Abstract
Conjugal transfer of pTiC58 requires two regions, tra which contains the oriT and several genes involved in DNA processing and a region of undefined size and function that is located at the 2-o’clock position of the plasmid. Using transposon mutagenesis with Tn3HoHo1 and a binary transfer system, we delimited this second region, called trb, to an 11-kb interval between the loci for vegetative replication and nopaline catabolism. DNA sequence analysis of this region identified 13 significant open reading frames (ORFs) spanning 11,003 bp. The first, encoding traI, already has been described and is responsible for the synthesis of Agrobacterium autoinducer (AAI) (I. Hwang, P.-L. Li, L. Zhang, K. R. Piper, D. M. Cook, M. E. Tate, and S. K. Farrand, Proc. Natl. Acad. Sci. USA 91:4639–4643, 1994). Translation products of the next 11 ORFs showed similarities to those of trbB, -C, -D, -E, -J, -K, -L, -F, -G, -H, and -I of the trb region of the octopine-type Ti plasmid pTi15955 and of the tra2 core region of RP4. In RP4, these genes encode mating-pair formation functions and are essential for the conjugal transfer of the IncP plasmid. Each of the trb gene homologues is oriented counterclockwise on the Ti plasmid. Expression of these genes, as measured by using the lacZ fusions formed by Tn3HoHo1, required the traI promoter and the transcriptional activator TraR along with its coinducer, AAI. While related to that of RP4, the trb system of pTiC58 did not allow propagation of the trb-specific bacteriophages PRD1, PRR1, and Pf3. The products of several trb genes of the Ti plasmid are similar to those of other loci that encode DNA transfer or protein secretion systems, all of which are members of the type IV secretion family.
The Ti plasmids of the plant pathogen Agrobacterium tumefaciens possess two DNA transfer systems. The first, called Vir, mediates the transfer of a segment of Ti plasmid DNA called T-DNA to the cells of a susceptible host plant. The T-DNA then incorporates into the plant chromosome, and the genes it encodes, when expressed, cause the unregulated growth of the plant cells that leads to the formation of a crown gall tumor. While it mediates trans-kingdom DNA transfer, the Vir system functions in a manner similar to that of the bacterium-to-bacterium transfer of conjugal plasmids (9, 34, 41, 67). Consistent with this, the Vir system also can mediate the transfer of DNA to bacteria (6). However, the conjugal transfer of the Ti plasmid between bacteria normally is dependent upon the second DNA transfer system, called Tra. Expression of the genes of the Tra system is tightly regulated through a complex circuitry that involves opines produced by the crown gall tumors, as well as a LuxR-LuxI type quorum-sensing regulatory mechanism (4, 18, 26, 35, 36, 48). Genetic and sequence analysis indicates that the Tra system is physically separated and functionally independent from the Vir system (15).
Beck von Bodman et al. localized the conjugal transfer system of the nopaline-type Ti plasmid pTiC58 to three distinct regions, tra1, tra2, and tra3 by Tn5 mutagenesis and trans-complementation (5). Mutations in any of these regions abolished conjugal transfer. The tra1 region contains only a single gene, traR, required for conjugal transfer (49). This gene codes for a homologue of LuxR and is the transcriptional activator responsible for the initiation of expression of the tra genes (4, 48). The tra2 region, now called tra, contains the origin of conjugal transfer (oriT) and two sets of genes organized as divergently expressed operons (14, 19). Three of the six genes flanking the oriT region are homologous to essential tra genes from IncP and IncQ plasmids. The products of some of these genes comprise the DNA transfer and replication function (Dtr) of the Ti plasmid Tra system and most probably are responsible for the formation of the relaxosome complex at the oriT site. With the possible exception of traF, which might code for a pilin processing protease (30), tra does not code for any identifiable mating-pair formation (Mpf) functions.
The tra3 region of pTiC58, is located at the 2-o’clock position on the plasmid and is flanked by noc, the locus conferring catabolism of nopaline and oriV/rep, the locus for vegetative replication (Fig. 1). In our previous studies we isolated six Tn5 insertions spanning approximately 3 kb within this region, all of which abolished conjugal transfer of the Ti plasmid (5) (see Fig. 2C). However, the region between noc and oriV/rep occupies more than 10 kb. Moreover, when compared to other systems, such as the Vir system of the Ti plasmid and the Tra system of IncP plasmids, a 3-kb region seems inadequate to encode the remaining Mpf functions that are essential to a conjugal transfer system. Our study of the Agrobacterium autoinducer (AAI) synthesis gene, traI, which is located in the region between noc and oriV/rep, also identified a partial open reading frame (ORF) just downstream from traI (36). This ORF showed significant similarity to trbB of RP4, which is one of the essential genes of the IncP Mpf system. traI and this partial ORF are located about 5 kb upstream from the Tn5 insertion mutations defining the minimal trb region (5). This finding led us to propose that the tra3 region of pTiC58 extends beyond 3 kb and encodes the Mpf function of the Ti plasmid conjugal transfer system. Sequence analysis of a corresponding region of the octopine-type Ti plasmid (1) and our recent description of a binary transfer system we developed to characterize the tra region of the nopaline-type Ti plasmid (15) further supported this hypothesis.
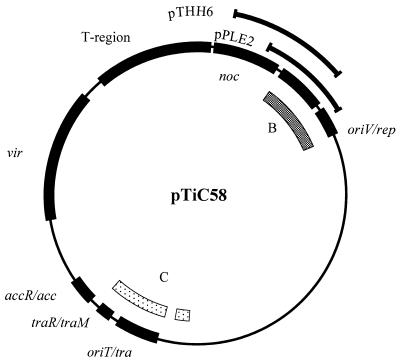
FIG. 1.
Functional map of pTiC58. The outer arcs represent the Ti plasmid DNA inserted in clones pTHH6 and pPLE2. The inner boxed arcs indicate two of the four regions of heteroduplex homology between the nopaline-type Ti plasmid and the octopine-type Ti plasmid as defined by Engler et al. (17). The shaded arc, labeled B, corresponds to the replication region, and the region that we have now defined as trb. The stippled arcs represent region C and code for the tra-Tra regulatory region (1, 19, 49).
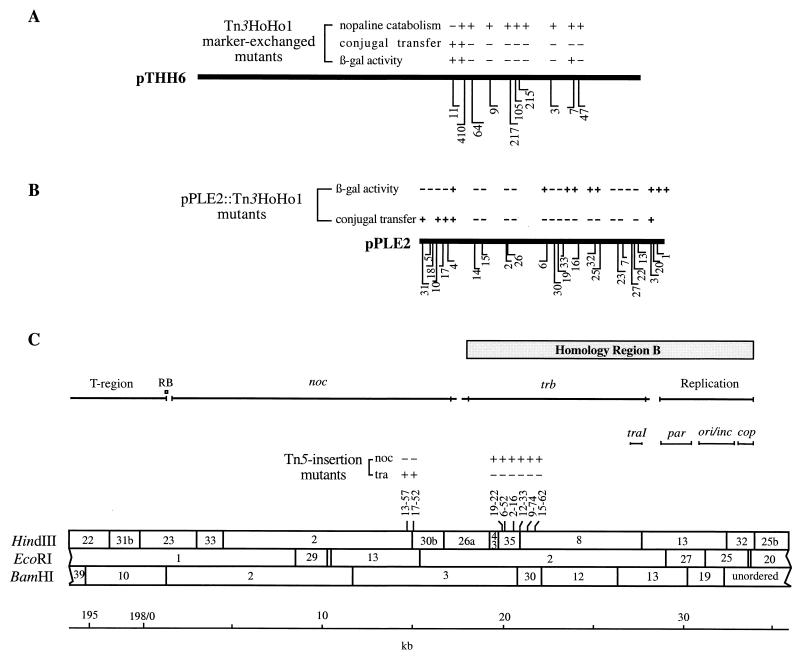
FIG. 2.
