Abstract
Introduction
A substantial proportion of patients with chronic coronary syndromes suffer from angina even after medical treatment and revascularization. Coronary microvascular dysfunction (CMD) is discussed as a potential mechanism.
Aim
To assess angina status in patients with chronic coronary syndromes undergoing functional assessment of coronary circulation regarding the presence of coronary microcirculatory dysfunction.
Material and methods
The study included 101 consecutive patients referred for coronary angiography requiring functional stenosis assessment, with median age of 66 years, 74% male, diagnosed or treated for dyslipidemia (91%) and diabetes type 2 (42%), 20% with a history of prior non-ST myocardial infarction. Fractional flow reserve (FFR), coronary flow reserve (CFR), resistive reserve ratio (RRR), and index of microcirculatory resistance (IMR) were measured. The diagnosis of CMD was defined by either IMR ≥ 25 units or CFR ≤ 2.0 in case of no significant stenosis. A change of one CCS class over 24 months follow-up was considered clinically significant.
Results
In patients without CMD diagnosis, there was a significant decrease in angina intensity (p < 0.001). Lack of angina improvement was associated with lower median RRR (2.30 (1.70, 3.30) vs. 3.05 (2.08, 4.10), p = 0.004) and lower median CFR (1.90 (1.40, 2.50) vs. 2.30 (IQR: 1.60, 3.00), p = 0.021), as compared to patients with angina improvement.
Conclusions
The presence of CMD is a risk factor for no angina improvement. Impaired coronary resistive reserve ratio and lower microvascular reactivity may be one of the pathomechanisms leading to the lack of angina improvement in patients with chronic coronary syndromes.
Keywords: coronary flow reserve, chronic coronary syndromes, coronary microcirculatory dysfunction, angina symptoms, resistive reserve ratio
Summary
Coronary microvascular dysfunction (CMD) is discussed as a potential mechanism of persistent angina in patients with chronic coronary syndrome, even after myocardial revascularization. Our study aimed to assess angina levels in patients undergoing functional testing due to moderate coronary lesions and CMD diagnosis. In patients without CMD diagnosis, there was a significant change in angina intensity. The presence of CMD, lower resistive reserve ratio and coronary flow reserve were a risk factor for no angina improvement. Impaired coronary resistive reserve ratio and lower microvascular reactivity may be one of the pathomechanisms leading to the lack of angina improvement in patients with chronic coronary syndromes.
Introduction
Chronic coronary syndromes are an important cause of morbidity and mortality, and their prevalence is increasing with age [1, 2]. Patients with chronic coronary syndromes may be treated with coronary revascularization and medical therapy [3]. Angina presence is attributed to ischemia caused by atherosclerotic lesions, but in a number of patients symptoms are present even without obstructive coronary lesions in invasive angiography [4–6]. Also after revascularization, persistent or recurrent angina is present in 25 to 35% of patients, even when additional imaging or functional ischemia testing (i.e. FFR) is used to optimize the treatment [7–10].
Several pathomechanisms of this phenomenon are suggested, including the suboptimal result of angioplasty, inadequate secondary preventive measures or the presence of coronary microcirculatory dysfunction (CMD) [8, 11].
Overall the prevalence of CMD in patients with chronic coronary syndromes is very high and reported in up to 64% of patients with ischemia with no obstructive coronary artery disease (INOCA) [12–14]. Physiological stratification of patients with CMD reported by Rahman suggests that both functional and structural changes can be attributed to the presence of angina [15]. Functional testing itself has been associated with angina improvement in patients with INOCA [16]. However, data on angina levels reported by the Canadian Cardiovascular Society angina scale in patients with CMD are scarce.
Aim
We aimed to assess angina status in patients with chronic coronary syndromes undergoing functional assessment of coronary circulation regarding the presence of coronary microcirculatory dysfunction.
Material and methods
Consecutive patients with chronic coronary syndromes who were referred for coronary angiography and had coronary stenosis requiring functional assessment underwent fractional flow reserve (FFR) measurements with hyperemia induced by an intravenous 140 μg/kg/min adenosine infusion [17, 18]. An additional, thermodilution-based assessment of coronary flow reserve (CFR), index of microcirculatory resistance (IMR), and resistive reserve ratio (RRR) with PressureWire X (Abbott, US) were performed [19]. All physiologic data were analyzed using CoroFlow v. 3.0 software (Abbott, US).
