Abstract
Purpose
To evaluate anti-VEGF treatment patterns and the influence of patient demographic and clinical characteristics on up to 6-year vision outcomes in neovascular age-related macular degeneration.
Design
Retrospective, multicenter, noninterventional registry study with up to 6 years of follow-up.
Participants
A cohort of 254 655 eyes (226 767 patients) with first anti-VEGF injection and at least 2 years of follow-up; 160 423 eyes had visual acuity (VA) data.
Methods
Anonymized patient data were collected in the United States through the IRIS® Registry (Intelligent Research in Sight).
Main Outcome Measures
Changes in VA from baseline; frequency of and gaps between intravitreal anti-VEGF injections; treatment discontinuations; switching anti-VEGF agents; and influence of baseline clinical and demographic characteristics on VA.
Results
After a mean VA increase of 3.0 ETDRS letters at year 1, annual decreases led to a net loss from baseline of 4.6 letters after 6 years. Patients with longer follow-ups had better baseline and follow-up VA. From a mean of 7.2 in year 1 and 5.6 in year 2, mean injections plateaued between 4.2 to 4.6 in years 3 through 6. Treatment was discontinued in 38.8% of eyes and switched in 32.3%. When adjusting for differences at baseline, every additional injection resulted in a 0.68 letter improvement from baseline to year 1; thus, multiple injections in a year have the potential to be clinically meaningful. Older age, male gender, Medicaid insurance, and not being treated by a retina specialist were associated with a higher likelihood of vision loss at year 1. Of the patients, 58.5% lost ≥ 10 letters VA at least once during follow-up, with 14.5% of patients experiencing sustained poor vision after a median of 3.4 years.
Conclusions
After modest mean VA improvement with intravitreal anti-VEGF injections at year 1, patients netted a loss of VA by year 6. Injection frequency decreased over time, and this was paired with a relatively high rate of discontinuation. Modeling suggested that more frequent injections were associated with better VA. Difficulty with continuous adherence to frequent intravitreal injections may have contributed to undertreatment resulting in less-than-optimal vision outcomes.
Financial Disclosure(s)
Proprietary or commercial disclosure may be found in the Footnotes and Disclosures at the end of this article.
Keywords: Antivascular endothelial growth factor, Intelligent Research in Sight Registry, Neovascular age-related macular degeneration, Visual acuity
Age-related macular degeneration (AMD) is a leading cause of blindness in people aged > 60 years.1, 2, 3, 4 Neovascular AMD (nAMD) is characterized by pathologic macular neovascularization with VEGF identified as a critical signal driving this process.5,6 Anti-VEGF agents, such as ranibizumab, bevacizumab, aflibercept, and brolucizumab, as well as the dual-pathway, anti-VEGF and angiopoietin-2 inhibitor faricimab, can inhibit the growth of neovascular lesions, resolve retinal edema, and have demonstrated positive vision outcomes in clinical trials7, 8, 9, 10, 11, 12, 13 and clinical practice studies in nAMD.14, 15, 16 To maintain the benefits of anti-VEGF, most patients must continue to receive regular anti-VEGF therapy; however, frequent injections and monitoring visits pose a substantial treatment burden, which often leads to undertreatment.14, 15, 16, 17
Many studies of clinical practice treatment patterns and outcomes in patients with nAMD have had limited follow-up (1–2 years)18, 19, 20 or have been impacted by a loss of patients to follow-up21,22 and also may not be generalizable to patients in the United States (US).23 The IRIS® Registry (Intelligent Research in Sight) database is the first US-based national comprehensive eye disease database and is the largest ophthalmic registry worldwide based on electronic health records (EHRs). The IRIS Registry is a representative data source of US patients because it collects information from approximately 70% of ophthalmology practices across all payers and all populations, even those without insurance.24 A 2021 study of IRIS Registry data by MacCumber et al25 in patients with nAMD with up to 3.5 years of follow-up found that overall, patients received 5.6 injections in year 1, 3.4 in year 2, and 3.1 in year 3.25 Visual acuity (VA) had increased slightly by a mean of 0.7 ETDRS letters from baseline at the end of year 1 (baseline mean 55.4 letters) but by the end of year 3, had decreased by a mean of 3.1 letters from baseline, and more than one third of eyes had discontinued treatment by the end of the year 3.25 The 2021 study was largely descriptive and did not investigate gaps and discontinuation of treatment, causes of sustained poor vision (SPV) or considerable vision loss (CVL), or evaluate the association of intravitreal anti-VEGF injection frequency or patient baseline characteristics on VA outcomes in nAMD.25
There is wide variability in clinical practice treatment patterns based on patients’ anatomic and visual response to injections, clinician and patient treatment preferences, agent options, and potentially conflicting expert opinions and evidence. Because nAMD is a chronic, life-long disease,7 it is important to describe patients’ persistence and VA over time with intravitreal anti-VEGF injections beyond 3 years in clinical practice. Furthermore, there is a need to identify factors that may lead to better or worse outcomes for these patients.
Methods
Study Design and Objectives
This was a retrospective, noncomparative, cohort study of patients with nAMD from the IRIS Registry receiving intravitreal anti-VEGF injections for nAMD in the US. The objective was to evaluate treatment patterns and outcomes after up to 6 years of follow-up. To meet this objective, we assessed treatment patterns and VA over time and evaluated the baseline demographic and clinical factors that influenced vision outcomes.
Data Source
To date, the IRIS Registry has integrated with up to 60 different EHR systems in the US. As of October 2021, the Registry included data on > 412 million patient visits from > 70.8 million unique patients, with approximately 16 000 eye clinicians reporting data.24 Importantly, the Registry captures a unique patient visiting multiple participating physicians. Data from January 1, 2013, to June 6, 2020, from the Registry, which included the most recent data available, were included in this study. Because the IRIS Registry data are deidentified, no patient-level consent or institutional review board approval was required. All research adhered to the tenets of the Declaration of Helsinki.
Study Population
The study population included patients with nAMD treated with intravitreal anti-VEGF injections between the index period of July 1, 2013, and June 30, 2018. The index date is defined as the date of the first documented anti-VEGF injection within the IRIS Registry during the index period.
Inclusion and Exclusion Criteria
Patients aged at least 50 years at their index date were required to have a first nAMD diagnosis within 6 months (180 days) before or on the date of their first anti-VEGF injection and no documented anti-VEGF injections before their index date in the IRIS Registry. Diagnostic International Classification of Diseases Ninth and Tenth Revision codes utilized to identify nAMD diagnoses in the Registry are listed in Table S1 (available at www.ophthalmologyscience.org). Inclusion in the study also required the practice where the patient was being treated to have an IRIS Registry data contribution for at least 6 months before the index date.
To be included in the VA study cohort, patients were required to have received 3 doses of intravitreal anti-VEGF within 180 days of starting treatment, a minimum of 2 years follow-up after their index date, VA measurement before the index date within 6 months (180 days), and at least 1 VA measurement after 1 year (± 60 days). Patients were not required to have a VA at each year time point to be included in each annual analysis (e.g., the patient could have missed VA at year 2 but still be included in VA analysis because VA reading was available at year 3). Patients were excluded from the study if they had unknown laterality of either nAMD diagnosis or of anti-VEGF treatment.
Outcomes
Visual acuity was evaluated at annual intervals for study eyes up to 6 years (± 60 days). Visual acuity change from baseline was calculated as best-documented visual acuity in approximate ETDRS letters from reported measures and were converted from either Snellen (ETDRS = 85 + 50 × log [Snellen fraction]) or logarithm of the maximum angle of resolution values.26
The frequency of intravitreal anti-VEGF injections by follow-up year and the duration of gaps between injections were determined for the overall cohort. At the time of the study, all anti-VEGF agents were indicated for treatment intervals ≤ 16 weeks; therefore, a period of > 18 weeks (up to 52 weeks) without an anti-VEGF injection was defined as a treatment gap. Not receiving anti-VEGF injection for > 52 weeks was defined as treatment discontinuation. Treatment discontinuations were evaluated for the overall cohort. The proportions of patients who switched between anti-VEGF agents, as well as subsequent switching back to the original agent, were also evaluated. For this outcome, switching was defined as at least 3 consecutive intravitreal injections of a different anti-VEGF agent from the original (or prior) agent.
Relationships between changes in VA from baseline to year 1 and baseline clinical and demographic characteristics were investigated. The characteristics examined included age, sex, race, clinician specialty, the historical presence of glaucoma, the historical presence of cataract, follow-up time, and injection intervals. Clinical specialty was defined as the treating provider at the first anti-VEGF injection.
