Abstract
Objectives: Vascular endothelium, the innermost monolayer of endothelial cells lining the vessel wall, plays a vital physiologic role in the functional integrity of the aorta. Endothelial-derived nitric oxide (NO) is an important molecule regulating vascular endothelial function by its vasodilatory properties and inhibiting pathological inflammatory and oxidative consequences of vascular aging and cardiovascular disorders. Sirtuin 1 (Sirt1), has recently emerged as an important regulator of vascular endothelial NO production. The effect of niacin on Sirt1 in human arterial tissue has not been studied. Methods: Using primary cultures of human aortic endothelial cells (HAEC), we examined the effect of niacin on endothelial Nicotinamide Adenine Dinucleotide+ (NAD+), Sirt1 and NO production. Results: In HAEC, we show that pharmacologically relevant doses of niacin at 0.2-0.3 mM for 24 h significantly increased cellular NAD+ levels, Sirt1 activity, and NO production as compared to controls. Using silencing of Sirt1 by siRNA, we observed that Sirt1 mediates niacin-induced NO production. Conclusions: Translationally, these findings suggest that Sirt1 activation by niacin may be one of the mechanisms of action of niacin acting on NO to improve endothelial function and mitigate human vascular aging and its deleterious cardiovascular consequences.
Keywords: Niacin, Nicotinic acid, Sirt1, nicotinamide adenosine dinucleotide (NAD+), nitric oxide (NO), endothelial function, vascular aging, cardiovascular disorders
Introduction
The aortic endothelium plays a vital role in maintaining arterial homeostasis and functional integrity of the aorta as well as pathophysiologic processes involved in cardiovascular diseases [1,2]. The healthy endothelium is the innermost monolayer of endothelial cells lining the vessel wall. It is able to respond optimally to physical and chemical signals by generating factors that regulate endothelial function, such as vascular tone, cellular adhesion, vessel wall inflammation, and thrombosis [3].
The Nobel Laureates, Furchgott, Murad, and Ignarro, showed that endothelial-derived relaxing factor nitric oxide (NO) is an essential regulator of endothelial function and vasodilation involved in various vascular disorders [4-6]. Endothelial nitric oxide synthase (eNOS) is a key enzyme in NO production from L-arginine. NO diffuses into vascular smooth muscle cells and activates guanylate cyclase, resulting in cGMP-mediated vasodilatation. NO has also been shown to exhibit anti-atherosclerotic properties such as mitigating leukocyte adhesion, vascular oxidative stress, and inflammation, platelet aggregation, and smooth muscle cell proliferation [7].
Aging is associated with a profound decline in vascular endothelial mass and function and is an independent risk factor for endothelial dysfunction and cardiovascular disorders [8,9]. The important observation and the discovery of sirtuins in the late 1990s as critical molecules involved in aging processes gained the attention of researchers to explore the critical role of sirtuin 1 (Sirt1; Silent Information Regulator 1) in aortic endothelial function, vascular aging, and its impact on atherosclerotic vascular disorders. The yeast Silent information regulator 2 (Sir2) gene was shown to extend lifespan in yeast and mice [10,11]. Sirtuins are nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylases that are found in organisms ranging from bacteria to humans. Seven mammalian sirtuins (Sirt 1-7) were identified and were shown to exhibit a wide variety of roles, including DNA repair and extending lifespan. Human Sirt 1 (an ortholog of Sirt2 in yeast) is the most extensively studied sirtuin for its role in biologic processes involved in aortic endothelial function and vascular disorders.
With reference to the current communication, emerging evidence indicates that the mammalian Sirt1, a ubiquitous protein deacetylase localized in nuclear/cytoplasmic compartments, deacetylates both histone and non-histone target proteins that are involved in vascular endothelial function, inflammation, and atherogenesis [12-14]. Mattagajasingh and colleagues have reported an important observation linking Sirt1 to endothelial function by showing that Sirt1, by deacetylating lysine 496 and 506 of eNOS, stimulates endothelial cell eNOS activity and NO production [15]. Inhibition of Sirt1 in the endothelium of the arteries inhibited endothelium-dependent vasodilation and decreased bioavailability of NO [15]. Using endothelial cell-specific Sirt1 transgenic mice, Zhang et al. showed that Sirt1 overexpression increased aortic eNOS, improved vasorelaxation, and decreased atherosclerosis in high-fat fed apoE-deficient mice [16].
