Abstract
Ischemia-reperfusion (I/R) injury paradoxically occurs during reperfusion following ischemia, exacerbating the initial tissue damage. The limited understanding of the intricate mechanisms underlying I/R injury hinders the development of effective therapeutic interventions. The Wnt signaling pathway exhibits extensive crosstalk with various other pathways, forming a network system of signaling pathways involved in I/R injury. This review article elucidates the underlying mechanisms involved in Wnt signaling, as well as the complex interplay between Wnt and other pathways, including Notch, phosphatidylinositol 3-kinase/protein kinase B, transforming growth factor-β, nuclear factor kappa, bone morphogenetic protein, N-methyl-D-aspartic acid receptor-Ca2+-Activin A, Hippo-Yes-associated protein, toll-like receptor 4/toll-interleukine-1 receptor domain-containing adapter-inducing interferon-β, and hepatocyte growth factor/mesenchymal-epithelial transition factor. In particular, we delve into their respective contributions to key pathological processes, including apoptosis, the inflammatory response, oxidative stress, extracellular matrix remodeling, angiogenesis, cell hypertrophy, fibrosis, ferroptosis, neurogenesis, and blood-brain barrier damage during I/R injury. Our comprehensive analysis of the mechanisms involved in Wnt signaling during I/R reveals that activation of the canonical Wnt pathway promotes organ recovery, while activation of the non-canonical Wnt pathways exacerbates injury. Moreover, we explore novel therapeutic approaches based on these mechanistic findings, incorporating evidence from animal experiments, current standards, and clinical trials. The objective of this review is to provide deeper insights into the roles of Wnt and its crosstalk signaling pathways in I/R-mediated processes and organ dysfunction, to facilitate the development of innovative therapeutic agents for I/R injury.
Subject terms: Cardiology, Molecular biology, Cardiovascular diseases
