Abstract
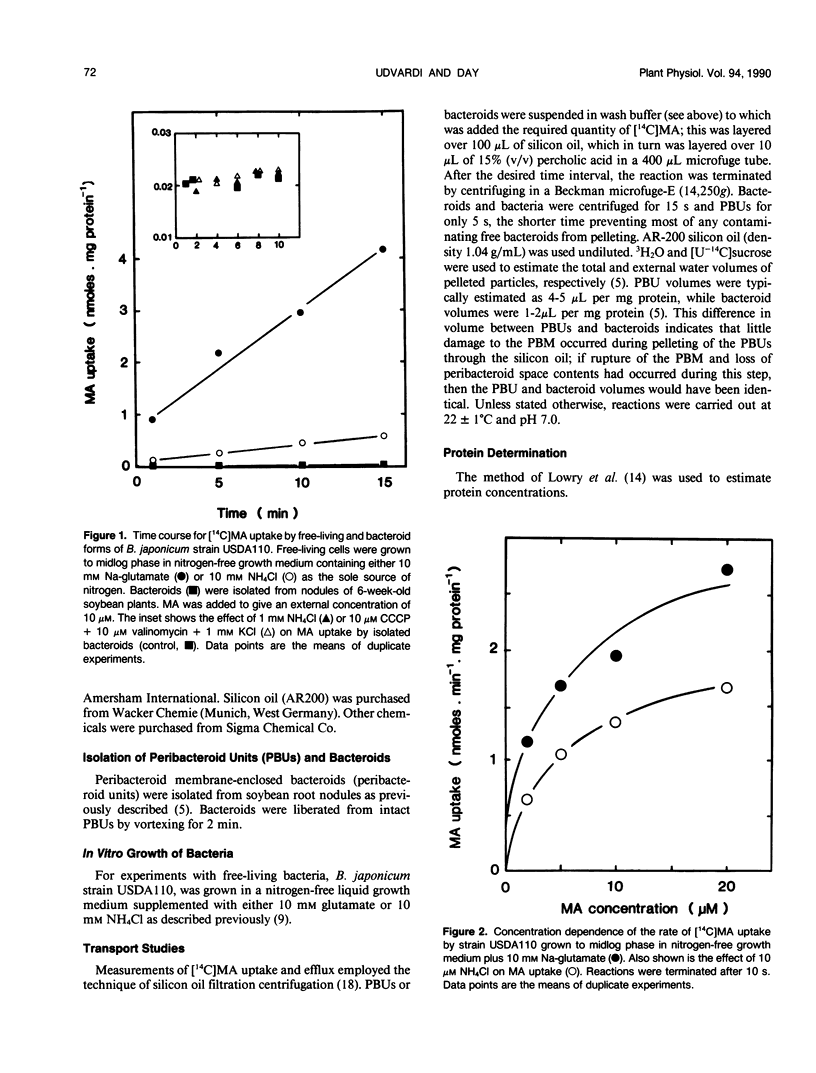
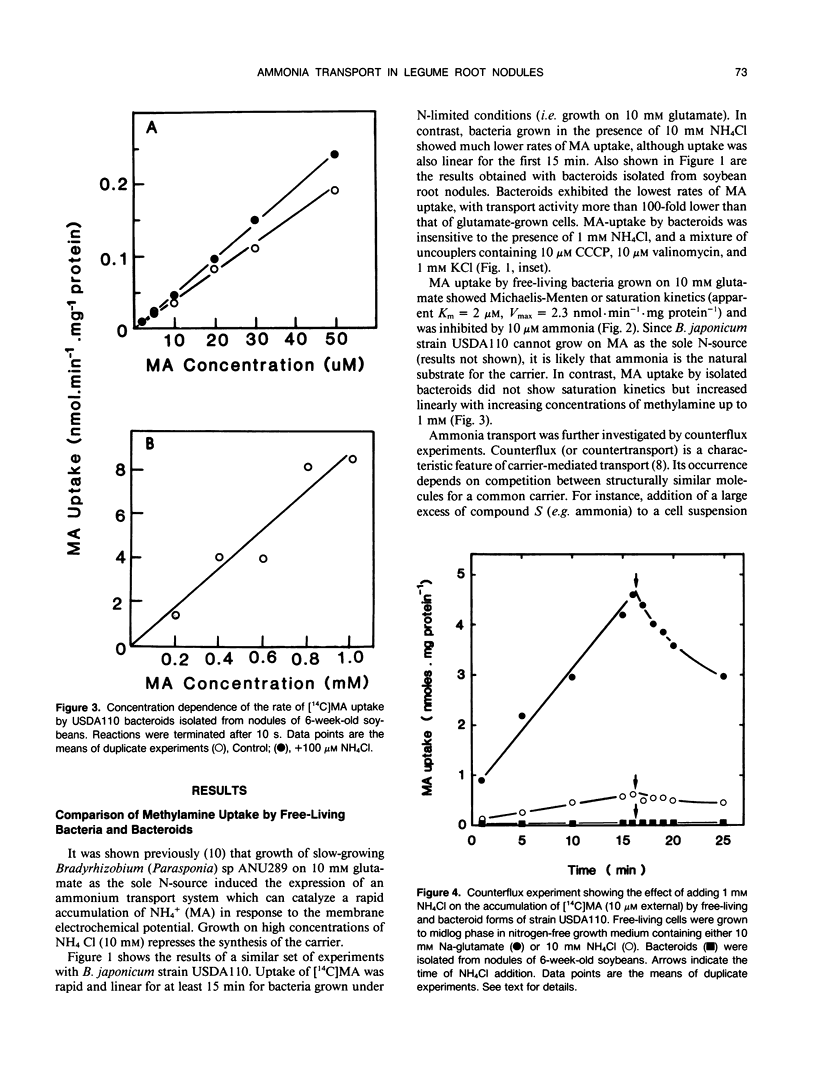
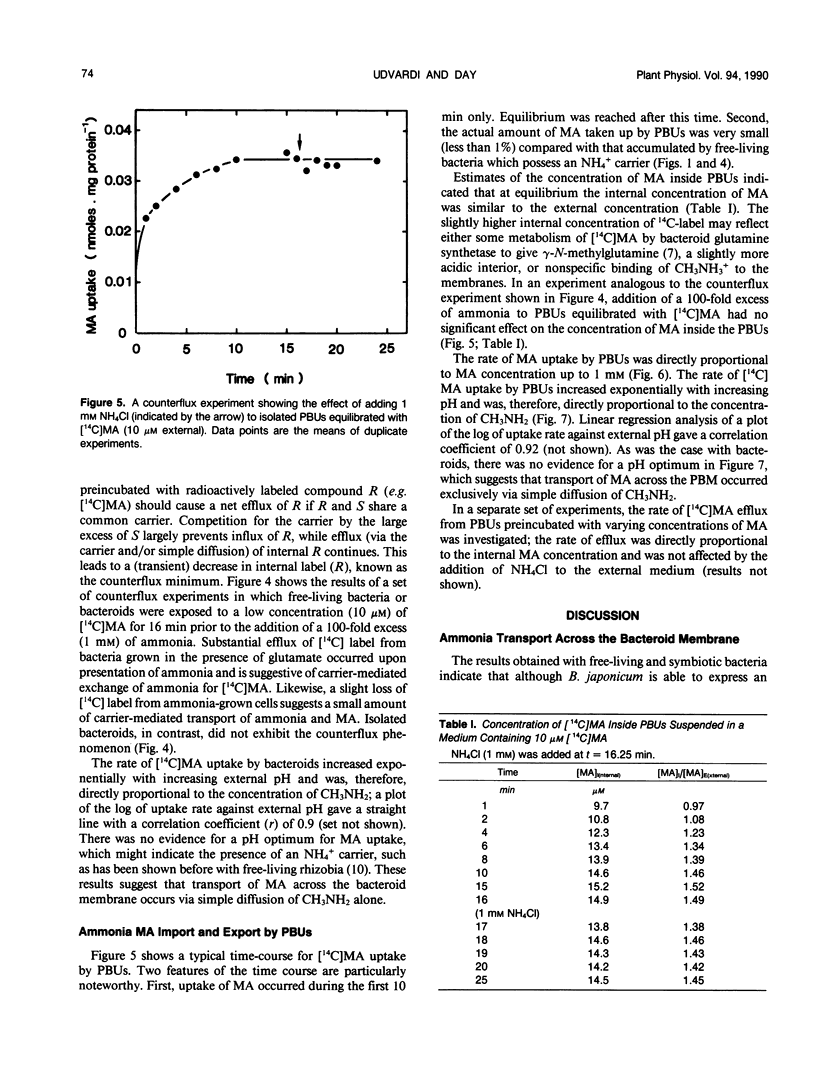
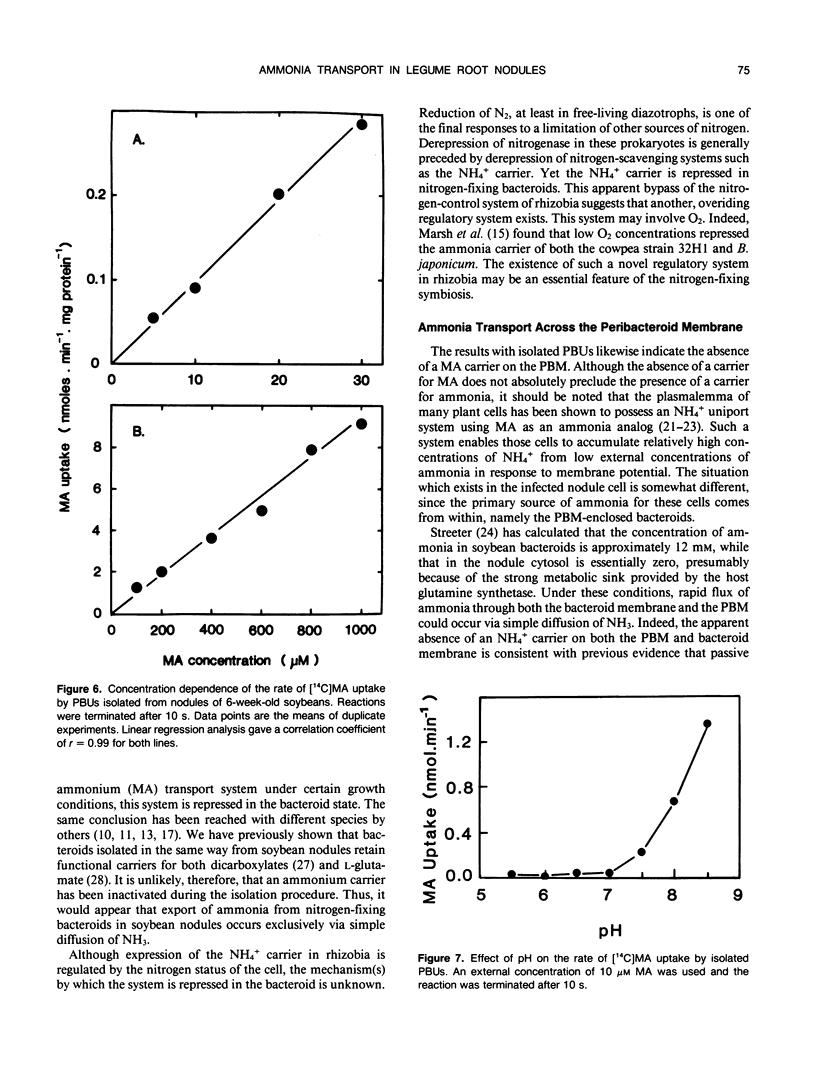
[14C]Methylamine (MA; an analog of ammonia) was used to investigate ammonia transport across the bacteroid and peribacteroid membranes (PBM) from soybean (Glycine max) root nodules. Free-living Bradyrhizobium japonicum USDA110 grown under nitrogen-limited conditions showed rapid MA uptake with saturation kinetics at neutral pH, indicative of a carrier. Exchange of accumulated MA for added ammonia occurred, showing that the carrier recognized both NH4+ and CH3NH3+. MA uptake by isolated bacteroids, on the other hand, was very slow at low concentrations of MA and increased linearly with increasing MA concentration up to 1 millimolar. Ammonia did not inhibit MA by isolated bacteroids and did not cause efflux of accumulated MA. PBM-enclosed bacteroids (peribacteroid units [PBUs]) were qualitatively similar to free bacteroids with respect to MA transport. The rates of uptake