A quantitative coronary angiography analysis was performed by an independent analyst at the core lab, blinded to functional assessment results. An edge detection system (CAAS 5.7 QCA system, PieMedical, Maastricht, The Netherlands) was used to calculate percent diameter stenosis (%DS). RadiAnt DICOM Viewer was used for the angiogram review.
All clinical data, including angina assessment with the Canadian Cardiovascular Society class, were acquired before coronary angiography and at a follow-up of 24 months during an office visit in the out-patient clinic by a physician blinded to a functional assessment result. A change of one CCS class was considered clinically significant. Medical treatment optimization was performed before the discharge, according to the diagnosis and advice about lifestyle improvement was provided in all cases.
Exclusion criteria were acute coronary syndrome as a clinical presentation, presence of any > 90% diameter stenosis in any major coronary artery (or > 50% narrowing of left main), and active inflammatory or neoplastic disease.
Normal range of physiologic measurements and definition of coronary microcirculatory dysfunction
The value of FFR ≤ 0.80 was considered significant and an indication for myocardial revascularization [2, 3]. Coronary flow reserve < 2.0 and IMR ≥ 25 units were defined as abnormal [2]. Coronary microcirculatory dysfunction (CMD) was diagnosed according to current guidelines when the IMR ≥ 25 units were present or, in vessels with FFR > 0.80, when CFR < 2.0 was measured [2]. Values of IMR were corrected according to the Yong formula to standardize for the presence of epicardial stenosis.
Statistical analysis
Data were presented as the mean with standard deviation or as the median with interquartile range (IQR) for non-normally distributed variables. The Shapiro-Wilk test was used to test for normality. Quantitative data were presented as a percentage of all groups. Comparisons for continuous data were performed using Student’s t-test for normally distributed data and with the Mann-Whitney U-test otherwise. Pre-post comparisons for repeated measures were performed with the Wilcoxon test.
Logistic regression was used to select risk factors of no angina improvement. Multivariate models were adjusted for sex and age as well as the vessel tested. All analyses were performed using R language for statistical computing (R-core team, Vienna) version 4.0.3 with the tidyverse ecosystem and ggstatsplot packages; the value of p < 0.05 was considered significant [20, 21].
All study procedures were approved by the Jagiellonian University Bioethics Committee (approval number 122.6120.262.2015 with further extensions). The research was funded by a Jagiellonian University statutory grant.
Results
Patient characteristics
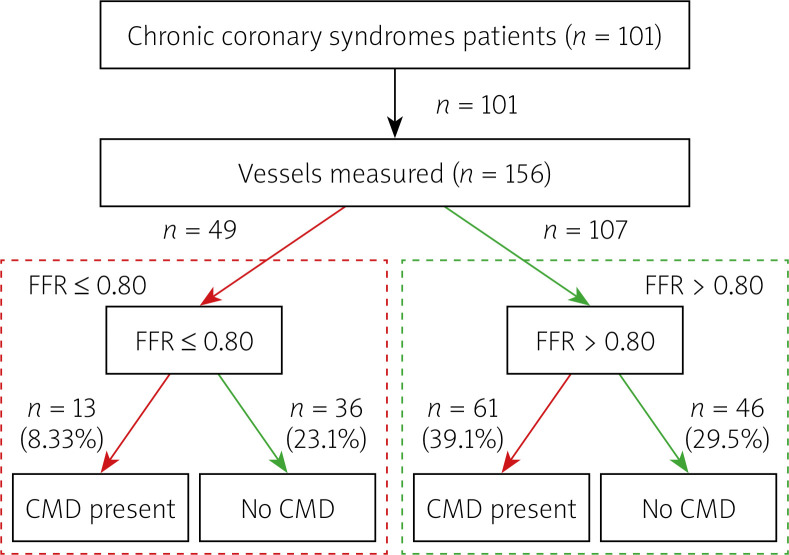
A group of 101 patients with chronic coronary syndromes, median age 66 years, 74% male, the majority diagnosed with or treated for arterial hypertension (96%), 91% with dyslipidaemia, 42% with diabetes type 2, was studied. Over 20% of patients had a history of prior non-ST myocardial infarction. Detailed data on patient characteristics and concomitant disease are presented in Table I. The flow chart of patients included in the study is presented in Figure 1.