Since NAD+ serves as an essential co-substrate required for Sirt1 activation, much research interest has focused on NAD+ boosting approaches to activate Sirt1 over the past decade. Cellular supply of NAD+ in the body is generated either by de novo synthesis from dietary tryptophan or via salvage pathways from precursors. In the heart and cardiovascular system, more than 99% of NAD+ is generated by the salvage pathway by mainly its precursors, including niacin and nicotinamide [NAM; 17]. Since NAD+ serves as an essential co-substrate for Sirt1, cellular NAD+ levels have been shown to play an important regulatory role in Sirt1 activation and its downstream biologic actions [18].
Niacin, at pharmacological doses, is the oldest currently used therapeutic agent for the treatment of dyslipidemia and atherosclerotic coronary artery disease [19,20]. Furthermore, niacin therapy has been shown to improve endothelium-dependent flow-mediated dilation, a surrogate biomarker for endothelial function, in healthy and CVD patients [21,22]. However, the mechanism of action of niacin on human endothelial function is not clearly understood.
During the past several years, our laboratory has been very active in defining mechanisms of action of niacin on lipid and lipoprotein metabolic pathways, aortic endothelial cell oxidative stress processes, and inflammatory processes that are key cellular events involved in atherosclerotic cardiovascular disease [23]. To gain further mechanistic knowledge on niacin, in this study, we hypothesized that in human aortic tissue, niacin by increasing endothelial NAD+, activates Sirt1 and NO production. Using primary cultures of human aortic endothelial cells, the findings reported in this communication show that pharmacological doses of niacin increased cellular NAD+ levels, Sirt1 activity, and NO production. Additionally, the data show that niacin-mediated Sirt1 activation regulates NO production, a key player in endothelial function.
Materials and methods
In-vitro model system
We used primary cultures of human aortic endothelial cells as an in-vitro model system. Normal human aortic endothelial cells (HAEC) were obtained from Invitrogen (Life Technologies Corp., Carlsbad, CA). Cells were provided as cryopreserved vials of a third passage in media containing 2% fetal bovine serum (FBS). These cells are shown to exhibit endothelial cell characteristics such as being positive for factor VIII-related antigen (von Willebrand’s factor) and LDL uptake and express adhesion molecules. Cells were grown in endothelial cell growth medium (M200, Invitrogen) with 2% fetal bovine serum in a 37°C, 5% CO2, 95% air incubator. Cells were used for in-vitro studies between 4-6 passages.
Niacin at concentrations of 0.1-0.3 mM were used for all studies for incubations with HAEC. In humans, the niacin’s plasma level is about 0.3 mM after oral ingestion of 2 g of niacin, a commonly used therapeutic dose in humans [24]. Thus, the doses of niacin used in our in-vitro experiments are clinically relevant and comparable to the niacin concentrations observed in human plasma.
For all in vitro studies, HAEC (passages 4-6) were treated with niacin (0-0.3 mM) for 24-48 h in the presence of 0.5% serum-containing media. After washing, cellular lysates were prepared by sonicating cells in a buffer containing 0.5 M NaCl, 20 mM Tris-HCl (pH 7.5), and 10% glycerol. Cellular lysates were used for various assays, as indicated below.
Measurement of cellular NAD content
NAD+ levels were measured using a very sensitive (in the range of 10-400 nM NAD+) bioluminescent-based NAD/NADH-Glo Assay kit (Promega) per manufacturer’s instructions. In this assay kit, the NAD cycling enzyme converts NAD+ to NADH. In the presence of NADH, Reductase enzymatically reduces a proluciferin reductase substrate to luciferin. Luciferin is detected using Ultra-Glo Luciferase, and the amount of luminescent light produced is proportional to the amount of NAD+.