Mutational analyses of the trb region of pTiC58. (A) Phenotypes of 10 Tn3HoHo1 insertional mutants in pTHH6. The indicated phenotypes are given for each insertion mutation tested following introduction into the Trac Ti plasmid pTiC58ΔaccR by marker exchange as described in the text. (B) Phenotypes of 25 Tn3HoHo1 mutants of pPLE2. Assays for conjugation were performed by using the binary transfer system UIA143(pFRtra, pPLE2-x) as the donor and C58C1RS as the recipient, as described by Cook et al. (15) and in Materials and Methods. (C) The restriction map of the region between 12- and 3-o’clock on pTiC58. The map, which is based on the nucleotide sequence of the region, differs somewhat from that of the standard map as reported by Depicker et al (16). The locations of Tn5 insertion mutations isolated in pTiC58ΔaccR and their phenotypes were described by Beck von Bodman et al. (5).
In this study we delineated the tra3 region of the nopaline-type Ti plasmid pTiC58 by transposon mutagenesis and trans-complementation. DNA sequence analysis identified this as the trb region of the Ti plasmid. Coupled with our previous findings with the binary transfer system (15), these results indicate that this locus of pTiC58 is responsible for the Mpf functions of the Ti plasmid conjugal transfer system.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and plasmids.
Strains of Agrobacterium tumefaciens and Escherichia coli, bacteriophages, and the plasmids used in this study are listed in Table 1. A. tumefaciens strains were grown at 28°C in L broth (LB) (53), in AB minimal medium (11), or on nutrient agar plates (Difco Laboratories, Detroit, Mich.). Mannitol or glucose, at a final concentration of 0.2%, was used as the sole carbon source in the minimal medium. E. coli strains were grown at 37°C in LB or on L agar plates. Antibiotics were added at the following concentrations when required: for A. tumefaciens, carbenicillin (100 or 200 μg/ml), gentamicin (30 μg/ml), kanamycin (Km, 100 μg/ml), rifampin (50 μg/ml), streptomycin (200 μg/ml), and tetracycline (Tc; 2 μg/ml); and for E. coli, ampicillin (100 μg/ml), Km (50 μg/ml), and Tc (10 μg/ml). X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; Gibco-BRL, Bethesda, Md.) was included in the media at 40 μg/ml to assess for the production of β-galactosidase.
TABLE 1.
Bacterial strains, bacteriophages, and plasmids used in this study
| Bacterial strain, phage, or plasmid | Relevant genotype, phenotype, or characteristic(s)a | Source or reference |
|---|---|---|
| E. coli | ||
| DH5α | supE44 ΔlacU169 (φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 53 |
| S17-1 | pro (r−m+) Mob+ Ampr Cmr Tpr Strr | 15 |
| S17-1(pSShe, pHoHo1) | pro (r− m+) Mob+ Ampr Cmr Tpr Strr, Tn3HoHo1 delivery strain | 19 |
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr)] | Strategene |
| C2110(pPH1JI) | met pro Gmr Spr, used to isolate homogenotes | 19 |
| HB101 | recA13 leuB6 proA2 | Our collection |
| A. tumefaciens | ||
| C58 | Wild-type pathogenic strain carrying pTiC58 | Our collection |
| NT1(pTiC58ΔaccR) | Derivative of C58 harboring a Trac mutant of pTiC58 | 5 |
| NT1 | Ti plasmid-cured C58 | 15 |
| UIA143 | recA, Ti plasmid-cured C58, Emr | 20 |
| C58C1RS | Ti plasmid-cured C58, Rifr Strr | 15 |
| Bacteriophages | ||
| PRD1 | Bacteriophage specific for IncP1, IncN, and IncW plasmids | E. Lanka |
| PRR1, Pf3 | Bacteriophages specific for IncP1 plasmids | E. Lanka |
| Plasmids | ||
| RP4 | IncP1α wild-type plasmid, Ampr Kmr Tcr | Our collection |
| pBluescript SK(+/−) | Cloning vector, Ampr | Strategene |
| pTZ18U/R | Cloning vector, Ampr | United States Biochemicals |
| pDSK519 | Broad-host-range IncQ cloning vector, Kmr | 38 |
| pRK415 | Broad-host-range IncP cloning vector, Tcr | 38 |
| pTHH6 | Cosmid clone containing a 22-kb insert of pTiC58 containing the trb region in pVK102, Tcr | 5 |
| pPLE2 | EcoRI fragment 2 of pTiC58 cloned in pDSK519 | 36 |
| pFRtra | tra region and traR from pTiC58 cloned in pRK415, Tcr | 15 |
| pSVB33 | EcoRI fragment 33 containing traR cloned into pSa152, Kmr Gmr | 48 |
| pTHE9 | Cosmid clone of EcoRI partial digest of pTiC58 carrying the trb region in pCP13, Tcr | 32 |
| pDE-xb | pTHH6::Tn3HoHo1 mutants, Cbr Tcr | This study |
| pDEK-x | pTiC58ΔaccR::Tn3HoHo1 marker-exchanged mutants, Cbr | This study |
| pPLE2-x | pPLE2::Tn3HoHo1 mutants, Cbr Kmr | This study |
Abbreviations: Rifr, rifampin resistance; Strr, streptomycin resistance; Tcr, Tc resistance; Cbr, carbenicillin resistance; Kmr, Km resistance; Ampr, ampicillin resistance; Gmr, gentamicin resistance; Emr, erythromycin resistance; Spr, spectinomycin resistance; Tmr, tremethoprim resistance.
“x” refers to the number of the independent insertion as shown in Fig. 2.
DNA manipulation and plasmid constructions.
Ti plasmids were isolated as described by Hayman and Farrand (32). Other plasmids were isolated by an alkaline lysis method (53). Standard recombinant DNA techniques were used as described by Sambrook et al. (53). Digestions with restriction endonucleases were conducted according to the manufacturers’ instructions, and digestion products were separated by electrophoresis in agarose gels by using Tris-borate-EDTA buffer.
Tn3HoHo1 mutagenesis and homogenotization.
Recombinant clones were mutagenized with Tn3HoHo1, and the sites of insertions were mapped as previously described (19). Insertion mutations of interest on clones were homogenotized into pTiC58ΔaccR by using pPH1JI as the eviction plasmid as previously described (19). Proper marker exchanges in the Ti plasmids were confirmed by restriction endonuclease analysis.
DNA sequence analysis.
DNA fragments were sequenced by the dideoxy method with the Sequenase kit (version 2; United States Biochemical Co., Cleveland, Ohio) or by automated sequencing using dye-terminator methodology by the Biotechnology Center at the University of Illinois at Urbana-Champaign. Nucleotide sequences were assembled and analyzed, and ORFs were identified and translated by using the DNA Strider program (43) and the Map program of the GCG software package (version 8.1; Genetic Computer Group, Madison, Wis.). Nucleotide and deduced amino acid sequences were compared to those in the databases by using the BLAST search protocol (2, 3). PileUp, Gap, and BestFit programs of the GCG package were used to compare sequences and to identify regions conserved among several protein sequences. The deduced amino acid sequences were analyzed for hydropathic properties, putative motifs, or domains by Motif, PepPlot, and PlotStructure programs of the GCG package. Potential secretion signal sequences were identified by the method described by von Heijine (62) and by the SignalP V1.1 World Wide Web Prediction Server (http://www.cbs.dtu.dk/) (46). Potential transmembrane (TM) domains were identified by using two TM domain prediction servers, each based on a different algorithm: PHDhtm (http://www.embl-heidelberg.de/predictprotein/) (52) and TMpred (http://ulrec3.unil.ch/software/TMPRED_form.html) (33).