Introduction
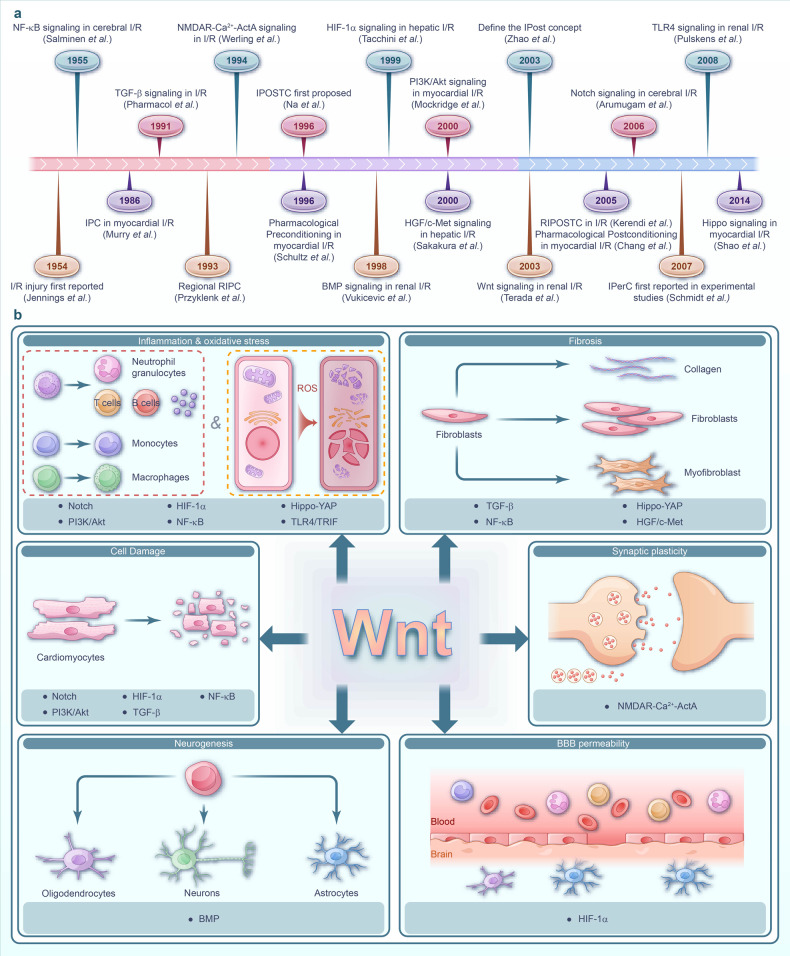
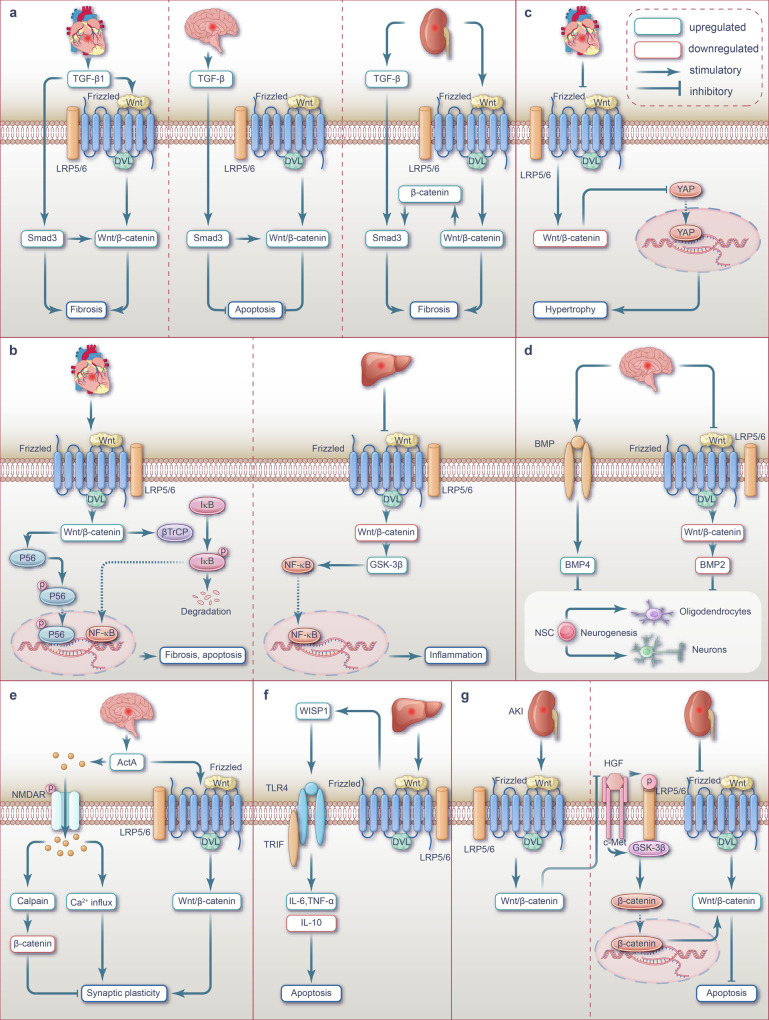
Ischemia of organs can have severe consequences such as myocardial infarction (MI) and cerebral infarction, leading to irreversible tissue damage.1,2 Tissue reperfusion is employed to prevent further ischemia; however, in some cases, it may worsen the injury through a process known as ischemia-reperfusion (I/R) injury,3,4 which can occur in many organs and result in additional disorders, disability, and even death.5 Multiple pathological processes are involved in I/R injuries, such as cell damage (apoptosis, necrosis, and ferroptosis), oxidative stress, inflammatory response, blood-brain barrier (BBB) breakdown, extracellular matrix (ECM) remodeling, angiogenesis, cardiomyocyte hypertrophy, and fibrosis.6–13 Extensive research has been dedicated to unraveling the mechanisms and therapeutic strategies associated with signaling pathways implicated in I/R injury. Several key pathways including Notch, phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt), transforming growth factor-β (TGF-β), nuclear factor kappa (NF-κB), bone morphogenetic protein (BMP), N-methyl-D-aspartic acid receptor (NMDAR)-Ca2+-Activin A, hippo-yes-associated protein (YAP), toll-like receptor 4/toll-interleukine-1 receptor domain-containing adapter-inducing interferon-β (TLR4/TRIF) and hepatocyte growth factor/mesenchymal-epithelial transition factor (HGF/c-Met), and Wnt, have emerged as crucial players in this context.14–24 Fig. 1a depicts the research milestones in the exploration of signaling pathways during I/R injury. Among these pathways, the Wnt signaling pathway, which has attracted attention, consists of multiple branches, with the canonical Wnt/β-catenin, non-canonical Wnt/PCP and Wnt/Ca2+ pathways being particular important for I/R injury. Evidence suggests that different branches of the Wnt pathway play distinct roles in various pathological processes.10,25–29 