Table I.
Patient characteristics
| Characteristic | Overall, n = 101 |
|---|---|
| Age [years] median (IQR) | 66 (59, 73) |
| Sex, n (%): | |
| Female | 26 (26) |
| Male | 75 (74) |
| BMI, median (IQR) [kg/m2] | 28.1 (26.0, 31.8) |
| Smoking, n (%): | |
| Current | 19 (20) |
| In the past | 22 (24) |
| Never | 52 (56) |
| Arterial hypertension, n (%) | 97 (96) |
| Dyslipidemia, n (%) | 92 (91) |
| Diabetes, n (%) | 42 (42) |
| Prior NSTEMI, n (%) | 18 (20) |
| Left ventricle ejection fraction, % median (IQR) | 55 (50, 60) |
| LDL cholesterol [mmol/l] median (IQR) | 2.22 (1.79, 2.86) |
| Acetylsalicylic acid, n (%) | 91 (90) |
| β-blockers, n (%) | 86 (85) |
| Dihydropyridine Ca blocker, n (%) | 33 (33) |
| Non-dihydropyridine Ca blocker, n (%) | 9 (9.0) |
| Angina at baseline, CCS class, n (%): | |
| 0 | 21 (20.8) |
| I | 30 (29.7) |
| II | 36 (35.6) |
| III | 14 (13.9) |
| Angina at follow-up, CCS class, n (%): | |
| 0 | 48 (47.5) |
| I | 22 (21.8) |
| II | 23 (22.8) |
| III | 7 (6.93) |
| IV | 1 (0.99) |
| Final diagnosis, n (%): | |
| No CMD | 19 (18.8) |
| CMD only | 32 (31.7) |
| Revascularization without CMD | 25 (24.8) |
| Revascularization with CMD | 25 (24.8) |
| Angina change at follow-up: | |
| Angina improvement | 52 (51.5) |
| No improvement or worsening | 49 (48.5) |
BMI – body mass index, NSTEMI – non-ST-elevation myocardial infarction, LDL – low-density lipoprotein.
Figure 1.
Flow chart of patients and vessels included in analysis
FFR – fractional flow reserve, CMD – coronary microvascular dysfunction.
Per vessel analysis
The analysis included 156 vessels, mainly left anterior descending (58%). Median artery diameter stenosis in QCA was 45% (IQR: 40, 50).
Coronary physiology measurements revealed a median FFR of 0.84 (IQR: 0.78; 0.91), FFR ≤ 0.80 was diagnosed in 49 vessels (31% of arteries tested). Thermodilution-based measurements revealed a median CFR of 2.1 (IQR: 1.5; 2.7) and IMR of 20 (IQR: 13;18) units. The RRR median value was 2.7 (IQR: 1.8; 3.7). The diagnosis of CMD was established in 74/156 vessels (47% tested), including 61/107 (57%) vessels with FFR > 0.80 and 13/49 (27%) vessels with FFR ≤ 0.80. The detailed data on angiographic and physiologic measurements are presented in Table II.
Table II.
Coronary vessel measurement results
| Characteristic | All vessels n = 156 |
|---|---|
| Vessel tested, n (%): | |
| LAD | 88 (58) |
| LCx | 38 (25) |
| RCA | 27 (18) |
| QCA diameter stenosis, median % (IQR) | 45 (40, 50) |
| Gensini score, median (IQR) | 10 (6, 14) |
| RFR, median (IQR) | 0.89 (0.84, 0.94) |
| FFR, median (IQR) | 0.84 (0.78, 0.91) |
| CFR, median (IQR) | 2.10 (1.50, 2.70) |
| IMR, median (IQR) | 20 (13, 28) |
| RRR, median (IQR) | 2.70 (1.80, 3.73) |
| CMD diagnosis – vessel level: | |
| No CMD | 62 (40%) |
| CMD | 94 (60%) |
CFR – coronary flow reserve, CMD – coronary microcirculatory dysfunction, FFR – fractional flow reserve, IMR – index of microcirculatory resistance, LAD – left anterior descending, LCx – left circumflex, QCA – quantitative coronary angiography, RCA – right coronary artery, RFR – resting full cycle ratio, RRR – resistive reserve ratio.