Measurements of Sirt1 activity
Cellular nuclear extracts were prepared using a nuclear and cytoplasmic extraction kit (Pierce Biotechnology, Rockford, IL). Sirt1 activity in nuclear extracts was assessed using Sirt1 direct fluorescent assay kit (Cayman Chemical) by measuring the deacetylation of p53 Sirt1-specific peptide according to the assay procedure described in the assay kit. In brief, this assay involves 2 steps, and both steps are performed in the same microplate. In the first step, the substrate, which comprises the acetylated p53 protein sequence Arg-His-Lys-Lys (acetyl)-tagged with a fluorescent probe aminomethyl coumarin is incubated with a cellular extract containing Sirt1 or human recombinant Sirt1 (as a standard) along with its co-substrate NAD+. In the second step, deacetylation sensitizes the p53 substrate such that the treatment with the developer releases a fluorescent deacetylated product. The fluorescence is measured in a fluorometer using an excitation wavelength of 350-360 nm and an emission wavelength of 450-465 nm.
Measurement of NO
The production of NO was measured by assessing stable NO metabolites nitrite (NO2) and nitrate (NO3) using a commercially available Nitrate/Nitrite Fluorescent assay kit (Cayman Chemicals) according to the manufacturer’s instructions. In brief, this assay consists of two steps: the first step is the conversion of nitrate to nitrite catalyzed by nitrate reductase, and the second step involves the addition of DAN (2,3, diaminonaphthalene), providing an acidic solution and followed by the addition of NaOH which enhances the detection of the fluorescent product 1(H)-naphthotriazole. Measurement of fluorescence using an excitation wavelength of 360-365 nm and an emission wavelength of 430 nm accurately measures NO concentration.
Transfection of cells with Sirt1-specific siRNA
For silencing Sirt1, HAEC were transfected with Sirt1 siRNA for 48 h using commercially available and validated Sirt1 siRNA: TTGGGTCTTCCCTCAAAGTAA and HiPerFect transfection reagent (Qiagen). Real-time PCR analysis of Sirt1 mRNA expression after normalization with β-actin indicated that siRNA transfection markedly silenced the mRNA expression of Sirt1 in HAEC (Sirt1 mRNA, change over control: control, untransfected cells = 1.0 ± 0.10, siRNA transfected cells = 0.19 ± 0.02).
Statistical analysis
Data presented are the mean ± SE of 3 separate experiments. Statistical analysis for two-group comparisons was performed by using an unpaired Student’s t-test (two-tailed), and a value of P<0.05 was considered significant.
Results
Niacin increases NAD+ levels in HAEC
HAEC were incubated in the absence (control) or presence of niacin (0.2-0.3 mM) for 24 h. As shown in Figure 1, treatment of HAEC with pharmacologically relevant doses of niacin at 0.2-0.3 mM significantly increased cellular NAD+ levels as compared to controls. Incubation of cells with niacin for 48 h also had similar effects on NAD+ levels as compared to 24 h incubation (data not shown). Niacin doses below 0.1 mM had no effect on NAD+ levels (data not shown).
Figure 1.

Niacin increases NAD+ levels in human aortic endothelial cells (HAEC). Cells were incubated in the presence or absence of niacin for 24-48 h. NAD+ levels in cellular extracts were measured as described in Methods, and NAD+ concentrations expressed as nmol/mg cellular protein. Data are mean ± SE of 3 experiments. P-values shown are compared to controls.
Niacin increases Sirt1 activity in HAEC
As shown in Figure 2, treatment of HAEC with niacin (0.2 mM and 0.3 mM) significantly augmented Sirt1 activity by about 30% when compared to control cells without a niacin treatment (Figure 2A).
Figure 2.

Niacin increases Sirt1 activity in HAEC. A: Cells (2×106) were incubated with niacin for 24 h. Sirt1 activity in cellular nuclear extracts was measured according to the assay kit noted in Methods. Sirt1 activity expressed per mg cellular protein. P-values shown are compared to the control. B: Cells (1×106) were incubated with either vehicle (Control), H2O2 (100 mM), or H2O2 + niacin (0.3 mM) for 24 h. Sirt1 activity expressed per 0.5 mg cellular protein. Data are mean ± SE of 3 experiments. The P-value shown on H2O2 is compared to control, and H2O2 + niacin is compared to H2O2 treated cells.