The Wnt pathway interacts with various key signaling pathways, creating an extensive network that collectively regulates I/R injury as shown in Fig. 1b. During and through I/R injury, the Wnt pathway interacts with NF-κB or HIF-1α signaling, thereby regulating inflammation and oxidative stress responses.30,31 Additionally, the crosstalk between the Wnt pathway and other signaling pathways, including Notch, PI3K/Akt, TGF-β, and NF-κB, is implicated in the regulation of apoptosis.32,33 Moreover, the Wnt/BMP signaling crosstalk is involved in regulating neurogenesis,34 while direct interaction between NMDAR-Ca2+-ActA and Wnt signaling modulates synaptic plasticity.35–41 Furthermore, the Wnt pathway crosstalk with Hippo-YAP, TGF-β, HGF/c-Met, NF-κB, and other signaling pathways regulate fibrosis in organs like the heart, kidney, and liver following I/R injury, which can potentially lead to adverse outcomes.35–44 The current treatment strategies include pre-ischemic preconditioning, post-ischemic preconditioning, and medicine preconditioning45–48 (Fig. 1a). However, the intricate complexity of I/R injury, along with the interconnections among various signaling pathways, remains significantly challenging. Therefore, there remains a lack of consensus in current research,38–43 which limits the advancement of treatment strategies. This article provides a comprehensive overview of the intricate interplay between Wnt signaling and other signaling pathways in the complex signaling network involved in I/R injury (Fig. 1b). The evidence encompasses studies conducted on patients, as well as findings from various animal and cell models. Additionally, by elucidating the underlying mechanisms, we outline current clinical and preclinical therapeutic strategies that target the Wnt pathway and interconnected signaling pathway networks (Fig. 1a). Considering the complex nature of organ damage in I/R injury, targeting network signaling pathways is crucial for effective interventions. Future studies should focus on developing strategies that effectively modulate these interconnected signals to mitigate the detrimental effects of I/R damage.
