Summary
Background
Leritrelvir is a novel α-ketoamide based peptidomimetic inhibitor of SARS-CoV-2 main protease. A preclinical study has demonstrated leritrelvir poses similar antiviral activities towards different SARS-CoV-2 variants compared with nirmatrelvir. A phase 2 clinical trial has shown a comparable antiviral efficacy and safety between leritrelvir with and without ritonavir co-administration. This trial aims to test efficacy and safety of leritrelvir monotherapy in adults with mild-to-moderate COVID-19.
Methods
This was a randomised, double-blind, placebo-controlled, multicentre phase 3 trial at 29 clinical sites in China. Enrolled patients were from 18 to 75 years old, diagnosed with mild or moderate COVID-19 and not requiring hospitalization. Patients had a positive SARS-CoV-2 nucleic acid test (NAT) and at least one of the COVID-19 symptoms within 48 h before randomization, and the interval between the first positive SARS-CoV-2 NAT and randomization was ≤120 h (5 days). Patients were randomly assigned in a 1:1 ratio to receive a 5-day course of either oral leritrelvir 400 mg TID or placebo. The primary efficacy endpoint was the time from the first dose to sustained clinical recovery of all 11 symptoms (stuffy or runny nose, sore throat, shortness of breath or dyspnea, cough, muscle or body aches, headache, chills, fever ≥37 °C, nausea, vomiting, and diarrhea). The safety endpoint was the incidence of adverse events (AE). Primary and safety analyses were performed in the intention-to-treat (ITT) population. This study is registered with ClinicalTrials.gov, NCT05620160.
Findings
Between Nov 12 and Dec 30, 2022 when the zero COVID policy was abolished nationwide, a total of 1359 patients underwent randomization, 680 were assigned to leritrelvir group and 679 to placebo group. The median time to sustained clinical recovery in leritrelvir group was significantly shorter (251.02 h [IQR 188.95–428.68 h]) than that of Placebo (271.33 h [IQR 219.00–529.63 h], P = 0.0022, hazard ratio [HR] 1.20, 95% confidence interval [CI], 1.07–1.35). Further analysis of subgroups for the median time to sustained clinical recovery revealed that (1) subgroup with positive viral nucleic acid tested ≤72 h had a 33.9 h difference in leritrelvir group than that of placebo; (2) the subgroup with baseline viral load >8 log 10 Copies/mL in leritrelvir group had 51.3 h difference than that of placebo. Leritrelvir reduced viral load by 0.82 log10 on day 4 compared to placebo. No participants in either group progressed to severe COVID-19 by day 29. Adverse events were reported in two groups: leritrelvir 315 (46.46%) compared with placebo 292 (43.52%). Treatment-relevant AEs were similar 218 (32.15%) in the leritrelvir group and 186 (27.72%) in placebo. Two cases of COVID-19 pneumonia were reported in placebo group, and one case in leritrelvir group, none of them were considered by the investigators to be leritrelvir related. The most frequently reported AEs (occurring in ≥5% of participants in at least one group) were laboratory finding: hypertriglyceridemia (leritrelvir 79 [11.7%] vs. placebo 70 [10.4%]) and hyperlipidemia (60 [8.8%] vs. 52 [7.7%]); all of them were nonserious.
Interpretation
Leritrelvir monotherapy has good efficacy for mild-to-moderate COVID-19 and without serious safety concerns.
Funding
This study was funded by the National Multidisciplinary Innovation Team Project of Traditional Chinese Medicine, Guangdong Science and Technology Foundation, Guangzhou Science and Technology Planning Project and R&D Program of Guangzhou Laboratory.
Keywords: Anti SARS-CoV-2 drug, Mono-therapy, COVID-19, Drug-drug interaction, RCT
Research in context.
Evidence before this study
Leritrelvir (400 mg TID) is a novel SARS-CoV-2 3CLpro inhibitor manufactured in China, which has been conditionally approved by the China's National Medical Products Administration for treating adults with mild-to-moderate COVID-19 without administration with ritonavir. We searched PubMed for clinical trials published in any language between Jan 1, 2019 and July 1, 2023, using the search terms (“COVID-19” or “SARS-CoV-2”) AND “leritrelvir” AND (“3-chymotrypsin-like protease inhibitor” or “3C-like protease inhibitor”) AND (“clinical trial” or “randomized controlled trial”). We identified no published clinical trials on leritrelvir in patients with COVID-19.
Added value of this study
To the best of our knowledge, this study is the first randomised, double-blind, placebo-controlled, multicentre phase 3 trial that was designed to assess the efficacy and safety of leritrelvir monotherapy for adults with mild-to-moderate COVID-19. In the intention-to-treat population, the primary endpoint–the time to sustained clinical recovery of all 11 symptoms through day 29 is significantly shorter in the leritrelvir group than placebo; leritrelvir can also effectively lower the SARS-CoV-2 viral load compared to placebo. The occurrence of any adverse events was similar in leritrelvir groups and placebo.
Implications of all the available evidence
This trial shows the effectiveness of leritrelvir monotherapy for adults with mild-to-moderate COVID-19 and without serious safety concern; leritrelvir use without ritonavir can avoid similar concerns of drug–drug interactions in other 3CL protease inhibitors (nirmatrelvir/ritonavir and SIM0417/ritonavir), potentially extending indications of leritrelvir to those multi-comorbid patients, and providing another treatment option for patients with mild-to-moderate COVID-19.
Introduction
The continuing evolution of the SARS-CoV-2 virus triggers immune escape and higher infectivity,1, 2, 3, 4, 5, 6 considerably challenging existing vaccines and therapeutic antibodies. Accessible and effective antiviral therapy is essential for combating COVID-19 in the aspects of blocking viral infection, reducing viral shedding, and improving prognosis.