Angina levels according to CCS scale and presence of CMD
Most of the patients suffered angina CCS class II at baseline, with no significant difference between groups based on CMD status (p = 0.34).
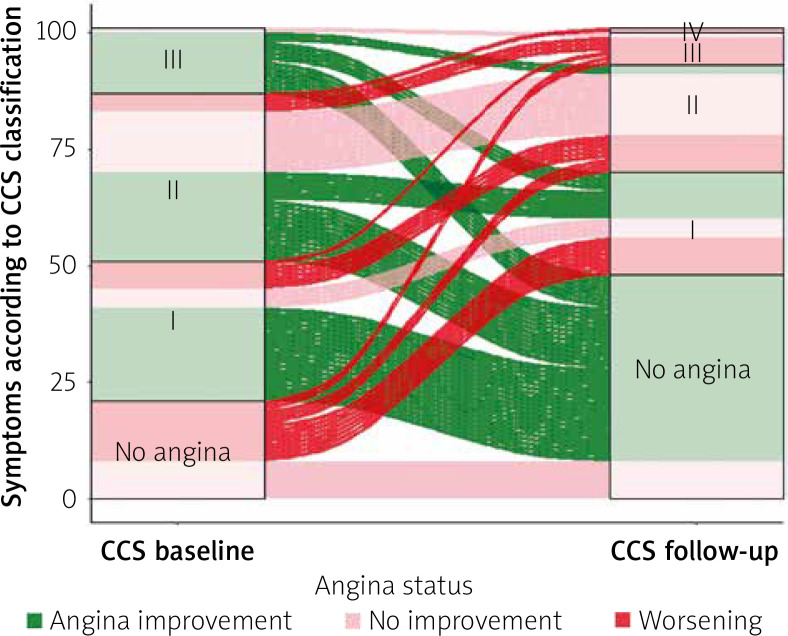
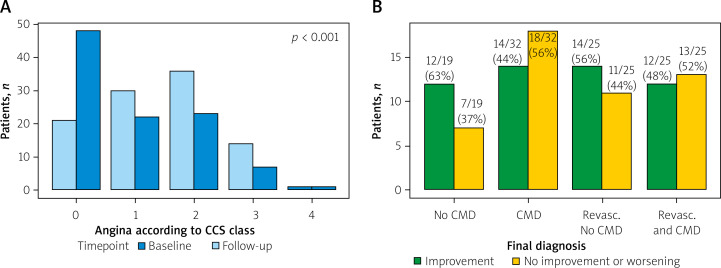
There were significant differences in CCS scale angina at baseline and after 24 months in particular patients (p < 0.001, Figures 2 and 3 A), with the improvement of at least 1 class according to CCS noted in 52/101 (51.4%) patients.
Figure 2.
Angina status according to CCS class in particular patients at baseline and follow-up
Figure 3.
A – Angina intensity according to the CCS scale. P < 0.001 for a change between baseline and follow-up. B – the proportion of patients with angina improvement of at least 1 class according to the final diagnosis
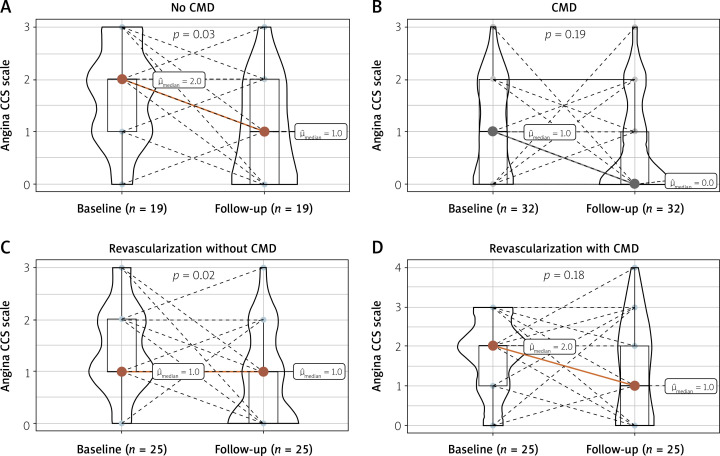
In a subgroup analysis, there was a significant decrease in median angina class in patients without CMD (p < 0.001 pre-post), and no significant difference in the CMD subgroup (pre-post p = 0.06). When the final patient diagnosis was considered (including both CMD status and presence of FFR ≤ 0.80), patients from the group diagnosed with CMD only (FFR > 0.80) and from the group revascularized with concomitant CMD had a lower improvement rate (44% and 48% respectively), as compared to groups of patients without any pathology (no CMD and FFR > 0.80) or from the group revascularized without CMD (63% and 56%, respectively). The differences between all four groups were not statistically significant (p-overall = 0.500). The improvement rate was similar in all groups based on the vessel tested (p = 0.8). Detailed results are presented in Figures 3 B and 4.