Fig. 1.
The intricate signaling network in I/R injury pathogenesis. a Timeline diagram of key milestones in I/R Injury research: revealing crucial discoveries and emphasizing complex signaling pathways. b The complexity of signaling pathways in I/R injury Pathology. Involvement of Wnt signaling and crosstalk with diverse signaling pathways in the pathological process of I/R injury: impact on cellular injury, inflammation, oxidation, fibrosis, neurogenesis, synaptic plasticity, and BBB permeability across organs I/R events. I/R ischemia-reperfusion, NF-κB nuclear factor-κB, IPC ischemic preconditioning, TGF-β transforming growth factor-β, RIPC remote ischemic preconditioning, NMDAR N-Methyl-D-Aspartate Receptor, ActA Activin A, IPOSTC ischemic postconditioning, BMP bone morphogenetic protein, HIF-1α hypoxia-inducible factor-1α, PI3K/Akt phosphoinositide-3 kinase/protein kinase B, HGF/c-Met hepatocyte growth factor receptor/mesenchymal-epithelial transition factor, RIPOSTC remote ischemic postconditioning, YAP Yes-associated protein, TLR4/TRIF toll-like receptor 4/toll-interleukine-1 receptor domain-containing adapter-inducing interferon-β, BBB blood-brain barrier
Wnt pathways and organ I/R Injury
Wnt pathways
The Wnt signaling pathway is an essential regulator49 involved in various cell activities, including proliferation, differentiation, migration, and development50–52 and consists of Wnt ligand proteins, Wnt receptors, and other signal transduction accessories such as scattered (Disheveled, Dsh/Dvl) proteins. The pathway can be divided into two categories base on its dependence on β-catenin, namely canonical and non-canonical pathways. Currently, 19 different Wnt ligand proteins have been identified, with some predominantly activating the canonical pathway (Wnt1, Wnt2, Wnt3, Wnt3a, Wnt8a, Wnt8b, Wnt10a, and Wnt10b) and others primarily activating the non-canonical pathway (Wnt4, Wnt5a, Wnt5b, Wnt6, Wnt7a, Wnt7b, and Wnt11).51,53 However, evidence suggests that some ligands (such as Wnt3a, Wnt5a, and Wnt9b) function in both the canonical and non-canonical Wnt pathways.53,54 Frizzled proteins serve as the primary receptors for Wnt signals,55 and function in conjunction with co-receptors such as low-density lipoprotein receptor-related protein 5/6 (LRP5/6)56 and tyrosine kinase co-receptors like recombinant receptor tyrosine kinase (RYK) like orphan receptor 1/2 (ROR1/2) and RYK.57 In the absence of Wnt ligands, β-catenin is targeted for degradation by a “destruction complex” consisting of Axin, adenomatous polyposis coli protein, Casein kinase 1α, and glucogen synthase kinase 3β (GSK-3β).58 However, in the presence of the Wnt ligands, the Wnt/β-catenin pathway is activated through binding the ligands to Frizzleds receptor and co-receptor LRP5/6. Then, β-catenin accumulates in the cytoplasm and is translocated to the nucleus where it binds to the T-cell factor and lymphoid enhancer factor (TCF/LEF),32,33,42–44 initiating the transcription of Wnt downstream target genes.59,60 Conversely, the non-canonical Wnt signaling pathway operates independently of β-catenin and includes the Wnt/planar cell polarity (PCP) and Wnt/Ca2+ pathways,61 both of which are activated when Wnt ligands bind to Frizzleds protein and ROR1/2.49,62–65 In the Wnt/PCP pathway, the activation of small G proteins Rho or Rac1 triggers the activation of c-Jun N-terminal kinase (JNK), which in turn plays a critical role in the rearrangement of the actin cytoskeleton and regulating cell polarity and promoting migration.51,66–69 In the Wnt/Ca2+ pathway, activated PLC induces IP3 production, leading to a substantial increase in intracellular Ca2+ levels. This triggers the activation of Ca2+-dependent effector molecules, including calmodulin-dependent protein kinase II (CaMKII), protein kinase-C (PKC), and calcineurin, and the nuclear factor of activated T cells (NFAT) to initiate the transcription of genes associated with Ca2+-related signaling.49,60,70–72
