Abstract
Background
Recent studies suggest that early tumor shrinkage (ETS) and depth of response (DpR) reflect outcomes of chemotherapy in various cancers. This study evaluated the association of ETS and DpR with clinical outcomes using data from JCOG1113, which demonstrated the non-inferiority of gemcitabine plus S-1 (GS) to gemcitabine plus cisplatin (GC) for chemotherapy-naïve advanced biliary tract cancer.
Material and Methods
In total, 354 (289 with measurable target lesions) patients enrolled in JCOG1113 were divided into ETS-unachieved and ETS-achieved groups (≥20% tumor reduction at week 6) and DpR-low and DpR-high groups (≥40% maximum shrinkage) until 12 weeks after enrollment. The impact of ETS and DpR on survival outcome was evaluated using the multivariable Cox proportional hazard model.
Results
The proportions of patients in the ETS-achieved and DpR-high groups were similar between the 2 treatment arms. The hazard ratios (HRs) of progression-free survival (PFS) and overall survival (OS) for the ETS-achieved group were 0.70 (95% confidence interval (CI), 0.52-0.93) and 0.60 (95%CI, 0.44-0.81), respectively. The HRs of PFS and OS for the DpR-high group were 0.67 (95%CI, 0.48-0.94) and 0.64 (95%CI, 0.46-0.90), respectively. In the subpopulation treatment effect pattern plot analysis, most patients in the ETS-achieved group in the GC arm did not experience disease progression after 12 weeks from the landmark.
Conclusion
As on-treatment markers, ETS and DpR were effective tools. ETS was clinically useful, because it can be used to evaluate the outcomes of treatment early at a specific time.
Keywords: gemcitabine, cisplatin, S-1, biliary tract neoplasms, randomized controlled trial, treatment outcome
This article evaluates the association between early tumor shrinkage and depth of response with clinical outcomes in patients with advanced biliary tract cancer using data from JCOG1113.
Implications for Practice.
JCOG1113 found gemcitabine plus S-1 to be non-inferior to gemcitabine plus cisplatin (GC). We examined early tumor shrinkage (ETS) and depth of response (DpR) as early predictors of progression-free survival (PFS) and overall survival (OS) using JCOG1113 data. ETS and DpR were associated with PFS and OS in multivariate analyses of the entire cohort. Moreover, most patients in an ETS-achieved group who belonged to the GC arm did not experience disease progression after 12 weeks from the landmark. ETS was considered clinically helpful owing to its ability to evaluate prognosis and treatment prognosis effectiveness at an early and specific time.
Introduction
Biliary tract cancer (BTC) includes intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, gallbladder cancer, and ampulla of Vater cancer. The incidence and etiology of BTC vary geographically. The incidence of BTC is generally high in Asian countries and relatively low in Western countries.1 However, the incidence of intrahepatic cholangiocarcinoma has recently increased in Western countries.2
Although radical resection is the only potentially curative treatment for BTC, patients often have unresectable tumors at diagnosis. Furthermore, patients who undergo radical resection frequently develop recurrence.3 Therefore, most patients with BTC require palliative chemotherapy. Based on the results of randomized controlled trials, gemcitabine plus cisplatin (GC) is the global standard of care for unresectable BTC.4,5 In contrast to GC, which requires at least 3 hours of hydration, gemcitabine plus S-1 (GS) does not require hydration. In addition, thrombocytopenia and gastrointestinal toxicity were less common in GS than in GC. Therefore, the Hepatobiliary and Pancreatic Oncology Group of the Japan Clinical Oncology Group (JCOG) conducted a randomized phase III trial (JCOG1113) to confirm the non-inferiority of GS to GC in terms of overall survival (OS) in patients with advanced BTC. The non-inferiority of GS to GC was confirmed.6 Based on this result, GS can be considered a treatment option for advanced BTC.
Early tumor shrinkage (ETS) and depth of response (DpR) are considered on-treatment markers that reflect the antitumor effect of chemotherapy and have been reported to be associated with survival in various cancers.7-12 A previous post hoc analysis of a phase III study showed an association between ETS and survival in patients with advanced BTC treated with gemcitabine plus oxaliplatin with or without erlotinib.13 In this study, we aimed to evaluate the association between ETS and DpR with clinical outcomes in patients with advanced BTC using data from JCOG1113.
Materials and Methods
The JCOG1113 Study
Details of JCOG1113, including the inclusion and exclusion criteria, and dosage schedule, have been published previously.6 Briefly, JCOG1113 was a prospective, open-label, multicenter, randomized phase III trial conducted at 33 institutions in Japan. Between May 2013 and March 2016, 354 chemotherapy-naïve patients with recurrent or unresectable BTC were enrolled and assigned to GC (n = 175) or GS (n = 179) arms. Measurable lesions were not mandatory. Cisplatin was administered up to 16 times (400 mg/m2).
Tumors were assessed every 6 weeks using computed tomography. Tumor responses were evaluated according to the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1.
Patients
Patients who had the data of measurable target lesions at 6 weeks for ETS and until 12 weeks for DpR were analyzed in this analysis. Measurable target lesions were evaluated based on RECIST version 1.1. In JCOG1113, written informed consent, including the secondary use of data, was obtained from all patients before enrollment, and the study protocol of JCOG1113, including the secondary use of data, was approved by the institutional review board of each participating institution.6
Definition of ETS and DpR
ETS was defined as tumor reduction in the sum of the diameters of the measurable target lesions at week 6 compared with that at baseline. Patients were divided into ETS-unachieved and ETS-achieved groups. The ETS-achieved group was defined as having reduction of 20% or more in the sum of the diameters of the measurable target lesions, whereas the ETS-unachieved group was defined as having reduction of less than 20%. The ETS-unachieved group also included patients with tumor growth.
DpR was defined as the maximum shrinkage in the sum of the diameters of the measurable target lesions within 12 weeks after enrollment. The patients were divided into DpR-low and DpR-high groups. The DpR-high group was defined as having a maximum shrinkage of ≥40%. The DpR-low group was defined as having a maximum shrinkage of <40%.