Figure 4.
Angina CCS scale change regarding revascularization and presence of CMD
Risk factors for no improvement
Data on angina status were obtained for all 101 patients. No angina improvement at the 24-month follow-up was observed in 49 (49%) patients. A detailed comparison of patient characteristics regarding angina improvement or no improvement is presented in Table III.
Table III.
Patient level univariate regression analysis of no angina improvement risk factors
| Characteristic | Overall, n = 101 | Angina improvement, n = 52 | No improvement or worsening, n = 49 | OR univariate (95% CI) | P-value1 |
|---|---|---|---|---|---|
| Age [years] median (IQR) | 66 (59, 73) | 66 (62, 70) | 64 (58, 74) | 0.99 [0.95;1.03] | 0.5 |
| Sex, n (%): | 0.2 | ||||
| Female | 26 (26) | 16 (31) | 10 (20) | - | |
| Male | 75 (74) | 36 (69) | 39 (80) | 1.72 [0.69;4.43] | |
| BMI, median (IQR) [kg/m2] | 28.1 (26.0, 31.8) | 28.1 (26.3, 32.1) | 28.0 (25.4, 30.4) | 1.00 [0.91;1.10] | 0.5 |
| Smoking, n (%): | 0.6 | ||||
| Current | 19 (20) | 9 (18) | 10 (24) | – | |
| In the past | 22 (24) | 11 (22) | 11 (26) | 0.90 [0.26;3.17] | |
| Never | 52 (56) | 31 (61) | 21 (50) | 0.62 [0.21;1.80] | |
| Arterial hypertension, n (%) | 97 (96) | 49 (94) | 48 (98) | 2.68 [0.30;78.9] | 0.6 |
| Dyslipidemia, n (%) | 92 (91) | 45 (87) | 47 (96) | 3.44 [0.76;26.5] | 0.2 |
| Diabetes, n (%) | 42 (42) | 21 (40) | 21 (43) | 1.11 [0.50;2.46] | 0.8 |
| Prior NSTEMI, n (%) | 18 (20) | 11 (25) | 7 (15) | 0.62 [0.22;1.76] | 0.37 |
| Left ventricle ejection fraction, % median (IQR) | 55 (50, 60) | 58 (50, 60) | 55 (50, 60) | 0.97 [0.93;1.01] | 0.2 |
| LDL [mmol/l] median (IQR) | 2.22 (1.79, 2.86) | 2.11 (1.74, 2.51) | 2.37 (1.95, 3.16) | 1.44 [1.01;2.17] | 0.045 |
| Acetylsalicylic acid, n (%) | 91 (90) | 46 (88) | 45 (92) | 1.45 [0.38;6.23] | 0.7 |
| B-adrenolytics, n (%) | 86 (85) | 48 (92) | 38 (78) | 0.30 [0.07;0.96] | 0.037 |
| Dihydropyridine Ca blocker, n (%) | 33 (33) | 17 (33) | 16 (33) | 1.00 [0.43;2.32] | > 0.9 |
| Non-dihydropyridine Ca blocker, n (%) | 9 (9.0) | 4 (7.8) | 5 (10) | 1.32 [0.32;5.90] | 0.7 |
BMI – body mass index, NSTEMI – non-ST-elevation myocardial infarction, LDL – low-density lipoprotein.
