Abstract
Background
In Bangladesh, the zoonotic transmission of anthrax from animals to humans poses substantial challenges for prevention and control programs, especially in resource-constrained settings. A comprehensive literature review was conducted focusing on anthrax infections in animals, humans, and the environment to enable better design of prevention and control strategies.
Materials and methods
We followed PRISMA guidelines to collect data on anthrax infection in animals and humans from reports between 1980 and January 2023. We used a standardized data extraction template to collect data on study location, year, hosts, deaths and risk factors responsible for anthrax occurrences at the animal, human and environmental sectors. Subsequently, we conducted a thorough analysis of the data gathered to identify the factors responsible for anthrax occurrences and to propose updated strategies for anthrax prevention and control.
Results
Of the 27 articles analyzed, 20 focused on animal or human anthrax, while seven addressed environmental contaminations. A total of 6354 cases of anthrax infection in animals were recorded, with 998 fatalities and an overall case fatality of 15.7 %. In humans, inadequate knowledge about anthrax and its transmission was a significant factor. Risk factors for human cutaneous anthrax included activities such as slaughtering diseased animals and contact with contaminated raw meat or blood. Risky practices such as disposal of animal carcasses in floodwaters or water bodies were observed in some areas, contributing to the persistence of the anthrax pathogen in the environment.
Conclusions
Our study highlights the necessity of a multisectoral One Health approach to effectively control and prevent anthrax outbreaks in both animals and humans. This approach should include comprehensive vaccination programs, social and behavioral change activities, environmental management, and the establishment of surveillance systems. Implementing these recommendations will be crucial in addressing the complex challenges posed by anthrax in low-resource settings.
Keywords: Animal anthrax, Human cutaneous anthrax, Risk factors, Multisectoral one health approach
1. Introduction
Anthrax is a highly contagious and deadly disease that primarily affects animals, but also has significant zoonotic impact [[1], [2], [3]]. It is caused by Bacillus anthracis, a Gram-positive, rod-shaped bacterium capable of forming spores under specific environmental conditions. While herbivores are considered the primary hosts, anthrax can affect a wide range of animal species, including humans, and has a high mortality rate [4,5]. The clinical presentation of anthrax can vary, including peracute, acute, subacute, and chronic stages [6]. In livestock, the incubation period usually ranges from 3 to 7 days [7].
Bacillus anthracis has a widespread distribution and can survive in the form of spores in harsh climatic conditions for over a century [8], rendering its eradication challenging. Despite being underreported, anthrax remains a significant concern in many parts of the world, including Asia, South America, and Africa, where people frequently slaughter and consume meat from animals infected with anthrax [9,10]. Moreover, sporadic cases of the disease have been reported in many countries worldwide, including Australia [11], Sweden [12], the United States [13], Italy [14], and several European countries.
Anthrax is highly prevalent in Southern Asian countries such as Bangladesh, where recurring outbreaks in both animals and humans have been well-documented [15,16]. In Bangladesh, rural communities often slaughter animals that are in a moribund state and infected with anthrax, subsequently selling the meat to compensate for financial losses [17]. This practice is strongly connected to their livelihoods, as many rely on livestock for their sustenance. Consequently, it substantially increases the risk of human exposure to anthrax in rural settings.
Between 2009 and 2010, a notable increase in both animal and human anthrax cases were reported in Bangladesh [15,16,18]. In most of these, human cutaneous anthrax seemed to be a result of animal anthrax occurrences, strongly suggesting transmission from animals to humans [16,19]. Specific geographical regions in Bangladesh − including Pabna, Sirajgang, Tangail, and Meherpur districts − are particularly vulnerable to outbreaks of both animal and human anthrax due to a combination of environmental, demographic, and cultural factors [20].
Although the World Health Organization [21] offers guidance about the methods of disposing dead animals in anthrax-endemic areas like Bangladesh, the lack of resources often makes it difficult for farmers to take appropriate action. The conventional methods of disposal of animal carcasses, such as burial or incineration, tend to be expensive [22]. Flooding causes inundation, and farmers often face difficulties burying carcasses in areas where anthrax poses a significant risk. This forces them to throw the carcasses into floodwaters [10]. In high-risk areas, these factors present serious obstacles to effectively controlling anthrax.
Several environmental factors make it difficult to control anthrax in animals. Anthrax outbreaks in animals have been associated with soil characteristics such as calcium and organic carbon content, together with extreme seasonal variations, such as periods of hot weather followed by precipitation [23,24]. In these conditions, the anthrax-causing bacteria are more likely to enter a vegetative cycle, resulting in the accumulation of anthrax spores on the soil surface or on contaminated grass. Animals become infected when they graze or eat this contaminated fodder [25].
To more effectively stop the transmission of anthrax, more knowledge synthesis articles outlining risk profiles from the perspectives of animals, humans, and the environment in Bangladesh are needed. To update the current anthrax control strategy, our primary objective was to provide a comprehensive overview of the anthrax situation in Bangladesh, with the aim of revising the current anthrax control strategy. In low-resource settings, the results of this review will form the foundation for customized prevention and control initiatives, especially under the OH approach.
2. Materials and methods
2.1. Ethical statement
The authors declare that they have adhered to the journal's ethical polices, as stated on author guidelines website, during the preparation and submission of this article. All the data used in this article were derived from secondary sources. Therefore, ethical approval for this study was not necessary.
2.2. Literature review strategy
We conducted a thorough search of relevant peer-reviewed journal articles, case reports, review articles, communications, abstracts, conference proceedings, book chapters, and letters, as well as other relevant documents, with a focus on the context of Bangladesh.
2.3. Search strategy
We strictly followed the guidelines specified in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement, which offers updated recommendations for reporting systematic reviews [26]. We gathered published documents, including articles, through searches of PubMed via NCBI, Google Scholar, and BanglaJOL (Bangladesh Journals Online) databases. Our search strategy involved the use of key search terms, including “animal or livestock anthrax AND Bangladesh”, “human anthrax or cutaneous anthrax AND Bangladesh”, and “zoonotic anthrax AND Bangladesh”. The search covered the period from 1980 to January 2023.
2.4. Inclusion criteria
In this study, we focused on peer-reviewed articles published between the 1980s and January 2023. We specifically considered for evaluation articles and reports written in English. Our inclusion criteria for selecting studies were as follows: (a) the research was conducted in Bangladesh; (b) it provided information on human, animal, and environmental aspects of anthrax, including factors responsible for its occurrence; and (c) the full text of the article was available.
2.5. Exclusion criteria
During the search process, a number of peer-reviewed articles were excluded based on the following criteria: (a) The full-text of the article was unavailable; (b) The article lacked essential epidemiological description, including information on the study unit, administrative location of the study, data on infected animals or humans, and deaths; (c) The study was conducted in geographical locations other than Bangladesh; (d) The study solely focused on genetic characterization without providing relevant epidemiological information; and (e) The article was not written in the English language.
2.6. Data extraction and management
We created a standardized template for data extraction to systematically evaluate each study. The template was designed to record and evaluate relevant data from research conducted in Bangladesh. The following data were retrieved and analyzed: outbreak location, animal and human infection, soil contamination, risk factors for animal-level occurrence, the test used for identifying causal agents, knowledge and practices, anthropological or human-level risk factors, ecological or environmental factors, vaccination coverage and lessons learned and the way forward [27].