Niacin blocks oxidative-stress mediated decrease in Sirt1 activity
Mediators of oxidative stress (such as hydrogen peroxide, H2O2) are shown to inhibit Sirt1 activity through promoting Sirt1 protein degradation and lowering Sirt1 gene expression [25]. This study tested whether niacin protects H2O2-mediated effects on Sirt1 activity. As shown in Figure 2B, stimulation of HAEC with H2O2 decreased Sirt1 activity as compared to controls. Treatment of cells with niacin protected H2O2-mediated reduction in Sirt1 activity (Figure 2B).
Niacin increases NO production
Since Sirt1 has been previously reported to regulate endothelial NO production, we tested whether niacin-mediated increased Sirt1 activation translates to NO production in HAEC. For this study, we examined the effect of niacin on NO production by measuring the stable NO metabolites nitrite (NO2) and nitrate (NO3). Treatment of HAEC with niacin (0.2 mM and 0.3 mM) markedly increased NO production by 40-76% when compared to controls without niacin treatment with (Figure 3).
Figure 3.

Niacin increases NO production in HAEC. NO production in cellular extracts was measured according to the procedures described in Methods, and concentrations were expressed as nmol/mg cellular protein. Data are mean ± SE of 3 experiments. P-values shown are compared to controls.
Niacin-induced Sirt1 mediates NO production by HAEC
Using silencing of Sirt1 by siRNA, we assessed the participation of Sirt1 in niacin-mediated effects on NO production. HAEC were transfected with Sirt1 siRNA for 48 h using commercially available and validated Sirt1 siRNA. As shown in Figure 4, niacin in non-Sirt1 siRNA transfected cells robustly increased NO production. In Sirt1 siRNA transfected cells, niacin (0.3 mM) failed to increase NO production to the same extent as seen in non-transfected cells (Figure 4). These data suggest that Sirt1 participates in niacin-mediated effects on NO production.
Figure 4.
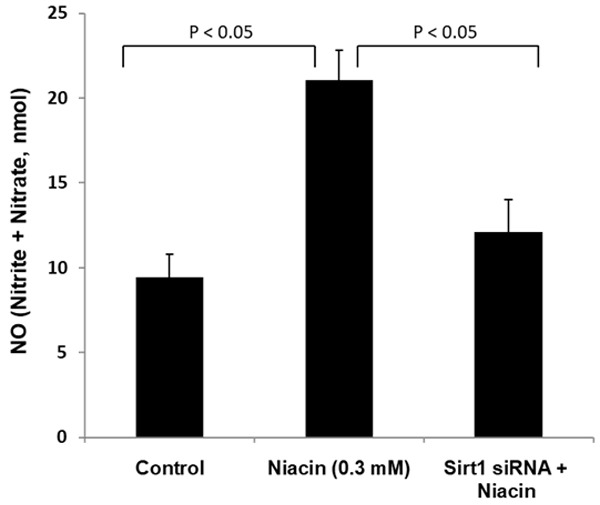
Silencing of Sirt1 by siRNA blocks niacin-mediated NO production. For silencing Sirt1, HAEC were transfected with Sirt1 siRNA for 48 h using commercially available and validated Sirt1 siRNA as described in Methods. Sirt1 siRNA was transfected, and control cells were incubated with niacin for 24 h. Cellular extracts were used for NO production assays. NO levels are expressed as nmol/0.5 mg cellular protein. Data are mean ± SE of 3 experiments. P-values shown are compared to the respective bar diagrams in the figure.
Effect of niacin on Sirt 1-targeted and marker genes relevant to human vascular endothelial cell function
In addition to Sirt 1-mediated NO production, NFκB and subsequent expression of vascular inflammatory genes including VCAM-1 (vascular cell adhesion molecule-1) and MCP-1 (monocyte chemotactic protein-1) are major cardiovascular relevant Sirt 1 targeted and marker genes involved in vascular endothelial cell function, early development of atherosclerotic lesions, and vascular aging. Stein and colleagues have shown that Sirt1 deacetylates RelA/p65 subunit of NFκB at K310 and suppresses its binding to the DNA and activity in human aortic endothelial cells [26]. Sirt1, by interfering with this key step in NFκB signaling, reduces vascular endothelial inflammation by inhibiting endothelial expression of key adhesion molecules such as VCAM-1, ICAM-1, and MCP-1 that regulate monocyte adhesion and migration into the endothelium and subsequent formation early atherosclerotic lesions [26]. In Sirt1 transgenic mouse models with apoE-deficiency, decreased aortic endothelial expression of VCAM-1 and ICAM-1 in Sirt1+/+apoE-/- was seen when compared to Sirt1-/+apoE-/- mice [27].