Wnt pathways during myocardial I/R injury
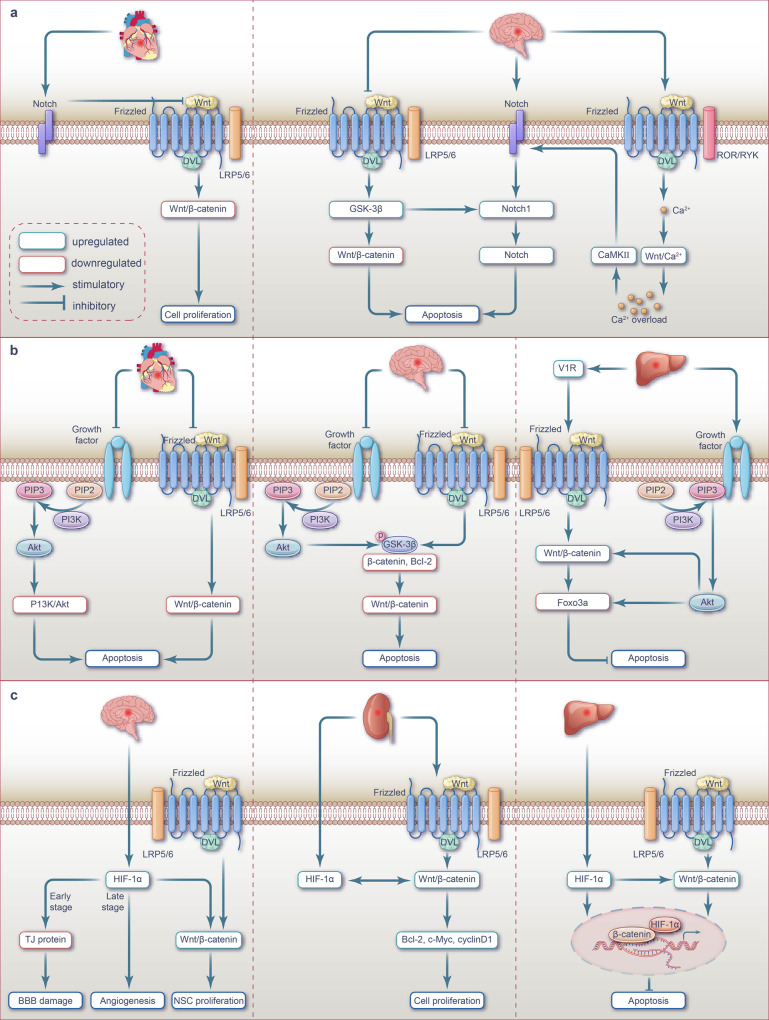
In the treatment of heart and vascular diseases, such as atherosclerosis, coronary artery disease, MI, arrhythmia, myocardial hypertrophy, and heart failure, timely myocardial reperfusion through thrombolysis or percutaneous coronary intervention therapy is crucial.73–76 It can salvage viable myocardium, limit the extent of MI, preserve left ventricular systolic function, and prevent heart failure,77 during which I/R injury often occurs, leading to various detrimental effects on the heart.73–76 The Wnt signaling pathway, initially involved in early heart development and typically inactive under normal conditions, plays a significant role in cardiovascular diseases.61 In the context of myocardial I/R injury, the Wnt pathway is engaged in various I/R-associated processes, including apoptosis,78 inflammatory responses, oxidative stress, ECM remodeling, angiogenesis, cardiac hypertrophy, and fibrosis.4,79–89 Various cell types within the heart, such as cardiac precursor cells, cardiomyocytes, fibroblasts, endothelial cells (ECs), epicardium, smooth muscle cells, adipocytes, and macrophages,85,86,90 play key roles in heart injury via cell-to-cell communication, in which the Wnt signaling pathway serve as a central regulator.85,91,92 With the increasing prevalence of cardiovascular diseases due to population aging, addressing the elevated risk of organ damage resulting from myocardial I/R is of utmost importance.
Apoptosis
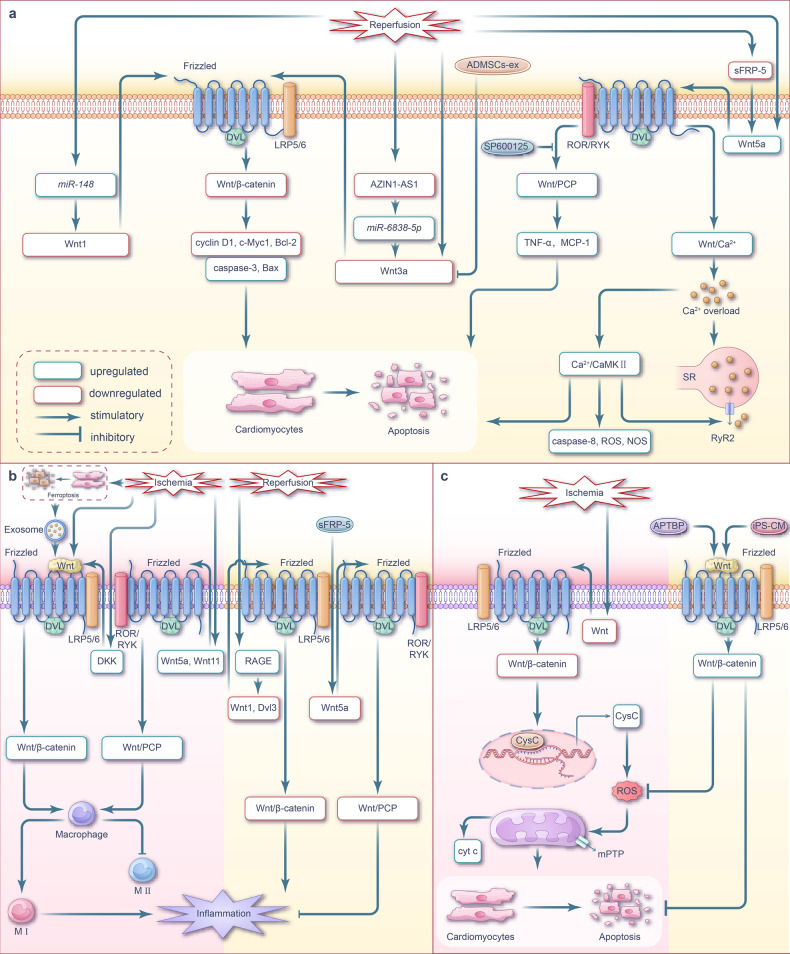
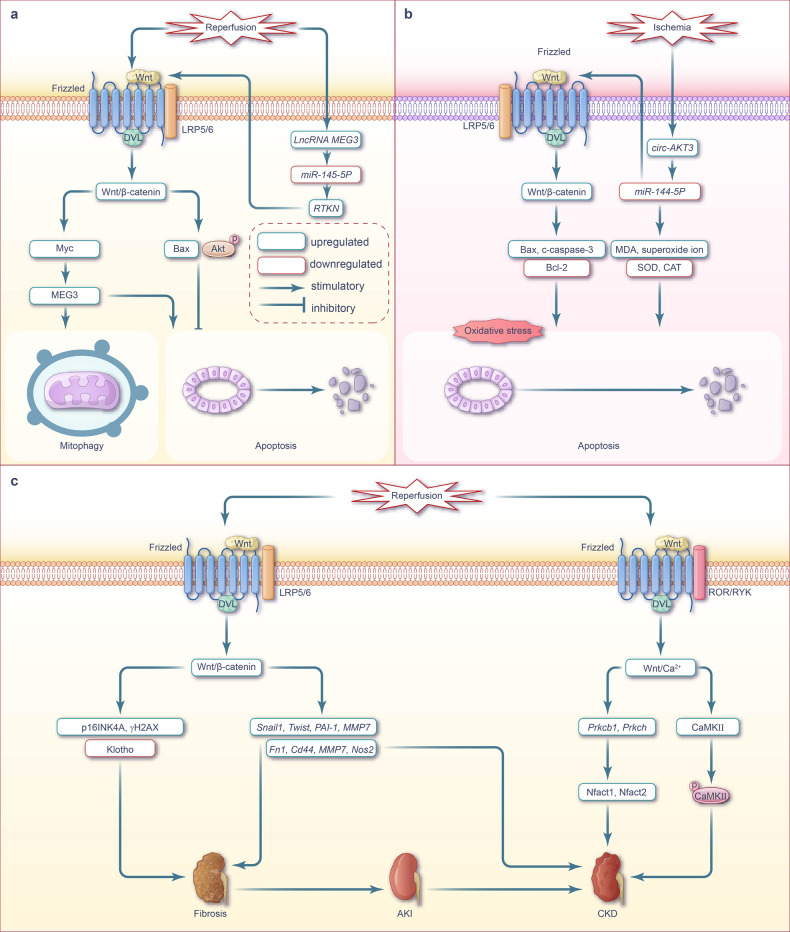
Myocardial I/R inhibits Wnt/β-catenin signaling and upregulates apoptosis; meanwhile, Wnt/PCP and Wnt/Ca2+ signaling pathways have also been implicated in apoptosis activation (Fig. 2a).
Fig. 2.