Various small-molecule anti-SARS-CoV-2 drugs have been developed ever since, nirmatrelvir/ritonavir is the first oral anti-SARS-CoV-2 drug recommended by WHO7 for the treatment of mild-to-moderate COVID-19 in adults who are at high risk for progression to severe COVID-19, including hospitalization or death. Nirmatrelvir is a peptidomimetic inhibitor of the SARS-CoV-2 main protease (Mpro, 3CL) and also a human cytochrome P450 (CYP) 3A4 substrate. Ritonavir, a strong cytochrome P450 (CYP) 3A4 inhibitor and a P-glycoprotein inhibitor, is co-administered with nirmatrelvir to increase the blood concentration of nirmatrelvir, thereby boosting it effective against SARS-CoV-2.8,9 Because many medications depend on CYP3A4 for clearance, precaution should be taken to avoid unexpected side effects due to drug–drug interaction when considering concomitant use with nirmatrelvir/ritonavir.10, 11, 12, 13, 14, 15, 16
Leritrelvir is a novel anti-SARS-CoV-2 drug targeting SARS-CoV-2 Mpro.17 Enzymatic inhibition kinetic analysis establishes that leritrelvir is a slow-tight inhibitor with a Ki of 8.6 nM; leritrelvir has a drug-target residence time of 104 min compared to 9 min of nirmatrelvir, suggesting approximately 12 times slower to dissociate from the protease–leritrelvir complex compared to nirmatrelvir. Crystal structure of SARS-CoV-2 Mpro: leritrelvir complex demonstrates that leritrelvir is covalently attached to the catalytic Cys 145 through the α-ketoamide warhead; more extensive interactions are identified between bound leritrelvir and Mpro active site compared to nirmatrelvir, consistent with a more stable acyl-enzyme inhibition complex for leritrelvir. In cell culture and human ACE2 transgenic mouse models, leritrelvir demonstrates comparable antiviral activity towards different SARS-CoV-2 variants as nirmatrelvir. Improvement in pharmacokinetics has been observed for leritrelvir over nirmatrelvir in various animal models, which may allow leritrelvir to be used without ritonavir. In the Phase 1 study, 400 mg leritrelvir (RAY1216) tablet TID for 5 consecutive days was well tolerated by participants and had a favorable safety profile. There are no Grade 3 or higher adverse events, serious adverse events, or dose-related adverse events. A phase 2 clinical study showed a rapid reduction in viral load and shorter virus shedding time either in the monotherapy (leritrelvir tablets 400 mg TID) or in ritonavir combination therapy (leritrelvir tablets 300 mg in co-administered with ritonavir tablets 100 mg BID); there was no statistical difference in both regimens.18 Given the evidence mentioned above, we conducted a multicentre, double-blind, randomized phase 3 clinical trial for adult patients with mild-to-moderate COVID-19 to further investigate the efficacy and safety of leritrelvir monotherapy.
Methods
Study design
This is a multicentre, randomised, double-blind, placebo-controlled phase 3 trial to assess the efficacy and safety of leritrelvir (RAY1216) monotherapy for mild-to-moderate COVID-19. The trial was done at 29 hospitals in mainland China.
The trial was conducted according to the Declaration of Helsinki, Good Clinical Practice guidelines, and the requirement of regulatory authority. The trial was approved by the National Human Genetic Resources Committee in China. Ethical approval was acquired by the institutional review board at each trial site before recruitment. This trial is registered with ClinicalTrials.gov, NCT05620160. Additional details are provided in the protocol and statistical analysis plan.
Participants
Non-hospitalized adults with mild-to-moderate COVID-19 were eligible; the definition of mild and moderate COVID-19 followed the Guidelines for the diagnosis and treatment of coronavirus disease 2019 (ninth trial version, China). Patients only showing mild clinical symptoms and no pneumonia signs on chest imaging were diagnosed as mild COVID-19. The main symptoms include fever, dry cough, and fatigue, may partially present with nasal congestion, runny nose, sore throat, decreased or lost sense of smell and/or taste, conjunctivitis, myalgia, and diarrhea. Patients were diagnosed as moderate COVID-19 when appearance of pneumonia signs on chest imaging in addition to clinical symptoms. Briefly, participants' ages were between 18 and 75 years old; the time from the first onset of symptoms of COVID-19 to randomization was ≤48 h; at least one of the symptoms of COVID-19 remained before randomization; SARS-CoV-2 nucleic acid test (NAT) remained positive within 48 h before randomization; the time from the first positive viral NAT to randomization was ≤120 h. Exclusion criteria include severe or critical patient's condition who required hospitalization at enrollment, or expected to develop severe or critical disease and required hospitalization within 48 h after randomisation; active liver disease or significant liver function abnormalities; undergoing dialysis or moderate to severe renal function impairment. Written informed consent was obtained from all participants. Full detailed inclusion and exclusion criteria are provided in Supplementary Appendix. Gender data were collected from self-report, and all participants provided informed consent.
Randomisation and masking
Eligible participants were randomly assigned in a 1:1 ratio to either leritrelvir group or placebo group. Randomization was stratified according to mild/moderate COVID-19 clinical classification and whether with high-risk developing into severe/critical condition (yes/no). The permuted block (6 patients per block) randomisation sequence, including stratification, was prepared and upload to the interactive Network Response System (IWRS) by non-blind statistician who had no involvement in the rest of the trial. Authorized persons assigned participants to the trial groups through the IWRS after participants were enrolled by investigators. Participants and investigators were masked, except the non-blind statistician, with the tablets with identical appearance. Interventions were executed based on the patients and drug randomized numbers. Additional details are provided in the Supplementary Appendix.
Procedures
Participants with mild-to-moderate COVID-19 were randomly to receive orally either leritrelvir 400 mg, TID or placebo for 5 days and a 24-day follow-up. Leritrelvir was manufactured and provided by Guangdong Raynovent Biotech Co., Ltd. (Figure S1 in the Supplementary Appendix)
Assessments at prespecified time points included vital sign recording, physical examination, 12-lead electrocardiogram examination, clinical laboratory examination (during the screening period, day 6 ± 1, and day 29 ± 3 after the first dose), collection of nasopharyngeal swabs to quantify the SARS-CoV-2 viral load with reverse transcriptase-polymerase chain reaction (RT-PCR) assay (at day 4, 6, 10 and 15), and reporting AEs (from signing the informed consent form to the last visit or within 28 days after the first dose for patients who were withdrawn early from the trial). Participants recorded scores of COVID-19-related clinical symptoms (on a scale of 0–3, the highest score indicates symptoms were the most severe) by using the questionnaire (Table S1). In detail, participants completed the first questionnaire before the first dose, then three times daily from day 1 to day 10, and once daily from day 11 to day 28. The full schedule of trial procedures is provided in the protocol. Chest imaging would be ordered by physician during the trial as required.