To gain further insight into the effect of niacin on Sirt 1-targeted and marker genes relevant to endothelial cell function, we examined the effect of niacin on NFκB, VCAM-1, and MCP-1 in HAEC and endothelial monocyte adhesion. Our data from these studies shown previously indicated that niacin at 0.25 mM concentration significantly inhibited p65/ NFκB binding activity stimulated by TNF-α by 46% [28]. Additionally, we showed previously that niacin significantly inhibited VCAM-1 and MCP-1 expression by 77% and 34% respectively and monocyte adhesion to endothelial cells by 41% [28]. Taken together, our findings show that niacin significantly inhibited Sirt 1 targeted and marker genes including NO production, NFκB, VCAM-1 and MCP-1 that are relevant to human vascular endothelial function and vascular aging.
Niacin had no effect on cell viability in HAEC
Using CellTiter-Glo cell viability assay kit, previously we have shown that niacin at these doses (0.2-0.3 mM) used in our studies had no toxic effect on HAEC viability [28].
Discussion
In pharmacologic doses, niacin remains a clinically used therapeutic agent with over half-century history of impressive broad-spectrum effects on plasma lipids, lipoproteins, and other pleiotropic anti-atherosclerotic actions. As monotherapy, it lowers atherogenic apo B-containing lipoproteins [e.g., LDL, VLDL, and Lp(a)] and raises apo A-containing high-density lipoprotein [19,20]. Importantly, in arteriographic studies, in patients with atherosclerotic cardiovascular disease (CVD), it reverses atherosclerosis in coronary, femoral, and carotid arteries when used with LDL-C lowering agents, bile acid sequestrants, or statins [19,20]. Additional studies have also showed that niacin therapy improves endothelium-dependent flow-mediated dilation, a surrogate biomarker for endothelial function, in healthy and CVD patients [21,22]. Nevertheless, the mechanism of action of niacin on human endothelial function is not clearly understood. To the best of our knowledge the effect of niacin on human aortic endothelium has not been reported.
In an attempt to shed light on the cellular mechanism of action of niacin on human endothelial function and its regulatory processes, our data clearly demonstrate that pharmacologically relevant doses of niacin markedly increased human aortic endothelial cell production of NO, a vital regulatory molecule involved in endothelial function. In mechanistic studies, we further showed that niacin by increasing cellular levels of NAD+ activates Sirt1, and niacin-induced Sirt1 regulates NO production in human aortic endothelial cells. Although the regulatory role of cellular NAD+ in Sirt1 activation and Sirt1-mediated NO production is well established, our findings showed for the first time that niacin (a precursor of NAD+) in primary cultures of human aortic endothelial cells activates Sirt1 and increases NO production by a Sirt1-mediated pathway.
Furthermore, Sirt1 has also emerged as a critical endogenous regulator of biological aging and aging of the vascular system [reviewed in 29]. As we age, there is a profound decline in vascular endothelial mass, NAD+ levels, and endothelial function, and aging has been thought to be an independent risk factor for increased incidence and prevalence of endothelial dysfunction and cardiovascular disorders in older population [30-32]. Our findings also suggest that niacin, through NAD+-Sirt1 and NO mediated mechanisms, improves vascular aging and its deleterious vascular consequences.
Based on the current and our previously reported studies, we suggest that the mechanism of action of niacin and Sirt 1-mediated processes to improve human vascular endothelial function and vascular aging include: 1) niacin, by increasing aortic endothelial NAD+ increases Sirt1 activity, and niacin in a Sirt1-dependent mechanism augments endothelial NO production, 2) niacin-induced Sirt1 inhibits NFκB, VCAM-1 and MCP-1 that are cardiovascular relevant Sirt 1 targeted and marker genes involved in vascular endothelial function, and endothelial inflammation. Translationally, these findings suggest that niacin, through Sirt1-mediated mechanism(s), beneficially improves endothelial function and aging of the cardiovascular system. Furthermore, our data suggest that niacin and its analogs may be included as one of the activators of Sirt1 alongside other natural and synthetic Sirt1 activating compounds (STACs) [33].