Wnt signaling pathway and targeted therapy for apoptosis, inflammation, and oxidative stress during myocardial I/R injury. a Wnt signaling pathway-mediated apoptosis during myocardial I/R injury. During myocardial I/R injury, the inhibition of Wnt/β-catenin signaling promotes cardiomyocytes apoptosis. However, the roles of non-canonical Wnt/PCP and Wnt/Ca2+ signaling pathways in myocardial I/R injury are the opposite. The activation of these two Wnt signaling pathways may exacerbate cardiomyocyte apoptosis through the activation of the JNK pathway or the induction of calcium overload. b Wnt signaling-mediated inflammation during myocardial I/R injury. Cardiomyocyte ferroptosis occurs during the ischemia phase, and the exosomes derived from the ferroptotic cells induce M1 macrophage transformation by activating the Wnt/β-catenin pathway. During myocardial ischemic phase, the activation of Wnt/β-catenin signaling promotes the polarization of macrophages towards the M1 phenotype while suppressing the M2 phenotype, ultimately exacerbating the inflammatory response. Upregulation of Wnt ligands and DKK family members in macrophages stimulates inflammation by activating the Wnt/β-catenin signaling pathway. During the process I/R injury, there is an upregulation of RAGE expression in the infarct border zone of rat cardiomyocytes, accompanied by downregulation of Wnt1 and Dvl3 expression. By inhibiting the Wnt/β-catenin signaling pathway, this leads to the promotion of inflammatory response, exacerbating cardiomyocyte apoptosis. Additionally, activation of the Wnt/PCP pathway in macrophages during the ischemic phase increases the expression of inflammatory cytokines, which aggravates cardiac inflammation. c Wnt signaling-mediated oxidative stress during myocardial I/R injury. During myocardial ischemia phase, the downregulation of Wnt protein inhibits Wnt/β-catenin signaling transduction, resulting in increased transcription of intracellular CysC. The elevated expression of CysC exacerbates intracellular oxidative stress and promotes the generation of ROS leading to cardiomyocyte apoptosis. Note: The pink background represents the ischemic phase, while the cream background represents the reperfusion phase. ADMSCs-ex exosomes isolated from adipose-derived mesenchymal stem cells, sFRP-5 secretory frizzled-related protein 5, LRP low-density lipoprotein receptor-related protein, ROR recombinant receptor tyrosine kinase like orphan receptor, RYK receptor tyrosine kinase, CaMKII calmodulin-dependent protein kinase II, ROS reactive oxygen species, NOS nitric oxide synthase, RyR ryanodine receptors, TNF-α tumor necrosis factor-α, MCP-1 monocyte chemoattractant protein-1, APTBP a peptide from tuna backbone protein, CysC cystatin C, mPTP mitochondrial permeability transition pore, cyt c cytochrome c, iPS-CM induced plenipotentiary stem cell-derived conditioned medium
In the myocardium of I/R-induced rats and hypoxia/reoxygenation (H/R)-induced H9C2 cells, a significant upregulation of miR-148b was observed, which inhibited the Wnt/β-catenin signaling by downregulating Wnt1 expression. Consequently, the expression of β-catenin, cyclin D1, C-myc, and the ratio of Bcl-2/Bax was downregulated, while the ratio of cleaved caspase 3 and p-GSK-3β/GSK-3β was upregulated. These changes ultimately increased ischemic area and cardiomyocyte apoptosis rate in I/R rats.93 Zhang et al. found that AZIN1-AS1 was significantly downregulated and miR-6838-5p was significantly upregulated in the myocardium of myocardial I/R rats and H/R H9C2 cells; this dysregulation ultimately led to the downregulation of Wnt3a expression, which in turn inhibited Wnt/β-catenin signaling and induced apoptosis.94 Additionally, Cui et al. established that the inhibition of Wnt/β-catenin signaling in I/R rat myocardium and H/R H9c2 cells, initiated by the downregulation of Wnt3a, promoted cardiomyocyte apoptosis.95
Within the non-canonical Wnt signaling pathway,96,97 secreted frizzled-related protein 5 (sFRP-5) acts as an extracellular inhibitor that counteracts Wnt5a-mediated signaling pathway.98,99 JNK, an essential component of Wnt/PCP signaling, is activated via the non-canonical Wnt pathway.98,100,101 Following I/R treatment, the downregulation of sFRP-5 transcription in the pericardial fat of mice activated macrophage in the injured heart. This activation subsequently upregulated Wnt5a expression, increased JNK phosphorylation, and elevated the expression levels of inflammatory cytokines IL-1β and TNF-α, as well as the chemokine MCP1, which ultimately promoted cardiomyocyte apoptosis via Wnt/PCP pathway activation.102