Outcomes
The primary outcome is the time from the first dose to sustained clinical recovery of all 11 symptoms through day 29 (stuffy or runny nose, sore throat, shortness of breath or dyspnea, cough, muscle or body aches, headache, chills, fever, nausea, vomiting, and diarrhea). Sustained clinical recovery is defined as the recovery of 11 symptoms to a total score of 0 for the sum of each symptom and recovery has to be maintained at least 72 h.
The secondary outcomes include all-cause mortality, the proportion of participants hospitalized or dying from disease progression, the proportion of participants hospitalized or dying from COVID-19-related disease progression, the change of viral load from baseline, the proportion of participants with the negative detection of SARS-CoV-2, time to sustained clinical remission [defined as the time from the first dose to the first observed clinical sustained remission, which refers to the reduction of severity of 11 COVID-19 symptoms one point from the initial records on a scale from 0 (normal) to 3 (most severe), at least 72 h], the proportion of participants with sustained clinical recovery/remission, time from the first dose to sustained recovery/remission for a single symptom. Safety outcome is the incidence of AEs, with severity determined according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0. Any AE that emerged or worsened from the time of informed consent to the last visit was actively recorded and reported to participants. Details of the endpoints are provided in the Supplementary Appendix (Table S2).
Statistical analysis
The original design required a total of 1049 events across both groups, which would provide 90% power under two-sided type I error of 0.05 if the median time to sustained clinical recovery is 264 h (11 days) and 216 h (9 days) in leritrelvir group, following an exponential distribution.
The primary efficacy was assessed in the intention-to-treat (ITT) population (all the participants assigned to treatment). We present adverse event data on the patients’ actual treatment exposure, coded using Medical Dictionary for Regulatory Activities.
Participants who taking contraindicated medications, switching to other treatments, early withdrawing, and disease progressed during the trial was considered as censored at day 28.
The primary outcome, time to sustained clinical recovery, was portrayed by Kaplan–Meier plot and compared with a log-rank test with baseline mild/moderate clinical classification, and with/without high risk factors for severe/critical COVID-19 as stratification factors. The HR and 95% CI for time to sustained clinical recovery was calculated by Cox proportional hazards model. Proportional hazards (PH) violations was assessed by scaled Schoenfeld residuals by using SAS9.4. For a given covariate, the PH assumption is performed by finding the correlation between the Schoenfeld residuals for a particular covariate and the ranking of individual failure times. If the PH assumption is met, then the correlation should be near zero. The null hypothesis is that the PH assumption is not violated. The results of unstratified Log-rank test and Per-Protocol Set (PPS) were displayed in the sensitivity analysis.
Subgroup analyses of the primary outcome were conducted by baseline clinical type (mild, common), whether at high risk for heavy/critical disease (yes or no), age (≤65 and 65–75),gender (male, female), presence of clinical symptoms in a system (respiratory system (yes or no), gastrointestinal system (yes or no), systemic symptoms (yes or no), vaccination status (yes or no), symptoms/signs duration of illness (≤24 h, 24–48 h), viral nucleic acid positive disease duration (≤72 h, 72–120 h).
Post hoc comparison on certain subgroups was carried out based on these baseline symptoms/signs: (a) single symptom score ≥2 or the score of shortness of breath or dyspnea ≥1; (b) respiratory rate ≥20 breaths/minute or heart rate ≥90 beats/minute, and saturation of oxygen (SpO 2) > 93% on room air at sea level. Fulfillment of both (a) and (b) denoted moderate condition otherwise was mild condition. Additional details were provided in the statistical analysis plan.
Interim analysis was monitored by the independent data monitoring committee. Statistical analyses were done using SAS software, version 9.4.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
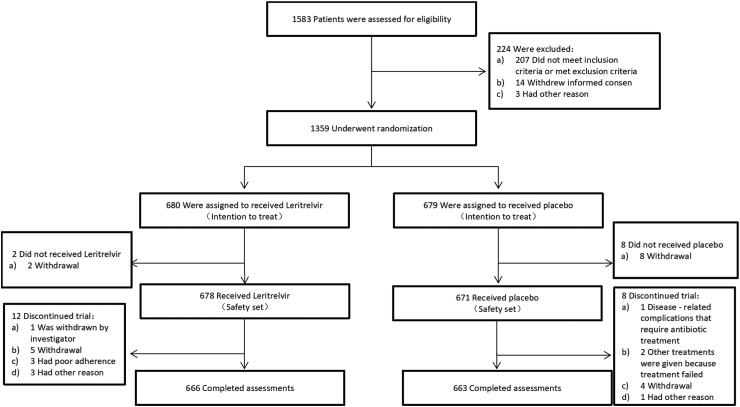
A total of 1583 SARS-CoV-2 infected participants were screened between Nov 12, 2022 and Dec 30, 2022 from 29 clinical sites in China, during the Omicron variant outbreak of COVID-19. A total of 1359 participants were enrolled and randomly assigned to receive leritrelvir (N = 680) or placebo (N = 679). The data-cutoff date for this report was March 28, 2023. After randomisation, ten people withdraw their informed consent before medication so that they were not included in the safety analysis. During the study, 12 participants in the leritrelvir group and 8 participants in the placebo group discontinued the trial (Fig. 1).
Fig. 1.
Trial profile. Eligible participants were randomly assigned in a 1:1 ratio to either the leritrelvir (RAY1216) group or the placebo group, and underwent a 5-day drug administration and 24-day follow-up. Assessments at prespecified time points included vital sign recording, physical examination, 12-lead electrocardiogram examination, clinical laboratory examination (during the screening period, day 6 ± 1, and day 29 ± 3 after the first dose), collection of nasopharyngeal swabs to quantify the SARS-CoV-2 viral load with reverse transcriptase-polymerase chain reaction (RT-PCR) assay (at day 4, 6, 10 and 15), and reporting AEs (from signing the informed consent form to the last visit or within 28 days after the first dose for patients who were withdrawn early from the trial).