Conjugation assays.
Conjugal transfer of pTiC58ΔaccR and of the oriT/tra plasmid, pFRtra, of the binary transfer system to the recipient strain, A. tumefaciens C58C1RS, were conducted either by the spot plate mating method as described by Beck von Bodman et al. (5) or by the filter mating method as described by Cook et al. (14). Transfer frequencies were expressed as numbers of transconjugants obtained per input donor cell.
Phage infection and adsorption assays.
About 107 exponential-phase bacteria were mixed with 3 ml of 0.7% melted agar (1× nutrient broth), and the suspension was layered over the surface of nutrient agar plates. Then, 10-μl volumes of serial 10-fold dilutions of a given phage suspension (stock concentration, ∼1010 PFU/ml) were spotted onto the soft agar surface, and the plates were incubated at the respective growth temperatures of the test strains until plaques appeared. Plaquing efficiency was calculated by dividing the relative titer of the phage suspension as determined on the test strains by the relative titer as determined on the reference strain, HB101(RP4). Adsorption of phage to various A. tumefaciens strains was assayed as follows. Exponential-phase bacteria were mixed with phages of known concentration at a multiplicity of infection (MOI) of ≅0.1. The mixtures were incubated for 15 min without shaking, followed by 15 min with gentle shaking. The cells were collected by centrifugation, and the number of phage remaining in the supernatant was determined by plaquing on the susceptible host strain HB101(RP4). Adsorption efficiency (ɛ) was expressed as ɛ = (x − n)/x, in which x is the titer of the input phage and n is the titer of the phage remaining in the supernatant.
Nucleotide sequence accession numbers.
The nucleotide sequence of the trb region of pTiC58 was deposited in the GenBank database under the accession number AF057718.
RESULTS
Tn3HoHo1-induced mutants of pTHH6 and the conjugation properties of pTiC58ΔaccR homogenotized with these mutants.
Cosmid clone pTHH6 overlaps the region previously identified as tra3. We mutagenized this clone with Tn3HoHo1, and 10 independent insertions were isolated and mapped (Fig. 2A). Each of the mutations was marker exchanged in pTiC58ΔaccR as described in Materials and Methods. Eight of the ten insertions spanning a region of about 6 kb completely abolished conjugal transfer (Fig. 2A and Table 2). Strains harboring these mutant Ti plasmids all were able to catabolize nopaline. Insertion 11, which is located at the leftmost end of the region, had no effect on conjugal transfer, but it did abolish the ability of the strain to utilize nopaline (Fig. 2A). Among the eight Tra− mutants, one, with the lacZ gene of the insertion oriented from right to left, expressed β-galactosidase activity (Fig. 2A). The remaining seven mutant plasmids, all with insertions oriented in the opposite direction, did not express detectable levels of the enzyme. However, none of the corresponding insertion derivatives of pTHH6, regardless of their orientation, expressed β-galactosidase activity when tested in strains with or without a Ti plasmid (data not shown).
TABLE 2.
Conjugal transfer frequencies of mutant derivatives of pTiC58ΔaccR
| Ti plasmid | Mutation | Conjugal transfer frequencya
|
|
|---|---|---|---|
| Without pPLE2 | With pPLE2 | ||
| pTiC58ΔaccR | 1.80 × 10−3 | 8.91 × 10−3 | |
| pDEK-47 | trbE | <10−7 | 1.42 × 10−3 |
| pDEK-7 | trbE | <10−7 | 8.28 × 10−2 |
| pDEK-3 | trbJ | <10−7 | 9.17 × 10−2 |
| pDEK-215 | trbG | <10−7 | 2.92 × 10−2 |
| pDEK-105 | trbG | <10−7 | 1.38 × 10−2 |
| pDEK-217 | trbG | <10−7 | 4.17 × 10−2 |
| pDEK-9 | trbI | <10−7 | 1.30 × 10−2 |
| pDEK-64 | trbI | <10−7 | 1.65 × 10−2 |
| pDEK-410 | ORF13 | 3.52 × 10−1 | 2.36 × 10−1 |
| pDEK-11 | nocR | 2.40 × 10−3 | 2.81 × 10−2 |
Frequencies are expressed as the number of transconjugants per input donor cell.
Cloning and mutagenesis of pPLE2.
During our analysis of the traI gene, which is responsible for the synthesis of AAI (36, 44), we also sequenced a portion of a gene, located immediately downstream of traI, that is homologous to trbB of RP4. Based on an analysis of the mutants described above, we cloned the 14-kb EcoRI fragment 2 from cosmid clone pTHE9 into pDSK519 to generate pPLE2 (15). Analysis with the binary system indicates that this clone encodes the entire trb region (15). Furthermore, when placed in trans, pPLE2 restored conjugal transfer to each of the Tn3HoHo1 trb mutants of pTiC58ΔaccR (Table 2).
We subsequently isolated and mapped 25 independent Tn3HoHo1 insertions in pPLE2 (Fig. 2B). Attempts to homogenotize these insertion mutations into pTiC58ΔaccR by marker exchange were not successful. As an alternative, we utilized the binary conjugal transfer system (15) to determine the effect of mutations in pPLE2 on the mobilization of the tra/oriT plasmid, pFRtra. This construct contains the tra/oriT region of pTiC58 and the traR gene cloned in an IncP1α vector, pRK415, and is mobilized at high frequency when a functional trb region is provided in trans (15). Nineteen independent pPLE2::Tn3HoHo1 insertion mutants were introduced into UIA143(pFRtra), and the resulting strains were mated with C58C1RS. Fourteen mutants, with insertions that span about 10 kb, abolished the conjugal mobilization of pFRtra (Fig. 2B). Of the five mutants that retained the ability to mobilize pFRtra, four contained inserts that mapped to the far left end of the clone while one insert mapped to the far right end. This defined an 11-kb interval between these two sets of insertions which contains genes required for conjugal transfer.
The Tn3HoHo1 mutant derivatives of pPLE2 were introduced into a series of A. tumefaciens strains to examine expression from any fusions of the lacZ reporter gene to genes within the region. These included: NT1, which lacks a Ti plasmid; NT1(pSVB33), which expresses traR from a recombinant plasmid (48); NT1(pTiC58ΔaccR), which contains a transfer-constitutive derivative of pTiC58 and expresses traR; and wild-type C58, in which traR expression is repressed by AccR in the absence of the conjugal opines (49). Only those clones with inserts oriented from right to left conferred production of β-galactosidase in their host strains (Fig. 2B). Furthermore, active reporters were expressed only when these mutant plasmids were tested in strains that constitutively express traR (Fig. 2B and data not shown). These results indicate that the entire trb region is expressed from right to left, or counterclockwise on the Ti plasmid, and that expression of this region requires TraR.
DNA sequence analysis of the trb region.