Wilcoxon rank sum test;
Pearson’s χ2 test; Fisher’s exact test.
Regression analysis at the patient level revealed that higher LDL concentrations at baseline were a risk factor for no angina improvement (OR+1mmol/l = 1.44; 95% CI: 1.01, 2.17, p = 0.045), whereas the use of β-blockers was a protective factor (OR = 0.30; 95% CI: 0.07, 0.96; p = 0.037) associated with lower risk of no improvement. The vessel-level analysis revealed that higher CFR (OR+1 unit = 0.75; 95% CI: 0.56, 1.00; p = 0.021) and higher RRR (OR+1 unit: 0.73; 95% CI: 0.58, 0.93, p = 0.004) values predicted angina improvement or no change at follow-up, whereas the presence of CMD was a risk factor for no angina improvement after 24 months (OR = 1.95; 95% CI: 1.02, 3.79; p = 0.042). The detailed univariate analysis is presented in Table III.
When the model was adjusted for sex and age, lower RRR values (adjusted OR+1unit = 0.72; 95% CI: 0.55, 0.91; p = 0.010) and the presence of CMD (adjusted OR = 2.07, 95% CI: 1.06, 4.13; p = 0.036) remained independent risk factors of no angina improvement at follow-up. The additional adjustment for vessel tested confirmed higher risk of no angina improvement in patients with CMD (vessel adjusted OR = 2.50, 95% CI: 1.11, 5.80; p = 0.030). On the other hand, the use of β-blockers was an independent protective factor (sex- and age-adjusted OR = 0.28, 95% CI: 0.07, 0.89; p = 0.041).
Discussion
Angina symptoms in patients with chronic coronary syndromes remain a significant clinical problem, especially when considering myocardial revascularization [8, 22]. Current guidelines recommend a patient-tailored approach, incorporating both medical treatment and revascularization, regarding patient comorbidities, anatomy, and function [2].
Presence of CMD and lack of angina improvement
The change of angina levels in patients with angina and no obstructive coronary artery disease (INOCA), assessed by the Seattle Angina Questionnaire, was reported by Ford et al. in the CorMicA trial results, where the introduction of functional testing into the diagnostic algorithm was associated with an improvement of symptoms and better patients’ quality of life [16]. To our best knowledge, currently, there are no studies quantifying a change in angina levels in patients with CMD after diagnosis, and reporting them in the Canadian Cardiovascular Society scale, the one most widely used in daily practice. Our study confirms a significant change in anginal symptoms in patients undergoing complex functional coronary physiology testing in a wide spectrum of chronic coronary syndromes.
We present data suggesting less improvement of symptoms in patients with CMD.
Currently available data suggest that this might be due to both structural and functional mechanisms, as reported in patients with recurrent angina after coronary revascularization [11, 23, 24]. For instance, Rahman et al., in a series of 46 patients with angina and CMD, reported a functional cause in 28 cases and a structural cause of CMD in further 18 patients; nevertheless, the definition of CMD in their study was based on CFR < 2.5 and heterogenous methodology of testing was used.
In our study, lower median CFR and RRR values measured invasively in the subgroup with no angina improvement suggest a functional pathophysiologic mechanism rather than a simple increase of coronary microcirculatory resistance. This finding may be interesting, as currently available publications are non-conclusive. Corcoran et al. reported decreased RRR values in over 32% of patients with obstructive coronary stenosis, but did not provide any data on patients’ symptoms [13]. On the other hand, lower RRR levels were reported in patients with angina and iFR < 0.89 but FFR > 0.80 (non-ischemic) discordant phenotype, compared to patients with high iFR and low FFR phenotype, but also without any data on RRR correlation with angina symptoms [25]. Further research regarding the functional status of coronary microcirculation in this discrepant setting should be performed.
Dyslipidemia and angina risk assessment
Higher LDL cholesterol values are an independent risk factor of an unfavorable outcome in cardiovascular disease and strict dyslipidemia targets are mandatory in patients with coronary artery disease, as proper treatment was associated with better survival in large-scale meta-analyses [26–28]. On the other hand, data on the relation between LDL cholesterol concentration and the level of symptoms are not available. In our analysis, high LDL cholesterol levels were associated with a significantly increased risk of no angina improvement after 24 months, which is another reason for achieving strict secondary prevention targets.