The clinical characteristics of the intention-to-treat population at baseline were balanced between leritrelvir and placebo (Table 1). The median age of participants in leritrelvir group was 32.0 years (range: 18.0–73.0 years, IQR 26.0–39.0 years), vs. 31.0 years in placebo (range: 18.0–70.0 years, IQR 26.0–38.0 years), and approximately half were female. 1252 participants (92.1%) were diagnosed as mild COVID-19 and 1338 participants (98.5%) were vaccinated (at least had one vaccination). The time from confirmed SARS-CoV-2 infection by RT-PCR to trial-randomization for most participants was ≤72 h (660 [97.1%] in leritrelvir group, 654 [96.3%] in placebo). The most common risk factors for potential progression to severe/critical COVID-19 were underlying diseases (54, 37.0%), mainly cardiovascular system diseases, respiratory diseases, hepatobiliary diseases, urinary system diseases), followed by obesity (body mass index ≥30 kg/m2) (37, 25.3%), heavy smoker (33, 22.6%), >60 years (15, 10.3%), and other risk factors (7, 4.8%). The median viral load at enrollment was 7.5 (IQR 6.2, 8.3) log10 Copies/mL (Table 1). Medication adherence was similar in two groups (Table S6).
Table 1.
Demographic and clinical characteristics of the intention-to-treat population.a
| Characteristic | Leritrelvir (N = 680) | Placebo (N = 679) | Total (N = 1359) |
|---|---|---|---|
| Median age at randomization (IQR) — years | 32.0 (26.0, 39.0) | 31.0 (26.0, 38.0) | 31.0 (26.0, 39.0) |
| Mean age (Range)— years | 33.7 (18, 73) | 33.4 (18, 70) | 33.6 (18, 73) |
| Sex — no. (%) | |||
| Male | 347 (51.0) | 323 (47.6) | 670 (49.3) |
| Female | 333 (49.0) | 356 (52.4) | 689 (50.7) |
| Ethnic group — no. (%) | |||
| Han | 653 (96.0) | 652 (96.0) | 1305 (96.0) |
| Other | 27 (4.0) | 27 (4.0) | 54 (4.0) |
| Vaccination status — no. (%) | |||
| Vaccinatedb | 672 (98.8) | 666 (98.1) | 1338 (98.5) |
| Unvaccinated | 8 (1.2) | 13 (1.9) | 21 (1.5) |
| COVID-19 severity — no. (%) | |||
| Mild | 627 (92.2) | 625 (92.0) | 1252 (92.1) |
| Moderate | 53 (7.8) | 54 (8.0) | 107 (7.9) |
| Time from onset of COVID-19 symptoms to randomization — no. (%)c | |||
| ≤24 h | 64 (9.4) | 59 (8.7) | 123 (9.1) |
| >24 h | 616 (90.6) | 620 (91.3) | 1236 (90.9) |
| Participants with potential progression to severe/critical COVID-19 — no. (%) | |||
| High-risk group | 71 (10.4) | 75 (11.0) | 146 (10.7) |
| Non-high-risk group | 609 (89.6) | 604 (89.0) | 1213 (89.3) |
| Risk factors for severe illness of COVID-19 — no. (%)d | |||
| Age of >60 years old | 8 (11.3) | 7 (9.3) | 15 (10.3) |
| Underlying diseasee | 22 (31.0) | 32 (42.7) | 54 (37.0) |
| Obesity (BMI ≥30) | 15 (21.1) | 22 (29.3) | 37 (25.3) |
| Heavy smoker | 21 (29.6) | 12 (16.0) | 33 (22.6) |
| Other risk factors | 5 (7.0) | 2 (2.7) | 7 (4.8) |
| Time from first RT-PCR confirmation of SARS-CoV-2 infection to trial randomization — no. (%) | |||
| ≤72 h | 660 (97.1) | 654 (96.3) | 1314 (96.7) |
| >72 h | 20 (2.9) | 25 (3.7) | 45 (3.3) |
| Viral load at baseline — log10 Copies/mL (IQR) | 7.5 (6.1–8.3) | 7.5 (6.4–8.3) | 7.5 (6.2–8.3) |
Shown are participants who underwent randomization. Participants were grouped according to treatment assignment. Summation of percentages may not be equal to 100 because of rounding. COVID-19 denotes coronavirus disease 2019, BMI body mass index, RT-PCR reverse-transcriptase–polymerase chain reaction, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
Patients have received at least one dose of COVID-19 vaccine.
The period was based on data collected at randomization.
Multiple high-risk factors could exist in the same participant. The percentage was calculated using the number of high-risk groups as the denominator. Heavy smoker was defined as patients with a smoking index (number of cigarettes smoked per day multiplied by the number of years smoked)≥ 400. Other risk factors: In addition to the factors mentioned above in Table 1, other medical conditions or factors judged by researchers that may cause patients to progress to severe/critical COVID-19.
Include cardiovascular and cerebrovascular diseases (including hypertension), chronic lung diseases, diabetes, chronic liver/kidney diseases, tumor, and other basic diseases.
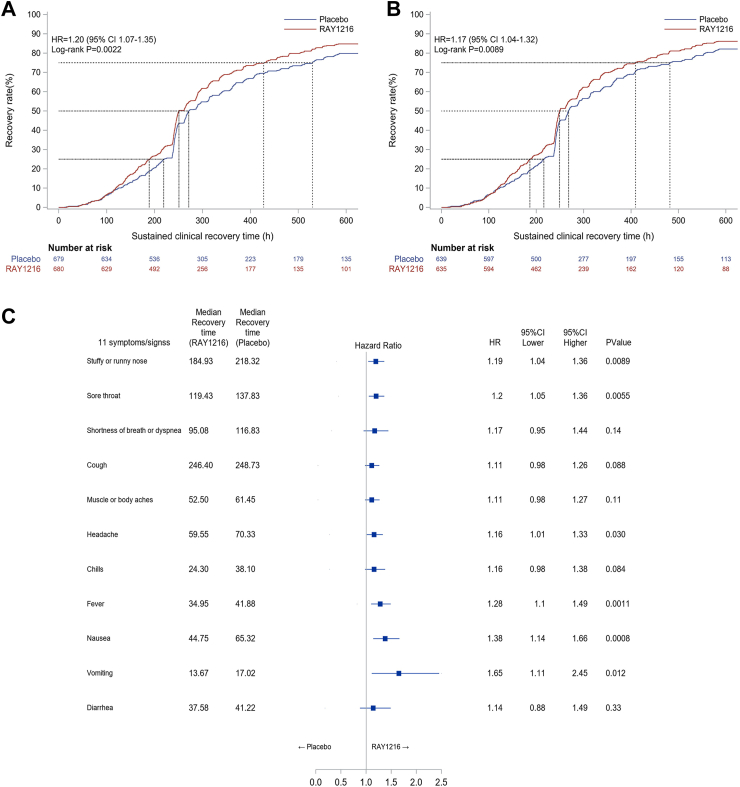
PH assumption was not violated. The primary outcome is the time from the first dose to sustained clinical recovery of all 11 symptoms through day 29. Time to sustained clinical recovery in patients assigned to leritrelvir vs. placebo was significantly different (median, 251.02 h vs. 271.33 h; difference: 20.31 h; HR: 1.20 [95% CI, 1.06–1.35]; P = 0.0022 by log-rank test; Fig. 2A, Table 2). Consistent results were also found in the sensitive analysis (Table S8). In the per-protocol population, data were analyzed for 635 participants in the leritrelvir group and 639 in placebo. A significant difference was also observed in time to sustained clinical recovery between the two groups (median: 249.18 h vs. 268.10 h, difference: 18.92 h; HR: 1.17 [95% CI, 1.04–1.32]; P = 0.0089 by log-rank test; Fig. 2B and Table S9).
