Abstract
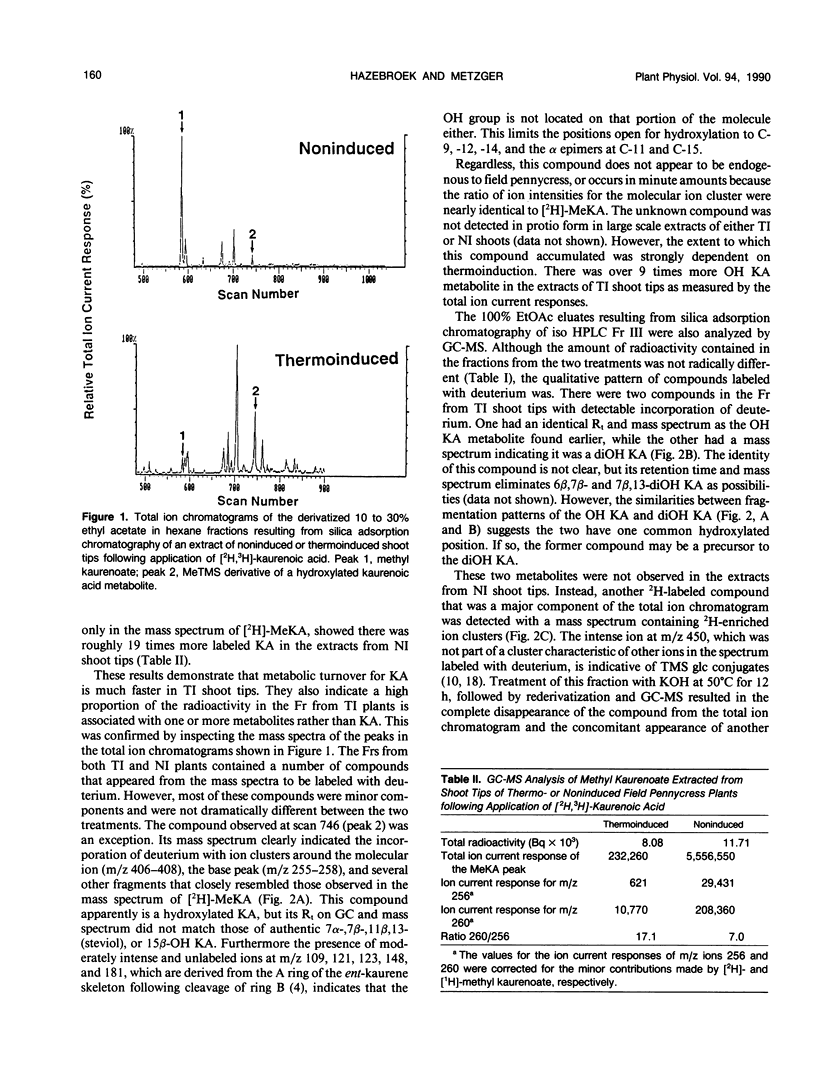
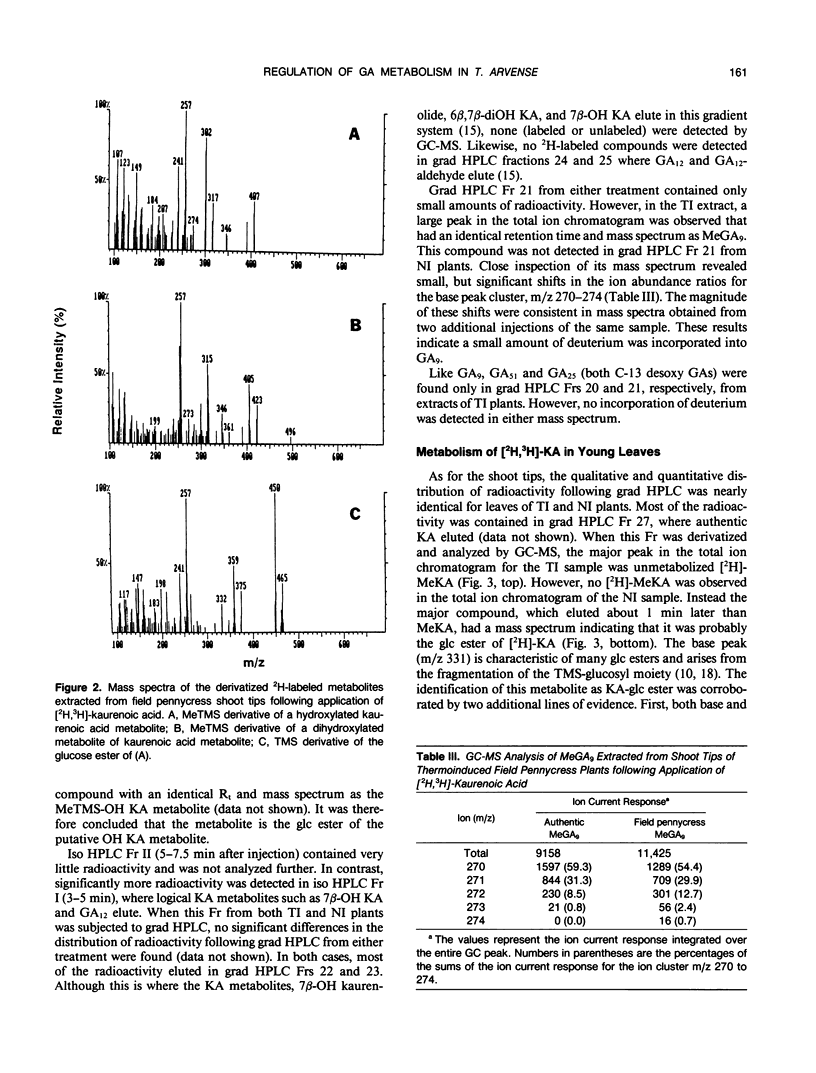
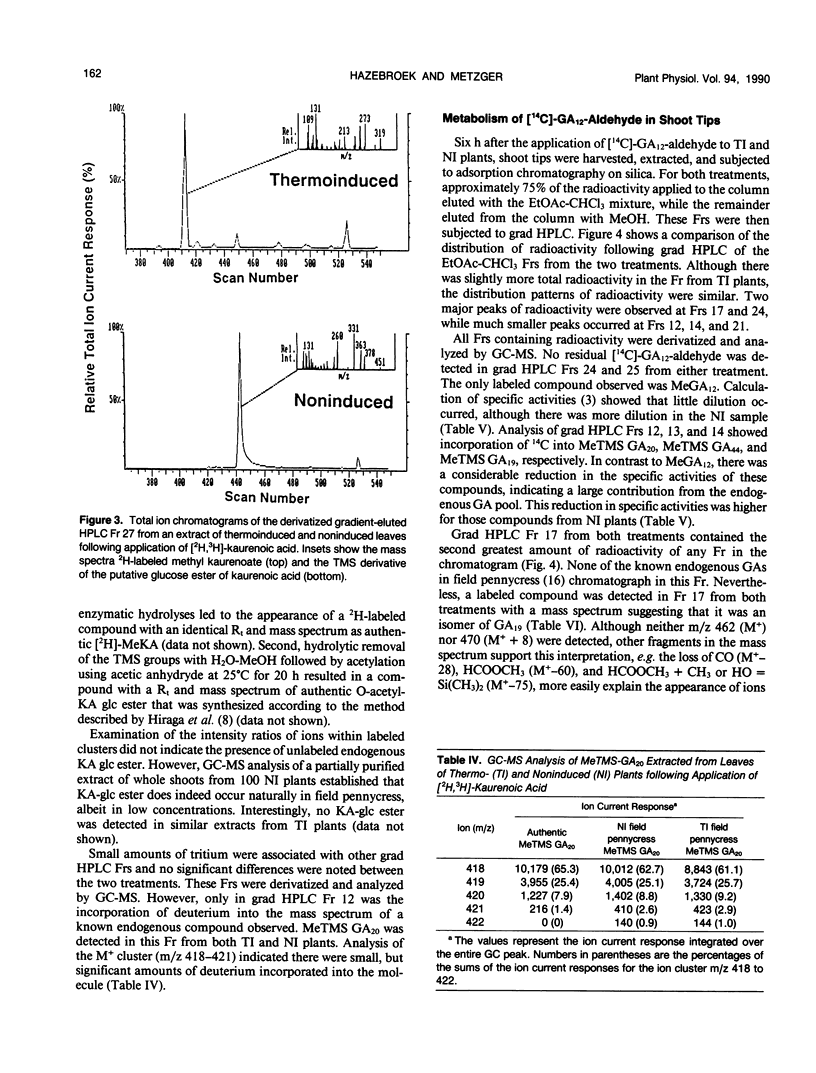
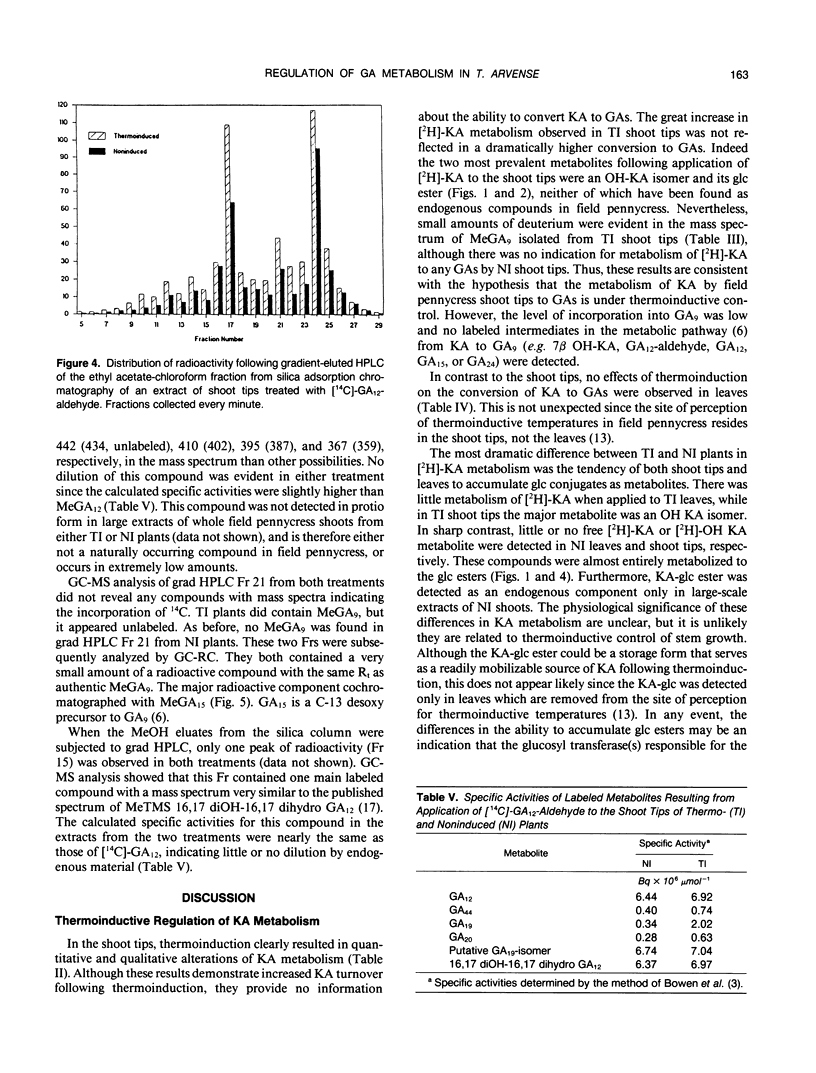
Field pennycress (Thlaspi arvense L.) is a winter annual crucifer with a cold requirement for stem elongation and flowering. In the present study, the metabolism of exogenous [2H]-ent-kaurenoic acid (KA) and [14C]-gibberellin A12-aldehyde (GA12-aldehyde) was compared in thermo- and noninduced plants. Thermoinduction greatly altered both quantitative and qualitative aspects of [2H]-KA metabolism in the shoot tips. The rate of disappearance of the parent compound was much greater in thermoinduced shoot tips. Moreover, there was 47 times more endogenous KA in noninduced than in thermoinduced shoot tips as determined by combined gas chromatography-mass spectrometry (GC-MS). The major metabolite of [2H]-KA in thermoinduced shoot tips was a monohydroxylated derivative of KA, while in noninduced shoot tips, the glucose ester of the hydroxy KA metabolite was the main product. Gibberellin A9 (GA9) was the only GA in which the incorporation of deuterium