Statistical Analysis
To summarize participant characteristics, median value and proportions are used. We performed Fisher’s exact test and Wilcoxon rank-sum test to compare continuous and categorical variables between the GC and GS arms, respectively. Survival curves were estimated using the Kaplan-Meier method. Progression-free survival (PFS) and OS were estimated based on ETS and DpR at weeks 6 and 12 (landmarks) after enrollment. Moreover, duration of response (DoR) was defined as the time from complete or partial response according to RECIST version 1.1. to progression or death from any cause. Multivariable analyses for PFS and OS were adjusted for treatment, sex, age (<65 and ≥65 years), performance status, primary site, biliary drainage, resection, the sum of the diameter of the measurable target lesions (<51 and≥51 mm) before treatment, and serum carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) levels. The sum of the diameter of the measurable target lesions CEA and CA19-9 levels was assessed as categorical variables based on median values in the multivariable Cox regression model. Furthermore, a time-dependent receiver operating characteristic (ROC) curve analysis was performed to evaluate the predictive accuracy of ETS for PFS and OS. A subpopulation treatment effect pattern plot (STEPP) analysis was then performed. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Time-dependent ROC curve and STEEP analyses were performed using the “steep” package in R 4.0.5. All statistical analyses were conducted at the JCOG Data Centre.
Results
Patients
The patient flow diagram is shown in Fig. 1. Among the 354 patients enrolled in JCOG1113, 289 had measurable target lesions. Among the 277 patients with available ETS data, 77 (27.8%) had achieved ETS ≥20% at week 6. The GC and GS arms had 25.4% and 30.4% of patients, respectively, met the criteria for the ETS-achieved group (Fig. 1A). Among 230 patients with available DpR data, 52 (22.6%) achieved DpR ≥40% within 12 weeks. The proportion of patients in the DpR-high group was 21.2% and 24.1% in the GC and GS arms, respectively (Fig. 1B).
Figure 1.
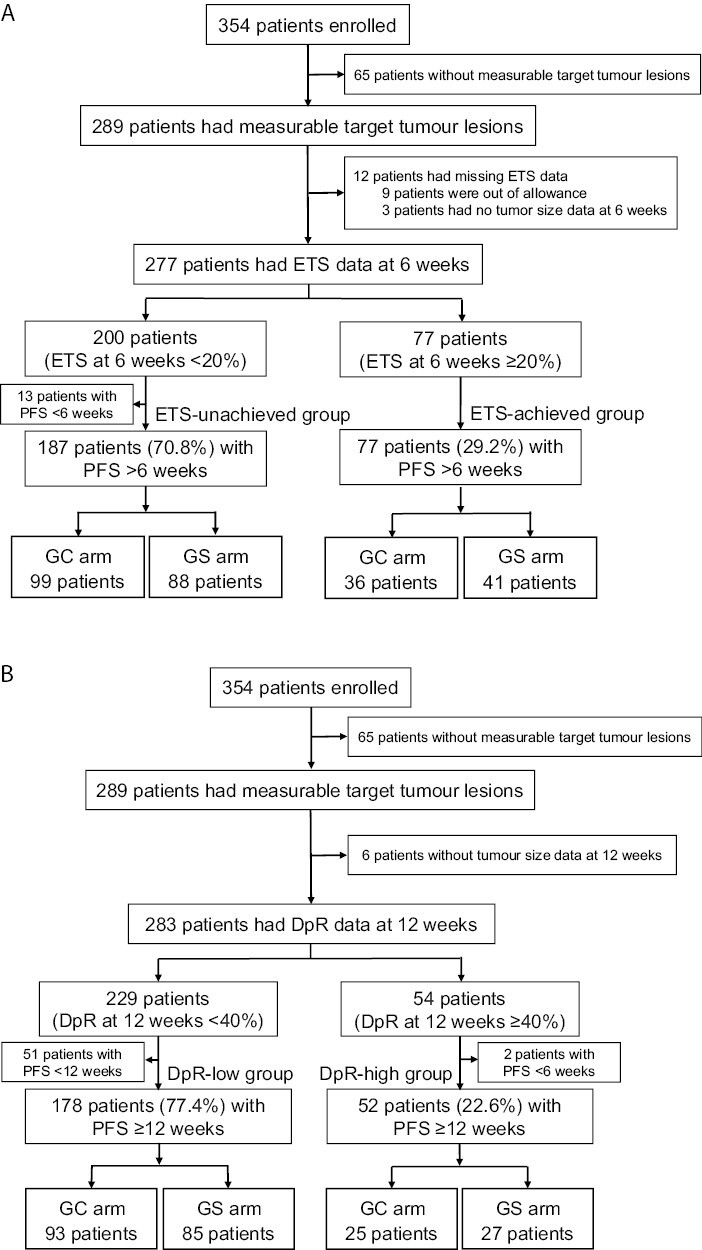
Patient flow diagram. (A) Definition of the assessable population and patient placement in the ETS-unachieved (ETS <20% at 6 weeks) and ETS-achieved groups (ETS ≥20% at 8 weeks) based on the treatment arm. (B) Definition of the assessable population and patient placement in the DpR-low (DpR <40% within 12 weeks) and DpR-high groups (DpR ≥40% within 12 weeks) based on the treatment arm. Abbreviations: ETS: early tumor shrinkage; GC: gemcitabine plus cisplatin; GS: gemcitabine plus S-1; PFS: progression-free survival; DpR: depth of response.
The patient characteristics are summarized in Table 1. The diameter of measurable target lesions was larger among patients of the GS arm than among those of the GC arm in the ETS-achieved group. The overall response at week 6 after enrollment, the best overall response according to RECIST version 1.1 and the duration to maximum tumor shrinkage are shown in Table 2. The associations among ETS, DpR, and best overall response (according to RECIST version 1.1.) are shown in Supplementary Tables S1 and S2.
Table 1.