3. Results
3.1. Search results
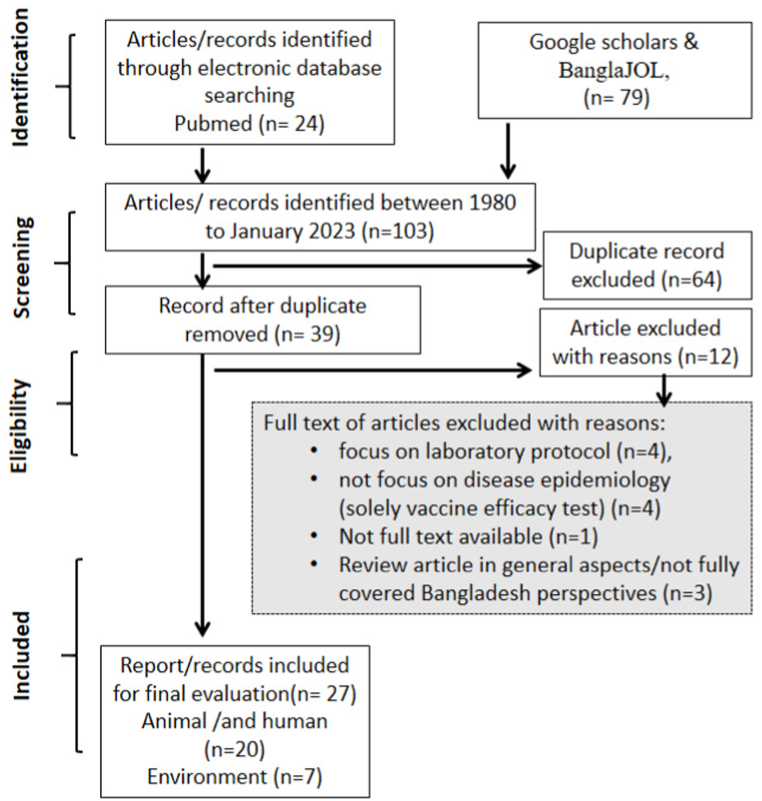
A primary screening of 103 articles was conducted using search terms in the abstracts, summaries, and key findings through the reference manager software EndNote (Thomson Reuters, Philadelphia, P. A. USA) to eliminate duplicates (n = 64). This process resulted in the selection of thirty-nine articles for secondary screening. From these, 12 articles were excluded for various reasons: focus on laboratory protocol (n = 4), non-disease epidemiology focus (vaccine efficacy test) (n = 4), unavailability of full text (n = 1), and review article with general aspects not fully covering Bangladesh perspectives (n = 3). Ultimately, 27 articles were included in the comprehensive review. The first and second authors of the manuscript reviewed the selected articles to ensure their suitability for this review article (Fig. 1).
Fig. 1.
Methodology of literature review process based on PRISMA framework.
3.2. Characteristics of study
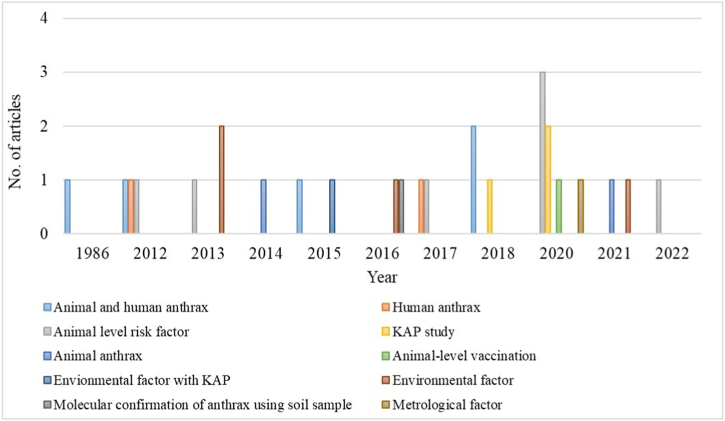
From 1980 to January 2023, a total of 27 articles met the inclusion criteria of this study (Fig. 1) and were included in the systematic review. Among these articles, 7.4 % (n = 2) focused solely on animal anthrax, 7.4 % (n = 2) focused solely on human anthrax, and 18.5 % (n = 5) examined both animal and human anthrax. Additionally, 26 % (n = 7) of the articles highlighted animal-level risk factors, 11 % (n = 3) focused on knowledge, attitude, and practice assessment, and 3.7 % (n = 1) discussed animal-level vaccination coverage. Furthermore, 18.5 % (n = 5) of the articles discussed environmental factors, while 3.7 % (n = 1) each focused on meteorological factors and molecular confirmation of anthrax in soil specimens (Fig. 2).
Fig. 2.
Number of articles included under this research by year and study type.
3.3. Disease situation
3.3.1. Animal anthrax occurrence
Anthrax outbreaks occur sporadically throughout Bangladesh, but they are highly prevalent in the anthrax-prone districts of the country and have consistently been underreported. As a notifiable disease of the World Organization for Animal Health (WOAH), Bangladesh follows standard procedures set by the WOAH and periodically submits anthrax outbreak data [28]. Outbreaks of anthrax are routinely confirmed in districts such as Sirajganj, Pabna, Tangail, Kushtia, Meherpur, and Rajshahi. However, several other districts − including Manikganj, Narayanganj, Laxmipur, Bogra, Chapai Nawabganj, Chuadanga, and Chattogram − have also reported multiple instances of anthrax outbreaks [29].
3.3.2. Case fatality
Six published articles from 1980 to 2022 presented quantitative data, especially on animal infections and deaths. These studies reported a total of 6354 confirmed cases of anthrax infection and 998 deaths. Based on these data, an overall case-fatality of 15.7 % (95 % CI: 14.8 % − 16.6 %) was calculated (Table 1).
Table 1.
Summary of confirmed cases of anthrax infections, deaths and case fatality from 1980 to January 2023.
| Year | Study type | Location/district | Number of outbreaks | Confirmed cases | Number of deaths | Case fatality (%) | Reference |
|---|---|---|---|---|---|---|---|
| 1986 | Cross-sectional study | Pubna milk shed area, Pubna | 1 | 62 | 43 | 69.3 (56.3–80.4) | [30] |
| 2012 | Cross-sectional/observational study | Sirajganj, Pabna and Tangail | 14 | 140 | 98 | 70.00 (61.7–77.4) | [15] |
| 2014 | Secondary surveillance | Whole Bangladesh (64 districts) | – | 5937 | 801 | 13.49 (12.62–14.37) | [31] |
| 2015 | Cross-sectional study | Sirajganj district | – | 159 | 48 | 30.2 (23.2–37.9) | [19] |
| 2018 | Cross-sectional study | Sirajganj, Tangail and Rajbari | 3 | 6 | a | – | [17] |
| 2021 | Qualitative exploratory study | Rajshahi, Meherpur, Kushtia, Sirajgonj, and Tangail |
19 suspected anthrax outbreaks | 50 | a | – | [32] |
| 6354 | 998 | 15.7 (14.8–16.6) |
Infected animals slaughtered and consumed; infection status was confirmed through retrospective testing of frozen meat sample.
3.3.3. Species distribution
Among the confirmed cases, cattle (84.12 %, n = 5233) were the most affected species, followed by goats (10.38 %, n = 643), buffaloes (4.73 %, n = 293), and sheep (0.77 %, n = 48).
3.3.4. Factors contributing to animal anthrax
Several studies conducted in Bangladesh have identified various risk factors for anthrax infection in animals (Table S1). These factors include inadequate supply of anthrax vaccine, low vaccination coverage, improper vaccine strategy, uncontrolled animal movements, and insufficient knowledge of anthrax epidemiology. Contaminated feed such as grass silage, and drinking water sources have also been found to contribute to the transmission dynamics of anthrax in animals [14,33].
In high-risk districts like Sirajganj, Pabna, and Tangail, farmers often face challenges in properly disposing of carcasses during the monsoon season, leading them to discard carcasses in rivers or floodwaters [10]. Additionally, approximately 98 % of farmers in both high and low-risk districts rely on laypersons for the treatment of sick animals, fail to wash grass, and experience frequent floods, all of which have been identified as significant factors contributing to anthrax outbreaks [34].