was detected by GC-MS, and this was observed only in thermoinduced shoot tips. The amount of incorporation was small as indicated by the large dilution by endogenous GA9. In contrast, thermo- and noninduced leaves metabolized exogenous [2H]-KA into GA20 equally well, although the amount of conversion was also limited. These results are consistent with the suggestion (JD Metzger [1990] Plant Physiol 94: 000-000) that the conversion of KA in to GAs is under thermoinductive control only in the shoot tip, the site of perception for thermoinductive temperatures in field pennycress. There were essentially no differences in the qualitative or quantitative distribution of metabolites formed following the application of [14C]-GA12-aldehyde to the shoot tips of thermo- or noninduced plants. Thus, the apparent thermoinductive regulation of the KA metabolism into GAs is probably limited to the two metabolic steps involved in converting KA to GA12-aldehyde.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnberg P. R., Brenner M. L., Mardaus M. C., Abe H., Pharis R. P. Metabolism of gibberellin a(12)-7-aldehyde by soybean cotyledons and its use in identifying gibberellin a(7) as an endogenous gibberellin. Plant Physiol. 1986 Sep;82(1):241–246. doi: 10.1104/pp.82.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnberg P. R., Maki S. L., Brenner M. L., Davis G. C., Carnes M. G. An improved enzymatic synthesis of labeled gibberellin A12-aldehyde and gibberellin A12. Anal Biochem. 1986 Feb 15;153(1):1–8. doi: 10.1016/0003-2697(86)90052-7. [DOI] [PubMed] [Google Scholar]
- Maki S. L., Brenner M. L., Birnberg P. R., Davies P. J., Krick T. P. Identification of Pea Gibberellins by Studying [C]GA(12)-Aldehyde Metabolism. Plant Physiol. 1986 Aug;81(4):984–990. doi: 10.1104/pp.81.4.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. D. Comparison of Biological Activities of Gibberellins and Gibberellin-Precursors Native to Thlaspi arvense L. Plant Physiol. 1990 Sep;94(1):151–156. doi: 10.1104/pp.94.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. D. Gibberellins and Light Regulated Petiole Growth in Thlaspi arvense L. Plant Physiol. 1988 Jan;86(1):237–240. doi: 10.1104/pp.86.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. D., Hazebroek J. P. Isolation of gibberellin precursors from heavily pigmented tissues. Plant Physiol. 1989 Dec;91(4):1488–1493. doi: 10.1104/pp.91.4.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. D. Localization of the Site of Perception of Thermoinductive Temperatures in Thlaspi arvense L. Plant Physiol. 1988 Oct;88(2):424–428. doi: 10.1104/pp.88.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. D., Mardaus M. C. Identification of Endogenous Gibberellins in the Winter Annual Weed Thlaspi arvense L. Plant Physiol. 1986 Feb;80(2):396–402. doi: 10.1104/pp.80.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. D. Role of Gibberellins in the Environmental Control of Stem Growth in Thlaspi arvense L. Plant Physiol. 1985 May;78(1):8–13. doi: 10.1104/pp.78.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane H., Fujioka S., Spray C. R., Phinney B. O., Macmillan J., Gaskin P., Takahashi N. Endogenous Gibberellins from Sporophytes of Two Tree Ferns, Cibotium glaucum and Dicksonia antarctica. Plant Physiol. 1988 Mar;86(3):857–862. doi: 10.1104/pp.86.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]