Finally, we sought to explore 2 separate measures of vision loss events over the 6-year period. To explore vision loss over time, 2 separate time-to-event analyses using Kaplan–Meier curves were performed to examine the time likelihood of (1) CVL and (2) SPV of VA over time. A loss of ≥ 10 ETDRS letters from baseline was used as an approximate measure of CVL and was evaluated for patients stratified by baseline VA. Development of SPV was defined as a Snellen VA of 20/200 or worse at 2 separate VA readings at least 3 months apart that subsequently did not improve beyond 20/100. Patients with a baseline VA of 20/200 or worse were not eligible for this outcome.
Statistical Analyses
Means, standard deviations (SDs), and medians were calculated for continuous variables, and counts and percentages for categorical variables. For comparative analyses, hypothesis testing; statistical modeling; t, chi-square, and Wilcoxon rank sum tests; and 95% confidence intervals (CIs) were used as was appropriate for each variable type. For statistical analyses of VA, Snellen values were converted to VA logarithm of the minimum angle of resolution values. Unadjusted linear regression analyses were utilized to identify demographic and clinical covariates of interest, with adjusted linear regression analyses then performed to examine the relationship between changes in VA at 1 year and both baseline characteristics and first-year treatment patterns (i.e., number of injections received).
For the Kaplan–Meier time-to-event analyses, patients were censored when the event was reached (time of either SPV or CVL or when data were no longer available, i.e., end of follow-up). Additionally, 2 Cox proportional hazards models stratified by baseline VA were applied to evaluate hazard ratios (HRs) of variables associated with the time-adjusted risk of CVL and SPV out to 6 years, with patients being censored at the end of their follow-ups. The variables included in the Cox proportional hazards models were baseline age, sex, race, payer type, physician specialty, historical presence of glaucoma, and historical presence of cataract. Patients with a baseline VA of 20/200 or worse were not included in the Kaplan–Meier or Cox proportional hazards model analyses for SPV. PySpark (Apache Spark), Amazon EMR (Amazon Web Services Inc) cluster, Python, and R statistical tools were utilized for the statistical analyses as appropriate.
Results
Patient Population
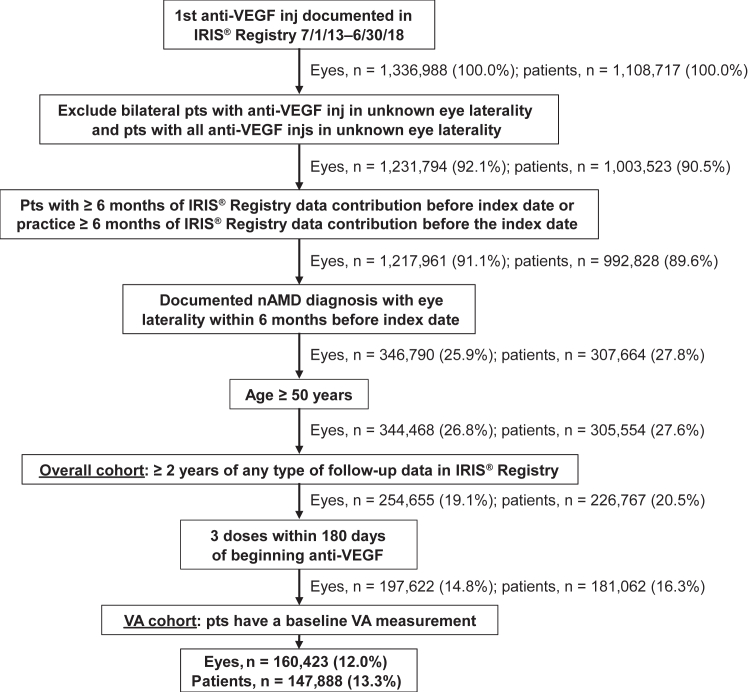
A total of 1 336 988 eyes from 1 108 707 patients with a first intravitreal anti-VEGF injection were documented in the IRIS Registry between July 1, 2013, and June 30, 2018. Of these, 254 655 eyes from 226 767 patients met the eligibility criteria for the overall study cohort. Within the overall study cohort, 197 622 eyes (78%) from 181 062 patients had received 3 injections within 180 days of starting treatment, and 160 423 of these eyes (147 888 patients) also had a baseline VA measurement and were therefore eligible for inclusion in the VA cohort (Fig 1). For the year 1 VA analysis, 135 384 patient eyes were available. At each of the following year’s VA analyses, an increasing number of patient eyes were excluded because of missing data or for not having reached the longer follow-up duration by the study cut-off date, resulting in only 6878 patient eyes having VA measurements at year 6.
Figure 1.
Patient eye/patient attrition. Index date defined as the date of the first documented anti-VEGF injection within the IRIS Registry (Intelligent Research in Sight) during the index period July 1, 2013, to June 6, 2018. inj = injection; nAMD = neovascular age-related macular degeneration; pts = patients; VA = visual acuity.
The baseline demographic and clinical characteristics of patients included in the VA cohort are shown in Table 2. The mean patient age at baseline was 79.6 (SD 8.7) years, 62.5% of patients were female, and the majority (89.6%) were White. Most patients (82.0%) had at least their first treatment provided by a retina specialist. The most common primary insurance or payer types were Medicare (45.5% of patients) and Medicare Advantage (31.4% of patients). The mean length of follow-up was 3.7 (SD 1.3) years from the index date (median, 3.3 years; interquartile range, 2.6–4.6 years). There were no meaningful differences in demographics between patients with and without VA measurement.
Table 2.
Demographic and Clinical Characteristics of Patients Included in the Visual Acuity Cohort
| Characteristic | Patients (N = 147 888)∗ |
|---|---|
| Age, yrs, mean (SD) | 79.55 (8.74) |
| Age group, yrs, n (%) | |
| 50–59 | 2966 (2.01) |
| 60–69 | 16 947 (11.46) |
| 70–79 | 48 403 (32.73) |
| 80–89 | 61 991 (41.92) |
| 90+ | 17 581 (11.89) |
| Sex, n (%) | |
| Female | 92 407 (62.48) |
| Male | 54 932 (37.14) |
| Unknown | 549 (0.37) |
| Race, n (%) | |
| White | 132 505 (89.60) |
| Unknown | 10 777 (7.29) |
| Asian | 1894 (1.28) |
| Black or African American | 1667 (1.13) |
| Other | 1045 (0.71) |
| Ethnicity, n (%) | |
| Non-Hispanic | 125 964 (85.18) |
| Unknown | 16 720 (11.31) |
| Hispanic | 5204 (3.52) |
| Ophthalmic conditions, n (%) | |
| Glaucoma | 11 376 (7.69) |
| Cataract | 55 418 (37.47) |
| US census region, n (%) | |
| South region: MD, DE, WV, VA, DC, NC, SC, KY, TN, FL, GA, AL, MS, LA, AR, OK, TX | 53 776 (36.36) |
| Midwest region: ND, SD, NE, KS, MO, MN, IA, WI, IL, IN, OH, MI | 37 837 (25.58) |
| West region: NM, AZ, CO, UT, WY, MT, ID, NV, CA, OR, WA, AK, HI | 28 809 (19.48) |
| North region: ME, NH, VT, PA, NY, NJ, MA, RI, CT | 26 687 (18.05) |
| Unknown region | 779 (0.53) |
| Insurance payers, n (%)† | |
| Medicare | 67 267 (45.49) |
| Medicare Advantage | 46 423 (31.39) |
| Commercial | 23 749 (16.06) |
| Other/Unknown/No insurance | 8829 (5.96) |
| Medicaid | 1620 (1.10) |
| Provider specialty, n (%)‡ | |
| Retina specialists | 121 205 (81.96) |
| Unknown/Other | 11 487 (7.77) |
| General ophthalmologist | 11 179 (7.56) |
| Nonretina specialist | 4017 (2.72) |
SD = standard deviation; US = United States.
To show demographic characteristics at the patient level, 1 eye was selected among patients with 2 eyes enrolled in this study. If eyes were enrolled on the same day, the worse eye was selected. If eyes were enrolled on different days, the first eye enrolled was selected.
Payer type at baseline.
At first anti-VEGF injection.