Studies have shown that animals feeding on root-out and unwashed (muddy) grass have 41.2 times higher odds of contracting anthrax compared to animals that do not eat such grass [18]. Feeding on water spinach from inundated areas and water hyacinth (Eichhornia crassipes) have also been identified as risk factors for anthrax in animals, with odds ratios of 22.2 and 12.0, respectively [18,35]. Moreover, farms that have infected animals or are located near farms that slaughter infected animals are more than 12 times more likely to have animal-level anthrax infections [36].
3.3.5. Animal-level vaccination coverage
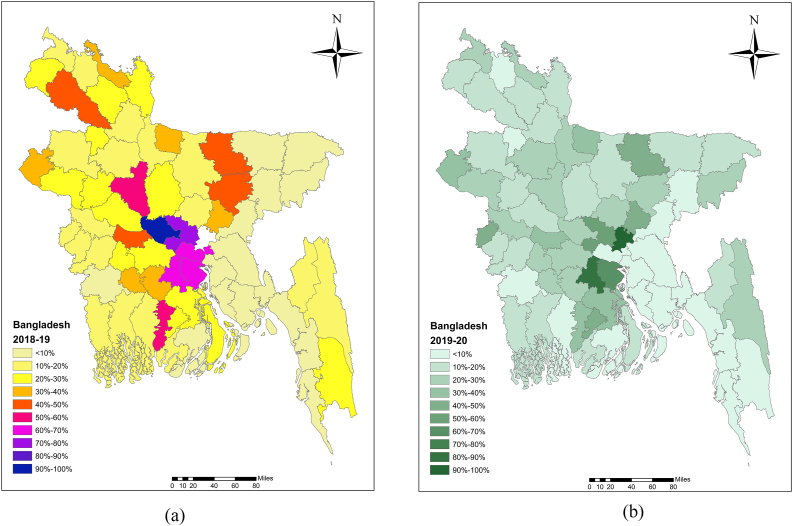
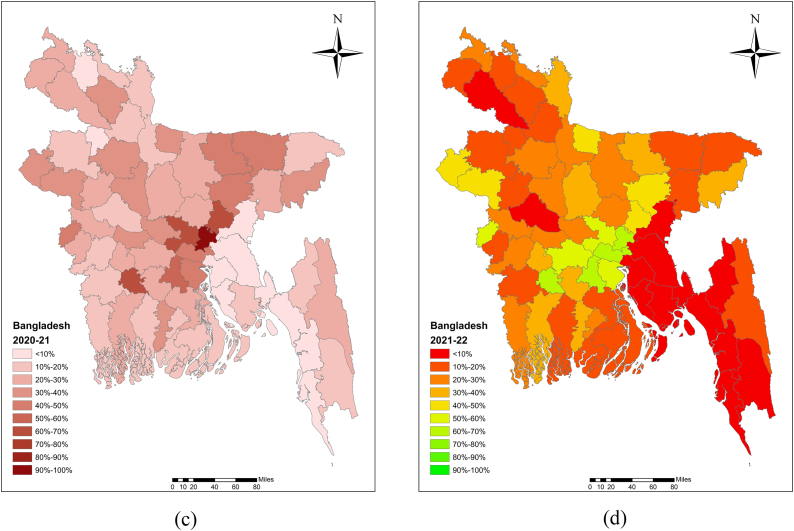
Various authors in Bangladesh have reported low levels of vaccination coverage levels [19,31,37]. Islam et al. [19] reported that the average vaccination coverage for large ruminants in the Sirajganj district of Bangladesh was 44.29 % in 2010, 46.23 % in 2011, and 37.88 % in 2012. A study conducted across Bangladesh from 2010 to 2012 documented only 7.31 % vaccination coverage [31]. The challenge of handling cattle during vaccination campaigns was notably recognized by the majority of farmers (54.0 %), who identified the absence of male members as a significant obstacle [38]. Additionally, insufficient vaccine supplies for animal immunization had also been identified [39] (Table S1). The average vaccination coverage for large ruminants (cattle and buffalo) was reported to be 22.7 % in 2018–19, 18.1 % in 2019–20, 23.0 % in 2020–21, and 20.8 % in 2021–22 (Fig. 3A–D).
Fig. 3.
Anthrax vaccination coverage at the animal level was visualized in Bangladesh map using ArcMap software for the periods (a) 2018–19, (b) 2019–20, (c) 2020–21, and (d) 2021–22.
3.3.6. Vaccine seed evaluation
Currently, the Department of Livestock Services (DLS) in Bangladesh produces approximately 5 million doses of Sterne Strain 34 F2 Bacillus anthracis vaccine annually to immunize susceptible animal populations [40]. These vaccine doses are sufficient to immunize all susceptible animals in high-risk districts where anthrax outbreaks occur frequently. It is worth noting that the quality of the vaccine has sometimes been criticized by various stakeholders in Bangladesh. However, several studies have evaluated the Sterne Strain 34 F2 Bacillus anthracis vaccine produced by the Livestock Research Institute (LRI) of DLS and have found it to be effective in protecting animals against anthrax under farming conditions [[41], [42], [43]]. Additionally, the transportation and cool chain facilities used for vaccine distribution did not significantly impact the immune response induced by this vaccine [43]. It is worth noting that the US-CDC laboratory conducted a recent assessment of the anthrax vaccine seed. Through the Multiple Locus Variable Number Tandem Repeat Analysis (MLVA-8), they confirmed the use of the Sterne strain as the vaccine seed [44].
3.4. Human anthrax in Bangladesh
The sequence of events for animal and human outbreaks indicates that human cutaneous anthrax usually follows animal anthrax, supporting the assumption that humans acquire anthrax from animals [18]. Samad and Hoque [30] confirmed 27 cases of human cutaneous anthrax in the milk shed areas of Pabna, Bangladesh, between 1980 and 1984. The Institute of Epidemiology, Disease Control and Research (IEDCR) reported 607, 278, and 176 cases of cutaneous anthrax in 2010, 2011, and 2012, respectively, in districts with significant animal anthrax outbreaks in Bangladesh [[45], [46], [47]]. In 2013, 327 cases of cutaneous anthrax were confirmed in Sirajganj, Tangail, Meherpur, and Chuadanga districts [48], and in 2014, 225 cases were confirmed in Sirajganj, Tangail, Meherpur, and Narayanganj districts [48]. However, in 2011, 15 cases of cutaneous anthrax were identified in the Outpatient Department (OPD) of Rajshahi Medical College Hospital in Bangladesh [49]. Between 2009 and 2010, 39 confirmed cases and 234 probable cases were identified from 14 documented outbreaks in anthrax-prone districts (Sirajganj, Tangail, Pabna) of Bangladesh [15]. In 2016–17, 70 people were infected with cutaneous anthrax in three outbreak sites in Sirajganj, Tangail, and Rajbari districts [17] (Table 2).
Table 2.