Fig. 2.
Time to Sustained Clinical Recovery. Panel A. Sustained Clinical Recovery, Intention-To-Treat Population (N = 1359). The 95% confidence intervals were estimated using the Greenwood method based on log–log transformation. Hazard ratios were calculated using Cox proportional hazards model. A lower boundary of the two-sided 95% confidence interval for the hazard ratio of more than 1 is considered to indicate superiority. Panel B. Sustained Clinical Recovery, Per-Protocol Population (N = 1274). Panel C. Forest plot of the time to sustained clinical recovery for separately calculated 11 Covid-19 related symptoms in intention-to-treat population. Shown are final analysis (data cutoff on May 15, 2023) of the estimated time to a negative transformation of SARS-CoV-2 by Kaplan–Meier method for each treatment group in the intention-to-treat population (N = 1359). 95% CI is estimated the Greenwood method with log-log transformation. Hazard ratio is calculated using the COX proportional hazards model. CI = confidence interval.
Table 2.
Outcome measures of primary and secondary endpoints (Intention to treat population).a
| Endpoint | Leritrelvir (N = 680) | Placebo (N = 679) | P-value |
|---|---|---|---|
| Primary endpointa | |||
| Time to sustained clinical recovery — hours, Median (IQR) | 251.02 (188.95, 428.68) | 271.33 (219.00, 529.63) | 0.0022 |
| Hazard ratio vs. placebo (95% CI) | 1.20 (1.07, 1.35) | ||
| Secondary endpoints | |||
| The disease progresses to hospitalization or death by day 29 — no. (%) | 0 | 0 | |
| All-cause mortality by day 29 — no. (%) | 0 | 0 | |
| Hospitalization or all-cause death from the progression of COVID-19-related illness by day 29 — no. (%) | 0 | 0 | |
| Change in SARS-CoV-2 viral load from baselineb | |||
| Viral load at baseline — Median (IQR), log10 Copies/mL | 7.5 (6.1–8.3) | 7.5 (6.4–8.3) | |
| Day 4 | <0.0001 | ||
| LSmean (SD), log10 Copies/mL | −2.25 (0.09) | −1.43 (0.09) | |
| Geometric mean ratio (95% CI) | 0.14 (0.10, 0.20) | ||
| Day 6 | <0.0001 | ||
| LSmean (SD), log10 Copies/mL | −3.28 (0.10) | −2.84 (0.10) | |
| Geometric mean ratio (95% CI) | 0.35 (0.24, 0.51) | ||
| Day 10 | 1.00 | ||
| LSmean (SD), log 10 Copies/mL | −4.61 (0.09) | −4.58 (0.09) | |
| Geometric mean ratio (95% CI) | 0.88 (0.63, 1.24) | ||
| Day 15 | 1.00 | ||
| LSmean (SD), log 10 Copies/mL | −5.44 (0.07) | −5.39 (0.07) | |
| Geometric mean ratio (95% CI) | 0.90 (0.65, 1.24) | ||
| Proportion of participants with SARS-CoV-2 virus turned negative (95% CI) (%) | |||
| Day 4 | 5.95 (4.24, 8.07) | 4.10 (2.70, 5.95) | 0.14 |
| Day 6 | 16.28 (13.50, 19.37) | 10.09 (7.86, 12.71) | 0.0011 |
| Day 10 | 36.93 (33.18, 40.81) | 32.33 (28.70, 36.13) | 0.084 |
| Day 15 | 66.04 (62.22, 69.71) | 59.46 (55.53, 63.31) | 0.016 |
| Time from the first dose to sustained clinical remissionc | |||
| Median time (IQR) — hours | 243.33 (158.58, 368.78) | 249.42 (171.43, 431.15) | 0.011 |
| Hazard ratio vs. placebo (95% CI) | 1.16 (1.03, 1.30) | ||
| Participants in clinical recovery (95% CI) by date (%) | |||
| Day 7 | 20.88 (17.89, 24.13) | 15.76 (13.10, 18.72) | 0.014 |
| Day 14 | 66.18 (62.48, 69.73) | 58.32 (54.51, 62.06) | 0.0029 |
| Day 21 | 79.12 (75.87, 82.11) | 72.90 (69.39, 76.21) | 0.0073 |
| Day 28 | 83.68 (80.68, 86.38) | 79.23 (75.99, 82.23) | 0.035 |
| Participants in clinical remission (95% CI) by date (%) | |||
| Day 7 | 29.12 (25.73, 32.69) | 23.86 (20.70, 27.25) | 0.028 |
| Day 14 | 70.44 (66.85, 73.85) | 64.51 (60.78, 68.11) | 0.021 |
| Day 21 | 82.94 (79.90, 85.69) | 78.06 (74.75, 81.12) | 0.024 |
| Day 28 | 86.91 (84.14, 89.36) | 83.21 (80.18, 85.95) | 0.057 |
The primary outcome, time to sustained clinical recovery, was portrayed by Kaplan–Meier plot and compared with a log-rank test with baseline mild/moderate clinical classification. The statistical method of secondary outcome could be seen in the protocol. CI. denotes confidence interval. Hazard ratios were calculated by means of a Cox proportional-hazards model. A hazard ratio of more than 1 suggests that participants receiving leritrelvir had a shorter time to sustained clinical recovery or sustained clinical remission than those receiving a placebo.
Change in SARS-CoV-2 viral load from baseline refers to difference in viral load (log) between the baseline and at each time point after drug administration. Difference was compared by mixed-effect models for repeated measures (MMRM) with the group, time-point, the interaction of group and time-point, baseline and randomized stratification factors as a fixed effect, and adjusted with Bonferroni correction.