VA
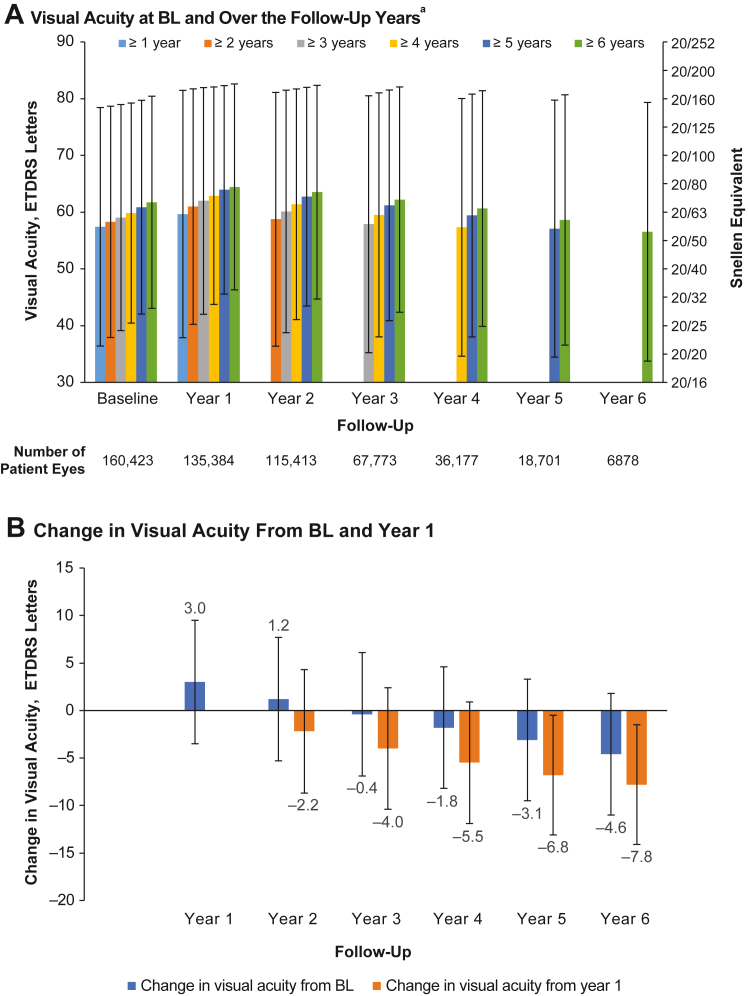
Mean VA at baseline and annually in the VA cohort are shown by length of follow-up in Figure 2A. Patients in the 1- and 6-year follow-up cohorts had baseline VA of 57.5 and 61.9 ETDRS letters (Snellen equivalents of approximately 20/70 and 20/60), respectively, with patients with longer total follow-up time having both better baseline and better follow-up VA. Analysis of changes in VA from baseline over time showed that although the mean change in VA after 1 year was an increase of 3.0 ETDRS letters, subsequent years showed decreases of approximately 1 to 2 letters each year (Fig 2B).
Figure 2.
Visual acuity (VA; ETDRS letters) in the VA cohort. A, Mean VA at baseline (BL) and over follow-up years 1 through 6 according to the number of years of follow-up. B, Mean changes in VA from BL and from year 1 over time. aThe number of patients' eyes included in each follow-up year was not mutually exclusive, i.e., patient eyes with 6 years of follow-up were included in all previous follow-up years. Patient eyes must have had consecutive VA measurements.
Injection Frequency
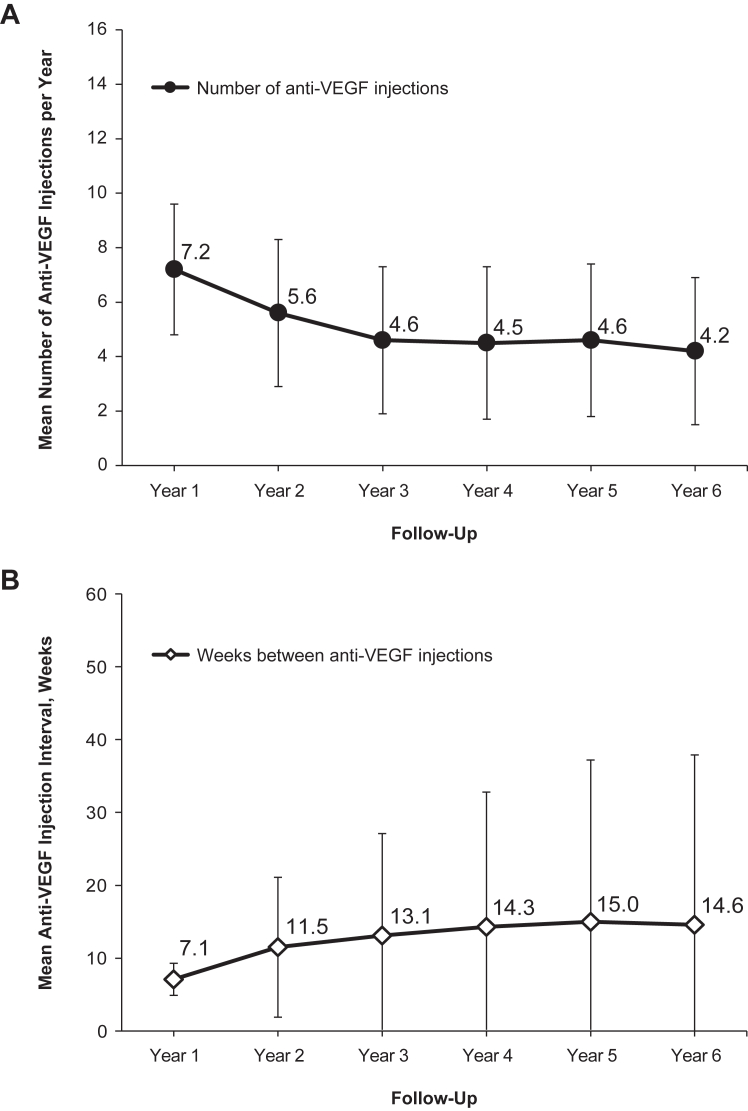
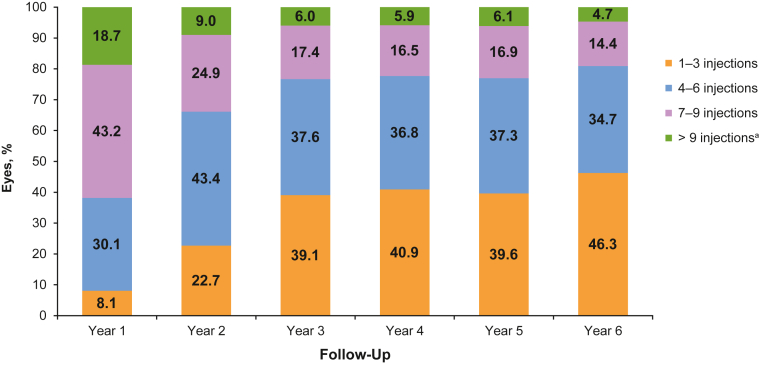
In the overall cohort, mean anti-VEGF injection frequency decreased over time. Mean SD injection frequency was 7.2 (2.4) per year in year 1, 5.6 (2.7) in year 2, and reached a plateau around year 3, with a mean injection frequency of 4.2 to 4.6 injections in years 3 through 6. Median injection frequency per year was 7 for year 1, 5 for year 2, and 4 for years 3 through 6. The mean time between intravitreal anti-VEGF injections was 7.1 (SD 2.2) weeks in year 1, increasing to 11.5 (9.6) weeks in year 2 and 13.1 to 15.0 weeks in years 3 to 6 (Fig 3). Injection frequency over time according to the number of years of follow-up is shown in Figure S4 (available at www.ophthalmologyscience.org.). In years 3 through 6 in the overall cohort, the most common injection frequency in patient eyes (39.1%–46.3%) was 1 to 3 injections per year, followed by 4 to 6 injections (34.7%–37.6% of patient eyes) and 7 to 9 injections (14.4%–17.4% of patient eyes) (Fig 5). The mean annual injection frequency by agent according to patients’ follow-up duration is shown in Figure S6 (available at www.ophthalmologyscience.org.). Patients with 1 to 2 years of follow-up had mean annual frequency 6.8 (SD 2.2) for aflibercept, 5.4 (SD 2.2) for bevacizumab, and 6.2 (SD 2.0) for ranibizumab; for 3 to 4 years' follow-up, 5.5 (SD 1.9) for aflibercept, 4.9 (SD 1.8) for bevacizumab, and 5.4 (SD 1.8) for ranibizumab, and for 5 to 6 years' follow-up, 5.5 (SD 2.0) for aflibercept, 4.7 (SD 1.8) for bevacizumab, and 5.2 (SD 1.9) for ranibizumab.