Summary of human cases and diagnostic tests used for confirmation of anthrax.
| Year | Location/District | Study type | Human infection | Lab. Test used | Reference |
|---|---|---|---|---|---|
| 1986 | Pubna milk shed area, Pubna | Cross-sectional study | 27 cutaneous anthrax cases | – | [30] |
| 2010 | Pubna, Sirajganj, Kushtia, Meherpur, Tangail, Manikganj, Satkhira, Lalmonirhat, Rajshahi, Narayanganj, Laxipur, and Chattogram | Passive Surveillance/extended surveillance and outbreak investigation | 607 cases | – | [45] |
| 2011 | Rajshahi, Sirajganj, Meherpur, Tangail, Bogra Pabna, and Chapai Nawabganj |
Surveillance and outbreak investigation | 278 cases | – | [46] |
| 2011 | Rajshahi medical college hospital | Case reports | 15 cutaneous anthrax cases | Gram-staining smear | [49] |
| 2012 | Sirajganj, Kushtia, Bogra, Tangail, and Meherpur | Surveillance and outbreak investigation | 176 cases | [47] | |
| 2012 | Sirajganj, Pabna, and Tangail | Exploratory investigation | 39 confirmed cases and 234 suspected cases identified | Loeffler's polychrome methylene blue stain MLVA |
[18] |
| 2013 | Tangail, Meherpur and Chuadanga | Surveillance and outbreak investigation | 327 cutaneous anthrax cases in Sirajganj, | [48] | |
| 2014 | Sirajgajganj, Mehepur, Narayanganj, and Tangail | Surveillance and outbreak investigation | 225 cases | – | [48] |
| 2016 | Rajshahi | Case reports | 13 infected cutaneous anthrax | – | [50] |
| 2018 | Sirajganj, Tangail and Rajbari | Outbreak investigation/cross-sectional study | 70 people got infection with cutaneous anthrax | Loeffler's polychrome methylene blue stain | [17] |
| 2018 | Rajbari | Case reports | 6 people got skin lesions | PCR | [35] |
3.4.1. Factors contributing to human anthrax
Multiple studies conducted in Bangladesh have confirmed the existence of several risk factors for the transmission of anthrax to humans. These risk factors include the slaughter and butchering of infected animals [14,32,35,49,51], as well as contact with contaminated raw meat, blood, skins, hides, and blood of infected animals [14,49]. These factors have been identified as significant sources of human infections and are key risk factors for cutaneous anthrax in Bangladesh. The research provides strong evidence that individuals engaged in the slaughter of sick animals and the handling of raw meat are at a significantly higher risk of anthrax infection. The study estimates the risk to be 21.9 times higher for slaughtering sick animals and 1.6 times higher for handling raw meat [15]. Additionally, certain risky behaviors, such as the improper disposal of waste materials in rivers, water bodies, bamboo bushes, or open land, can contribute to the persistence of anthrax pathogens in the environment, thereby increasing the likelihood of infecting animals [32].
3.5. Anthrax knowledge and practices
Various studies conducted in Bangladesh have highlighted the inadequate knowledge and practices related to anthrax within the population. The decision to slaughter a sick animal is often driven by financial constraints and a lack of understanding about the disease [49]. Livestock raisers have been found to have insufficient awareness about the transmission dynamics of anthrax from animals to humans, which further exacerbates the spread of the disease [15,19,50]. Importantly, a considerable proportion (37.26 %) of cattle raisers in high-risk districts have no knowledge of anthrax [39]. Another study revealed that most of the population (90 %) lacks knowledge about anthrax, although around 40 % recognized the term ‘Anthrax’ or its Bangla name ‘Torka’, and 25 % were aware of the potential of vaccines to protect animals [17]. Moreover, about 51 % of the respondents showed a lack of knowledge regarding the proper disposal of carcasses, often throwing them in open space or rivers [38]. However, it was noted that households with educated family members showed better practices, which enhanced overall awareness and knowledge about zoonotic diseases, including anthrax, within the community [25]. In humans living in low- and medium-risk districts of Bangladesh, the level of knowledge regarding anthrax zoonosis, including animal vaccination, is typically very low [34]. The summary of human-level risk factors including anthrax knowledge and practices are provided in Table S2.
3.6. Surveillance
Following the identification of major human outbreaks in 2009, an enhanced surveillance system was implemented to strengthen early detection, diagnosis, and response. Risk communication is included in this system as an essential component to prevent and control both human and animal health issues. In the context of animal health, the real-time surveillance systems have expanded the capabilities of field and laboratory operations, with a specific focus on sample collection, transfer, and testing facilities that have been established with support from the US CDC [40]. In both human and animal health domains, comprehensive protocols, standard operating procedures (SOPs), questionnaires, consent forms, and other essential documents have been developed to investigate anthrax outbreaks. Training initiatives have been conducted to equip both field and laboratory staff with the necessary skills for sample collection and testing. The surveillance efforts in both human and animal health are summarized in Table 3.
Table 3.
Surveillance initiatives for anthrax in both animal and human populations in Bangladesh.
| Surveillance type | Location | Duration | Collected sample | Test | Reference |
|---|---|---|---|---|---|
| Human health | |||||
| Active surveillance (IEDCR) | Endemic districts | 2009 to ongoing | Swabs | PCR | This study |
| Active surveillance (IEDCR) | 5 sentinel sites | March 2018 to ongoing | Swabs | PCR | [48] |
| Animal Health | |||||
| Passive surveillance/secondary (DLS) | 64 districts | 2007 to 2017 | Blood from infected animal | Polychrome methylene blue test (PMB) | This study |
| Passive surveillance (online): Bangladesh Animal Health Intelligence System (BAHIS: FAO-DLS) | 64 districts | 2017 to onwards | Blood from infected animal | PBM | This study |
| Active surveillance under PARB project USCDC-DLS |
Sirajganj and Meherpur districts | 2019 to 2021 | Blood samples from infected animals | PMB and PCR | This study |
| Active surveillance (Strengthening Veterinary Public health capacity)-DLS | All over the country | 2020 to onwards | Blood samples | PMB and PCR | This study |
3.7. Anthrax in environment
Several studies conducted in Bangladesh have consistently revealed high levels of soil contamination in high-risk districts, ranging from 11.7 % to 80 % [14,25,33]. Consequently, soil is a prominent reservoir for the dissemination of B. anthracis, thereby contributing to animal-level infections within natural settings [52]. Furthermore, a separate study, focused on environmental factors within three districts (Dhaka, Khulna, and Rajshahi) of Bangladesh, confirmed the presence of anthrax bacteria in various samples. Specifically, it reported that 85.7 % (6/8) of bone samples, 33.3 % (1/3) of water samples, 30 % (3/10) of feed samples, and 100 % (3/3) of rumen ingesta samples tested positive for anthrax bacteria [33]. Molecular confirmation of B. anthracis was also conducted using soil samples collected from Sirajganj and Tangail districts in 2013 [53].
3.8. Environmental factors
Several authors confirmed that environmental factors play a vital role in the occurrence of anthrax spores in the soil. Among these factors, loamy-type soils in anthrax-prone areas, soils with elevated levels of calcium and an organic carbon component, high temperature and precipitation, and poor vaccination coverage are of paramount importance [54]. It was revealed that 77.08 % of loamy soils and 22.92 % of clay soils were contaminated with anthrax spores. The average pH of the anthrax-spore contaminated soil was acidic (6.38). However, the burial, removal, or cleaning of infected carcasses, as well as contaminated premises, are pivotal factors contributing to the persistence of the pathogen in the environment [52]. As a result, soil in high-risk areas contain pathogenic Bacillus anthracis spores, which can lead to animal-level infections during grazing [43].