and efflux of MA by PBUs were linearly dependent on the imposed concentration gradient and unaffected by NH4Cl. MA uptake by PBUs increased exponentially with increasing pH, confirming that the rate increased linearly with increasing CH3NH2 concentration. The results are consistent with other evidence that transfer of ammonia from the nitrogen-fixing bacteroid to the host cytosol in soybean root nodules occurs solely by simple diffusion of NH3 across both the bacteroid and peribacteroid membranes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergersen F. J., Turner G. L. Activity of nitrogenase and glutamine synthetase in relation to availability of oxygen in continuous cultures of a strain of cowpea Rhizobium sp. supplied with excess ammonium. Biochim Biophys Acta. 1978 Feb 1;538(3):406–416. doi: 10.1016/0304-4165(78)90402-6. [DOI] [PubMed] [Google Scholar]
- Bergersen J. F., Turner G. L. Nitrogen fixation by the bacteroid fraction of breis of soybean root nodules. Biochim Biophys Acta. 1967 Aug 29;141(3):507–515. doi: 10.1016/0304-4165(67)90179-1. [DOI] [PubMed] [Google Scholar]
- Bishop P. E., Guevara J. G., Engelke J. A., Evans H. J. Relation between Glutamine Synthetase and Nitrogenase Activities in the Symbiotic Association between Rhizobium japonicum and Glycine max. Plant Physiol. 1976 Apr;57(4):542–546. doi: 10.1104/pp.57.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. M., Dilworth M. J. Ammonia assimilation by rhizobium cultures and bacteroids. J Gen Microbiol. 1975 Jan;86(1):39–48. doi: 10.1099/00221287-86-1-39. [DOI] [PubMed] [Google Scholar]
- Gober J. W., Kashket E. R. Methylammonium uptake by Rhizobium sp. strain 32H1. J Bacteriol. 1983 Mar;153(3):1196–1201. doi: 10.1128/jb.153.3.1196-1201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laane C., Krone W., Konings W., Haaker H., Veeger C. Short-term effect of ammonium chloride on nitrogen fixation by Azotobacter vinelandii and by bacteroids of Rhizobium leguminosarum. Eur J Biochem. 1980 Jan;103(1):39–46. doi: 10.1111/j.1432-1033.1980.tb04286.x. [DOI] [PubMed] [Google Scholar]
- O'Gara F., Shanmugam K. T. Regulation of nitrogen fixation by Rhizobia. Export of fixed N2 as NH+4. Biochim Biophys Acta. 1976 Jul 21;437(2):313–321. doi: 10.1016/0304-4165(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Palmieri F., Klingenberg M. Direct methods for measuring metabolite transport and distribution in mitochondria. Methods Enzymol. 1979;56:279–301. doi: 10.1016/0076-6879(79)56029-7. [DOI] [PubMed] [Google Scholar]
- Raven J. A., Farquhar G. D. Methylammonium Transport in Phaseolus vulgaris Leaf Slices. Plant Physiol. 1981 Apr;67(4):859–863. doi: 10.1104/pp.67.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubb R. S. Regulation of nitrogen fixation in Rhizobium sp. Appl Environ Microbiol. 1976 Oct;32(4):483–488. doi: 10.1128/aem.32.4.483-488.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udvardi M. K., Day D. A. Electrogenic ATPase Activity on the Peribacteroid Membrane of Soybean (Glycine max L.) Root Nodules. Plant Physiol. 1989 Jul;90(3):982–987. doi: 10.1104/pp.90.3.982. [DOI] [PMC free article] [PubMed] [Google Scholar]