Figure 3.
Treatment patterns in the overall cohort for patients with at least 2 years of follow-up. A, Injection frequency over time as the mean number of anti-VEGF intravitreal injections. B, Interval between treatments as the mean number of weeks between injections over follow-up years 1 through 6.
Figure 5.
Injection frequency in patient eyes with aflibercept, bevacizumab, or ranibizumab by years of follow-up treatment (overall cohort). aMore than 9 injections includes those eyes with 10 to 13 and > 13 injections.
Treatment Duration and Discontinuation
The mean duration of treatment in the VA cohort was 148 (SD 83) weeks. Visual acuity cohort patients persisted with treatment for 61.2% (98 235/160 423) of eyes, with 21.8% (34 988/160 423) of eyes having at least 1 treatment gap. The mean time from starting treatment to the first gap in treatment was 97 (SD 57) weeks. Treatment was discontinued (i.e., the patient had > 1 year in the Registry without an injection) for the remaining 38.8% (62 188/160 423) of eyes, with a mean time to discontinuation of 89 (SD 68) weeks after starting treatment.
Treatment Switching
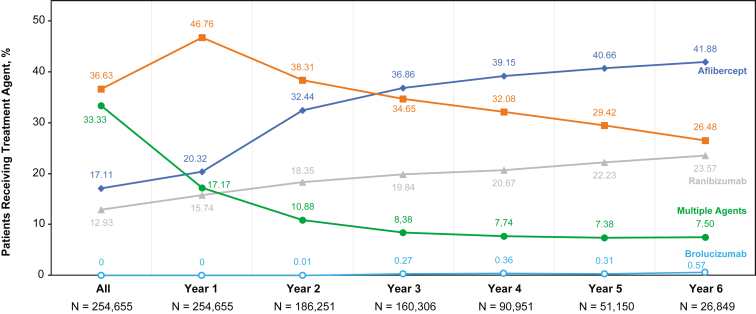
A switch between anti-VEGF agents occurred in 32.3% (51 756/160 423) of eyes in the VA cohort, with a time from treatment initiation to the first switch of 81.2 (SD 64.1) days. The proportion of patients treated with multiple agents decreased from year 1 to year 4, whereas the proportion of patients receiving aflibercept increased over the years of follow-up (Fig 7). Switching from bevacizumab to aflibercept or ranibizumab occurred in 21.1% (33 781/160 423) of eyes in this cohort with a mean time to first switch of 74.7 (SD 60.9) days. A switch from aflibercept or ranibizumab to bevacizumab occurred in 5.9% (9485/160 423) of eyes with a mean time to first switch of 81.9 (SD 63.0) days. Among those who switched, 11.8% (6111/51 756) of eyes were subsequently switched back to their original agent.
Figure 7.
Percent of patients receiving a treatment agent over the follow-up period in the overall cohort.aaNote: The patients within each cohort changed over time. This graph serves to illustrate the relative change in the proportions of patients receiving different treatment agents at each time point (data are not continuous).
Influence of Baseline Demographic and Clinical Characteristics on Changes in VA
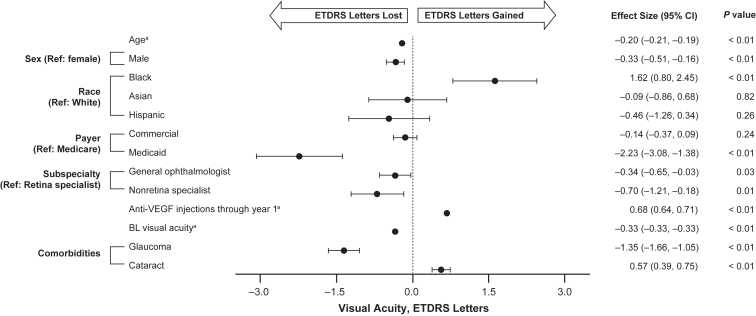
The relationships between change in VA from baseline to year 1 and baseline demographic and clinical characteristics are shown in Figure 8. Older age (effect size [95% CI] –0.20 [–0.21, –0.19], P < 0.01); male sex (–0.33 [–0.51, –0.16], P < 0.01); receiving Medicaid rather than Medicare insurance (–2.23 [–3.08, –1.38], P < 0.01); not being treated by a retina specialist for at least their first treatment, but by a general ophthalmologist (–0.34 [–0.65, –0.03], P = 0.03) or nonretina specialist (–0.70 [–1.21, –0.18], P = 0.01); worse baseline VA (–0.33 [–0.33, –0.33], P < 0.01); and history of having glaucoma (–1.35 [–1.66, –1.05], P < 0.01) were all found to be significantly associated with a loss of VA (loss of ETDRS letters). Conversely, Black race (1.62 [0.80, 2.45], P < 0.01) and the history of cataracts (0.57 [0.39, 0.75], P < 0.01) were found to be significantly associated with higher VA. The number of anti-VEGF injections was also positively associated with better VA outcomes when adjusted for other baseline characteristics, with a 0.68 ETDRS letter improvement for every additional anti-VEGF injection.
Figure 8.
Adjusted analysis of the association of change in visual acuity (VA) from baseline (BL) to year 1 with BL clinical and demographic characteristics for eyes included in the VA cohort.baContinuous variables. bGeneralized linear model adjusted for sex, race (White, Black, Asian, Hispanic, and other), payer type (Medicare, commercial, Medicaid, and other/unknown), provider subspecialty (retina specialist, general ophthalmologist, nonretina specialist, and other/unknown), BL VA, and comorbidities. Variables assessed included age (continuous), sex, race (White, Black, Asian, Hispanic, other coefficient [95% confidence interval {CI}] 0.03 [–0.98, 1.04], P = 0.95, and unknown coefficient [95% CI] –0.25 [–0.60, 0.10], P = 0.16), payer type (Medicare, commercial, Medicaid, and other/unknown coefficient [95% CI] –0.26 [–0.62, 0.09], P = 0.15), subspecialty (retina specialist, general ophthalmologist, nonretina specialist, and other/unknown coefficient [95% CI] –0.37 [–0.68, –0.05], P = 0.02), intravitreal anti-VEGF injections/anti-VEGF-1 injections through year 1, BL VA, and historical comorbidities (glaucoma and cataract). Ref = reference.
Time to Vision Loss of ≥ 10 ETDRS Letters (CVL) and to SPV
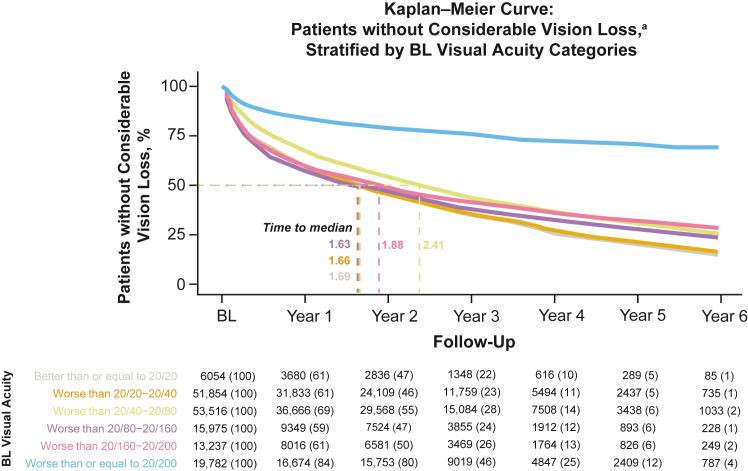
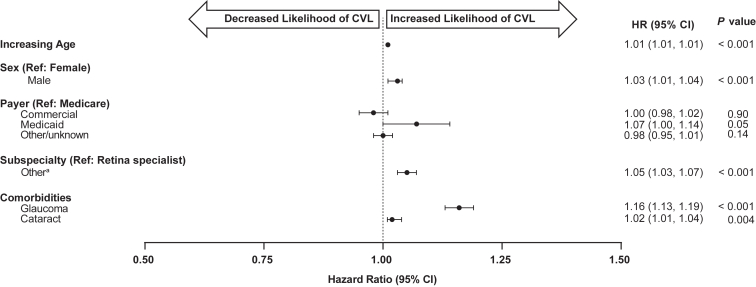
Kaplan–Meier curves for time to CVL, defined as loss of ≥ 10 ETDRS letters at any time during follow-up, are shown in Figure 9 (stratified by baseline VA category). Of the patients, 58.5% experienced CVL, with a median time-to-event of < 3 years and < 2 years when excluding the 20/200 or worse baseline VA cohort. Median (95% CI) time-to-event for eyes with baseline VA of 20/20 or better was 1.69 (1.57, 1.78) years, worse than 20/20 to 20/40 was 1.66 (1.63, 1.69) years, worse than 20/40 to 20/80 was 2.41 (2.37, 2.46), worse than 20/80 to 20/160 was 1.63 (1.56, 1.71) years, and worse than 20/160 was 1.88 (1.79, 1.97) years. Patients with a baseline 20/200 or worse did not experience a median time-to-event. Adjusted Cox proportional hazards models for estimating the risk of CVL showed that older age at baseline (HR [95% CI] 1.01 [1.01, 1.01], P < 0.001), male gender (1.03 [1.01, 1.04], P < 0.001), receiving Medicaid rather than Medicare insurance (1.07 [1.00, 1.14], P = 0.05), being treated by a nonretina rather than a retina specialist (1.05 [1.03, 1.04], P < 0.001), and the history of glaucoma (1.16 [1.13, 1.19], P < 0.001) and cataracts (1.02 [1.01, 1.04], P = 0.004) were significantly associated with an increased risk of > 10 letter VA loss (Fig 10, Table S3, available at www.ophthalmologyscience.org.).