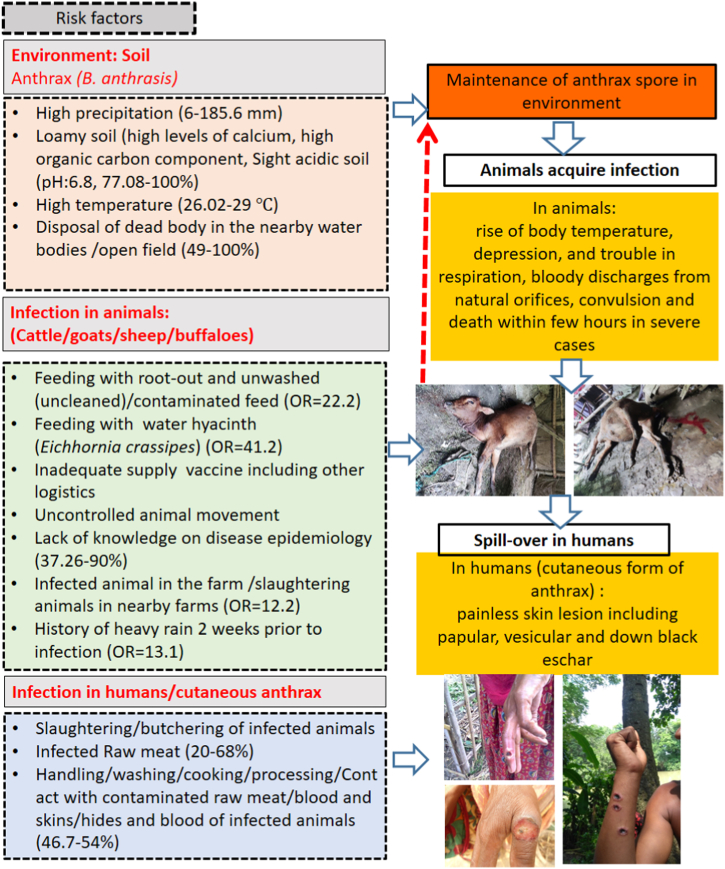
Furthermore, a study investigated the association between climatic changes and the incidence of anthrax in Bangladesh using meteorological data and secondary surveillance data from the DLS spanning from 2010 to 2014. The study revealed that with every 1 mm increase in precipitation, 1 additional hour of daily cloud coverage, and a 1-knot increase in wind speed, anthrax outbreaks increased by 9.45, 36.69, and 107 cases, respectively [36]. Ecological factors identified in this study are summarized in Table S3 and presented in Fig. 4.
Fig. 4.
Human, animal and environmental factors responsible for the sustenance of anthrax spore in soil, infection in animals and humans, respectively in Bangladesh as per literature review of this article.
3.9. Laboratory evaluation of the samples
3.9.1. Animal samples
In the majority of cases anthrax diagnosis is primarily based on clinical signs, which include rise in body temperature, depression, respiratory distress, bloody discharges from body openings, tremors, and sudden death (within a few hours). This diagnosis is usually performed in Upazila veterinary hospitals [19]. This is because animal health laboratories across the country are generally not equipped to conduct comprehensive testing of animal samples at the field level.
To confirm anthrax cases, basic diagnostic techniques like the polychrome methylene blue (PMB) stain method (McFadyean reaction) are used in field disease investigation laboratories (FDILs) and veterinary hospitals located in anthrax-prone districts [15,17,19]. More advanced molecular-based testing methods, such as PCR, are performed at the Central Disease Investigation Laboratory (CDIL) with support from the US CDC. Another study involving animals also utilized PCR testing [43]. In some instances, more sophisticated molecular-based assays, like the MLVA, have been used for the evaluation of animal samples [18].
3.9.2. Human samples
In humans, clinical manifestations that include painless skin lesions (papular, vesicular) and the development of a dark black eschar, are considered as tentative anthrax cases. To confirm these, skin swab samples (taken from the exudates of the skin lesions) are subjected to various diagnostic techniques. These techniques range from basic (PMB) methods like gram-staining smear [49], Loeffler's polychrome methylene blue stain [15], to more advance molecular assays such as PCR [35] and MLVA [15]. It is worth noting that each of these tests has varying levels of sensitivity (Se) and specificity (Se), and they require different technological capabilities and laboratory facilities. In many low-resource settings, clinical manifestations and basic microbiological techniques, including culture, are often relied upon for outbreak investigation. Nevertheless, in several countries, advanced testing competencies like PCR and bacterial culture have been developed to identify and confirm the anthrax pathogen from clinical samples. It is important to note that for the isolation of B. anthracis, the lack of expensive Biosafety Level 3 laboratory facilities is not a limiting factor [55]. Skilled laboratory personnel working within Biosafety Level 2 cabinets can conduct bacterial culture and molecular diagnosis, making these capabilities available in low-resource settings like Bangladesh [56].
3.9.3. Environmental samples
The detection of Bacillus anthracis in soil samples is usually conducted using Gram's Method, which reveals the presence of blue or purple-colored bacteria. Additionally, the McFadyean McFadyean reaction has also been used to identify short chains of B. anthracis cells, which are often found among amorphous disintegrated capsular materials in PMB test [54]. However, the analysis of Variable Number Tandem Repeats (VNTR) using MLVA is commonly employed for biomolecular evaluations of B. anthracis in soil samples [52]. Moreover, several advanced methods have been developed for the molecular evaluation of B. anthracis isolated from soil samples, including the Ground Anthrax Bacillus Refined Isolation (GABRI) method, Canonical Single Nucleotide Polymorphism (CanSNP) Analysis, Multi-Locus Variable Number of Tandem Repeats (VNTRs) Analysis incorporating Single Nucleotide Repeats (SNRs) Analysis [33], and whole genome sequencing [53].
4. Discussion
Anthrax, a zoonotic disease, is endemic in Bangladesh, presenting a critical challenge. The primary concern is to prevent the slaughter of anthrax-infected animals and related activities, as well as the sale of infected meat at lower prices to consumers, which has led to focal outbreaks of human cutaneous anthrax in the country. In this context, health education of the people in high risk areas is essential. Given the significant challenges posed by anthrax in Bangladesh, which involve several factors related to animals, humans, and the environment, we propose recommending interventions using the Theory of Change framework. This approach should be aligned with a One Health strategy to effectively address and prevent anthrax in both animals and humans [57].
It has been observed that most cases of human cutaneous anthrax transmissions in Bangladesh have occurred after animal anthrax outbreaks [15,19,30]. Because of financial constraints, farmers frequently slaughter infected animals and sell the meat to sustain their livelihoods.
The presence of human anthrax in Bangladesh is closely linked to the endemic distribution of animal anthrax. A lower proportion of vaccine coverage has been identified as a critical risk factor for the occurrence of anthrax at the animal-level in Bangladesh [15,31,54]. In rural settings, people live close to their animals, facilitating contact with infected animals. Risky behaviors like slaughtering and butchering of infected animals [2,19,35,49,51], as well as contact with contaminated raw meat, blood [2,49], and skins or hides of infected animals [2], create pathways for the anthrax bacterium to come into contact with the human skin, leading to the development of human cutaneous anthrax.
Over the past decade, various interventions like mass vaccination, awareness campaigns, and motivational programs involving training and the distribution of Social and behavior change (SBC) materials under the OH framework have been implemented in high-risk districts through different projects and programs. These efforts have significantly reduced the occurrence of animal and human anthrax outbreaks. Through a program funded by the US-CDC, the DLS has developed several guidelines and protocols for the prevention and control of anthrax in Bangladesh, including surveillance guidelines, laboratory protocols for anthrax detection, and vaccination strategies for animals. Currently, DLS provides ongoing support, including compensation for anthrax-infected animals and covering relevant expenses for the safe disposal of a carcass [40] (Fig. 5A and B). In addition, activities related to outbreak investigation, data sharing, awareness-raising, and implementation in selected high-risk districts (Sirajganj and Meherpur) of Bangladesh are being conducted through coordination between animal and human health.
Fig. 5.
(a) A crossbred cow affected by anthrax in Sirajganj district, Bangladesh, (b) demonstrates the safe disposal of the infected carcass through the deep burial method, ensuring a burial depth of 6 feet.
At the field level in upazila and district veterinary hospitals, there are inadequacies in animal health laboratories concerning diagnosis of anthrax from clinical samples. As a result, only basic tests such as the polychrome methylene blue (PMB) staining technique (McFadyen reaction) are currently being used to confirm anthrax samples as a reliable diagnostic test [58]. However, the molecular method PCR has been routinely practiced at the Department of Livestock Services' Central Disease Investigation Laboratory (CDIL).