Patient characteristics of ETS-unachieved and ETS-achieved groups.
| ETS-unachieved group | P-value | ETS-achieved group | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All (n = 200) | GC (n = 106) | GS (n = 94) | All (n = 77) | GC (n = 36) | GS (n = 41) | ||||
| Age (years) | Median (range) | 66 (37-79) | 66.5 (45-78) | 66 (37-79) | .67 | 68 (27-78) | 68.5 (52-78) | 68 (27-78) | .93 |
| Sex | Male/female, n (%) | 111/89 (56/45) | 59/47 (56/44) | 52/42 (55/45) | 1.00 | 35/42 (46/55) | 17/19 (47/53) | 18/23 (44/56) | .82 |
| ECOG PS | 0/1, n (%) | 150/50 (75/25) | 80/26 (76/25) | 70/24 (75/26) | .88 | 46/31 (60/40) | 21/15 (58/42) | 25/16 (61/40) | .82 |
| Primary site | Gall bladder, n (%) | 75 (38) | 41 (39) | 34 (36) | 40 (52) | 16 (44) | 24 (59) | ||
| Others, n (%) | 125 (63) | 65 (61) | 60 (64) | .77 | 37 (48) | 20 (56) | 17 (42) | .26 | |
| Primary site | ICC, n (%) | 66 (33) | 34 (32) | 32 (34) | .89 | 21 (28) | 11 (31) | 10 (24) | .47 |
| ECC, n (%) | 47 (24) | 24 (23) | 23 (25) | 16 (21) | 9 (25) | 7 (17) | |||
| Others, n (%) | 87 (44) | 48 (45) | 39 (42) | 40 (52) | 16 (44) | 24 (59) | |||
| Resection | Present, n (%) | 43 (22) | 21 (20) | 22 (23) | .61 | 16 (21) | 9 (25) | 7 (17) | .41 |
| Stage | M1, n (%) | 124 (62) | 66 (62) | 58 (62) | .76 | 51 (66) | 23 (64) | 28 (68) | .74 |
| Diameter of measurable target lesions (mm) | Median (range) | 51.5 (10.0-210.0) | 56.1 (10.0-210.0) | 46.0 (11.0-192.0) | .15 | 52.8 (11.0-184.9) | 45.1 (11.0-184.9) | 60.1 (17.0-180.2) | .04 |
| CEA (ng/mL) | Median (range) | 4.3 (0.8-3706.5) | 3.8 (0.8-3706.5) | 4.6 (0.8-1731.0) | .62 | 3.9 (0.9-2747.8) | 3.0 (0.9-681.4) | 5.3 (1.2-2747.8) | .12 |
| CA19-9 (U/mL) | Median (range) | 175.1 (0.0-1367000.0) | 126.0 (0.9-1367000.0) | 236.0 (0.0-111100.0) | .64 | 170.2 (1.0-76460.0) | 151.6 (1.0-62525.0) | 295.5 (1.0-76460.0) | .13 |
Abbreviations: ETS: early tumor shrinkage; GC: gemcitabine plus cisplatin; GS: gemcitabine plus S-1; ECOG PS: Eastern Cooperative Oncology Group performance status; CEA: serum carcinoembryonic antigen; CA19-9: serum carbohydrate antigen 19-9; ICC: intrahepatic cholangiocarcinoma; ECC: extrahepatic cholangiocarcinoma.
Table 2.
Overall response, best overall response, and duration to maximum tumor shrinkage.
| All n = 289 (%) |
GC n = 148 (%) |
GS n = 141 (%) |
|
|---|---|---|---|
| Overall response at 6 weeks | |||
| CR | 0 | 0 | 0 |
| PR | 43 (14.9) | 21 (14.2) | 22 (15.6) |
| SD | 201 (69.6) | 102 (68.9) | 99 (70.2) |
| PD | 37 (12.8) | 20 (13.5) | 17 (12.1) |
| NE | 3 (1.0) | 2 (1.4) | 1 (0.7) |
| Missing data | 5 (1.7) | 3 (2.0) | 2 (1.4) |
| Best overall response | |||
| CR | 2 (0.7) | 0 | 2 (1.4) |
| PR | 88 (30.4) | 48 (32.4) | 40 (28.4) |
| SD | 150 (51.9) | 74 (50.0) | 76 (53.9) |
| PD | 40 (13.8) | 21 (14.2) | 19 (13.5) |
| NE | 9 (3.1) | 5 (3.4) | 4 (2.8) |
| Duration to maximum tumor shrinkage | |||
| 6 weeks | 132 (45.7) | 64 (43.2) | 68 (48.2) |
| 12 weeks | 61 (21.1) | 28 (18.9) | 33 (23.4) |
| 18 weeks | 48 (16.6) | 28 (18.9) | 20 (14.2) |
| 24 weeks | 48 (16.6) | 28 (18.9) | 20 (14.2) |
Abbreviations: GC: gemcitabine plus cisplatin; GS: gemcitabine plus S-1; CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease; NE: not evaluated.
Multivariable Analyses for PFS and OS
The results of the multivariable analysis for PFS and OS based on ETS are shown in Table 3. The HRs of PFS and OS for the ETS-achieved group were 0.70 (95%CI, 0.52-0.93; P = .01) and 0.60 (95%CI, 0.44-0.81; P < .01), respectively. The results of the multivariable analysis of PFS and OS based on DpR are shown in Table 4. The HRs of PFS and OS for the DpR-high group were 0.67 (95%CI, 0.48-0.94; P = .02) and 0.64 (95%CI, 0.46-0.90; P < .01), respectively.
Table 3.