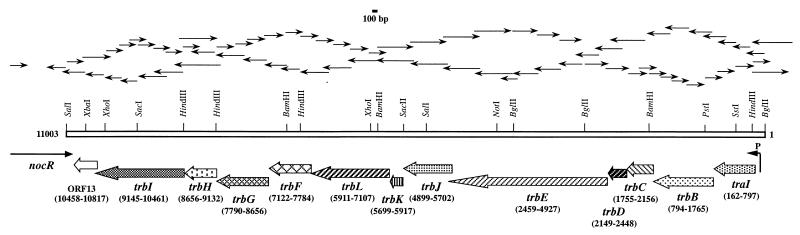
We determined the complete double-stranded DNA sequence of an 11,003-bp segment between the rep/oriV region (27) and the noc operon (54) by using the sequencing strategy shown in Fig. 3. Analysis of this sequence allowed us to make some corrections in the standard map of pTiC58 (16) with respect to the order of BamHI and HindIII fragments within this region of the plasmid (Fig. 2C). The region between repA and nocR of pTiC58 is 68% identical at the nucleotide sequence level to the trb region of the octopine-type Ti plasmid pTi15955 (1). This region of the Ti plasmid also is highly related to the putative trb region of pNGR234a, the 536-kb Sym plasmid present in Rhizobium sp. NGR234 (21). We identified 13 complete ORFs and 1 partial ORF in this 11-kb region. All, except the partial ORF, are translatable in the counterclockwise direction. The first ORF corresponds to traI, which we have described previously (36). Limited sequence from the region upstream of traI (42) showed strong homology to the repA genes from an octopine-type Ti plasmid pTiB6S3 (59) and the newly reported Ti plasmid pTi-SAKURA (58). No identifiable ORFs are present between traI and repA. At the left end of the sequenced region, the partial ORF, which is oriented from left to right, is identical at the nucleotide sequence level to the 3′ end of the known sequence of nocR, the transcriptional activator required for expression of the nopaline catabolism operon of pTiC58 (63). The 3′ ends of nocR and ORF13 are separated by 3 bp.
FIG. 3.
Organization of the trb region of pTiC58. Each arrow in the upper part of the figure represents the length of a single sequencing reaction. Relevant restriction sites are shown as vertical lines. Shaded arrows at the bottom represent the ORFs which are named after their RP4 homologues. The coordinates of the start and stop codons of each ORF are with respect to the BglII cleavage site in the region just upstream of traI as position 1. The arrow labeled P just before traI represents the location of the traI/trb promoter. The level of each ORF arrow indicates its reading frame.
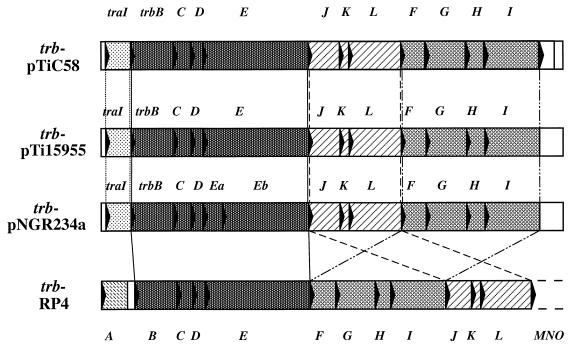
Of the remaining ORFs, the translational product of the last, ORF13, does not show significant similarity to those of any known genes in the databank. The deduced amino acid sequences of the remaining 11 ORFs between traI and ORF13 are related at the amino acid sequence level to the gene products of trbB, -C, -D, -E, -J, -K, -L, -F, -G, -H, and -I of the trb region of pTi15955 and of the tra2 core region of RP4 (1, 39, 47) (Table 3). Like that of pTi15955, the gene organization of the trb region of pTiC58 is very similar to that of RP4, except that the trb region of pTiC58 has trbJKL placed between trbE and trbF (Fig. 4). The translational products of the trb genes of the Ti plasmid also are similar at the deduced amino acid sequence level to those of genes associated with other mating systems and with protein secretion systems (Table 3). These include virB of the Ti plasmid (56), tra of plasmid F (23), and ptl of Bordetella pertussis (37, 66) (Table 3).
TABLE 3.
Relatedness among the predicted gene products of the Ti plasmid trb region and those of the other mating bridge or secretion systemsa
| Trb of pTiC58 | Trb of pTi15955 | Trb of pNGR234a | Trb of IncP plasmids
|
VirB of pTiC58 | Ptl of B. pertussis | Tra of plasmid F | ||
|---|---|---|---|---|---|---|---|---|
| Trb | α (RP4) | β (R751) | ||||||
| TrbB | 93/89 | 89/81 | TrbB | 66/49 | 67/49 | VirB11 (58/32) | PtlH (54/32) | TraG (43/19) |
| TrbC | 79/70 | 82/68 | TrbC | 59/38 | 64/38 | VirB2 (54/27) | ||
| TrbD | 91/82 | 86/72 | TrbD | 63/45 | 66/47 | VirB3 (38/18) | ||
| TrbE | 94/89 | 87/81b | TrbE | 71/52 | 69/51 | VirB4 (47/24) | PtlC (50/23) | TraC (49/21); TraB (46/21) |
| TrbJ | 91/84 | 79/68 | TrbJ | 48/25 | 48/25 | |||
| TrbK | 76/49 | 61/39 | TrbK | 43/24 | 45/21 | |||
| TrbL | 92/83 | 87/76 | TrbL | 56/28 | 55/28 | |||
| TrbF | 97/93 | 89/78 | TrbF | 58/37 | 61/34 | VirB5 (33/18) | ||
| TrbG | 90/84 | 84/74 | TrbG | 67/48 | 64/44 | |||
| TrbH | 81/70 | 68/56 | TrbH | 43/33 | 49/31 | |||
| TrbI | 91/83 | 78/66 | TrbI | 54/33 | 53/32 | VirB10 (50/25) | PtlG (50/26) | TraF (43/19) |
Values of relatedness are percentage of similarities/percentage of identities for the pairs of amino acid sequences.
Since TrbE of pNGR234a consists of two separate parts, a and b (21), the score here represents the amino acid sequence of TrbE of pTiC58 aligned with the combined amino acid sequences of TrbEa and TrbEb of pNGR234a.
FIG. 4.
Comparison of the gene organizations among the trb regions of pTiC58, pTi15955, pNGR234a, and the tra2 core region of RP4. Regions shaded in the same pattern represent homologous segments.
That the trb genes all are transcribed in the counterclockwise orientation on the Ti plasmid is consistent with the transposon mutagenesis studies described above. We could not locate any identifiable promoter elements between any of these genes suggesting that they form a single transcriptional unit. In certain of the consecutive ORFs, the stop codon of the preceeding ORF overlaps the start codon of the succeeding ORF, with no identifiable ribosomal binding site present before the start codon. This characteristic suggests that these ORFs, which include trbB-C, trbC-D, trbK-L, and trbF-G, are translationally coupled.
Properties of the putative trb gene products.
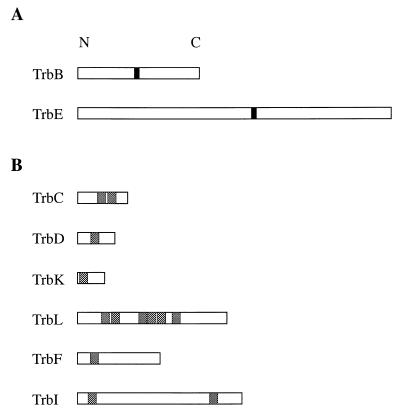
We analyzed the amino acid sequence of each of the putative trb gene products for their properties and searched for any significant domains or motifs by using several computer programs (Table 4). Two proteins, TrbB and TrbE, contain a domain related to the sequence of the canonical nucleoside triphosphate (NTP)-binding motif A (Fig. 5A). TrbH contains a putative membrane lipoprotein lipid attachment site.
TABLE 4.