Specific geographical locations, such as Sirajganj, Pabna, and Tangail districts, have been identified as high-risk areas due to the recurrent occurrence of anthrax outbreaks in both animals and humans each year [[15], [16], [17], [18]]. This association is supported by epidemiological evidence. The presence of loamy soils with high levels of calcium and organic carbon, combined with high temperatures and precipitation, creates an environment which promote the survival of anthrax spores in these locations [54]. Studies have shown that loamy soil is more than three times as likely to be contaminated with anthrax spores compared to clay soil with a slightly acidic pH (6.38) [52]. Furthermore, the burial of anthrax-infected carcasses without proper disinfection of the contaminated premises contributes to the persistence of the pathogen in the environment [52]. A high proportion of anthrax spores found in soil samples strongly suggests that soil is a key contributor to the spread of B. anthracis and may be associated with animal infections. The current practices of burying dead livestock or improperly removing infected carcasses, including infected materials, are plausible causes of contamination and the continued presence of pathogens in the environment.
The data from other research studies indicate a notable tendency of low vaccination coverage [31,54]. Additionally, a recent outbreak in previously vaccinated zones has been linked to unrestrained animal movement [17]. These contribute to recurrent outbreaks each year in the endemic settings of Bangladesh.
The Sterne 34 F2 vaccine, which contains a non-capsulated attenuated B. anthracis strain, has been associated with vaccine reactions. These include localized edema, pain during injection, and a rise in body temperature. However, despite these side effects, the vaccine has been found to be highly effective in immunizing animals for up to one year [59]. Due to the side effects associated with the Sterne 34 F2 vaccine, farmers, particularly goat raisers, have shown reluctance to vaccinate their livestock, which may leave the animals vulnerable to infection. In response to this challenge, the DLS has developed a standard protocol for goat vaccination against anthrax with assistance from the US-CDC. However, this protocol is yet to be implemented at the field level [40].
4.1. Lesson learned and way forwards
Addressing recognized risk factors in animal, human, and environmental contexts is crucial to minimize anthrax exposures in animals and prevent spill-over to humans. Controlling animal anthrax by addressing these risk factors at all levels is highly challenging [18]. To prevent animal-level occurrences, addressing anthropological factors among animal raisers is essential. This includes mitigating agro-climatic risk factors, managing uncontrolled animal movements, ensuring adequate vaccination, and fostering knowledge about anthrax epidemiology [31].
Ensuring a regular and sufficient supply of animal anthrax vaccine for all susceptible herbivore animals once a year, supported by logistic capabilities, is imperative [19]. Thoroughly washing grass before feeding it to animals, particularly during outbreak seasons, is highly beneficial in interrupting the transmission pathway [60].
Nevertheless, there is a need for the rapid identification of anthrax cases in animals through community-based participatory surveillance. Testing animal samples at field-level laboratories is challenging. There is a need for basic diagnostic facilities (such as gram stain and PMB stain) and training for laboratory personnel in sample collection and testing.
Infected animals should be treated by registered veterinarians following standard treatment protocols. Creating awareness and motivating farmers through training sessions and the distribution of SBC materials regarding disease transmission and safe disposal of infected animal carcasses are crucial [19].
Appropriate carcass disposal and disinfection of contaminated premises and materials will minimize the pathogen load in the environment. To address financial constraints hindering proper burial of animal carcasses and waste materials, financial support should be ensured for farmers facing losses.
Providing thorough vaccination coverage over an extended period for animal immunization can decrease the prevalence of anthrax in animals, consequently reducing transmission to humans [32,61]. Annual mass animal vaccination, coupled with efforts to discourage the slaughter of infected animals, would be highly commendable.
Prioritizing anthrax vaccination in animals, especially in flooded areas, is crucial to avert new outbreaks [35]. Government intervention should guarantee doorstep animal vaccination services and vaccination campaigns [43]. During outbreak seasons, meat inspection services would help prevent human cutaneous anthrax in rural communities [61].
Considering that Bangladesh's previous anthrax control strategy lacked comprehensive coverage, there is a pressing requirement to incorporate the findings from this research into the document. This will permit a ‘One Health’ multi-sectoral approach, including animal, human and environmental perspectives, to be reinforced. Emphasis should be on infection control and prevention, conducting surveillance, risk communication, animal immunization, outbreak investigation, and response, ensuring health benefits for all species.
4.2. Limitations
It is essential to interpret the findings cautiously as the study has some inherent limitations. Firstly, the review encompassed a limited number of reports studies in each subject area, with the majority of available studies (1980–January 2023) being qualitative in nature. The absence of data on occurrences at both the animal and human levels, the susceptible animal population, and laboratory diagnostic information necessitates the conduct of more robust meta-analysis. Animal anthrax confirmation often relies on clinical features and PMB staining techniques, while human cases are typically confirmed through clinical observations and epidemiological links. However, there are instances where the validation of all confirmed cases was not possible. Finally, our analysis depended exclusively on information extracted from published articles. Incorporating grey literature and accessing national anthrax data for both humans and animals could have significantly enhance the strength and comprehensiveness of our analysis.
5. Conclusions
To effectively break the life cycle of the anthrax bacterium and prevent its transmission to humans, it is crucial to invest in One-Health financing. This approach allows for coordination among various stakeholders to ensure the health of humans, animals, and the environment. By adopting a One Health perspective, significant progress can be made in addressing the burden of anthrax in both animal and human health. This approach should include comprehensive vaccination programs, social and behavioral change activities, environmental management, and the establishment of surveillance systems. By implementing these measures, Bangladesh can effectively control and prevent anthrax outbreaks in both animals and humans.
Funding
This research did not require any funding support.
Data availability statement
The data utilized to support the findings of this study can be found within the text, tables, and supplementary materials included in this manuscript.
CRediT authorship contribution statement