Figure 9.
Time-to-event analysis for vision loss of ≥ 10 ETDRS letters stratified by baseline (BL) visual acuity (VA cohort).
Figure 10.
Adjusted Cox proportional hazards model for estimating time to visual acuity 10-ETDRS letter loss (considerable vision loss [CVL]) over 6 years for all eligible patient eyes (n = 160 423). aIncludes general ophthalmologist, nonretina specialist, optometrist, unknown. CI = confidence interval; HR = hazard ratio; Ref = reference.
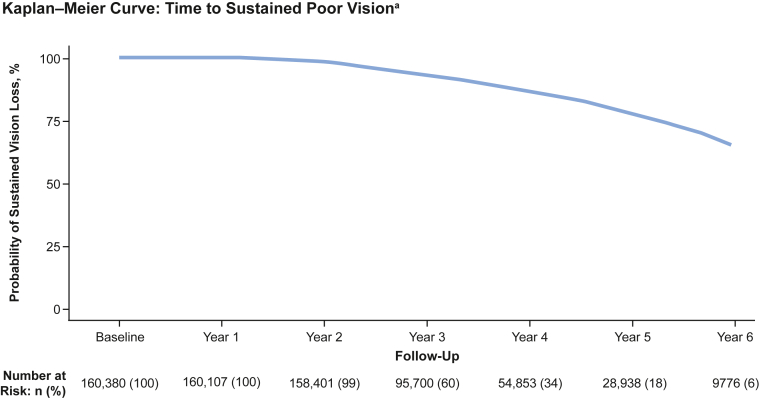
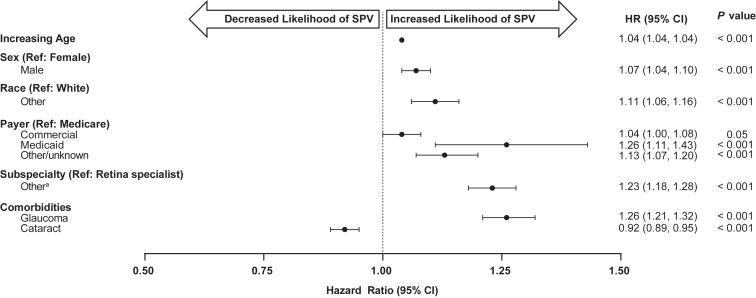
The results of a Kaplan–Meier analysis for SPV (defined as a Snellen VA of 20/200 or worse at 2 separate VA readings at least 3 months apart that subsequently did not improve beyond 20/100) are shown in Figure 11. Sustained poor vision was observed in 14.5% (23 220/160 380) of eyes, with a median (interquartile range) of 3.44 (2.55–4.71) years to SPV. Adjusted Cox proportional hazards models for estimating the time-adjusted risk of SPV showed that older age at baseline (HR [95% CI] 1.04 [1.04, 1.04], P < 0.001); male gender (1.07 [1.04, 1.10], P < 0.001); race other than White (1.11 [1.06, 1.16], P < 0.001); payer or insurance types other than Medicare, in particular Medicaid (1.26 [1.11, 1.43], P = 0.001); not being treated by a retina specialist (1.23 [1.18, 1.28]; P < 0.001); and the history of glaucoma (1.26 [1.21, 1.32], P < 0.001) were all associated with an increased risk of SPV during the follow-up period (Fig 12, Table S4, available at www.ophthalmologyscience.org.). The presence of cataract at baseline was the only variable evaluated associated with a significantly lower risk of SPV (0.92 [0.89, 0.95], P < 0.001).
Figure 11.
Time-to-event analysis for the probability of sustained poor vision (SPV) over time (visual acuity [VA] cohort). SPV was defined as a Snellen VA of 20/200 or worse at 2 separate visits ≥ 3 months apart that subsequently did not improve beyond 20/100. Cohort excludes patients with VA worse than 20/200 at baseline.
Figure 12.
Adjusted Cox proportional hazards model for estimating the time-adjusted risk of sustained poor vision (SPV) over 6 years for all eligible patient eyes (n = 160 423). aIncludes general ophthalmologist, nonretina specialist, optometrist, unknown. CI = confidence interval; HR = hazard ratio; Ref = reference.
Discussion
This study, which evaluated treatment patterns and vision outcomes over a follow-up period of up to 6 years, represents the largest and longest follow-up study to date in patients with nAMD from the IRIS Registry, following > 160 000 patients with baseline VA measures for up to 6 years. This study utilized statistical models to evaluate predictors of VA change and assess the impact of baseline characteristics and the number of anti-VEGF injections received on vision outcomes and investigated long-term treatment patterns. It also evaluated the likelihood of CVL (loss of ≥ 10 letters) and SPV (20/200 or worse), stratified by baseline VA over a 6-year period, as well as variables contributing to SPV.
Visual acuity decreased over time after the first year of treatment, as had been reported from other clinical practice studies of anti-VEGF therapy in nAMD.27, 28, 29 In clinical practice, visual outcomes with anti-VEGF therapy may be worse than those observed in clinical trials, possibly as a result of less frequent and less consistent treatment in routine clinical practice.23,30,31 Treatment interruptions of > 6 months can result in a permanent VA loss even after treatment is restarted.32 It is also likely that many patients treated in clinical practice may not have been eligible for clinical trials.28,29,33, 34, 35 Similar to previously reported clinical practice studies, in the current study, average VA improved over the first year of treatment, but VA then worsened in each follow-up year. Intravitreal anti-VEGF therapy can place a considerable burden on patients due to the need for regular injections and associated clinic visits.14,15,17 A safe anti-VEGF treatment modality that would meaningfully reduce this burden represents a substantial unmet need.
Mean anti-VEGF injection frequency decreased over time. This may reflect the increasing use of treat-and-extend protocols, which gradually increase the interval between injections. Overall, patients received relatively few intravitreal anti-VEGF injections in years 3 through 6 (a mean of approximately 4 injections), with an increasing proportion of eyes receiving 3 or fewer injections per year and the proportion of eyes treated with 7 or more injections decreasing. It may be that patients requiring more frequent treatments experience more rapid declines, leading to their eventual discontinuation of treatment. When evaluating injection frequency by agent, patients generally received a similar number of injections annually. Numerically, patients on aflibercept received the most injections and experienced the shortest intervals between injections, whereas patients on bevacizumab received the fewest injections and experienced the longest intervals between injections at each follow-up year (Fig S6). Over time, the variance from the mean increased for each agent, suggesting that treatment intervals became more customized with prolonged treatment.