Characteristics of the predicted Trb proteins of pTiC58
| Proteina | Predicted protein size
|
Related RP4 trb gene product | Putative motif or domain | Proposed function or activity | |
|---|---|---|---|---|---|
| No. of amino acids | Molecu-lar wt | ||||
| TraI | 211 | 23,452 | NAb | AAI synthesis (36) | |
| TrbB | 323 | 34,746 | TrbB | NTP-binding motif A | ATPase, autophosphorylase (13), Mpf (31) |
| TrbC | 133 | 13,334 | TrbC | Sec-dependent export signal; TM domain | Pilus formation (30) |
| TrbC* (29–133) | 105 | 10,521 | |||
| TrbD | 99 | 11,265 | TrbD | TM domain | Mpf (31) |
| TrbE | 822 | 91,788 | TrbE | NTP-binding motif A | ATPase (55), Mpf (31) |
| TrbJ | 267 | 29,571 | TrbJ | Sec-dependent export signal | Mpf (31) |
| TrbJ* (29–267) | 239 | 26,480 | |||
| TrbK | 72 | 26,480 | TrbK | TM domain | Entry exclusion (29, 31) |
| TrbL | 398 | 40,665 | TrbL | Sec-dependent export signal; TM domain | Mpf (31) |
| TrbL* (30–398) | 369 | 37,479 | |||
| TrbF | 220 | 24,709 | TrbF | TM domain | Mpf (31) |
| TrbG | 288 | 31,019 | TrbG | Sec-dependent export signal | Mpf (31) |
| TrbG* (39–288) | 250 | 27,047 | |||
| TrbH | 158 | 16,075 | TrbH | Sec-dependent export signal; membrane lipoprotein lipid attachment site | Mpf (31) |
| TrbH* (35–158) | 124 | 12,601 | |||
| TrbI | 438 | 47,678 | TrbI | TM domain | Mpf (31) |
Asterisks indicate the predicted mature proteins after removal of their signal peptides (Table 5). Parenthetic values indicate the residue numbers of the predicted mature protein.
NA, not applicable.
FIG. 5.
Significant motifs and domains of Trb proteins. (A) Filled areas represent the locations of the NTP-binding motif A in TrbB and TrbE. (B) Shaded areas represent the putative TM domains in six Trb proteins as predicted by PHDhtm and TMpred (see Materials and Methods). Only the regions that are predicted by both programs are considered significant. The protein export signal peptide (Table 5) often is predicted as a TM domain by these programs. Therefore, in these representations we have excluded those that also could be signal peptides. N, amino terminus; C, carboxy terminus.
We also examined the deduced amino acid sequences of each of the trb gene products for Sec-dependent export signal peptides and cleavage sites by using the “−3,−1” rule described by von Heijne (62) and the signal peptide prediction server, SignalP V1.1 (46). Due to the inherent error in both methods (46, 62), we considered as significant only those sequences that gave strong matches using both algorithms. Of the 11 trb gene products of pTiC58, 5 contain a potential signal peptide (Table 5). We also analyzed the Trb proteins for possible TM domains with two programs, PHDhtm (52) and TMpred (33), which utilize different databases and algorithms. Of the 11 proteins, 6 contain at least one potential TM domain (Fig. 5B).
TABLE 5.
Potential protein export signal sequences of five Trb proteins
| Protein | Amino-terminal sequencea |
|---|---|
| +++ − | |
| TrbC | MSHKIRIAIALAAFAAGSFIGLADPAFA / SSG |
| +++ − | |
| TrbG | MAPNMNIQLRRFSATLTAATIVAVIVPFSASLSSRALA / ADG |
| + + + +− | |
| TrbH | MRSLLSHPQPSHAIRSPAAACALALFLSGCQSLG / TDG |
| ++ + − + | |
| TrbJ | MPRHFSRPLEAARVAMITAIVILVAFPTYA / GGV |
| + ++ − | |
| TrbL | MVVVRPSRRLEMTFIVMAIVLLATAPAFA / QQG |
The predicted cleavage sites are indicated by a diagonal stroke. Hydrophobic core regions are underlined. Positively and negatively charged amino acids are indicated by a plus or a minus sign above the residue.
Phage plaquing and adsorption on A. tumefaciens strains expressing Tra functions.
Propagation of donor-specific phages is one of the criteria that serve to define the Mpf system of RP4 (31). We tested three such phages, PRD1, PRR1, and Pf3 (Table 1), for their ability to plaque on four Agrobacterium strains: NT1, C58, NT1(pTiC58ΔaccR), and NT1(pFRtra, pPLE2). As controls, we also tested NT1(RP4) and E. coli HB101(RP4). All three bacteriophages plaqued on NT1(RP4) with an efficiency of about 10% compared to HB101(RP4). None of these phages produced visible plaques on any of the other A. tumefaciens strains, even at very high MOIs (data not shown). We then determined if PRD1, which recognizes the RP4 mating bridge complex (28), adsorbs to A. tumefaciens strains expressing the trb components of pTiC58 by using strains NT1(pTiC58ΔaccR) and NT1(pFRtra, pPLE2). Less than 1% of the input bacteriophage were adsorbed to these strains, whereas adsorption levels for HB101(RP4) and NT1(RP4) were about 60 and 42%, respectively (data not shown).
DISCUSSION
Identification and sequence analysis of the trb region.
Our genetic and sequence analyses clearly show that the 11-kb region between noc and oriV/rep of pTiC58 codes for trb, the major portion of the Mpf component of the conjugal mating apparatus of this Ti plasmid. As predicted by the heteroduplex analysis of Engler et al. (17), the trb region of pTiC58 is strongly related at the nucleotide sequence level to the trb region of pTi15955, an octopine-type Ti plasmid (1). Moreover, this region from pTiC58 is 99% identical to a recently reported segment of a new Ti plasmid, pTi-SAKURA (reference 58 and data not shown). Although no information is available concerning the phenotypic characteristics of this plasmid, given the high degree of homology we predict that this region encodes the Mpf for the conjugal transfer system of pTi-SAKURA.
The trb region of pTiC58 consists of 13 significant ORFs. The first, corresponding to traI, is responsible for the synthesis of AAI [N-(3-oxooctanoyl)-l-homoserine lactone] and is essential for the quorum-sensing mediated regulation of the conjugal transfer of the Ti plasmid (26, 36, 48, 68). Of the remaining 12 ORFs, 11 could encode proteins that are similar to those from genes of several DNA-transfer and protein-secretion systems (Table 3). Among these systems, the tra2 core region of RP4 showed significant relatedness with the trb region from pTiC58 in several aspects. First, the trb region of pTiC58 contains homologues of all of the essential trb genes of RP4, suggesting that the trb system of pTiC58 codes for an Mpf system very similar to that of RP4. Second, the Trb proteins from the two systems show degrees of relatedness ranging from identities of 30 to 50% and similarities of 50 to 70% (Table 3), indicating that these two systems are closely related. Third, the homology between trb of pTiC58 and tra2 of RP4 exists not only at the individual gene level but extends to the gene organization (Fig. 4). Both of these systems are organized with a leading regulatory gene followed by 11 trb genes, trbB through trbI. Only the order of three of the genes, trbJ, -K, and -L, differs between these two plasmids (Fig. 4).
In RP4, all of the 11 shared trb genes except trbK are required for conjugal transfer of the plasmid (31). While our insertion mutations in the trb region of pTiC58 abolish transfer, the possibility of polarity precludes speculation on the requirements for most of these genes in the conjugal process. However, transposon insertions in the distal ORF, ORF13 do not affect conjugal transfer of the Ti plasmid (Table 2) or the mobilization of pFRtra in the binary transfer system (Fig. 2B). Moreover, a smaller trb clone, pPLtrb, which lacks ORF13, remains fully functional in the binary transfer system (data not shown). Thus, this ORF, even if translated into a protein product, is not essential for conjugal transfer. In a similar fashion, several genes located downstream of trbL in the trb operon of RP4 are not required for conjugal transfer of this IncP1 plasmid (40). Homologs of these genes, including trbM, trbN, trbO, and trbP are not present in the Ti plasmid trb region. Finally, ORF13 is not related to any of these genes, and we could not detect a similar ORF in the sequences of the trb regions from pTi15955 and pNGR234a. We conclude that ORF13, while perhaps part of the trb operon, is not required for conjugal transfer. Transposon insertions in trbI, the gene that precedes ORF13, abolished conjugal transfer when tested in both the whole Ti plasmid system and in the binary transfer system (Fig. 2; Table 2). Since there can be no polar effect resulting from these insertions except on ORF13, trbI may be essential for conjugal transfer of pTiC58. Similarly, trbI is required for the transfer of RP4 (31). However, pTiA6NC, which contains a deletion of most of trbI, can still undergo conjugal transfer, albeit at a severely reduced frequency (1). Hence, the essential nature of trbI in conjugal transfer of the Ti plasmids remains to be elucidated.