The time to sustained clinical remission was defined as the time from the first dose to the first observation of sustained clinical remission. Sustained clinical remission was defined as the remission of 11 symptoms to normal (the symptoms that were severe or moderate at baseline were relieved to mild or normal, and the symptoms that were mild at baseline were returned to normal; those that were evaluated as 0 times or none were considered normal), and the duration is at least 72 h.
Compared with the placebo group, participants in leritrelvir group also reduced time to sustained recovery of 11 symptoms in these subgroups: (a) without risk factors for developing into severe/critical COVID-19 (250.88 h vs. 270.93 h, difference: 20.05 h, HR = 1.20 [95% CI: 1.06–1.36]); (b) with a positive viral NAT results within 72 h (251.02 h vs. 284.92 h, difference: 33.90 h, HR = 1.21 [95% CI: 1.08–1.37]); (c) baseline viral load >8 log10 Copies/mL (262.22 h vs. 313.55 h, difference: 51.33 h, HR = 1.36 [95% CI: 1.10–1.67]); (d) the full vaccinated (250.18 h vs. 290.12 h, difference: 39.94 h, HR = 1.35 [95% CI: 1.04–1.75]) and received booster shot (261.83 h vs. 268.65 h, difference: 6.82, hours HR = 1.17 [95% CI: 1.02–1.34]. In the subgroup of participants with risk factors for developing into severe/critical COVID-19 and the subgroup of participants with symptoms onset ≤24 h, leritrelvir demonstrated a trend toward shorter median time to sustained clinical recovery relative to placebo, although not statistically significant (Figure S3).
No participants in either group were progressed to severe/critical COVID-19, hospitalized, or died (Table 2). Compared with placebo, leritrelvir reduced viral load by 0.82 log10 Copies/mL on day 4. The proportion of participants with negative SARS-CoV-2 tests were higher in leritrelvir group than that in placebo on day 6 and day 15 (Table 2). The sustained clinical recovery rate after 1 week treatment in leritrelvir group was higher than placebo (142 [20.88%] vs. 107 [15.76%], P = 0.014), and much higher was seen after two weeks treatment (450 [66.18%] vs. 396 [58.32%], P = 0.0029) (Table 2). Further looking into individual symptoms, compared with placebo, leritrelvir effectively shortened the time from the first dose to sustained clinical recovery (P < 0.05) on stuffy or running nose, sore throat, headache, fever, nausea, and vomiting (Fig. 2C and Table S10).
The percentage of patients experiencing any AEs were similar in two groups: 315 (46.46%) in leritrelvir group and 292 (43.52%) in placebo (Table 3). Similar results were seen in the treatment-relevant AEs: 218 (32.15%) in leritrelvir group and 186 (27.72%) in placebo (Table 3). Three serious AEs (COVID-19 pneumonia and scalp hematoma) were reported in three participants in placebo group; one case of COVID-19 pneumonia was reported in the leritrelvir group; none of them were considered by the investigators to be related to leritrelvir (Table 3). The most frequently reported AEs (occurring in ≥5% participants in at least one group) were laboratory finding: hypertriglyceridemia (79 [11.7%] with the leritrelvir and 70 [10.4%] with placebo), hyperlipidemia (60 [8.8%] and 52 [7.7%], respectively) (Table S14); all of them were nonserious.
Table 3.
Adverse events safety population.a
| Adverse Event-no. (%) | Leritrelvir (N = 678) | Placebo (N = 671) |
|---|---|---|
| Adverse events overall | ||
| Any adverse event | 315 (46.46) | 292 (43.52) |
| Serious adverse event | 1 (0.15) | 3 (0.45) |
| The adverse event leading to the discontinuation of the trial regimen | 2 (0.29) | 1 (0.15) |
| Adverse events considered by the investigator to be related to the assigned regimen | ||
| Any adverse event | 218 (32.15) | 186 (27.72) |
| Serious adverse event | 0 | 0 |
All the adverse events as coded according to the Medical Dictionary for Regulatory Activities, version 25.1 (Chinese), were recorded from the time of signing the consent form through 28-day follow-up. Participants were those who received at least one dose of the study intervention.
We further performed some post hoc analyses. Participants with mild condition (see definition in Statistical analysis section) in leritrelvir group only had 1/43 patients (2.33%) presenting pneumonia imaging progression compared to 5/57 patients in placebo (8.77%). Patients with moderate condition in leritrelvir group had shorter median time to sustained clinical recovery than those in placebo (267.45 h vs. 296.65 h; difference: 29.20 h; HR: 1.22 [95% CI 1.03–1.44]; Table S13).
Discussion
Although the number of COVID-19 cases and casualty worldwide has recently declined greatly, there is still considerable number of infections and deaths.19 More safe and efficacious COVID-19 antiviral drugs are urgently needed.
Our results demonstrated that this oral leritrelvir regimen without ritonavir booster (400 mg three times a day for 5 days) significantly shortened the time to sustained clinical recovery of all 11 COVID-19 clinical symptoms by nearly 1 day (20.3 h) for mild-to-moderate COVID-19 patients compared with placebo. The effectiveness of leritrelvir is also supported from the time to sustained clinical recovery of individual symptoms: compared with placebo, the time to sustained clinical recovery in leritrelvir group was significantly shorter for systemic symptoms (fever, headache), respiratory symptoms (stuffy or runny nose, sore throat), and digestive symptoms. No participants with high risk of potential progression to severe/critical COVID-19 (71, 10%) developed severe disease by day 29 in both leritrelvir and placebo groups.
Treatment with leritrelvir showed significantly reduction of SARS-CoV-2 viral load by nearly 10 times after 3-day treatment compared with placebo; this result is similar to that in nirmatrelvir/ritonavir trial,20 and is one day shorter achieving the same goal. In the subgroup analysis, we noticed that patients with higher baseline viral load (>8 log10 Copies/mL) had shorter time to sustained clinical recovery; leritrelvir treatment of the patients with symptoms onset ≤24 h showed a propensity (although not statistically significant, probably due to the subgroup small size) toward shorter median time to sustained clinical recovery relative to placebo, suggesting that leritrelvir could help mitigate symptoms and shorten the course of disease if starting treatment promptly.