Sk Shaheenur Islam: Writing – review & editing, Formal analysis, Data curation, Conceptualization. Md Samun Sarker: Writing – original draft, Formal analysis, Data curation. A.H.M Taslima Akhter: Writing – original draft, Data curation. Ireen Sultana Shanta: Writing – review & editing. A.K.M. Anisur Rahman: Writing – review & editing, Formal analysis. Md. Abu Sufian: Writing – review & editing, Methodology, Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors express their appreciation to Prof. Michael P. Ward, Sydney School of Veterinary Science, The University of Sydney in Camden, New South Wales, Australia for his valuable contributions in thoroughly editing the manuscript. The authors would like to express their gratitude to Dr. Shovon Chakma, UQ Spatial Epidemiology Laboratory, School of Veterinary Science, University of Queensland, Gatton, Australia for providing important insights and feedback on the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e23481.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Fukao T. Immune system paralysis by anthrax lethal toxin: the roles of innate and adaptive immunity. Lancet Infect. Dis. 2004;4:166–170. doi: 10.1016/S1473-3099(04)00940-5. [DOI] [PubMed] [Google Scholar]
- 2.Fasanella A., Adone R., Hugh-Jones M. Classification and management of animal anthrax outbreaks based on the source of infection. Ann. Ist. Super Sanita. 2014;50:192–195. doi: 10.4415/ANN_14_02_14. [DOI] [PubMed] [Google Scholar]
- 3.Mwakapeje E.R., Høgset S., Fyumagwa R., Nonga H.E., Mdegela R.H., Skjerve E. Anthrax outbreaks in the humans - livestock and wildlife interface areas of Northern Tanzania: a retrospective record review 2006-2016. BMC Publ. Health. 2018;18:106. doi: 10.1186/s12889-017-5007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss M.M., Weiss P.D., Weiss J.B. Anthrax vaccine and public health policy. Am. J. Publ. Health. 2007;97:1945–1951. doi: 10.2105/AJPH.2006.102749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.OIE . 2017. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2017. [Google Scholar]
- 6.Hungerford T. Sidney. ninth ed. McGraw-Hill; 1990. Disease of livestock. [Google Scholar]
- 7.Wilson D. ELSEVIER Health sciences; 2010. Clinical Veterinary Advisor: the Horse. [Google Scholar]
- 8.Halvorson H.O. Two generations of spore research: from father to son. Microbiologia. 1997;13:131–148. [PubMed] [Google Scholar]
- 9.Brown M. Manson's tropical diseases. Lancet Infect. Dis. 2009;9:407–408. doi: 10.1016/S1473-3099(09)70170-7. [DOI] [Google Scholar]
- 10.Islam M.S., Hossain M.J., Mikolon A., Parveen S., Khan M.S.U., Haider N., Chakraborty A., Titu A.M.N., Rahman M.W., Sazzad H.M.S., Rahman M., Gurley E.S., Luby S.P. Risk practices for animal and human anthrax in Bangladesh: an exploratory study. Infect. Ecol. Epidemiol. 2013;3 doi: 10.3402/iee.v3i0.21356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durrheim D.N., Freeman P., Roth I., Hornitzky M. Epidemiologic questions from anthrax outbreak, Hunter Valley, Australia. Emerg. Infect. Dis. 2009;15:840–842. doi: 10.3201/eid1505.081744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewerin S.S., Elvander M., Westermark T., Hartzell L.N., Norström A.K., Ehrs S., Knutsson R., Englund S., Andersson A.-C., Granberg M., Bäckman S., Wikström P., Sandstedt K. Anthrax outbreak in a Swedish beef cattle herd--1st case in 27 years: case report. Acta Vet. Scand. 2010;52:7. doi: 10.1186/1751-0147-52-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mongoh M.N., Dyer N.W., Stoltenow C.L., Khaitsa M.L. Risk factors associated with anthrax outbreak in animals in North Dakota, 2005: a retrospective case-control study. Publ. Health Rep. 2008;123:352–359. doi: 10.1177/003335490812300315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fasanella A., Garofolo G., Hossain M.J., Shamsuddin M., Blackburn J.K., Hugh-Jones M. Bangladesh anthrax outbreaks are probably caused by contaminated livestock feed. Epidemiol. Infect. 2013;141:1021–1028. doi: 10.1017/S0950268812001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakraborty A., Khan S.U., Hasnat M.A., Parveen S., Islam M.S., Mikolon A., Chakraborty R.K., Ahmed B.-N., Ara K., Haider N., Zaki S.R., Hoffmaster A.R., Rahman M., Luby S.P., Hossain M.J. Anthrax outbreaks in Bangladesh, 2009-2010. Am. J. Trop. Med. Hyg. 2012;86:703–710. doi: 10.4269/ajtmh.2012.11-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed B.-N., Sultana Y., Fatema D.S.M., Ara K., Begum N., Mostanzid S.M., Jubayer S. Anthrax: an emerging zoonotic disease in Bangladesh, Bangladesh. J. Med. Microbiol. 2011;4:46–50. doi: 10.3329/bjmm.v4i1.8470. [DOI] [Google Scholar]
- 17.Islam S.S., Chakma S., Taslima Akhter A.H.M., Ibrahim N., Talukder F., Chowdhuary G.A. Investigation of animal anthrax outbreaks in the human-animal interface at risky districts of Bangladesh during 2016-2017. J. Adv. Vet. Anim. Res. 2018;5:397–404. doi: 10.5455/javar.2018.e290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biswas P.K., Islam M.Z., Shil S.K., Chakraborty R.K., Ahmed S.S.U., Christensen J.P. Risk factors associated with anthrax in cattle on smallholdings. Epidemiol. Infect. 2012;140:1888–1895. doi: 10.1017/S0950268811002408. [DOI] [PubMed] [Google Scholar]
- 19.Islam S.S., Castellan D.M., Akhter A.H.M.T., Hossain M.M., Hasan M.Z. Animal anthrax in Sirajganj district of Bangladesh from 2010 to 2012. Asian J. Med. Biol. Res. 2016;1:387–395. doi: 10.3329/ajmbr.v1i3.26444. [DOI] [Google Scholar]
- 20.Sitali D.C., Twambo M.C., Chisoni M., Bwalya M.J., Munyeme M. Lay perceptions, beliefs and practices linked to the persistence of anthrax outbreaks in cattle in the Western Province of Zambia. Onderstepoort J. Vet. Res. 2018;85 doi: 10.4102/ojvr.v85i1.1615. e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Who . 1998. Guidelines for the Surveillance and Control of Anthrax in Humans and Animals. [Google Scholar]
- 22.Kock R., Haider N., Mboera L.E., Zumla A. A One-Health lens for anthrax. Lancet Planet. Health. 2019;3 doi: 10.1016/S2542-5196(19)30111-1. e285–e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moazeni Jula G.R., Jabbari A.R., Malek B. Isolation of anthrax spores from soil in endemic regions of isfahan, Iran. Arch. Razi Inst. 2004;58:29–38. doi: 10.22092/ari.2004.103823. [DOI] [Google Scholar]
- 24.Dey R., Hoffman P.S., Glomski I.J. Germination and amplification of anthrax spores by soil-dwelling amoebas. Appl. Environ. Microbiol. 2012;78:8075–8081. doi: 10.1128/AEM.02034-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahman M.M., Hossain M.S., Haque M.S., Nabi M.R., Morshed M.G., Ahsan G.U. Knowledge and attitude towards anthrax at the anthrax belt sirajgonj district in Bangladesh. J. Vet. Med. One Heal. Res. 2020;2:417–426. doi: 10.36111/jvmohr.2020.2(2).0023. [DOI] [Google Scholar]
- 26.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hróbjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., McGuinness L.A., Stewart L.A., Thomas J., Tricco A.C., Welch V.A., Whiting P., Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh H.-F., Shannon S.E. Three approaches to qualitative content analysis. Qual. Health Res. 2005;15:1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 28.WOAH, Bangladesh: Six-Monthly Report on the Notification of OIE-Listed Diseases. infections and infestations; 2020. [Google Scholar]
- 29.Ferdous Z., McPherson R., Mahjabeen M. Landscape of zoonoses clusters in Bangladesh from 2001 to 2016: impact to national health. Asian J. Res. Infect. Dis. 2022;11:1–14. doi: 10.9734/ajrid/2022/v11i230305. [DOI] [Google Scholar]
- 30.Samad M.A., Hoque M.E. Anthrax in man and cattle in Bangladesh. J. Trop. Med. Hyg. 1986;89:43–45. [PubMed] [Google Scholar]
- 31.Mondal S.P., Yamage M. A retrospective study on the epidemiology of anthrax, foot and mouth disease, haemorrhagic septicaemia, peste des petits ruminants and rabies in Bangladesh. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104435. 2010-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Islam M.S., Hasan S.M.M., Salzer J.S., Kadzik M., Haque F., Haider N., Hossain M.B., Islam M.A., Rahman M., Kennedy E., Gurley E.S. Human exposures to by-products from animals suspected to have died of anthrax in Bangladesh: an exploratory study. Transbound. Emerg. Dis. 2021;68:2514–2520. doi: 10.1111/tbed.13921. [DOI] [PubMed] [Google Scholar]