Acknowledgements
This research was supported by the Southern California Institute for Research and Education and the Department of Veterans Affairs Healthcare System, Long Beach, California, USA.
Disclosure of conflict of interest
Dr. Kashyap has received research grants from AbbVie, Amgen, Amylin, Arisaph, AstraZeneca, Bristol Meyers Squibb, Eli-Lilly, Kos, Sanofi-Aventis, Merck, and has been an advisor and/or speaker for AbbVie, Amarin, Kos, Bristol Meyers Squibb, and Merck. Shaan Kamanna, Shobha Ganji and Vaijinath Kamanna has no disclosures.
Abbreviations
- CVD
Cardiovascular disease
- eNOS
Endothelial nitric oxide synthase
- FBS
Fetal bovine serum
- HAEC
Human aortic endothelial cells
- MCP-1
Monocyte chemotactic protein-1
- NAD
Nicotinamide adenine dinucleotide
- NO
Nitric oxide
- Sirt1
Sirtuin 1
- STACs
Sirtuin1 activating compounds
- VCAM-1
Vascular cell adhesion molecule-1
References
- 1.Vita JA, Keaney JF Jr. Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106:640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 2.Moncada S, Higgs EA. Nitric oxide and the vascular endothelium. Handb Exp Pharmacol. 2006:213–254. doi: 10.1007/3-540-32967-6_7. [DOI] [PubMed] [Google Scholar]
- 3.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 4.Furchgott RF. Endothelium-derived relaxing factor: discovery, early studies, and identification as nitric oxide. Biosci Rep. 1999;19:235–251. doi: 10.1023/a:1020537506008. [DOI] [PubMed] [Google Scholar]
- 5.Murad F. The 1996 Albert Lasker Medical Research Awards. Signal transduction using nitric oxide and cyclic guanosine monophosphate. JAMA. 1996;276:1189–1192. [PubMed] [Google Scholar]
- 6.Ignarro LJ, Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovasc Pharmacol. 1999;34:879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation. 2004;109(Suppl 1):II27–II33. doi: 10.1161/01.CIR.0000129501.88485.1f. [DOI] [PubMed] [Google Scholar]
- 8.Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, Kim LJ, Osborne B, Joshi S, Lu Y, Treviño-Villarreal JH, Kang MJ, Hung TT, Lee B, Williams EO, Igarashi M, Mitchell JR, Wu LE, Turner N, Arany Z, Guarente L, Sinclair DA. Impairment of an endothelial NAD+-H2S signaling network is a reversible cause of vascular aging. Cell. 2018;173:74–89. e20. doi: 10.1016/j.cell.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part 1: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 10.Sinclair DA, Guarente L. Extrachromosomal rDNA circles - a cause of aging in yeast. Cell. 1997;91:1033–1042. doi: 10.1016/s0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 11.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stein S, Matter CM. Protective roles of SIRT1 in atherosclerosis. Cell Cycle. 2011;10:640–647. doi: 10.4161/cc.10.4.14863. [DOI] [PubMed] [Google Scholar]
- 13.Borradaile NM, Pickering JG. NAD+, sirtuins, and cardiovascular disease. Curr Pharm Des. 2009;15:110–117. doi: 10.2174/138161209787185742. [DOI] [PubMed] [Google Scholar]
- 14.Winnik S, Stein S, Matter CM. SIRT1 - an anti-inflammatory pathway at the crossroads between metabolic disease and atherosclerosis. Curr Vasc Pharmacol. 2012;10:693–696. doi: 10.2174/157016112803520756. [DOI] [PubMed] [Google Scholar]
- 15.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, Liu G, Wei YS, Cai H, Liu DP, Liang CC. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res. 2008;80:191–199. doi: 10.1093/cvr/cvn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogan KL, Brenner C. Nicotinic acid, nicotinamide, nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr. 2008;28:115–130. doi: 10.1146/annurev.nutr.28.061807.155443. [DOI] [PubMed] [Google Scholar]