The rate of treatment discontinuation was substantial at 38.8% of eyes within 2 years, although this was consistent with the findings of 39% to 42% after 3 years and lower than 62% after 18 months reported in other analyses of data from clinical practice.25,36 However, the eligibility requirement for a minimum of 3 injections within the first 180 days may have skewed discontinuation rates in the current analysis because prior studies may have reported on patients who discontinued after just 1 or 2 injections. Although the precise reasons for discontinuation in the current analysis are not known, prior studies have reported patients not seeing value in the treatments because of stable or good vision, the occurrence of an adverse event, the development of other ocular and systemic comorbidities, and simply the burden that patients experience with repeated injections.16,37, 38, 39, 40 It is also possible that there was no recurrence of fluid requiring treatment when the physician elected pro re nata treatment. For some patients, the high rate of discontinuation may underscore the need for less burdensome approaches to nAMD management.
Switching between anti-VEGF agents occurred in approximately one third of patient eyes evaluated in this study. As with discontinuation, it is not possible to know the exact reasons for medication switching. However, a lack of response or an incomplete response to treatment, patient or physician preference, or desire to reduce injection frequency have been cited as important factors in guiding the decision to switch agents.41 There is, however, limited evidence for substantial improvements in outcomes after switching among the agents evaluated in this study.41, 42, 43, 44 Payer and formulary restrictions may also play a role in the decision to switch treatments.41,45,46 Additional research is needed to provide a better understanding of the clinical reasoning, physician treatment strategies, and patient preferences that underlie decisions to switch between anti-VEGF agents in nAMD.
Statistical models identified several factors associated with loss of VA after 1 year of anti-VEGF treatment, with poorer baseline VA supportive of prior analyses.47 In the adjusted model, there was an association of an increasing number of injections leading to improved vision, with each additional anti-VEGF injection leading to an additional 0.68 ETDRS letters of VA gained in year 1. Patients who receive 8 injections per year are thus likely to experience a clinically meaningful increase in ETDRS letters (i.e., 0.68 × 8 = 5.44). Improvement in VA with an increasing number of injections was also reported in a smaller retrospective study in nAMD.29 Black race and the history of cataracts at baseline were the only variables significantly associated with a gain of ETDRS letters after 1 year, with the mechanisms driving these findings being unknown. Notably, compared with White patients, Black patients experienced a significant increase in ETDRS letters gained after treatment initiation. When considering the impact of the history of cataracts, it should be noted that it is unclear how long it has been since such a diagnosis was received; it is possible that treatment for cataracts during this time period resulted in the observed minor improvement of ETDRS letters.
A large majority of patients lost 10 or more ETDRS letters (CVL) at least 1 time within 3 years of follow-up, and 14.5% experienced a drop in VA to 20/200 or worse SPV after about 3.5 years. Factors associated with an increased risk of CVL were insurance/payer type, not being treated by a retina specialist, and a history of glaucoma and cataracts. For SPV, factors associated with increased risk were older age, male gender, insurance/payer type, not being treated by a retina specialist, and the historical presence of glaucoma. Interestingly, a history of cataracts was fairly protective of patients experiencing SPV (even though it was not protective in those experiencing CVL). Race other than White was also a significant factor associated with SPV; however, non-White patients represented < 10% of the study cohort. The association of certain modifiable risk factors with increased risk of these 2 vision outcomes found in these modeling exercises suggests that patients could benefit from earlier intervention, treatment of other ocular conditions, and improved access to specialist care.
The findings of this up to 6-year study build upon previous studies of patients with nAMD who had up to 5 years of follow-up. A previous IRIS Registry study involving > 100 000 eyes with up to 3.5 years follow-up reported that VA was slightly better at the end of the first year of treatment but then declined through the remainder of the study.25 The SIERRA-AMD clinical practice US cohort study of nAMD included anonymized EHRs for approximately 80 000 patients from clinical practices that utilized the Vestrum Health Retina database. Overall, the mean year 1 letter gain in SIERRA-AMD was 1.1 letters from a mean baseline of 53.1 letters,28 compared with an increase of 3.0 letters in our analysis from baseline VA of up to 61.9 ETDRS letters in the current analysis. In both studies, both VA and anti-VEGF injection frequency declined among patients with 4 years of follow-up.28 Similarly, a study using the Vestrum database that included 5208 eyes with nAMD with 5 years of follow-up reported a year 1 gain of 3.1 letters from a baseline of 54 to 57 letters but then losses of 0.2 and 2.2 letters at years 3 and 5, respectively.29
The current study had several meaningful differences compared with these previous studies, including the databases utilized, follow-up durations, inclusion criteria, and evaluation of clinical and demographic characteristics associated with vision loss or improvement using linear regression and Cox proportional hazards models. Most studies have not required a minimum of 3 injections in the first 180 days to be included. Nevertheless, the year 1 means of 5.6 (SD 3.0) and 7.6 (2.1) injections in the previous IRIS Registry study and in SIERRA, respectively,25,28 were comparable with the year 1 mean of 7.2 (SD 2.4) in the current study.
The current work highlights some of the challenges with our current approach to nAMD management using relatively short-duration intravitreal anti-VEGF treatments. Most patients do not seem to be realizing the maximal potential benefit of anti-VEGF therapy through 6-years of follow-up, some of which could be due to chronic undertreatment arising from poor patient adherence, limited availability of physicians or clinics, and insufficient patient awareness that their chronic disease will require prolonged therapy to maintain their vision.16 Other possible reasons for not reaching maximal benefit include poor response to treatment and the development of additional ocular conditions, such as geographic atrophy or glaucoma that contribute to loss of vision.41,48 New approaches that require fewer treatments over time and/or those that provide continuous delivery of an anti-VEGF therapeutic over at least several months may have the potential to improve patient outcomes relative to the current management paradigm.
Limitations
This was a retrospective analysis of patient registry data from routine clinical practice and is therefore associated with multiple inherent limitations that preclude definitive identification of the cause of declining VA. As a registry of clinical practice data, by year 3, only 42% of the initial cohort reported VA, limiting the results presented beyond year 2. There are inherent flaws in all general practice measures of VA, such as the large gaps in VA at the lower end of the Snellen chart and the increased time required for nonophthalmic specialists to perform the ETDRS chart.49 Additionally, provider reporting of VA was not standardized in the Registry, and values used were approximate ETDRS letters for the VA analyses.
Major limitations of this analysis were that it was not known if patients included had either missed visits or had treatment extensions planned and that reasons for treatment discontinuation or treatment gaps, such as poor or no treatment response, were also not known. A further limitation is that it is not possible in all cases to know the reasons for medication switching when it occurred. Because injection treatment patterns (including the number of anti-VEGF injections, treatment intervals, and switching of agents) were extremely variable from year to year; they therefore could not be reliably associated with the outcome at the end of the follow-up period. Thus, it was not possible to determine if increased frequency of treatment gaps was associated with worse visual outcomes nor if any of the factors for potentially worse or better outcomes were associated with greater or lower frequency of treatment gaps. Additionally, the timing of historical diagnoses of comorbidities (e.g., presence of glaucoma and presence of cataracts) and related treatment are unknown, complicating the interpretation of results. Furthermore, with regard to VA, there are conditions other than AMD that could have led to poor VA that may not have been captured in the current study. Finally, information on anatomic outcomes, such as macular fluid status by OCT, is not available.
It was possible that a patient could have been treated in a previous practice setting before inclusion in the Registry. In addition, the EHR look-back period was only 6 months before the patient’s index date. Both factors could have led to the previous treatment not being captured resulting in a potential risk that eyes that were not treatment-naive at baseline could have been included in this study. Outcomes were also not evaluated separately for patients with bilateral and unilateral nAMD.
Although the sample size of self-identified Black and non-White patients with nAMD included in this analysis was low compared with self-identified White patients, estimates reflect previous population-based studies in which the incidence of nAMD has been shown to be lower in Black patients compared with White patients.50 Additional research into racial and ethnic variations among patients with nAMD and their response to treatment should be explored and may lead to more accurate model estimates. However, given the size and inclusivity of the IRIS Registry, these findings are generalizable to the US population.
This retrospective study of data from a US-wide registry of patients in routine clinical practice demonstrates that eyes with nAMD that were treated with intravitreal anti-VEGF injections showed initial modest gains in vision in the first year of treatment, followed by a high rate of vision loss over a period of up to 6 years of treatment, with a net loss from baseline of 4.6 letters. Of the patients, 58.5% experienced CVL at least once, and 14.5% experienced SPV. Injection frequency decreased from a mean of 5.6 injections in year 2 and plateaued at 4.2 to 4.6 injections in years 3 through 6; of the patients, 38.8% discontinued treatment. Statistical modeling suggested, however, that patients who received more frequent anti-VEGF injections in the first year had better vision outcomes. Overall, these findings suggest that most patients with nAMD may find it difficult to adhere to the requirement for frequent intravitreal anti-VEGF injections and could therefore be at risk of experiencing poor vision outcomes. New therapies, including those with different modes of action and/or new routes of administration, which safely reduce treatment burden by extending the duration between retreatments while maintaining optimal efficacy outcomes, could result in improved visual outcomes for patients in routine clinical practice.