Our analyses suggest that all of the 12 genes in the trb region of pTiC58 are expressed from the promoter located immediately upstream of traI. Correctly inserted Tn3HoHo1 insertions in pTHH6, the insert of which lacks the 5′ end of traI, do not express β-galactosidase activity under any conditions. However, properly oriented fusions formed by these insertions express enzyme activity when marker exchanged into pTiC58Δ-accR (Fig. 2A). Similar insertions in pPLE2, which contains the promoter region upstream of traI, do express β-galactosidase activity, but this expression requires TraR (Fig. 2). Therefore, the traI/trb operon is regulated as part of the TraR-AAI quorum-sensing regulon. Fuqua and Winans reported that traI of the octopine-type Ti plasmid also requires TraR for expression, but there is no information available concerning expression of the rest of the trb region from this octopine-type Ti plasmid (25). Given the similar gene organization, we predict that expression of the entire trb region of the pTiR10/pTi15955 also requires TraR and AAI.
As is the case for their RP4 homologues, the putative products of trbC, -D, -K, -L, -F, and -I of pTiC58 contain potential transmembrane domain(s) (Fig. 5). This finding is consistent with this locus encoding the mating bridge. Several of the putative gene products also contain amino-terminal Sec-dependent signal peptides (Table 5). In addition, TrbB and TrbE contain the NTP-binding motif A, “GXXXXGKT/S” (64) (Fig. 5A). This domain is conserved in other homologues, including TrbB and TrbE of RP4 (41), and in VirB11 and VirB4 of the Ti plasmid Vir transfer system. VirB11 and VirB4 exhibit ATPase activities (13, 55), and the NTP-binding motifs are essential to their functions (7, 24, 51, 57). Unlike all of the other TrbE homologues so far described, the trbE gene of pNGR234a is composed of two ORFs: trbEa and trbEb (21). These two proteins of pNGR234a may function cooperatively in the same way as the single full-length protein by a LacZα-like complementation mechanism. It also is possible that only one of the two trbE gene products is required for the Mpf. Alternatively, trbE of pNGR234a may be defective, with its function being provided by some other conjugal system present in Rhizobium sp. NGR234. All transport systems to which trb of pTiC58 is related contain a TrbE homologue, and the function encoded by this gene is essential for conjugal transfer of RP4 (31).
Differences between the trb systems from pTiC58 and RP4.
Despite the sequence and organizational similarities between trb from RP4 and the Ti plasmid, these two systems differ in several ways. Bacteria expressing trb of IncP1 plasmids plaque bacteriophages PRD1, PRR1, and Pf3 (for a review, see reference 22). However, these bacteriophages do not propagate on cells expressing trb of pTiC58. This failure is not due to some defect in A. tumefaciens; strain NT1(RP4) plaques all three bacteriophages at reasonable efficiencies. Furthermore, PRD1 fails even to adsorb to strains of A. tumefaciens expressing trb. These results suggest that the epitopes of the Trb proteins of RP4 responsible for phage sensitivity are not conserved in the Trb proteins of pTiC58. Mutational analysis implicates TrbC, TrbE, and especially TrbL in sensitivity to PRD1 conferred by RP4 (28). TrbL is one of the two least-conserved gene products encoded by the trb operons of pTiC58 and RP4 (Table 3). Whether any one of the differences in the three proteins of the two systems is responsible for the inability of PRD1 to adsorb to strains harboring the Ti plasmid remains to be determined.
As a further measure of the disparity between the two trb systems, the IncP Mpf system cannot substitute for the Ti plasmid Mpf system. Ti plasmids with a mutation in trb do not transfer from donors also harboring pPH1JI, a self-conjugal derivative of R751 containing the intact IncP1β tra1 and tra2 regions (data not shown). Moreover, RP4 does not mobilize a clone containing the Ti plasmid tra/oriT region, nor can the tra system of pTiC58 mobilize vectors based on IncP1α plasmids (14). Thus, the two Mpf components are specific to their respective transfer systems. However, these results demonstrate only that the Mpf systems of the IncP and Ti plasmids are not cross-functional as a whole. We have not ruled out the possibility that one or more of the individual trb genes are interchangeable between the two systems.
Although most of the trb genes are closely related, some of the genes show significant divergence. The most notable of these is trbK. In RP4, trbK is necessary and sufficient for the entry exclusion function of the plasmid (29, 31). Although TrbK of pTiC58 contains neither a cysteine residue nor a detectable secretion signal peptide, both of which are important for the functionality of TrbK of RP4 (29), our preliminary results indicate that cells containing pTiC58 exhibit a Ti plasmid-specific entry exclusion phenotype. Moreover, a clone expressing the trbK gene from pTiC58 confers entry exclusion (45).
trb of the Ti plasmid and the type IV secretion superfamily.
We previously proposed (1, 15, 19) that the extant Ti plasmid, which contains both a conjugal transfer system and the Vir system, arose from the fusion of two conjugal plasmids. Our results indicate that the conjugal transfer system itself is chimeric, being composed of both IncP-like elements, which include TraG, TraF, and the Trb proteins, and also IncQ-like elements, which include TraA and oriT (1, 19).
In the larger context, the Trb proteins of pTiC58 are related to proteins from several secretion systems (Table 3). These type-IV secretion systems (12) transport DNA or protein substrates, or perhaps both (8, 61). They include the VirB system of the Ti plasmids (56, 65), the Trb system of the IncP plasmids (39), the Trw system of the IncW plasmids (12), the Tra system of the IncN plasmids (50), the Ptl system of B. pertussis (66), the Dot system of Legionella pneumophila (61), and perhaps the Cag system of Helicobacter pylori (10, 60). These systems can be further grouped into subfamilies based on their degrees of similarity. Given the extensive sequence and organizational similarities among Trb from the Ti plasmids, Trb from pNGR234a, and Trb from IncP plasmids RP4 and R751, we propose that these Mpf systems belong to a common DNA transporter family with the Trb system from RP4 as the representative. Similarly, the VirB operon of the Ti plasmids, along with Tra of the IncN plasmids and Ptl of B. pertussis, form a second family within this type IV superfamily, with VirB as the representative.
ACKNOWLEDGMENTS
We are grateful to Audra Smyth for helpful discussion and critical reading of the manuscript. We also thank Susanne Beck von Bodman, Ingyu Hwang, and David Cook for helpful discussion.
This work was supported by grants R01GM52465 from the NIH and AG92-3312-8231 from the USDA to S.K.F. P.-L.L. was supported in part by HATCH project 15-0326 to S.K.F.