- 18.Kane AE, Sinclair DA. Sirtuins and NAD+ in the development and treatment of metabolic and cardiovascular diseases. Circ Res. 2018;123:868–885. doi: 10.1161/CIRCRESAHA.118.312498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyers CD, Kamanna VS, Kashyap ML. Niacin therapy in atherosclerosis. Curr Opin Lipidol. 2004;15:659–665. doi: 10.1097/00041433-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Lavigne PM, Karas RH. The current state of niacin in cardiovascular disease prevention: a systematic review and meta-regression. J Am Coll Cardiol. 2013;61:440–446. doi: 10.1016/j.jacc.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 21.Kaplon RE, Gano LB, Seals DR. Vascular endothelial function and oxidative stress are related to dietary niacin intake among healthy middle-aged and older adults. J Appl Physiol (1985) 2014;116:156–163. doi: 10.1152/japplphysiol.00969.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahebkar A. Effect of niacin on endothelial function: a systematic review and meta-analysis of randomized controlled trials. Vasc Med. 2014;19:54–66. doi: 10.1177/1358863X13515766. [DOI] [PubMed] [Google Scholar]
- 23.Kamanna VS, Ganji SH, Kashyap ML. Recent advances in niacin and lipid metabolism. Curr Opin Lipidol. 2013;24:239–245. doi: 10.1097/MOL.0b013e3283613a68. [DOI] [PubMed] [Google Scholar]
- 24.Menon RM, Gonzalez MA, Adams MH, Tolbert DS, Leu JH, Cefali EA. Effect of the rate of niacin administration on the plasma and urine pharmacokinetics of niacin and its metabolites. J Clin Pharmacol. 2007;47:681–688. doi: 10.1177/0091270007300264. [DOI] [PubMed] [Google Scholar]
- 25.Zhao X, Jin Y, Yang L, Hou Z, Liu Y, Sun T, Pei J, Li J, Yao C, Wang X, Chen G. Promotion of SIRT1 protein degradation and lower SIRT1 gene expression via reactive oxygen species is involved in Sb-induced apoptosis in BEAS-2b cells. Toxicol Lett. 2018;296:73–81. doi: 10.1016/j.toxlet.2018.07.047. [DOI] [PubMed] [Google Scholar]
- 26.Stein S, Schafer N, Breitenstein A, Besler C, Winnik S, Lohmann C, Heinrich K, Brokopp CE, Handschin C, Landmesser U, Tanner FC, Luscher TF, Matter CM. SIRT1 reduces endothelial activation without affecting vascular function in apoE-/- mice. Aging (Albany NY) 2010;2:353–360. doi: 10.18632/aging.100162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stein S, Lohmann C, Schafer N, Hofmann J, Rohrer L, Besler C, Rothgiesser KM, Becher B, Hottiger MO, Boren J, McBurney MW, Landmesser U, Luscher TF, Matter CM. SIRT1 decreases Lox-1 mediated foam cell formation in atherogenesis. Eur Heart J. 2010;31:2301–2309. doi: 10.1093/eurheartj/ehq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganji SH, Qin S, Zhang L, Kamanna VS, Kashyap ML. Niacin inhibits vascular oxidative stress, redox-sensitive genes, and monocyte adhesion to human aortic endothelial cells. Atherosclerosis. 2009;202:68–75. doi: 10.1016/j.atherosclerosis.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 29.Marian AJ. Genetic basis of cardiovascular aging is at the core of human longevity. J Cardiovasc Aging. 2022;2:25. doi: 10.20517/jca.2022.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donato AJ, Machin DR, Lesniewski LA. Mechanisms of dysfunctions in the aging vasculature and role in age-related diseases. Circ Res. 2018;123:825–848. doi: 10.1161/CIRCRESAHA.118.312563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitada M, Ogura Y, Koya D. The protective role of Sirt1 in vascular tissue: its relationship to vascular aging and atherosclerosis. Aging (Albany NY) 2016;8:2290–2307. doi: 10.18632/aging.101068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Man AWC, Li H, Xia N. The role of sirtuin 1 in regulating endothelial function, arterial remodeling and vascular aging. Front Physiol. 2019;10:1173. doi: 10.3389/fphys.2019.01173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]