It is worth noting that previous interim analyses of nirmatrelvir/ritonavir in the EPIC-SR (Evaluation of Protease Inhibition for COVID-19 in Standard-Risk Patients) phase 2/3 study, in which unvaccinated adults with low risk of hospitalization or death and vaccinated adults with one or more risk factors for progressing to severe illness were enrolled, showed that nirmatrelvir/ritonavir treatment achieved sustained alleviation of all symptoms for four consecutive days, but compared to placebo, was not significantly different.21
With regard to safety, there were no leritrelvir-related severe AEs. Participants in leritrelvir group had a similar incidence of AEs to those in placebo. Patients in leritrelvir group did not have dysgeusia, and dry mouth as listed in nirmatrelvir/ritonavir fact sheet,22 and the main AEs of the enrolled patients were mild and no intervention required. During the trial period, hypertriglyceridemia, hyperlipidemia and hyperuricemia were found in both leritrelvir group and placebo without significant difference. Dyslipidemia is not uncommon during acute and after SARS-CoV-2 infection. Dyslipidemia is also found in another clinical trial with ensitrlvir, a small-molecule antiviral targeting the 3C-like (3CL) protease of SARS-CoV-2.23,24 The underlying causes of these laboratory findings might be SARS-CoV-2 infection-related,25 and further investigation is required.
At present, all 3CL protease inhibitors, conditionally approved in China (nirmatrelvir/ritonavir and SIM0417/ritonavir)26 require co-administration with ritonavir. Drug-drug interactions (DDIs) are a major constraint due to ritonavir's potent inhibition of CYP3A4 and, to a lesser extent, of CYP2D6.27 Therefore, many drugs are prohibited or under vigilant supervision when concomitant use with nirmatrelvir/ritonavir, such as some cardiovascular system drugs, anti-infective agents, immunosuppressants, antineoplastic agents, digestive system drugs, bronchodilators, and etc.27; individuals who use these drugs are those at high risk for progression to severe COVID-19. Leritrelvir without booster ritonavir exhibits good pharmacokinetic characteristics in both plasma stability and metabolism in vivo, thus avoiding similar concerns of DDIs and potentially extending indications to those patients with multiple comorbidities.
The trial was executed during the first outbreak of COVID-19 after strict prevention and control measures were abandoned in China. Participants in both leritrelvir and placebo groups were well balanced in terms of age, vaccination, symptoms baseline and SARS-CoV-2 viral load, etc. The trial has some limitations. Firstly, most participants were relatively young (≤65 years old), vaccinated, and with mild COVID-19, it is therefore difficult to compare these data with other anti-SARS-COV-2 drugs in the literature. The reasons underlying this observation might be related to participants in these subgroups had better immunity and less inflammatory responses to the infection, thus making them more benefit from leritrelvir. Secondly, the proportion of high-risk participants for severe/critical COVID-19 is small (146, 10.7%); although no significant difference was observed in time to sustained clinical recovery between leritrelvir and placebo groups, but the propensity existed (leritrelvir, 264.83 h vs. placebo, 289.22 h). Thirdly, all participants are Asian. Although the result is statistically significant, the clinical improvements efficacy of leritrelvir are predominantly for COVID-19 patients with mild symptoms and low risk for adverse consequences for now. Further study of leritrelvir efficacy on high-risk population, patients with long COVID and other races in a larger scale will be initiated in the near future.
In summary, leritrelvir monotherapy with the defined regimen in this trial not only reveals good efficacy for treating mild-to moderate COVID-19 patients, but also has significant antiviral efficacy. Indications of leritrelvir can expand to include those COVID-19 patients having DDI concerns with ritonavir.
Contributors
Conceived study: Nanshan Zhong, Jingping Zheng, Zifeng Yang, Designed study and experiments: Yangqing Zhan, Zifeng Yang, Nanshan Zhong.
Performed experiments: Yangqing Zhan, Nanshan Zhong, Jingping Zheng, Yueping Li, Hongzhou Lu, Ling Lin, Liang Su, Tianxin Xiang, Hongqiu Pan, Chaolin Huang, Ying Deng, Furong Wang, Ruhong Xu, Bingliang Lin, Ruilin Sun, Fangqi Ge, Dexiong Chen, Ping Zhang, Jianlin Tong, Xifu Wang, Qingwei Meng, Zhigang Zheng, Shuqiang Ou, Xiaoyun Guo, Herui Yao, Tao Yu, Weiyang Li, Yu Zhang.
Interpreted data: Zhengshi Lin, Nanshan Zhong, Yangqing Zhan, Jingyi Liang, Zhonghao Fang, Shiwei Liang, Haijun Li.
Manuscript preparation: Zhengshi Lin, Chuanmeizi Tu, Qianying Li, Zifeng Yang, Nanshan Zhong, Ruifeng Chen, Jingyi Liang, Zhonghao Fang, Jincan Luo, Yudi Song, Changyuan Kang, Mei Jiang.
Jingyi Liang, Zhonghao Fang, Shiwiei Liang, Zhengshi Lin have accessed and verified the data, and Zifeng Yang, Jingping Zheng, Nanshan Zhong were responsible for the decision to submit the manuscript.
Data sharing statement
After approval from Human Genetic Resource Adminstration of China and Guangdong Raynovent Biotech Co., Ltd., this trial data can be shared with qualifying researchers who submit a valuable research question.
Declaration of interests
No potential conflicts of interest were reported by the authors.
Acknowledgements
We thank the trial participants, their families, and all investigators involved in this trial (listed in the Supplementary Appendix) for their contributions in conducting this trial. This work was supported by National Multidisciplinary Innovation Team Project of Traditional Chinese Medicine (ZYYCXTD-D-202201); Guangdong Science and Technology Foundation (2022B1111060003); Guangzhou Science and Technology Planning Project (2022B01W0001 and 202102100003); R&D Program of Guangzhou Laboratory (TL22-13). We would like to acknowledge the following additional collaborative institutes: Jiangmen Central Hospital, Guangdong Province, P.R. China. (Huang Yanming, M.M); Guangzhou Chest Hospital, Guangdong Province, P.R. China. (Dong Haiping, M.D.); Nanfang Hospital, Southern Medical University, Guangdong Province, P.R. China. (Jinlin Hou, M.M); Jinan Central Hospital, Shandong Province, P.R. China. (Shao Lei, M.D.); Peking University Third Hospital, Bejing City, P.R. China. (Li Xiaoguang, M.D.); Peking University People's Hospital, Bejing City, P.R. China. (Gao Yan, M.M.)