Acknowledgments
The authors would like to thank Edward Neuberger, PharmD, for his contributions to this study.
Manuscript no. XOPS-D-23-00141R1.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Portions of these data were presented at the 39th Annual Meeting of the American Society of Retina Specialists (San Antonio, Texas; October 8–12, 2021) and the 2021 Annual Meeting of the American Academy of Ophthalmology (New Orleans Louisiana; November 12–13, 2021).
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s):
C.C.W.: Grants/Contracts – 4DMT, Adverum, Aerie, AffaMed, Alexion, Alimera, Alkahest, Allergan, Aldeyra, Allgenesis, Amgen, Annexin, Annexon, Apellis, Asclepix, Bayer, Boehringer Ingelheim, Chengdu Kanghong, Clearside, Curacle, EyePoint, Gemini, Genentech, GlaxoSmithKline, Graybug, Gyroscope, IONIS, iRENIX, IVERIC bio, Janssen, Kodiak, LMRI, Nanoscope, Neurotech, NGM, Novartis, Ocular Therapeutix, Ocuphire, OcuTerra, OliX, Ophthotech, Opthea, Outlook Therapeutics, Oxurion, Oxular, Oyster Point, PerceiveBio, RecensMedical, Regeneron, RegenXBio, Rezolute, Roche, SamChunDang Pharm, Sandoz, Senju, Shanghai Henlius, Taiwan Liposome Co., UNITY, Verily, Xbrane; Consulting Fees – 4DMT, AbbVie, Adverum, Aerie, AGTC, Alcon, Alimera, Allergan, Allgenesis, Alnylam, Annexon, Apellis, Arrowhead, Bausch + Lomb, Bayer, Bionic Vision Technologies, Boehringer Ingelheim, Cholgene, Clearside, Curacle, EyePoint, Foresite, Frontera, Genentech, Gyroscope, IACTA, IVERIC Bio, Janssen, Kato, Kiora, Kodiak, Kriya, Merck, Nanoscope, NGM, Notal Vision, Novartis, OccuRx, Ocular Therapeutix, Ocuphire, Ocuterra, OliX, ONL, Opthea, Oxular, Palatin, PerceiveBio, Perfuse, PolyPhotonix, Ray, RecensMedical, Regeneron, RegenXBio, Resonance, Roche, Sanofi, SciNeuro, Stealth, Surrozen, Suzhou Raymon, Takeda, THEA, TissueGen, Valo, Verana; Advisory/Data Safety Monitoring Board Participation – Kato, Aerie; Leadership or Fiduciary Role in Other Board, Society, Committee or Advocacy Group – ASRS, Vit-Buckle Society; Stock Options – ONL, PolyPhotonix, RecensMedical, TissueGen, Visgenx, Vitranu.
V.G.: Employee Salary; Stock – Genentech.
D.T.: Employee Salary; Stock – Genentech.
A.M.: Employee Salary; Stock – Genentech.
E.K.: Employee Salary – Genentech; Stock – Roche.
H.B.F.: Employee Salary; Stock or Stock Options – Verana Health.
A.L.: Employee Salary; Stock or Stock Options – Verana Health.
T.L.: Grants/Contracts – Astellas; Consulting Fees – Alcon, Astellas, Boehringer Ingelheim, Graybug, Kanaph, Protagonist, Roche/Genentech, Verana Health; Advisory/Data Safety Monitoring Board Participation – Nanoscope, Regeneron, Apellis.
Supported by funding from Genentech, Inc., a member of the Roche Group, for the study and third-party writing assistance, which was provided by Annie Rowe, PhD, and Jeannine Delwiche, PhD of Envision Pharma Group.
HUMAN SUBJECTS: Human subjects are included in this study. Because the IRIS Registry data are deidentified, no patient-level consent or institutional review board approval was required. All research adhered to the tenets of the Declaration of Helsinki.
No animal subjects were used in this study.
Author Contributions:
Conception and design: Garmo, Tabano, Wykoff, Fevrier, LaPrise, Leng
Data collection: Fevrier, LaPrise, Leng
Analysis and interpretation: Wykoff, Garmo, Tabano, Menezes, Kim, Fevrier, LaPrise, Leng
Obtained funding: Garmo. Study was performed as part of regular employment duties at Genentech, Inc. No additional funding was provided.
Overall responsibility: Wykoff, Garmo, Tabano, Kim, Fevrier, LaPrise, Leng
Supplementary Data
References
- 1.Gottlieb J.L. Age-related macular degeneration. JAMA. 2002;288:2233–2236. doi: 10.1001/jama.288.18.2233. [DOI] [PubMed] [Google Scholar]
- 2.Nowak J.Z. Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep. 2006;58:353–363. [PubMed] [Google Scholar]
- 3.Ambati J., Fowler B.J. Mechanisms of age-related macular degeneration. Neuron. 2012;75:26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong W.L., Su X., Li X., et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell P., Liew G., Gopinath B., Wong T.Y. Age-related macular degeneration. Lancet. 2018;392:1147–1159. doi: 10.1016/S0140-6736(18)31550-2. [DOI] [PubMed] [Google Scholar]
- 6.Apte R.S., Chen D.S., Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176:1248–1264. doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holekamp N.M. Review of neovascular age-related macular degeneration treatment options. Am J Manag Care. 2019;25(10 suppl):S172–S181. [PubMed] [Google Scholar]
- 8.Rosenfeld P.J., Brown D.M., Heier J.S., et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 9.Brown D.M., Kaiser P.K., Michels M., et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 10.Dugel P.U., Koh A., Ogura Y., et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127:72–84. doi: 10.1016/j.ophtha.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Heier J.S., Brown D.M., Chong V., et al. Intravitreal aflibercept (VEGF Trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Heier J.S., Khanani A.M., Quezada Ruiz C., et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and Lucerne): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399:729–740. doi: 10.1016/S0140-6736(22)00010-1. [DOI] [PubMed] [Google Scholar]
- 13.Wykoff C.C., Khurana R.N., Nguyen Q.D., et al. Risk of blindness among patients with diabetes and newly diagnosed diabetic retinopathy. Diabetes Care. 2021;44:748–756. doi: 10.2337/dc20-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prenner J.L., Halperin L.S., Rycroft C., et al. Disease burden in the treatment of age-related macular degeneration: findings from a time-and-motion study. Am J Ophthalmol. 2015;160:725–731.e1. doi: 10.1016/j.ajo.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 15.Varano M., Eter N., Winyard S., et al. Current barriers to treatment for wet age-related macular degeneration (wAMD): findings from the wAMD patient and caregiver survey. Clin Ophthalmol. 2015;9:2243–2250. doi: 10.2147/OPTH.S92548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monés J., Singh R.P., Bandello F., et al. Undertreatment of neovascular age-related macular degeneration after 10 years of anti-vascular endothelial growth factor therapy in the real world: the need for a change of mindset. Ophthalmologica. 2020;243:1–8. doi: 10.1159/000502747. [DOI] [PubMed] [Google Scholar]
- 17.Gohil R., Crosby-Nwaobi R., Forbes A., et al. Caregiver burden in patients receiving ranibizumab therapy for neovascular age related macular degeneration. PLOS ONE. 2015;10 doi: 10.1371/journal.pone.0129361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira A., Sagkriotis A., Olson M., et al. Treatment frequency and dosing interval of ranibizumab and aflibercept for neovascular age-related macular degeneration in routine clinical practice in the USA. PLoS One. 2015;10 doi: 10.1371/journal.pone.0133968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao P., Lum F., Wood K., et al. Real-world vision in age-related macular degeneration patients treated with single anti-VEGF drug type for 1 year in the IRIS registry. Ophthalmology. 2018;125:522–528. doi: 10.1016/j.ophtha.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Ho A.C., Kleinman D.M., Lum F.C., et al. Baseline visual acuity at wet AMD diagnosis predicts long-term vision outcomes: an analysis of the IRIS registry. Ophthalmic Surg Lasers Imaging Retina. 2020;51:633–639. doi: 10.3928/23258160-20201104-05. [DOI] [PubMed] [Google Scholar]