Multivariable analysis for progression-free survival and overall survival, including early tumor shrinkage.
| Progression-free survival | Overall survival | |||
|---|---|---|---|---|
| Variable | Hazard ratio (95% CI) |
P-value | Hazard ratio (95% CI) |
P-value |
| Treatment | .12 | .07 | ||
| GC | 1 | 1 | ||
| GS | 0.82 (0.47-1.79) | 0.78 (0.59-1.02) | ||
| Sex | .40 | .35 | ||
| Male | 1 | 1 | ||
| Female | 0.89 (0.68-1.16) | 0.88 (0.67-1.15) | ||
| Age | .08 | .77 | ||
| <65 years | 1 | 1 | ||
| ≥65 years | 0.79 (0.61-1.03) | 0.96 (0.73-1.27) | ||
| ECOG PS | .49 | <.01 | ||
| 0 | 1 | 1 | ||
| 1 | 1.11 (0.82-1.51) | 1.54 (1.13-2.09) | ||
| Primary tumor site | .34 | .20 | ||
| Gall bladder | 1 | 1 | ||
| Non-gall bladder | 0.88 (0.67-1.15) | 0.84 (0.63-1.10) | ||
| Biliary drainage | .39 | .08 | ||
| Absent | 1 | 1 | ||
| Present | 1.13 (0.86-1.48) | 0.71 (0.48-1.04) | ||
| Prior surgical resection | .37 | .08 | ||
| Absent | 1 | 1 | ||
| Present | 0.85 (0.59-1.22) | 0.71 (0.48-1.04) | ||
| Diameter of measurable target lesions | .73 | .78 | ||
| <51 mm | 1 | 1 | ||
| ≥51 mm | 0.95 (0.71-1.27) | 0.96 (0.71-1.29) | ||
| CEA | <.01 | <.01 | ||
| <3.8 ng/mL | 1 | 1 | ||
| ≥3.8 ng/mL | 1.50 (1.15-1.97) | 1.72 (1.29-2.29) | ||
| CA19-9 | .03 | <.01 | ||
| <174.7 U/mL | 1 | 1 | ||
| ≥174.7 U/mL | 1.36 (1.03-1.78) | 1.65 (1.25-2.19) | ||
| ETS at 6 weeks | .01 | <.01 | ||
| <20% | 1 | 1 | ||
| ≥20% | 0.70 (0.52-0.93) | 0.60 (0.44-0.81) | ||
Abbreviations: CI: confidence interval; GC: gemcitabine plus cisplatin; GS: gemcitabine plus S-1; ECOG PS: Eastern Cooperative Oncology Group performance status; CEA: serum carcinoembryonic antigen; CA19-9: serum carbohydrate antigen 19-9; ETS: early tumor shrinkage.
Table 4.
Multivariate analysis for progression-free survival and overall survival, including depth of response.
| Progression-free survival | Overall survival | |||
|---|---|---|---|---|
| Variable | Hazard ratio (95% CI) |
P-value | Hazard ratio (95% CI) |
P-value |
| Treatment | .11 | .14 | ||
| GC | 1 | 1 | ||
| GS | 0.80 (0.61-1.05) | 0.82 (0.62-1.07) | ||
| Sex | .43 | .99 | ||
| Male | 1 | 1 | ||
| Female | 0.89 (0.67-1.19) | 1.00 (0.76-1.31) | ||
| Age | .07 | .61 | ||
| <65 years | 1 | 1 | ||
| ≥65 years | 0.77 (0.58-1.02) | 0.93 (0.71-1.22) | ||
| ECOG PS | .92 | .06 | ||
| 0 | 1 | 1 | ||
| 1 | 1.02 (0.73-1.42) | 1.34 (0.99-1.80) | ||
| Primary tumor site | .32 | .27 | ||
| Gallbladder | 1 | 1 | ||
| Non-gallbladder | 0.86 (0.65-1.16) | 0.86 (0.65-1.13) | ||
| Biliary drainage | .50 | .18 | ||
| Absent | 1 | 1 | ||
| Present | 1.11 (0.83-1.49) | 1.22 (0.91-1.62) | ||
| Prior surgical resection | .40 | .19 | ||
| Absent | 1 | 1 | ||
| Present | 0.85 (0.58-1.25) | 0.77 (0.53-1.13) | ||
| Diameter of measurable target lesions | .42 | .75 | ||
| <51 mm | 1 | 1 | ||
| ≥51 mm | 0.88 (0.65-1.20) | 0.95 (0.71-1.28) | ||
| CEA | <.01 | <.01 | ||
| <3.8 ng/mL | 1 | 1 | ||
| ≥3.8 ng/mL | 1.48 (1.11-1.96) | 1.52 (1.15-2.01) | ||
| CA19-9 | <.01 | <.01 | ||
| <174.7 U/mL | 1 | 1 | ||
| ≥174.7 U/mL | 1.51 (1.12-2.04) | 1.79 (1.35-2.37) | ||
| DpR within 12 weeks | .02 | <.01 | ||
| <40% | 1 | 1 | ||
| ≥40% | 0.67 (0.48-0.94) | 0.64 (0.46-0.90) | ||
Abbreviations: CI: confidence interval; GC: gemcitabine plus cisplatin; GS: gemcitabine plus S-1; ECOG PS: Eastern Cooperative Oncology Group performance status; CEA: serum carcinoembryonic antigen; CA19-9: serum carbohydrate antigen 19-9; DpR: depth of response.
Effect of ETS and DpR on PFS and OS in the GC and GS Arms
Supplementary Fig. S1 shows the Kaplan-Meier curves for PFS and OS based on ETS. ETS-achieved was identified as a prognostic factor for PFS in the GC arm (HR, 0.64; 95%CI, 0.43-0.95) (Supplementary Fig. S1A and S1B). The HR for the effect of ETS on OS was 0.67 (95%CI, 0.44-1.03) in the GC arm and 0.95 in the GS arm (95%CI, 0.64-1.42), respectively (Supplementary Fig. S1C and S1D).
The Kaplan-Meier curves for PFS and OS based on DpR are shown in Supplementary Fig. S2. DpR-high was identified as a prognostic factor for PFS in the GC arm (HR 0.63; 95%CI, 0.40-0.998) (Supplementary Fig. S2A and S2B). The HR for the effect of DpR on OS was 0.69 (95%CI, 0.43-1.11) in the GC arm and 0.88 (95%CI, 0.56-1.37) in the GS arm, respectively (Supplementary Fig. S2C and S2D).
Effect of ETS on DoR
Supplementary Fig. S3 shows the Kaplan-Meier curves for DoR based on ETS. DoR of the ETS-achieved group was longer than that of the ETS-unachieved groups. The HR for the effect of ETS on DoR was 0.76 (95%CI, 0.26-2.37).