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102359.
Contributor Information
Jingping Zheng, Email: jpzhenggy@163.com.
Nanshan Zhong, Email: nanshan@vip.163.com.
Zifeng Yang, Email: jeffyah@163.com.
Appendix A. Supplementary data
References
- 1.Davies N.G., Abbott S., Barnard R.C., et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;(6538):372. doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao Y., Wang J., Jian F., et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602(7898):657–663. doi: 10.1038/s41586-021-04385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao Y., Yisimayi A., Jian F., et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022;608(7923):593–602. doi: 10.1038/s41586-022-04980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui Z., Liu P., Wang N., et al. Structural and functional characterizations of infectivity and immune evasion of SARS-CoV-2 Omicron. Cell. 2022;185(5):860–871.e13. doi: 10.1016/j.cell.2022.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Q., Guo Y., Iketani S., et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature. 2022;608(7923):603–608. doi: 10.1038/s41586-022-05053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Y., Jian F., Wang J., et al. Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. Nature. 2023;614(7948):521–529. doi: 10.1038/s41586-022-05644-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NIH . 2022. Therapeutic management of nonhospitalized adults with COVID-19.https://www.covid19treatmentguidelines.nih.gov/ Available from: [Google Scholar]
- 8.Owen D.R., Allerton C.M.N., Anderson A.S., et al. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374(6575):1586–1593. doi: 10.1126/science.abl4784. [DOI] [PubMed] [Google Scholar]
- 9.Lamb Y.N. Nirmatrelvir plus ritonavir: first approval. Drugs. 2022;82(5):585–591. doi: 10.1007/s40265-022-01692-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young C., Papiro T., Greenberg J.H. Elevated tacrolimus levels after treatment with nirmatrelvir/ritonavir (Paxlovid) for COVID-19 infection in a child with a kidney transplant. Pediatr Nephrol. 2023;38(4):1387–1388. doi: 10.1007/s00467-022-05712-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li G.F., Yu G. Drug-induced liver injury with ritonavir-boosted nirmatrelvir: evidence from coronavirus disease 2019 emergency use authorization adverse event reporting system. Gastroenterology. 2023;165:305. doi: 10.1053/j.gastro.2023.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hopkins A.M., Sorich M.J., McLachlan A.J., et al. Understanding the risk of drug interactions between ritonavir-containing COVID-19 therapies and small-molecule kinase inhibitors in patients with cancer. JCO Precis Oncol. 2023;7 doi: 10.1200/PO.22.00538. [DOI] [PubMed] [Google Scholar]
- 13.Haque O.I., Mahar S., Hussain S., Sloane P. Pharmacokinetic interaction between verapamil and ritonavir-boosted nirmatrelvir: implications for the management of COVID-19 in patients with hypertension. BMJ Case Rep. 2023;16(1) doi: 10.1136/bcr-2022-252677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girardin F., Manuel O., Marzolini C., Buclin T. Evaluating the risk of drug-drug interactions with pharmacokinetic boosters: the case of ritonavir-enhanced nirmatrelvir to prevent severe COVID-19. Clin Microbiol Infect. 2022;28(8):1044–1046. doi: 10.1016/j.cmi.2022.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heskin J., Pallett S.J.C., Mughal N., et al. Caution required with use of ritonavir-boosted PF-07321332 in COVID-19 management. Lancet. 2022;399(10319):21–22. doi: 10.1016/S0140-6736(21)02657-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Focosi D., McConnell S., Shoham S., Casadevall A., Maggi F., Antonelli G. Nirmatrelvir and COVID-19: development, pharmacokinetics, clinical efficacy, resistance, relapse, and pharmacoeconomics. Int J Antimicrob Agents. 2023;61(2) doi: 10.1016/j.ijantimicag.2022.106708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X., Huang X., Ma Q., et al. Inhibition mechanism and antiviral activity of an α-ketoamide based SARS-CoV-2 main protease inhibitor. bioRxiv. 2023 doi: 10.1101/2023.03.09.531862. [DOI] [Google Scholar]
- 18.Wang B., Li H.J., Cai M.M., et al. Antiviral efficacy of RAY1216 monotherapy and combination therapy with ritonavir in patients with COVID-19: a phase 2, single centre, randomised, double-blind, placebo-controlled trial. eClinicalMedicine. 2023;63 doi: 10.1016/j.eclinm.2023.102189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO . 2023. China: WHO coronavirus (COVID-19) dashboard. [Google Scholar]
- 20.Hammond J., Leister-Tebbe H., Gardner A., et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19. N Engl J Med. 2022;386(15):1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfizer announces additional phase 2/3 study results confirming robust efficacy of novel COVID-19 oral antiviral treatment candidate in reducing risk of hospitalization or death. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-additional-phase-23-study-results Available from:
- 22.Fact Sheet For Healthcare Providers . 2023. Emergency use authorization for paxlovid. [Google Scholar]
- 23.Mukae H., Yotsuyanagi H., Ohmagari N., et al. Efficacy and safety of ensitrelvir in patients with mild-to-moderate COVID-19: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study. Clin Infect Dis. 2022;76:1403. doi: 10.1093/cid/ciac933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukae H., Yotsuyanagi H., Ohmagari N., et al. A randomized phase 2/3 study of ensitrelvir, a novel oral SARS-CoV-2 3C-like protease inhibitor, in Japanese patients with mild-to-moderate COVID-19 or asymptomatic SARS-CoV-2 infection: results of the phase 2a part. Antimicrob Agents Chemother. 2022;66(10) doi: 10.1128/aac.00697-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu E., Xie Y., Al-Aly Z. Risks and burdens of incident dyslipidaemia in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2023;11(2):120–128. doi: 10.1016/S2213-8587(22)00355-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Another domestic COVID-19 oral drug was conditionally approved for marketing, which is the same 3CL protease inhibitor route as Pfizer Paxlovid 2023. https://baijiahao.baidu.com/s?id=1761163369102573940&wfr=spider&for=pc Available from:
- 27.Marzolini C., Kuritzkes D.R., Marra F., et al. Recommendations for the management of drug-drug interactions between the COVID-19 antiviral nirmatrelvir/ritonavir (Paxlovid) and comedications. Clin Pharmacol Ther. 2022;112(6):1191–1200. doi: 10.1002/cpt.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.