- 21.Mehta H., Tufail A., Daien V., et al. Real-world outcomes in patients with neovascular age-related macular degeneration treated with intravitreal vascular endothelial growth factor inhibitors. Prog Retin Eye Res. 2018;65:127–146. doi: 10.1016/j.preteyeres.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Obeid A., Gao X., Ali F.S., et al. Loss to follow-up among patients with neovascular age-related macular degeneration who received intravitreal anti-vascular endothelial growth factor injections. JAMA Ophthalmol. 2018;136:1251–1259. doi: 10.1001/jamaophthalmol.2018.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finger R.P., Wiedemann P., Blumhagen F., et al. Treatment patterns, visual acuity and quality-of-life outcomes of the WAVE study – a noninterventional study of ranibizumab treatment for neovascular age-related macular degeneration in Germany. Acta Ophthalmol. 2013;91:540–546. doi: 10.1111/j.1755-3768.2012.02493.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee C.S., Blazes M., Lorch A., et al. American Academy of Ophthalmology Intelligent Research in Sight (IRIS®) Registry and the IRIS Registry Analytic Center Consortium. Ophthalmol Sci. 2022;2 doi: 10.1016/j.xops.2022.100112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacCumber M.W., Yu J.S., Sagkriotis A., et al. Antivascular endothelial growth factor agents for wet age-related macular degeneration: an IRIS registry analysis. Can J Ophthalmol. 2023;58:252–261. doi: 10.1016/j.jcjo.2021.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Gregori N.Z., Feuer W., Rosenfeld P.J. Novel method for analyzing snellen visual acuity measurements. Retina. 2010;30:1046–1050. doi: 10.1097/IAE.0b013e3181d87e04. [DOI] [PubMed] [Google Scholar]
- 27.Maguire M.G., Martin D.F., Ying G.S., et al. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123:1751–1761. doi: 10.1016/j.ophtha.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khanani A.M., Skelly A., Bezlyak V., et al. Sierra-AMD: a retrospective, real-world evidence study of patients with neovascular age-related macular degeneration in the United States. Ophthalmol Retina. 2020;4:122–133. doi: 10.1016/j.oret.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Ciulla T.A., Hussain R.M., Taraborelli D., et al. Longer-term anti-VEGF therapy outcomes in neovascular age-related macular degeneration, diabetic macular edema, and vein occlusion-related macular edema: clinical outcomes in 130 247 eyes. Ophthalmol Retina. 2022;6:796–806. doi: 10.1016/j.oret.2022.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Holz F.G., Tadayoni R., Beatty S., et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99:220–226. doi: 10.1136/bjophthalmol-2014-305327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ziemssen F., Wachtlin J., Kuehlewein L., et al. Intravitreal ranibizumab therapy for diabetic macular edema in routine practice: two-year real-life data from a non-interventional, multicenter study in Germany. Diabetes Ther. 2018;9:2271–2289. doi: 10.1007/s13300-018-0513-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soares R.R., Mellen P., Garrigan H., et al. Outcomes of eyes lost to follow-up with neovascular age-related macular degeneration receiving intravitreal anti-vascular endothelial growth factor. Ophthalmol Retina. 2020;4:134–140. doi: 10.1016/j.oret.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Kim L.N., Mehta H., Barthelmes D., et al. Metaanalysis of real-world outcomes of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration. Retina. 2016;36:1418–1431. doi: 10.1097/IAE.0000000000001142. [DOI] [PubMed] [Google Scholar]
- 34.Ciulla T.A., Bracha P., Pollack J., Williams D.F. Real-world outcomes of anti-vascular endothelial growth factor therapy in diabetic macular edema in the United States. Ophthalmol Retina. 2018;2:1179–1187. doi: 10.1016/j.oret.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Ciulla T.A., Hussain R.M., Pollack J.S., Williams D.F. Visual acuity outcomes and anti-vascular endothelial growth factor therapy intensity in neovascular age-related macular degeneration patients: a real-world analysis of 49 485 eyes. Ophthalmol Retina. 2020;4:19–30. doi: 10.1016/j.oret.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 36.Curtis L.H., Hammill B.G., Qualls L.G., et al. Treatment patterns for neovascular age-related macular degeneration: analysis of 284 380 medicare beneficiaries. Am J Ophthalmol. 2012;153:1116–1124.e1. doi: 10.1016/j.ajo.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 37.Bakri S.J., Karcher H., Andersen S., Souied E.H. Anti-vascular endothelial growth factor treatment discontinuation and interval in neovascular age-related macular degeneration in the United States. Am J Ophthalmol. 2022;242:189–196. doi: 10.1016/j.ajo.2022.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Basilious A., Smuck B., Duncan J., et al. Patterns of anti-vascular endothelial growth factor discontinuation in neovascular age-related macular degeneration. Can J Ophthalmol. 2023 March doi: 10.1016/j.jcjo.2023.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Gomi F., Toyoda R., Yoon A.H., Imai K. Factors of anti-vascular endothelial growth factor therapy withdrawal in patients with neovascular age-related macular degeneration: implications for improving patient adherence. J Clin Med. 2021;10:3106. doi: 10.3390/jcm10143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okada M., Mitchell P., Finger R.P., et al. Nonadherence or nonpersistence to intravitreal injection therapy for neovascular age-related macular degeneration: a mixed-methods systematic review. Ophthalmology. 2021;128:234–247. doi: 10.1016/j.ophtha.2020.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pikkel J., Attas S. ‘What should I inject next?’ Challenging treatment decisions in the multiple anti-VEGF: a review of publications exploring anti-VEGF switching for nAMD. Int Ophthalmol. 2018;38:2031–2039. doi: 10.1007/s10792-017-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Lancker L., Petrarca R., Moutsouris K., et al. Clinical experience of switching anti-VEGF therapy from ranibizumab to aflibercept in age-related choroidal neovascularization. Eur J Ophthalmol. 2017;27:342–345. doi: 10.5301/ejo.5000861. [DOI] [PubMed] [Google Scholar]
- 43.Salcedo-Villanueva G., Feria-Anzaldo E., Romo-Aguas J.C., et al. Anti-VEGF treatment switch in neovascular age-related macular degeneration: a comparison of aflibercept versus ranibizumab after a single-dose switch. Int Ophthalmol. 2019;39:2023–2031. doi: 10.1007/s10792-018-1038-4. [DOI] [PubMed] [Google Scholar]
- 44.Empeslidis T., Storey M., Giannopoulos T., et al. How successful is switching from bevacizumab or ranibizumab to aflibercept in age-related macular degeneration? A systematic overview. Adv Ther. 2019;36:1532–1548. doi: 10.1007/s12325-019-00971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karagiannis D.A., Ladas I.D., Parikakis E., et al. Changing from bevacizumab to ranibizumab in age-related macular degeneration. Is it safe? Clin Interv Aging. 2009;4:457–461. doi: 10.2147/cia.s8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Randazzo A., Raimondi R., Fossati G., et al. Outcomes of abrupt switch to bevacizumab of patients undergoing aflibercept intravitreal injections for neovascular age-related macular degeneration in a tertiary center in Lombardy, Italy: a real-life retrospective analysis. J Ophthalmol. 2021;2021 doi: 10.1155/2021/7940297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Writing Committee for the UK Age-Related Macular Degeneration EMR Users Group The neovascular age-related macular degeneration database: multicenter study of 92 976 ranibizumab injections: report 1: visual acuity. Ophthalmology. 2014;121:1092–1101. doi: 10.1016/j.ophtha.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 48.Eshtiaghi A., Issa M., Popovic M.M., et al. Geographic atrophy incidence and progression after intravitreal injections of anti-vascular endothelial growth factor agents for age-related macular degeneration: a meta-analysis. Retina. 2021;41:2424–2435. doi: 10.1097/IAE.0000000000003207. [DOI] [PubMed] [Google Scholar]
- 49.Perera C., Chakrabarti R., Islam F.M., Crowston J. The eye phone study: reliability and accuracy of assessing Snellen visual acuity using smartphone technology. Eye (Lond) 2015;29:888–894. doi: 10.1038/eye.2015.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fisher D.E., Klein B.E., Wong T.Y., et al. Incidence of age-related macular degeneration in a multi-ethnic United States population: the multi-ethnic study of atherosclerosis. Ophthalmology. 2016;123:1297–1308. doi: 10.1016/j.ophtha.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.