Antianginal treatment and CMD
Typical antianginal drugs proposed by chronic coronary syndrome guidelines do not influence patients’ survival, and the use of first- or second-line treatment is mainly based on the comorbidity profile, with no specific evidence base available [1, 29]. In our analysis, the use of β-blockers, but not calcium channel blockers, was an independent protective factor, associated with angina improvement. In terms of β-blockers use, data are consistent with currently available data, but on the other hand, the lack of improvement with calcium channel blockers can be partially attributed to a small number of patients using the drug. Interestingly, a significant placebo effect was suggested by Gallone et al. in their meta-analysis of randomized control trials in patients with coronary artery disease treated either pharmacologically or by any sort of procedure [30]. In our observational study, there was no sham procedure. Also data on a change of pharmacotherapy during the follow-up were unavailable; therefore it is possible that the angina improvement may be partially attributed to the placebo effect or an escalation of medical therapy.
Our study has some further limitations. Firstly, only 101 patients were included in our study. However, thermodilution-based in-depth functional assessment of coronary microcirculation was performed in all patients, with methodology endorsed by current chronic coronary syndromes guidelines. Secondly, our study was a non-randomized, observational study, with no sham procedure, no provocative vasospasm testing and without an intravascular imaging for confirmation of the optimal PCI result. Nevertheless, our data are consistent with available randomized trial results in terms of change of angina symptoms after the introduction of functional assessment. Thirdly, anginal symptoms were assessed using only the Canadian Cardiovascular Society scale; however, this tool is widely used in daily practice and the results can be easily interpreted. Lastly, as the presented data are derived from a relatively small cohort of patients and are mainly hypothesis-generating, they should be confirmed in larger clinical studies.
Conclusions
The presence of CMD is a risk factor of no angina improvement in patients with chronic coronary syndromes undergoing functional assessment of epicardial stenosis. Lower coronary microcirculatory ability to relax (i.e. lower resistive reserve ratio) may be one of the pathomechanisms of no angina improvement in these patients.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Sorbets E, Steg PG, Young R, et al. β-blockers, calcium antagonists, and mortality in stable coronary artery disease: an international cohort study. Eur Heart J 2019; 40: 1399-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020; 41: 407-77. [DOI] [PubMed] [Google Scholar]
- 3.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019; 40: 87-165. [DOI] [PubMed] [Google Scholar]
- 4.Perera D, Berry C, Hoole SP, et al. Invasive coronary physiology in patients with angina and non-obstructive coronary artery disease: a consensus document from the coronary microvascular dysfunction workstream of the British Heart Foundation/National Institute for Health Research Partnership. Heart 2022; 109: 88-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rieckmann N, Neumann K, Feger S, et al. Health-related qualify of life, angina type and coronary artery disease in patients with stable chest pain. Health Qual Life Outcomes 2020; 18: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford TJ, Stanley B, Good R, et al. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA Trial. J Am Coll Cardiol 2018; 72: 2841-55. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z, Jones P, Weintraub WS, et al. Predicting the benefits of percutaneous coronary intervention on 1-year angina and quality of life in stable ischemic heart disease: risk models from the COURAGE Trial (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation). Circ Cardiovasc Qual Outcomes 2018; 11: e003971. [DOI] [PubMed] [Google Scholar]
- 8.De Luca L, Rosano GMC, Spoletini I. Post-percutaneous coronary intervention angina: from physiopathological mechanisms to individualized treatment. Cardiol J 2022; 29: 850-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pijls NHJ, Fearon WF, Tonino PAL, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease. J Am Coll Cardiol 2010; 56: 177-84. [DOI] [PubMed] [Google Scholar]