Predictive Accuracy of ETS for PFS and OS
Time-dependent ROC curves assessing the ability of ETS to predict PFS at 12 weeks and OS at 36 weeks from the landmarks are shown in Fig. 2. ETS moderately predicted PFS at 12 weeks and OS at 36 weeks in the GC arm: area under the curve (AUC) was 0.72 and 0.74, respectively (Fig. 2A and 2C).
Figure 2.
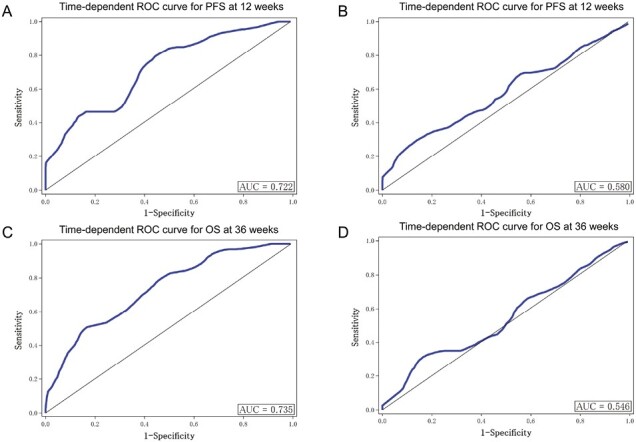
ROC curve analysis assessing the ability of ETS to predict PFS at 12 weeks and OS at 36 weeks in the GC and GS arms. (A) Time-dependent ROC curve for PFS at 12 weeks in the GC arm. (B) Time-dependent ROC curve for PFS at 12 weeks in the GS arm. (C) Time-dependent ROC curve for OS at 36 weeks in the GC arm. (D) Time-dependent ROC curve for OS at 36 weeks in the GS arm. Abbreviations: PFS: progression-free survival; OS: overall survival; ETS: early tumor shrinkage; GC: gemcitabine plus cisplatin; GS: gemcitabine plus S-1; ROC: receiver operating characteristic; AUC: area under the curve.
The STEPP analyses for PFS and OS from the landmarks are shown in Fig. 3. Most patients in the ETS-achieved group who belonged to the GC arm did not experience disease progression after 12 weeks from the landmark. Conversely, approximately 25% of patients from the GS arm showed disease progression at 12 weeks from the landmark, including some patients in the ETS-achieved group (Fig. 3A). Furthermore, there was no association between the degree of ETS and the 36-week OS in the GS arm (Fig. 3B).
Figure 3.
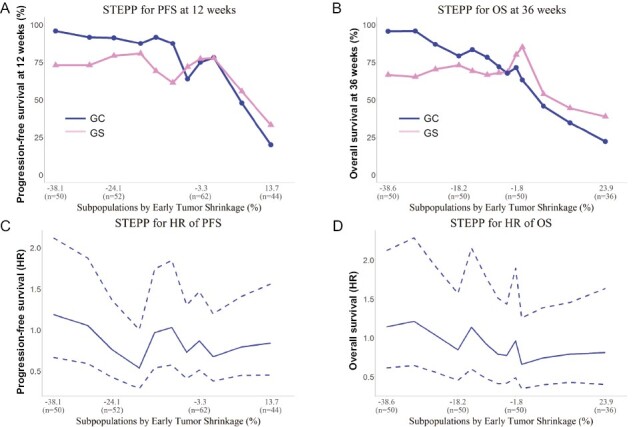
STEPP analysis of PFS and OS based on ETS in the GC and GS arms. (A) STEPP analysis of PFS at 12 weeks. (B) STEPP analysis of OS at 36 weeks. (C) STEPP analysis of the HR for PFS. (D) STEPP analysis of the HR for OS. The HRs for PFS and OS in the GS arm were comparable to those in the GC arm. Abbreviations: PFS: progression-free survival; OS: overall survival; ETS: early tumor shrinkage; STEPP: subpopulation treatment effect pattern plot; HR: hazard ratio; GC: gemcitabine plus cisplatin; GS: gemcitabine plus S-1.
Based on ETS, the HRs for PFS and OS in the GS arm compared with those in the GC arm were near 1.0 for each subpopulation (Fig. 3C and 3D).
Discussion
To the best of our knowledge, the present study is the first report to evaluate the association between ETS and DpR with survival in chemotherapy-naïve patients, with advanced BTC, treated with GC or GS. Using multivariable analyses, we demonstrated that ETS at week 6 and DpR within 12 weeks were associated with PFS and OS from the postenrollment landmarks. Furthermore, the risk of progression and death in patients with ETS-achieved was reduced by 30% and 40%, when adjusted with prognostic factors, including performance status and tumor markers, respectively. Moreover, most patients in the ETS-achieved group who belonged to the GC arm did not experience disease progression after 12 weeks from the landmark.
Although palliative chemotherapy, including second-line chemotherapy, has gradually developed for advanced BTC,14-22 the prognosis remains poor. Moreover, approximately 50% of patients do not receive second-line chemotherapy in clinical practice due to poor physical conditions after tumor progression.23 Physicians treating this dismal disease should be able to guide patients and their families toward appropriate treatment choices, goals of care, and quality end of life. However, the optimal timing for these discussions presents challenges for the following reasons: discussions too early may cause patients and their families to feel fearful or distressed,24 while delayed conversations, may not enable physicians to meet the wishes of patients and their families regarding end-of-life decisions. Early predictions of the prognosis and response to first-line chemotherapy may help decide when to begin these discussions. Therefore, we consider ETS to be a valuable on-treatment prognostic factor in patients with advanced BTC. Overall, this study suggests that a more careful evaluation of signs of tumor progression should be carried out, especially in patients belonging to the ETS-unachieved group.
