal bone to its tip, was measured using a CT image analysis workstation. In cases where there was ossification in the stylohyoid ligament, this additional length was added to the measurement of the process [16].
Angio-CT and angio-MRI are the preferred diagnostic tools for carotid artery dissection. Exploring the supra-aortic trunks with CT without and after injection of contrast product provides both direct and indirect evidence through a simultaneous study of the arterial wall and vascular lumen. In an unenhanced CT, in cases of acute dissection, a crescent-shaped hyperattenuating region at the upper section of the cervical internal carotid artery corresponds to a wall hematoma. However, when the appropriate CT angiography settings are used, the intramural hematoma appears isoattenuating compared to the surrounding muscles and cannot be distinguished from atherosclerotic thickening or thrombus [17]. After injection of contrast product, the vasa varum in the adventitial layer may enhance although, defining <<the target sig>> corresponding narrow eccentric lumen surrounded by crescent-shaped mural thickening and thin annular enhancement [18].
Angio-MRI remains more sensitive, especially with the time-of-flight (TOF) sequence. Characteristics of intramural hematoma are variables depending on different parameters mainly the age and size of the hematoma (size, shape and age), surrounding structures (fat venous plexus, skull base, cerebrospinal fluid), and MR imaging sequences (matrix, section thickness, pulse sequence) [19].
The aspect of hematomas depends on the evolution of hemoglobin breakdown [17]. Subacute hematoma is more clearly visualized on T1-weighted images with fat saturation and appears characteristically as a crescent-shaped hyperintense area around an eccentric flow void corresponding to the vessel lumen [20]. Time-of-flight (TOF) sequence is very sensitive showing a flame sign corresponding to luminal stenosis, but when the lumen is less compressed and still partially patent you may get the "string sign." [21]
Main complications of internal carotid dissection are ischemic stroke and pseudoaneurysm. Differential diagnosis of carotid dissection is mainly made with other causes of arterial wall thickening such as atherosclerosis, radiation treatment, and vasculitis.
There is limited information available regarding the management of carotid artery dissection secondary to Eagle syndrome. Treatment with endovascular approaches in the acute phase, followed by elective styloidectomy, may help reduce the risk of recurrent vascular complications.
In conclusion, Eagle syndrome is a rare cause of bilateral internal carotid artery dissection. Given the significant cerebrovascular consequences and the potential for recurrent events in this condition, healthcare professionals need to be aware of this rare disorder.
Patient consent
Written informed consent for publication was obtained from patient.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Eagle WW. Elongated styloid processes: report of two cases. Arch Otolaryngol. 1937;25:584–587. [Google Scholar]
- 2.Eagle WW. Elongated styloid process; further observations and a new syndrome. Arch Otolaryngol. 1948;47:630–640. doi: 10.1001/archotol.1948.00690030654006. [DOI] [PubMed] [Google Scholar]
- 3.Zammit M., Chircop C., Attard V., D'Anastasi M. Eagle's syndrome: a piercing matter. BMJ Case Rep. 2018;11(1) doi: 10.1136/bcr-2018-226611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gozil R, Yener N, Calguner E, Araç M, Tunç E, et al. Morphological characteristics of styloid process evaluated by computerized axial tomography. Ann Anat. 2001;183:527–535. doi: 10.1016/S0940-9602(01)80060-1. [DOI] [PubMed] [Google Scholar]