REFERENCES
- 1.Alt-Mörbe J, Stryker J L, Fuqua C, Li P-L, Farrand S K, Winans S C. The conjugal transfer system of Agrobacterium tumefaciens octopine-type Ti plasmids is closely related to the transfer system of an IncP plasmid and distantly related to Ti plasmid vir genes. J Bacteriol. 1996;178:4248–4257. doi: 10.1128/jb.178.14.4248-4257.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck von Bodman S, Hayman G T, Farrand S K. Opine catabolism and conjugal transfer of the nopaline Ti plasmid pTiC58 are coordinately regulated by a single repressor. Proc Natl Acad Sci USA. 1992;89:643–647. doi: 10.1073/pnas.89.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck von Bodman S, McCutchan J E, Farrand S K. Characterization of conjugal transfer functions of Agrobacterium tumefaciens Ti plasmid pTiC58. J Bacteriol. 1989;171:5281–5289. doi: 10.1128/jb.171.10.5281-5289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beijersbergen A, Dulk-Ras A D, Schilperoort R A, Hooykaas P J J. Conjugative transfer by the virulence system of Agrobacterium tumefaciens. Science. 1992;256:1324–1327. doi: 10.1126/science.256.5061.1324. [DOI] [PubMed] [Google Scholar]
- 7.Berger B R, Christie P J. The Agrobacterium tumefaciens virB4 gene product is an essential virulence protein requiring an intact nucleoside triphosphate-binding domain. J Bacteriol. 1993;175:1723–1734. doi: 10.1128/jb.175.6.1723-1734.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binns A N, Beaupré C E, Dale E M. Inhibition of VirB-mediated transfer of diverse substrates from Agrobacterium tumefaciens by the IncQ plasmid RSF1010. J Bacteriol. 1995;177:4890–4899. doi: 10.1128/jb.177.17.4890-4899.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Binns A N, Thomashow M F. Cell biology of Agrobacterium infection and transformation of plants. Annu Rev Microbiol. 1988;42:575–606. [Google Scholar]
- 10.Censini S, Lange C, Xiang Z, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chilton M D, Currier T C, Farrand S K, Bendich A J, Gordon M P, Nester E W. Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc Natl Acad Sci USA. 1974;71:3672–3676. doi: 10.1073/pnas.71.9.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christie P J. Agrobacterium tumefaciens T-complex transport apparatus: a paradigm for a new family of multifunctional transporters in eubacteria. J Bacteriol. 1997;179:3085–3094. doi: 10.1128/jb.179.10.3085-3094.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christie P J, Ward J E, Jr, Gordon M P, Nester E W. A gene required for transfer of T-DNA to plants encodes an ATPase with autophosphorylating activity. Proc Natl Acad Sci USA. 1989;86:9677–9681. doi: 10.1073/pnas.86.24.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook D M, Farrand S K. The oriT region of the Agrobacterium tumefaciens Ti plasmid pTiC58 shares DNA sequence identity with the transfer origins of RSF1010 and RK2/RP4 and with T-region borders. J Bacteriol. 1992;174:6238–6246. doi: 10.1128/jb.174.19.6238-6246.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook D M, Li P-L, Ruchaud F, Padden S, Farrand S K. Ti plasmid conjugation is independent of vir: reconstitution of the tra functions from pTiC58 as a binary system. J Bacteriol. 1997;179:1291–1297. doi: 10.1128/jb.179.4.1291-1297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Depicker A, de Wilde M, de Vos G, de Vos R, Van Montagu M, Schell J. Molecular cloning of overlapping segments of the nopaline Ti-plasmid pTiC58 as a means to restriction endonuclease mapping. Plasmid. 1980;3:193–211. doi: 10.1016/0147-619x(80)90109-2. [DOI] [PubMed] [Google Scholar]
- 17.Engler G, Depicker R, Maenhaut R, Villarroel R, Van Montagu M, Schell J. Physical mapping of DNA base sequence homologies between an octopine and a nopaline Ti plasmid of Agrobacterium tumefaciens. J Mol Biol. 1981;152:183–208. doi: 10.1016/0022-2836(81)90239-4. [DOI] [PubMed] [Google Scholar]
- 18.Farrand S K. Conjugation of Agrobacterium plasmids. In: Clewell D B, editor. Bacterial conjugation. New York, N.Y: Plenum Press, Inc.; 1993. pp. 255–291. [Google Scholar]
- 19.Farrand S K, Hwang I, Cook D M. The tra region of the nopaline-type Ti plasmid is a chimera with elements related to the transfer systems of RSF1010, RP4, and F. J Bacteriol. 1996;178:4233–4247. doi: 10.1128/jb.178.14.4233-4247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farrand S K, O’Morchoe S P, McCutchan J. Construction of an Agrobacterium tumefaciens C58 recA mutant. J Bacteriol. 1989;171:5314–5321. doi: 10.1128/jb.171.10.5314-5321.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freiberg C, Fellay R, Bairoch A, Broughton W J, Rosenthal A, Perret X. Molecular basis of symbiosis between Rhizobium and legumes. Nature (London) 1997;387:394–401. doi: 10.1038/387394a0. [DOI] [PubMed] [Google Scholar]
- 22.Frost L S. Conjugative pili and pilus-specific phages. In: Clewell D B, editor. Bacterial conjugation. New York, NY: Plenum Press, Inc.; 1993. pp. 189–221. [Google Scholar]
- 23.Frost L S, Ippen-Ihler K, Skurray R A. An analysis of the sequence and gene products of the transfer region of the F sex factor. Microbiol Rev. 1994;58:162–210. doi: 10.1128/mr.58.2.162-210.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fullner K J, Stephens K M, Nester E W. An essential virulence protein of Agrobacterium tumefaciens, VirB4, requires an intact mononucleotide binding domain to function in transfer of T-DNA. Mol Gen Genet. 1994;245:704–715. doi: 10.1007/BF00297277. [DOI] [PubMed] [Google Scholar]
- 25.Fuqua C, Winans S C. Conserved cis-acting promoter elements are required for density-dependent transcription of Agrobacterium tumefaciens conjugal transfer genes. J Bacteriol. 1996;178:435–440. doi: 10.1128/jb.178.2.435-440.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuqua W C, Winans S C, Greenberg E P. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallie D R, Hagiya M, Kado C I. Analysis of Agrobacterium tumefaciens plasmid pTiC58 replication region with a novel high-copy-number derivative. J Bacteriol. 1985;161:1034–1041. doi: 10.1128/jb.161.3.1034-1041.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grahn A M, Haase J, Lanka E, Bamford D H. Assembly of a functional phage PRD1 receptor depends on 11 genes of the IncP plasmid mating pair formation complex. J Bacteriol. 1997;179:4733–4740. doi: 10.1128/jb.179.15.4733-4740.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haase J, Kalkum M, Lanka E. TrbK, a small cytoplasmic membrane lipoprotein, functions in entry exclusion of the IncPα plasmid RP4. J Bacteriol. 1996;178:6720–6729. doi: 10.1128/jb.178.23.6720-6729.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haase J, Lanka E. A specific protease encoded by the conjugative DNA transfer systems of IncP and Ti plasmids is essential for pilus synthesis. J Bacteriol. 1997;179:5728–5735. doi: 10.1128/jb.179.18.5728-5735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haase J, Lurz R, Grahn A M, Bamford D H, Lanka E. Bacterial conjugation mediated by plasmid RP4: RSF1010 mobilization, donor-specific phage propagation, and pilus production require the same Tra2 core components of a proposed DNA transport complex. J Bacteriol. 1995;177:4779–4791. doi: 10.1128/jb.177.16.4779-4791.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayman G T, Farrand S K. Characterization and mapping of the agrocinopine-agrocin 84 locus on the nopaline Ti plasmid pTiC58. J Bacteriol. 1988;170:1759–1767. doi: 10.1128/jb.170.4.1759-1767.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofmann K, Stoffel W. TMBASE-A database of membrane spanning protein segments. Biol Chem Hoppe-Seyler. 1993;374:166. [Google Scholar]
- 34.Hooykaas P J J, Beijersbergen A G M. The virulence system of Agrobacterium tumefaciens. Annu Rev Phytopathol. 1994;32:157–179. [Google Scholar]
- 35.Hwang I, Cook D M, Farrand S K. A new regulatory element modulates homoserine lactone-mediated autoinduction of Ti plasmid conjugal transfer. J Bacteriol. 1995;177:449–458. doi: 10.1128/jb.177.2.449-458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hwang I, Li P-L, Zhang L, Piper K R, Cook D M, Tate M E, Farrand S K. TraI, a LuxI homologue, is responsible for production of conjugation factor, the Ti plasmid N-acylhomoserine lactone autoinducer. Proc Natl Acad Sci USA. 1994;91:4639–4643. doi: 10.1073/pnas.91.11.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson F D, Burns D L. Detection and subcellular localization of three Ptl proteins involved in the secretion of pertussis toxin from Bodetella pertussis. J Bacteriol. 1994;176:5350–5356. doi: 10.1128/jb.176.17.5350-5356.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 39.Lessl M, Balzer D, Pansegrau W, Lanka E. Sequence similarities between the RP4 Tra2 and the Ti VirB region strongly support the conjugation model for T-DNA transfer. J Biol Chem. 1992;267:20471–20480. [PubMed] [Google Scholar]
- 40.Lessl M, Balzer D, Weyrauch K, Lanka E. The mating pair formation system of plasmid RP4 defined by RSF1010 mobilization and donor-specific phage propagation. J Bacteriol. 1993;175:6415–6425. doi: 10.1128/jb.175.20.6415-6425.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lessl M, Lanka E. Common mechanisms in bacterial conjugation and Ti-mediated T-DNA transfer to plant cells. Cell. 1994;77:321–324. doi: 10.1016/0092-8674(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 42.Li, P.-L., and S. K. Farrand. Unpublished data.