Previously, pooled analysis of clinical trials showed a correlation between the response rate according to RECIST criteria and time to tumor progression and OS.25 The RECIST is a global standard for evaluating antitumor effects. Furthermore, the RECIST is commonly used to decide whether chemotherapy should be continued, even though it was not intended by the original RECIST guidelines and prediction of prognosis. However, in the RECIST, time point for evaluating tumor shrinkage, such as complete or partial response, is not defined. Indeed, there was a difference between the overall response at 6 weeks after enrollment (14.9%) and the best overall response (31.1%) in JCOG1113. Alternatively, ETS was evaluated at the initial tumor assessment, defined as 6 weeks after enrollment in this study. ETS has the advantage of earlier assessment than the RECIST for evaluating response to chemotherapy and prognosis. As with the RECIST, the time required to reach maximum shrinkage may be long and unpredictable. Indeed, in this study, the time until maximum tumor shrinkage was unpredictable, because it could occur in approximately 30% of patients even after 18 weeks. Therefore, ETS may be more useful than the RECIST criteria as an on-treatment marker for prognosis.
Conversely, performance status and tumor markers such as CEA and CA19-9 were identified as prognostic factors in this study. However, these factors were already included in patient characteristics at starting of treatment, and they did not differ between the ETS-achieved and ETS-unachieved groups. Therefore, these factors cannot predict whether patients will achieve ETS by treatment with GC or GS.
ETS may be useful as a clinical decision-making tool, particularly for patients receiving GC. For example, most of the ETS-achieved patients, treated with GC at 6 weeks, did not show tumor progression at 12 weeks. To plan the monitoring intervals and the timing for the subsequent treatment, especially conversion surgery or other local therapies, information that assures that the tumor will not progress for a relatively long time is clinically valuable. The importance of tumor shrinkage rates at 6 weeks after starting first-line chemotherapy starting should also be recognized in clinical practice to predict clinical outcomes.
DpR is one of the indicators used to evaluate the nature of tumor shrinkage. It was reported that the proportion of patients with DpR-high was greater in patients treated with the anti-epidermal growth factor receptor antibody than in those treated with anti-vascular endothelial growth factor antibody in metastatic colorectal cancer. The difference may impact the subsequent course of the disease and refer to tailored therapy approaches.7 This study suggests that physicians may not need to determine whether to use GC or GS depending on factors such as locally advanced stage and high tumor burden, as there are no differences between GC and GS regarding tumor shrinkage effects. Therefore, GS can be considered a treatment option for advanced BTC due to its convenience and lack of hydration requirements. Recently, as the combination of GC with immune checkpoint inhibitors (such as durvalumab or pembrolizumab) can be considered as the new standard of first-line treatment for advanced BTC,14,22 ETS and DpR need to be investigated further in these new regimens before they are generally accepted for treating advanced BTC.
This study has some limitations. First, this was an exploratory analysis with insufficient statistical power. More specifically, the effects of ETS in the GC and GS arms should be interpreted with caution because of subgroup analysis within exploratory analyses. This means that this analysis included many biases. For example, among patient characteristics, the diameter of measurable target lesions of the GS arm was larger than that of the GC arm. Therefore, further studies are needed to establish whether there is any difference between the GC and GS treatments. Second, this study was limited to patients with measurable lesions based on the RECIST, as ETS and DpR cannot be evaluated in patients without measurable lesions. Third, to avoid lead-time bias, this study excluded patients with PFS <6 weeks and <12 weeks from analyses of ETS and DpR, respectively. To date, there are no appropriate screening tools for these patients. Finally, the optimal cutoff values and time points for ETS in patients with advanced BTC are controversial. Although this study adopted a cutoff value of 20% for ETS, as has been the case in many studies of colorectal cancer and other gastrointestinal cancers, one study that showed an association of ETS with PFS and OS in advanced BTC adopted a cutoff value of 10%.13 Moreover, the cutoff value for DpR varied in previous gastrointestinal cancer studies.26 Therefore, cutoff values of 20% for ETS and 40% for DpR could be a reference for further investigation in patients with advanced BTC.
Conclusions
This study suggests that ETS and DpR offer valuable tools for prognostication in chemotherapy-naïve advanced BTC. In particular, ETS was considered clinically helpful due to its ability to evaluate the prognosis and effectiveness of treatment at an early and specific time.
Supplementary Material
Acknowledgment
We would like to thank the 33 participating institutions. We are also grateful to the members of the JCOG Data Center and JCOG Operations Office for their support in preparing the manuscript, data management, and oversight of the management of the study.
Contributor Information
Naohiro Okano, Department of Medical Oncology, Kyorin University Faculty of Medicine, Tokyo, Japan.
Chigusa Morizane, Department of Hepatobiliary and Pancreatic Oncology, National Cancer Center Hospital, Tokyo, Japan.
Takuji Okusaka, Department of Hepatobiliary and Pancreatic Oncology, National Cancer Center Hospital, Tokyo, Japan.
Ryo Sadachi, JCOG Data Center/Operations Office, National Cancer Center Hospital, Tokyo, Japan.
Tomoko Kataoka, JCOG Data Center/Operations Office, National Cancer Center Hospital, Tokyo, Japan.
Satoshi Kobayashi, Department of Gastroenterology, Kanagawa Cancer Center, Yokohama, Japan.
Masafumi Ikeda, Department of Hepatobiliary and Pancreatic Oncology, National Cancer Center Hospital East, Kashiwa, Japan.
Masato Ozaka, Hepato-Biliary-Pancreatic Medicine Department, Cancer Institute Hospital of Japanese Foundation for Cancer Research, Tokyo, Japan.
Tomonori Mizutani, Department of Medical Oncology, Kyorin University Faculty of Medicine, Tokyo, Japan.
Kazuya Sugimori, Gastroenterological Center, Yokohama City University Medical Center, Yokohama, Japan.
Akiko Todaka, Division of Gastrointestinal Oncology, Shizuoka Cancer Center, Shizuoka, Japan.
Satoshi Shimizu, Department of Gastroenterology, Saitama Cancer Center, Saitama, Japan.
Nobumasa Mizuno, Department of Gastroenterology, Aichi Cancer Center Hospital, Nagoya, Japan.
Tomohisa Yamamoto, Department of Surgery, Kansai Medical University Hospital, Osaka, Japan.
Keiji Sano, Department of Surgery, Teikyo University School of Medicine, Tokyo, Japan.