- 5.Okabe S, Morimoto Y, Ansai T, et al. Clinical significance and variation of the advanced calcified stylohyoid complex detected by panoramic radiographs among 80-year-old subjects. Dentomaxillofac Radiol. 2006;35:191–199. doi: 10.1259/dmfr/12056500. [DOI] [PubMed] [Google Scholar]
- 6.Ramadan SU, Gokharman D, Tuncbilek I, et al. Assessment of the stylohoid chain by 3D-CT. Surg Radiol Anat. 2007;29:583–588. doi: 10.1007/s00276-007-0239-8. [DOI] [PubMed] [Google Scholar]
- 7.Chuang WC, Short JH, McKinney AM, Anker L. Knoll B.et al. Reversible left hemispheric ischemia secondary to carotid compression in Eagle syndrome: surgical and CT angiographic correlation. AJNR Am J Neuroradiol. 2007;28:143–145. [PMC free article] [PubMed] [Google Scholar]
- 8.Zamboni P., Scerrati A., Menegatti E., et al. The eagle jugular syndrome. BMC Neurol. 2019;19:333. doi: 10.1186/s12883-019-1572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eagle WW. Elongated styloid processes: report of two cases. Arch Otolaryngol. 1937;25:584–587. [Google Scholar]
- 10.Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898–906. doi: 10.1056/NEJM200103223441206. [DOI] [PubMed] [Google Scholar]
- 11.Ye W, Fang W, Fan Z, Fisher M. Li Det al. High-resolution magnetic resonance imaging of cervicocranial artery dissection: imaging features associated with stroke. Stroke. 2019;50(11):3101–3107. doi: 10.1161/STROKEAHA.119.026362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramadan SU, Gokharman D, Tuncbilek I, Kacar M, Kosar P, Kosar U. Assessment of the stylohyoid chain by 3D-CT. Surg Radiol Anat. 2007;29:583–588. doi: 10.1007/s00276-007-0239-8. [DOI] [PubMed] [Google Scholar]
- 13.Başekim CC, Mutlu H, Güngör A, Silit E, Pekkafali Z, Kutlay M, et al .Evaluation of styloid process by three dimensional computed tomography. Eur Radiol, 15: 134–139. morphologica, 72(4), 318-321. [DOI] [PubMed]
- 14.Jung T, Tschernitschek H, Hippen H, Schneider B, Borchers L. Elongated styloid process: when is it really elongated? Dentomaxillofac Radiol. 2004;33:119–124. doi: 10.1259/dmfr/13491574. [DOI] [PubMed] [Google Scholar]
- 15.Cullu N, Deveer M, Sahan M, Tetiker H, Yilmaz M. Radiological evaluation of the styloid process length in the normal population. Folia Morphol (Warsz) 2013;72(4):318–321. doi: 10.5603/fm.2013.0053. [DOI] [PubMed] [Google Scholar]
- 16.Shayganfar A, Golbidi D, Yahay M, Nouri S, Sirus S. Radiological evaluation of the styloid process length using 64-row multidetector computed tomography scan. Adv Biomed Res. 2018;7:85. doi: 10.4103/2277-9175.233479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuber M, Meary E, Meder JF, Mas JL. Magnetic resonance imaging and dynamic CT scan in cervical artery dissections. Stroke. 1994;25(3):576–581. doi: 10.1161/01.str.25.3.576. [DOI] [PubMed] [Google Scholar]
- 18.Rodallec M, Véronique M, Gerber S, Desmottes L, Zins M, et al. Craniocervical arterial dissection: spectrum of imaging findings and differential diagnosis. Radiographics. 2008;28(6):1711–1728. doi: 10.1148/rg.286085512. [DOI] [PubMed] [Google Scholar]
- 19.Kitanaka C, Tanaka J, Kuwahara M, Teraoka A. Magnetic resonance imaging study of intracranial vertebrobasilar artery dissections. Stroke. 1994;25(3):571–575. doi: 10.1161/01.str.25.3.571. [DOI] [PubMed] [Google Scholar]
- 20.Chen CJ, Tseng YC, Lee TH, Hsu HL, See LC. Multisection CT angiography compared with catheter angiography in diagnosing vertebral artery dissection. AJNR Am J Neuroradiol. 2004;25(5):769–774. [PMC free article] [PubMed] [Google Scholar]
- 21.Leclerc X, Lucas C, Godefroy O, Nicol L, Moretti A. Leys Det al. Preliminary experience using contrast-enhanced MR angiography to assess vertebral artery structure for the follow-up of suspected dissection. AJNR Am J Neuroradiol. 1999;20(8):1482–1490. [PMC free article] [PubMed] [Google Scholar]