- 43.Mark C. “DNA Strider”: a “C” program for the fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res. 1988;16:1829–1836. doi: 10.1093/nar/16.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moré M I, Finger L D, Stryker J L, Fuqua C, Eberhard A, Winans S C. Enzymatic synthesis of a quorum-sensing autoinducer through use of defined substrates. Science. 1996;272:1655–1658. doi: 10.1126/science.272.5268.1655. [DOI] [PubMed] [Google Scholar]
- 45.Nelson, C. D., C. E. Bratis, and S. K. Farrand. Unpublished data.
- 46.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 47.Pansegrau W, Lanka E, Barth P T, Figurski D H, Guiney D G, Haas D, Helinski D R, Schwab H, Stanisich V A, Thomas C M. Complete nucleotide sequence of Birmingham IncPα plasmids. Compilation and comparative analysis. J Mol Biol. 1994;239:623–663. doi: 10.1006/jmbi.1994.1404. [DOI] [PubMed] [Google Scholar]
- 48.Piper K R, Beck von Bodman S, Farrand S K. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature (London) 1993;362:448–450. doi: 10.1038/362448a0. [DOI] [PubMed] [Google Scholar]
- 49.Piper, K. R., S. Beck von Bodman, I. Hwang, and S. K. Farrand. Submitted for publication.
- 50.Pohlman R F, Genetti H D, Winans S C. Common ancestry between IncN conjugal transfer genes and macromolecular export systems of plant and animal pathogens. Mol Microbiol. 1994;14:655–668. doi: 10.1111/j.1365-2958.1994.tb01304.x. [DOI] [PubMed] [Google Scholar]
- 51.Rashkova S, Spudich G M, Christie P J. Characterization of membrane and protein interaction determinants of the Agrobacterium tumefaciens VirB11 ATPase. J Bacteriol. 1997;179:583–591. doi: 10.1128/jb.179.3.583-591.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rost B, Casadio R, Fariselli P. Refining neural network predictions for helical transmembrane protein by dynamic programming. In: States D, et al., editors. Fourth International Conference on Intelligent Systems for Molecular Biology, St. Louis, Mo. Menlo Park, Calif: AAAI Press; 1996. pp. 192–200. [PubMed] [Google Scholar]
- 53.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1989. [Google Scholar]
- 54.Schröder J, von Lintig J, Zanker H. Functional organization of the regions responsible for nopaline and octopine catabolism in Ti plasmids of Agrobacterium tumefaciens. In: Hennecke H, Verma D P S, editors. Advances in molecular genetics of plant-microbe interactions. Vol. 1. Amsterdam, The Netherlands: Kluwer Academic Publishers; 1990. pp. 28–31. [Google Scholar]
- 55.Shirasu K, Koukolikova-Nicola Z, Hohn B, Kado C I. An inner-membrane-associated virulence protein essential for T-DNA transfer from Agrobacterium tumefaciens to plants exhibits ATPase activity and similarities to conjugative transfer genes. Mol Microbiol. 1994;11:581–588. doi: 10.1111/j.1365-2958.1994.tb00338.x. [DOI] [PubMed] [Google Scholar]
- 56.Shirasu K, Morel P, Kado C I. Characterization of the virB operon of an Agrobacterium tumefaciens Ti plasmid: nucleotide sequence and protein analysis. Mol Microbiol. 1990;4:1153–1163. doi: 10.1111/j.1365-2958.1990.tb00690.x. [DOI] [PubMed] [Google Scholar]
- 57.Stephens K M, Roush C, Nester E. Agrobacterium tumefaciens VirB11 protein requires a consensus nucleotide-binding site for function in virulence. J Bacteriol. 1995;177:27–36. doi: 10.1128/jb.177.1.27-36.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki K, Ohta N, Hattori Y, Uraji M, Kato A, Yoshida K. Novel structural difference between nopaline- and octopine-type trbJ genes: construction of genetic and physical map and sequencing of trb/traI and rep gene clusters of a new Ti plasmid pTi-SAKURA. Biochim Biophys Acta. 1998;1396:1–7. doi: 10.1016/s0167-4781(97)00182-6. [DOI] [PubMed] [Google Scholar]
- 59.Tabata S, Hooykaas P J J, Oka A. Sequence determination and characterization of the replicator region in the tumor-inducing plasmid pTiB6S3. J Bacteriol. 1989;171:1665–1672. doi: 10.1128/jb.171.3.1665-1672.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tummuru M K R, Sharma S A, Blaser M J. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995;18:867–876. doi: 10.1111/j.1365-2958.1995.18050867.x. [DOI] [PubMed] [Google Scholar]
- 61.Vogel J P, Andrews H L, Wong S K, Isberg R R. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- 62.von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.von Lintig J, Zanker H, Schröder J. Positive regulators of opine-inducible promoters in the nopaline and octopine catabolism regions of Ti plasmids. Mol Plant-Microbe Interact. 1991;4:370–378. doi: 10.1094/mpmi-4-370. [DOI] [PubMed] [Google Scholar]
- 64.Walker J E, Saraste M, Runswick M J, Gay N J. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ward J E, Akiyoshi D E, Regier D, Datta A, Gordon M P, Nester E W. Characterization of the virB operon from an Agrobacterium tumefaciens Ti plasmid. J Biol Chem. 1988;263:5804–5814. [PubMed] [Google Scholar]
- 66.Weiss A A, Johnson F D, Burns D L. Molecular characterization of an operon required for pertussis toxin secretion. Proc Natl Acad Sci USA. 1993;90:2970–2974. doi: 10.1073/pnas.90.7.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zambryski P C. Chronicles from the Agrobacterium-plant cell DNA transfer story. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:465–490. [Google Scholar]
- 68.Zhang L, Murphy P J, Kerr A, Tate M E. Agrobacterium conjugation and gene regulation by N-acyl-l-homoserine lactones. Nature (London) 1993;362:446–448. doi: 10.1038/362446a0. [DOI] [PubMed] [Google Scholar]