Kazutoshi Tobimatsu, Division of Gastroenterology, Department of Internal Medicine, Kobe University Graduate School of Medicine, Kobe, Japan.
Akio Katanuma, Center for Gastroenterology, Teine Keijinkai Hospital, Sapporo, Japan.
Kunihito Gotoh, Department of Surgery, National Hospital Organization Osaka National Hospital, Osaka, Japan.
Hironori Yamaguchi, Department of Clinical Oncology, Jichi Medical University, Tochigi, Japan.
Hiroshi Ishii, Clinical Research Center, Chiba Cancer Center, Chiba, Japan.
Akihiro Ohba, Department of Hepatobiliary and Pancreatic Oncology, National Cancer Center Hospital, Tokyo, Japan.
Junji Furuse, Department of Medical Oncology, Kyorin University Faculty of Medicine, Tokyo, Japan; Department of Gastroenterology, Kanagawa Cancer Center, Yokohama, Japan.
Makoto Ueno, Department of Gastroenterology, Kanagawa Cancer Center, Yokohama, Japan.
Funding
The work was supported by the National Cancer Center Research and Development Funds (grant nos 23-A-22, 26-A-4, 29-A-3, 2020-J-3, 2023-J-03), Japan Agency for Medical Research and Development (grant no. JP19ck0106350), and the Grant-in-Aid for Clinical Cancer Research (H22-ganrinsho-ippan-013) from the Ministry of Health, Labor, and Welfare of Japan.
Conflict of Interest
N.O. reports personal fees from Taiho Pharmaceutical, Eli Lilly Japan, Eisai, Bayer Yakuhin, Chugai Pharma, GlaxoSmithKline, Ono Pharmaceutical, Takeda, and Daiichi Sankyo. C.M. reports grants from Eisai, Yakult Honsha, Ono Pharmaceutical, J-Pharma, AstraZeneca, Merck biopharma, Daiichi Sankyo, and HITACHI and personal fees from Yakult Honsha, MSD, Servier, Novartis, Teijin Pharma, Taiho Pharmaceutical, and Eisai. T.O. reports grants from AstraZeneca, Syneos Health, EP-CRSU, Eisai, MSD, and Incyte Japan and personal fees from AstraZeneca, Eisai, Nihon Servier, Dainippon Sumitomo Pharma, Bristol-Myers, FUJIFILM Toyama Chemical, Incyte Japan, Ono Pharmaceutical, Yakult Honsha, Johnson & Johnson, Daiichi Sankyo, Taiho Pharmaceutical, Chugai Pharmaceutical, Nippon Shinyaku, Eli Lilly Japan, Pfizer Japan, and Novartis Pharma. S.K. reports personal fees from AstraZeneca, Bayer Pharmaceutical, Boston Scientific, Chugai Pharmaceutical, Eisai, Eli Lilly, Takeda Pharmaceutical, Taiho Pharmaceutical, and Yakult Honsha. M.I. reports grants from Eisai, Merck biopharma, Eli Lilly Japan, Yakult, Ono, ASLAN, J-Pharma, AstraZeneca, Pfizer, Merus N.V., Nihon Servier, Delta-Fly Pharma, Chiome Bioscience, Chugai, Bristol-Myers Squibb, Novartis, Bayer, Takeda, MSD, and Syneos Health and personal fees from Eisai, MSD, Eli Lilly Japan, Yakult, Teijin Pharma, Astellas, Sumitomo Dainippon, Otsuka, Nihon Servier, Taiho, Chugai, Bristol-Myers Squibb, Novartis, Bayer, Takeda, EA Pharma, AstraZeneca, AbbVie, Abbott Japan, Fujifilm Toyama Chemical, Incyte Biosciences Japan. M.O. reports personal fees from Taiho Pharmaceutical, Yakult Honsha, MSD, and Pfizer. M.Z. reports personal fees from Chugai Pharmaceutical and AstraZeneca. K.S. reports grants from Taiho Pharmaceutical. A.T. reports from personal fees from Ono Pharmaceutical, Yakult Honsha, Nihon Servier, and Taiho Pharmaceutical. S.S. reports grants from AstraZeneca, Incyte Corporation, and Delta-Fly Pharma. N.M. reports grants from Yakult Honsha, Novartis, MSD, ASLAN Pharmaceuticals, Incyte, Ono Pharmaceutical, Seagen, Taiho Pharmaceutical, and Dainippon Sumitomo Pharma and personal fees from Yakult Honsha, AstraZeneca, Novartis, FUJIFILM Toyama Chemical, and MSD. H.Y. reports grants and personal fees from Taiho Pharmaceutical and Nippon Kayaku. H.I. reports from personal fees from Yakult Honsha, Taiho Pharmaceutical, Eli Lilly Japan, Novartis, Chugai Pharmaceutical, Incyte Biosciences Japan, Ono Pharmaceutical. A.O. reports grants from Ono Pharmaceutical, Daiichi Sankyo, and Chugai Pharmaceutical and personal fees from Ono Pharmaceutical, Servier, and Yakult. J.F. reports grants from Astellas, Astra Zeneca, Incyte Biosciences Japan, Eisai, MSD, Ono Pharmaceutical, Sanofy, J-Pharma, Daiichi Sankyo, Sumitomo Dainippon, Taiho Pharmaceutical, Takeda, Delta-Fly-Pharma, and Chugai Pharma and personal fees from Fuji film, Astellas, Onco Therapy Science, Delta-Fly-Pharma, Merck Bio, Ono Pharmaceutical, MSD, Taiho Pharmaceutical, Chugai Pharma, Astra Zeneca, Incyte Biosciences Japan, J-Pharma, Eisai, Eli Lilly Japan, Yakult Honsha, Servier Japan, Novartis Pharma, Takeda, Bayer, EA Pharma, Teijin pharma, Daiichi Sankyo, and Terumo. U.M. reports grants from Taiho Pharmaceutical, AstraZeneca, Merck Biopharma, MSD, Astellas Pharma, Eisai, Ono Pharmaceutical, Incyte, Chugai Pharmaceutical, DFP, Daiichi Sankyo, Novartis, Boehringer Ingelheim, and J-Pharma and personal fees from Taiho Pharmaceutical, AstraZeneca, Yakult Honsha, MSD, Nihon Servier, Ono Pharmaceutical, Incyte, Chugai Pharmaceutical, Boehringer Ingelheim, and J-Pharma.