- 33.Galante D., Manzulli V., Serrecchia L., Di Taranto P., Hugh-Jones M., Hossain M.J., Rondinone V., Cipolletta D., Pace L., Iatarola M., Tolve F., Aceti A., Poppa E., Fasanella A. vol. 10. Pathog.; Basel, Switzerland: 2021. (Investigation on Anthrax in Bangladesh during the Outbreaks of 2011 and Definition of the Epidemiological Correlations). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nazir K.H.M.N.H., Islam M.A. Knowledge and awareness of anthrax among the community people at high, medium and low risk areas of Bangladesh. Emerg. Res. Med. Sci. 2020;3:41–48. https://stm1.bookpi.org/index.php/erms-v3/article/view/39 [Google Scholar]
- 35.Talukdar F., Sabrina M., Sultana R., Sazzad M. Outbreak of cutaneous anthrax in kalukhali upazilla, Rajbari district, Bangladesh 2017. Iproceedings. 2018;4 doi: 10.2196/10644. [DOI] [Google Scholar]
- 36.Rume F.I., Karim M.R., Ahsan C.R., Yasmin M., Biswas P.K. Risk factors for bovine anthrax in Bangladesh, 2010-2014: a case-control study. Epidemiol. Infect. 2020;148:e67. doi: 10.1017/S0950268820000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hassan J., Ahsan M.M., Rahman M.B., Chowdhury S.M.Z.H., Parvej M.S., Nazir K.N.H. Factors associated with repeated outbreak of anthrax in Bangladesh: qualitative and quantitative study. J. Adv. Vet. Anim. Res. 2015;2:158–164. doi: 10.5455/javar.2015.b72. [DOI] [Google Scholar]
- 38.Sarker M.S.A., El Zowalaty M.E., Shahid M.A.H., Sarker M.A., Rahman M.B., Järhult J.D., Nazir K.H.M.N.H. Maximization of livestock anthrax vaccination coverage in Bangladesh: an alternative approach. Vaccines. 2020;8 doi: 10.3390/vaccines8030435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dutta P.K., Biswas H., Ahmed J.U., Shakif-Ul-Azam M., Ahammed B.M.J., Dey A.R. Knowledge, attitude and practices (KAP) towards Anthrax among livestock farmers in selected rural areas of Bangladesh. Vet. Med. Sci. 2021;7:1648–1655. doi: 10.1002/vms3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DLS, Veterinary Public Health Service Strengthening and Protection Project. 2023. [Google Scholar]
- 41.Dipti M., Rashid M., Ferdoush M., Roy P., Khan M., Hossain M. Morphological and immunological characterization of anthrax vaccine in cattle. Bangladesh J. Vet. Med. 2014;11:43–49. doi: 10.3329/bjvm.v11i1.17732. [DOI] [Google Scholar]
- 42.Roy P.R., Rashid M., Ferdoush M., Dipti M., Chowdury M., Mostofa M., Roy S., Khan M., Hossain M. Biochemical and immunological characterization of anthrax spore vaccine in goat. Bangladesh J. Vet. Med. 2014;11:151–157. doi: 10.3329/bjvm.v11i2.19140. [DOI] [Google Scholar]
- 43.Sarker M.S.A., Shahid M.A.H., Hoque M.N., Sarker M.A., Rahman M.B., Islam S.S. The the rich mapping: Be a supplementary approach for anthrax control at community level. J. Adv. Vet. Res. 2021;11:41–46. https://advetresearch.com/index.php/AVR/article/view/609 [Google Scholar]
- 44.DLS, Evaluation of Anthrax Vaccine from USCDC Support. Preventing Anthrax and Rabies in Bangladesh by Enhancing Surveillance and Response Project. PARB); 2021. [Google Scholar]
- 45.IEDCR, Anthrax outbreak . 2010. Anthrax Update.http://www.iedcr.org/pdf/files/anthrax/Anthrax_Update_Last.pdf 2010. [Google Scholar]
- 46.IEDCR, Anthrax outbreak . 2011. Anthrax Update.http://www.iedcr.org/pdf/files/anthrax/Anthrax_2011_final.pd 2011. [Google Scholar]
- 47.IEDCR, Anthrax outbreak . 2012. Anthrax Update 2012.http://www.iedcr.org/pdf/files/anthrax/Anthrax_graph_04.10.12.pdf [Google Scholar]
- 48.IEDCR . 2022. Anthrax Surveillance. [Google Scholar]
- 49.Siddiqui M.A., Khan M.A.H., Ahmed S.S., Anwar K.S., Akhtaruzzaman S.M., Salam M.A. Recent outbreak of cutaneous anthrax in Bangladesh: clinico-demographic profile and treatment outcome of cases attended at Rajshahi Medical College Hospital. BMC Res. Notes. 2012;5:464. doi: 10.1186/1756-0500-5-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haque M.A., Khan M.M.R., Sharmin L.S., Alam K.M.F., Rahman M.K., Alam M.S. Cutaneous anthrax outbreak in Rajshahi district. TAJ J. Teach. Assoc. 2018;30:17–20. doi: 10.3329/taj.v30i1.39116. [DOI] [Google Scholar]
- 51.Karim M.R., Samad M.A., Ali M.Z., Rahman M.H., Hassan M.Z., Yousuf M.A., Kabir M.H., Swapnil A.M., Giasuddin M. Attitude and perception toward anthrax among cattle owners in selected rural communities in Bangladesh, Bangladesh. J. Livest. Res. 2020;21–25:168–172. doi: 10.3329/bjlr.v0i0.45460. [DOI] [Google Scholar]
- 52.Rume F.I., Ahsan C.R., Biswas P.K., Yasmin M., Braun P., Walter M.C., Antwerpen M., Grass G., Hanczaruk M. Unexpected genomic relationships between Bacillus anthracis strains from Bangladesh and Central Europe. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2016;45:66–74. doi: 10.1016/j.meegid.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 53.Rume F.I., Antwerpen M., Braun P., Biswas P.K., Yasmin M., Grass G., Ahsan C.R., Hanczaruk M. Genome sequence of Bacillus anthracis strain tangail-1 from Bangladesh. Genome Announc. 2016;4 doi: 10.1128/genomeA.00748-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahsan M.M., Khan M.F.R., Rahman M.B., Hassan J., Chowdhury S.M.Z.H., Parvej M.S., Jahan M., Nazir K.H.M.N.H. Investigation into Bacillus anthracis spore in soil and analysis of environmental parameters related to repeated anthrax outbreak in Sirajganj, Bangladesh. Thai J. Vet. Med. 2013;43:449–454. [Google Scholar]
- 55.Rume F.I., Karim M.R., Biswas P.K., Yasmin M., Ahsan C.R. Climate change and its influence on occurrence and distribution of Anthrax in Bangladesh. Int. J. Infect. Dis. 2020;101:411. doi: 10.1016/j.ijid.2020.09.1078. [DOI] [Google Scholar]
- 56.Vieira A.R., Salzer J.S., Traxler R.M., Hendricks K.A., Kadzik M.E., Marston C.K., Kolton C.B., Stoddard R.A., Hoffmaster A.R., Bower W.A., Walke H.T. Enhancing surveillance and diagnostics in anthrax-endemic countries. Emerg. Infect. Dis. 2017;23:S147–S153. doi: 10.3201/eid2313.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Who . 2004. WHO & Food and Agriculture Organization of the United Nations. Vitamin and Mineral Requirements in Human Nutrition. [Google Scholar]
- 58.Bhattacharya D., Kshatri J.S., Choudhary H.R., Parai D., Shandilya J., Mansingh A., Pattnaik M., Mishra K., Padhi S.P., Padhi A., Pati S. One health approach for elimination of human anthrax in a tribal district of Odisha: study protocol. PLoS One. 2021;16:1–11. doi: 10.1371/journal.pone.0251041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Owen M.P., Schauwers W., Hugh-Jones M.E., Kiernan J.A., Turnbull P.C.B., Beyer W. A simple, reliable M'Fadyean stain for visualizing the Bacillus anthracis capsule. J. Microbiol. Methods. 2013;92:264–269. doi: 10.1016/j.mimet.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 60.Adone R., Sali M., Francia M., Iatarola M., Donatiello A., Fasanella A. Development of a sterne-based complement fixation test to monitor the humoral response induced by Anthrax vaccines. Front. Microbiol. 2016;7:1–7. doi: 10.3389/fmicb.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Islam M., Mahmud M., Yesmin S., Islam M., Sarker M., Nazir K. Risk factors assessment of zoonotic anthrax among the people at risk (PAR) in selected areas of Bangladesh. Asian J. Med. Heal. 2017;4:1–7. doi: 10.9734/ajmah/2017/32369. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data utilized to support the findings of this study can be found within the text, tables, and supplementary materials included in this manuscript.