- 10.De Bruyne B, Pijls NHJ, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012; 367: 991-1001. [DOI] [PubMed] [Google Scholar]
- 11.Crea F, Bairey Merz CN, Beltrame JF, et al. Mechanisms and diagnostic evaluation of persistent or recurrent angina following percutaneous coronary revascularization. Eur Heart J 2019; 40: 2455-62. [DOI] [PubMed] [Google Scholar]
- 12.Sara JD, Widmer RJ, Matsuzawa Y, et al. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv 2015; 8: 1445-53. [DOI] [PubMed] [Google Scholar]
- 13.Corcoran D, Young R, Adlam D, et al. Coronary microvascular dysfunction in patients with stable coronary artery disease: the CE-MARC 2 coronary physiology sub-study. Int J Cardiol 2018; 266: 7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Legutko J, Niewiara L, Guzik B, et al. The impact of coronary microvascular dysfunction on the discordance between fractional flow reserve and resting full-cycle ratio in patients with chronic coronary syndromes. Front Cardiovasc Med 2022; 9: 1003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahman H, Demir OM, Khan F, et al. Physiological stratification of patients with angina due to coronary microvascular dysfunction. J Am Coll Cardiol 2020; 75: 2538-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford TJ, Stanley B, Good R, et al. Stratified medical therapy using invasive coronary function testing in angina. J Am Coll Cardiol 2018; 72: 2841-55. [DOI] [PubMed] [Google Scholar]
- 17.Legutko J, Dziewierz A, Rzeszutko L, et al. Comparison of hyperemic efficacy between femoral and antecubital fossa vein adenosine infusion for fractional flow reserve assessment. Adv Interv Cardiol 2019; 15: 52-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Legutko J, Kleczyński P, Dziewierz A, et al. Adenosine intracoronary bolus dose escalation versus intravenous infusion to induce maximum coronary hyperemia for fractional flow reserve assessment. Kardiol Pol 2019; 77: 610-7. [DOI] [PubMed] [Google Scholar]
- 19.Pijls NHJ, De Bruyne B, Smith L, et al. Coronary thermodilution to assess flow reserve: validation in humans. Circulation 2002; 105: 2482-6. [DOI] [PubMed] [Google Scholar]
- 20.Wickham H, Averick M, Bryan J, et al. Welcome to the tidyverse. J Open Source Softw 2019; 4: 1686-92. [Google Scholar]
- 21.Ben-Shachar M, Lüdecke D, Makowski D. effectsize: estimation of effect size indices and standardized parameters. J Open Source Softw 2020; 5: 2815-22. [Google Scholar]
- 22.Crea F, Noel Bairey Merz C, Beltrame JF, et al. Mechanisms and diagnostic evaluation of persistent or recurrent angina following percutaneous coronary revascularization. Eur Heart J 2019; 40: 2455-62. [DOI] [PubMed] [Google Scholar]
- 23.Ajmal M, Chatterjee A, Acharya D. Persistent or recurrent angina following percutaneous coronary revascularization. Curr Cardiol Rep 2022; 24: 1837-48. [DOI] [PubMed] [Google Scholar]
- 24.Rahman H, Demir OM, Khan F, et al. Physiological stratification of patients with angina due to coronary microvascular dysfunction. J Am Coll Cardiol 2020; 75: 2538-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SH, Choi KH, Lee JM, et al. Physiologic characteristics and clinical outcomes of patients with discordance between FFR and iFR. JACC Cardiovasc Interv 2019; 12: 2018-31. [DOI] [PubMed] [Google Scholar]
- 26.Navarese EP, Robinson JG, Kowalewski M, et al. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis. JAMA 2018; 319: 1566-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis 2019; 290: 140-205. [DOI] [PubMed] [Google Scholar]
- 28.Visseren FLJ, MacH F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021; 42: 3227-337. [DOI] [PubMed] [Google Scholar]
- 29.Ferrari R, Pavasini R, Camici PG, et al. Anti-anginal drugs – beliefs and evidence: systematic review covering 50 years of medical treatment. Eur Heart J 2019; 40: 190-4. [DOI] [PubMed] [Google Scholar]
- 30.Gallone G, Baldetti L, Angelini F, et al. The placebo effect on symptoms, quality of life, and functional outcomes in patients with angina pectoris: a meta-analysis of randomized placebo-controlled trials. Can J Cardiol 2022; 38: 113-22. [DOI] [PubMed] [Google Scholar]