Author Contributions
Conception/design: N.O. and R.S. Provision of study material or patients: N.O., C.M., T.O., S.K., M.I., M.O., T.M., K.S., A.T., S.S., N.M., T.Y., K.S., K.T., A.K., K.G., H.Y., H.I., A.O., J.F. and M.U. Collection and/or assembly of data: N.O., R.S. and T.K. Data analysis and interpretation: N.O., R.S., T.K. and T.M. Manuscript writing: all authors. Final approval of manuscript: all authors.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Ouyang G, Liu Q, Wu Y, et al. The global, regional, and national burden of gallbladder and biliary tract cancer and its attributable risk factors in 195 countries and territories, 1990 to 2017: a systematic analysis for the Global Burden of Disease Study 2017. Cancer. 2021;127(13):2238-2250. 10.1002/cncr.33476. [DOI] [PubMed] [Google Scholar]
- 2. Bertuccio P, Malvezzi M, Carioli G, et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71(1):104-114. 10.1016/j.jhep.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 3. Ishihara S, Horiguchi A, Miyakawa S, et al. Biliary tract cancer registry in Japan from 2008 to 2013. J Hepatobiliary Pancreat Sci. 2016;23(3):149-157. 10.1002/jhbp.314. [DOI] [PubMed] [Google Scholar]
- 4. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273-1281. 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 5. Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103(4):469-474. 10.1038/sj.bjc.6605779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morizane C, Okusaka T, Mizusawa J, et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann Oncol. 2019;30(12):1950-1958. 10.1093/annonc/mdz402 [DOI] [PubMed] [Google Scholar]
- 7. Heinemann V, Stintzing S, Modest DP, et al. Early tumour shrinkage (ETS) and depth of response (DpR) in the treatment of patients with metastatic colorectal cancer (mCRC). Eur J Cancer. 2015;51(14):1927-1936. 10.1016/j.ejca.2015.06.116 [DOI] [PubMed] [Google Scholar]
- 8. Piessevaux H, Buyse M, Schlichting M, et al. Use of early tumor shrinkage to predict long-term outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol. 2013;31(30):3764-3775. 10.1200/JCO.2012.42.8532 [DOI] [PubMed] [Google Scholar]
- 9. Cremolini C, Loupakis F, Antoniotti C, et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann Oncol. 2015;26(6):1188-1194. 10.1093/annonc/mdv112 [DOI] [PubMed] [Google Scholar]
- 10. Nishina T, Azuma M, Nishikawa K, et al. Early tumor shrinkage and depth of response in patients with advanced gastric cancer: a retrospective analysis of a randomized phase III study of first-line S-1 plus oxaliplatin vs. S-1 plus cisplatin. Gastric Cancer. 2019;22(1):138-146. 10.1007/s10120-018-0845-7 [DOI] [PubMed] [Google Scholar]
- 11. Kaga Y, Sunakawa Y, Kubota Y, et al. Early tumor shrinkage as a predictor of favorable outcomes in patients with advanced pancreatic cancer treated with FOLFIRINOX. Oncotarget. 2016;7(41):67314-67320. 10.18632/oncotarget.12007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vivaldi C, Fornaro L, Cappelli C, et al. Early tumor shrinkage and depth of response evaluation in metastatic pancreatic cancer treated with first line chemotherapy: an observational retrospective cohort study. Cancers (Basel). 2019;11(7):939. 10.3390/cancers11070939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim ST, Jang KT, Lee SJ, et al. Tumour shrinkage at 6 weeks predicts favorable clinical outcomes in a phase III study of gemcitabine and oxaliplatin with or without erlotinib for advanced biliary tract cancer. BMC Cancer. 2015;15:530. 10.1186/s12885-015-1552-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oh D-Y, He AR, Qin S, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1(8). 10.1056/EVIDoa2200015 [DOI] [PubMed]
- 15. Ioka T, Kanai M, Kobayashi S, et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 (GCS) versus gemcitabine, cisplatin (GC) for advanced biliary tract cancer (KHBO1401-MITSUBA). J Hepatobiliary Pancreat Sci. 2023;30(1):102-110. 10.1002/jhbp.1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671-684. 10.1016/S1470-2045(20)30109-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abou-Alfa GK, Macarulla T, Javle MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(6):796-807. 10.1016/S1470-2045(20)30157-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38(1):1-10. 10.1200/JCO.19.02105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21(2):271-282. 10.1016/S1470-2045(19)30691-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hong DS, DuBois SG, Kummar S, et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21(4):531-540. 10.1016/S1470-2045(19)30856-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lamarca A, Palmer DH, Wasan HS, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22(5):690-701. 10.1016/S1470-2045(21)00027-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kelley RK, Ueno M, Yoo C, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401(10391):1853-1865. 10.1016/S0140-6736(23)00727-4 [DOI] [PubMed] [Google Scholar]
- 23. Okano N, Kasuga A, Kawai K, et al. The modified Glasgow prognostic score in patients with gemcitabine-refractory biliary tract cancer. Anticancer Res. 2018;38(3):1755-1761. 10.21873/anticanres.12412 [DOI] [PubMed] [Google Scholar]
- 24. Johnson S, Butow P, Kerridge I, Tattersall T.. Advance care planning for cancer patients: a systematic review of perceptions and experiences of patients, families, and healthcare providers. Psychooncology. 2016;25(4):362-386. https://doi.10.1002/pon.3926. [DOI] [PubMed] [Google Scholar]
- 25. Eckel F, Schmid RM.. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96(6):896-902. 10.1038/sj.bjc.6603648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xie X, Li X, Yao W.. A narrative review: depth of response as a predictor of the long-term outcomes for solid tumors. Transl Cancer Res. 2021;10(2):1119-1130. 10.21037/tcr-20-2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