Zhou et al. demonstrated that the protein levels of Wnt5a and Frizzled2 were elevated, along with the increased intracellular calcium concentration in the myocardium of cardiac I/R rats and H/R H9C2 cells.103 It has been postulated that the Wnt/Ca2+ pathway mediates Ca2+ accumulation and promotes apoptosis. Indeed, Ca2+ overload during myocardial I/R triggers the production of caspase 8, oxygen free radicals, and nitric oxide, which alters the redox environment of calcium channel proteins and transporters. Therefore, the Wnt-associated Ca2+ channels, known as ryanodine receptors, undergo a series of changes during myocardial I/R, including redox modification, phosphorylation, and nitrosation, thereby inducing the dysfunctional opening of diastolic ryanodine receptors channels, leading to ventricular remodeling, arrhythmia, and untimely heart failure.104–106
These findings suggest that targeting the upstream molecules of the Wnt signaling pathway or Wnt itself can inhibit apoptosis and ameliorate myocardial injury in I/R by reversing Wnt signaling (Fig. 2a). For instance, targeting miR-148b93 or miR-6838-5p,94 or adding Wnt3a protein78 before hypoxia has been reported to inhibit cardiomyocyte apoptosis by upregulating Wnt/β-catenin signaling. Cui et al. utilized adipose-derived mesenchymal stem cell exosomes (ADMSC-ex) to treat myocardial I/R. These exosomes upregulated Wnt3a, p-GSK-3β (Ser9), and β-catenin, activated Wnt/β-catenin signaling, upregulated Bcl-2 and cyclin D1, inhibited Bax expression and caspase3 activity, antagonized I/R-induced cardiomyocyte apoptosis, and increased the cell survival rates.95 Alternatively, treatment with recombinant sFRP-5 protein and the JNK inhibitor SP600125 has been reported to inhibit apoptosis by downregulating Wnt/PCP signaling.102
Inflammatory response
An intense surge in cell death over a short period can trigger an inflammatory response and activate cell repair–related pathways. Inflammation serves as an adaptive cellular response to injury. Immune inflammatory pathways play an important role in cardiac injury and repair. However, excessive inflammatory responses can cause severe and irreversible damage to cardiomyocytes.107 The Wnt signaling pathway has emerged as a key regulator of inflammatory responses in myocardial injury, particularly in acute myocardial infarction (AMI) models. Previous studies have shown that myocardial I/R activates Wnt/β-catenin signaling to promote inflammatory responses; however, contrasting studies have reported that inhibition of Wnt/β-catenin signaling promotes such responses. Nonetheless, there is also evidence that non-canonical Wnt signal activation promotes inflammatory responses61 (Fig. 2b).
Following MI, macrophages play distinct roles in left ventricular remodeling. Macrophage polarization and classification are critical for their diverse roles in immune function. An imbalance between pro-inflammatory macrophage (M1) and anti-inflammatory macrophage (M2) activities reflects the inflammatory state of the local cardiac tissue microenvironment.108,109 “In MI stage phase 1 (inflammatory phase,110–112 i.e., 1-4 days after infarction110–113), the macrophages recruited in the infarct area are predominantly M1 type, which secrete pro-inflammatory factors such as TNFα, IL1β, IL6, IL10 to remove cell debris; In the stage phase 2 of MI (reparative phase,110–112 i.e., the 5 -7days after infarction), M2 macrophages are predominantly recruited in the infarct area. The transformation of macrophages helps to promote the regression of inflammation and the repair of damaged myocardium.113,114 However, persistent induction of macrophage M1 phenotype polarization aggravates the inflammatory response through the secretion of IFN-γ,115 leading to cardiomyocyte apoptosis and ECM degradation.116, thereby aggravating myocardial injury.”
Further, both canonical and non-canonical Wnt signaling pathways can promote the polarization of macrophages toward the M1 phenotype and inhibit M2 phenotype polarization.117,118 Therefore, Wnt signaling activation in cardiomyocytes following ischemia may induce cell death in a macrophage-dependent manner, ultimately aggravating myocardial injury (Fig. 3b). Wnt/β-catenin signaling is activated by the inflammatory response during ischemia.119 Sun et al. demonstrated that the malondialdehyde content and Fe2+ concentration in the hearts of mice significantly increased following MI, while the expression of ischemia-susceptibility marker NOS2 was upregulated and that of the M2-polarization marker IL-10 was downregulated in macrophages. The authors suggested that cardiomyocyte ferroptosis occurs during the ischemia phase, accompanied by macrophage polarization toward the M1 phenotype and verified that hypoxic HL-1 cells undergo ferroptosis in vitro and the exosomes derived from ferroptotic HL-1 cells induce M1 macrophage transformation in RAW264.7 cells by activating the Wnt/β-catenin pathway.120
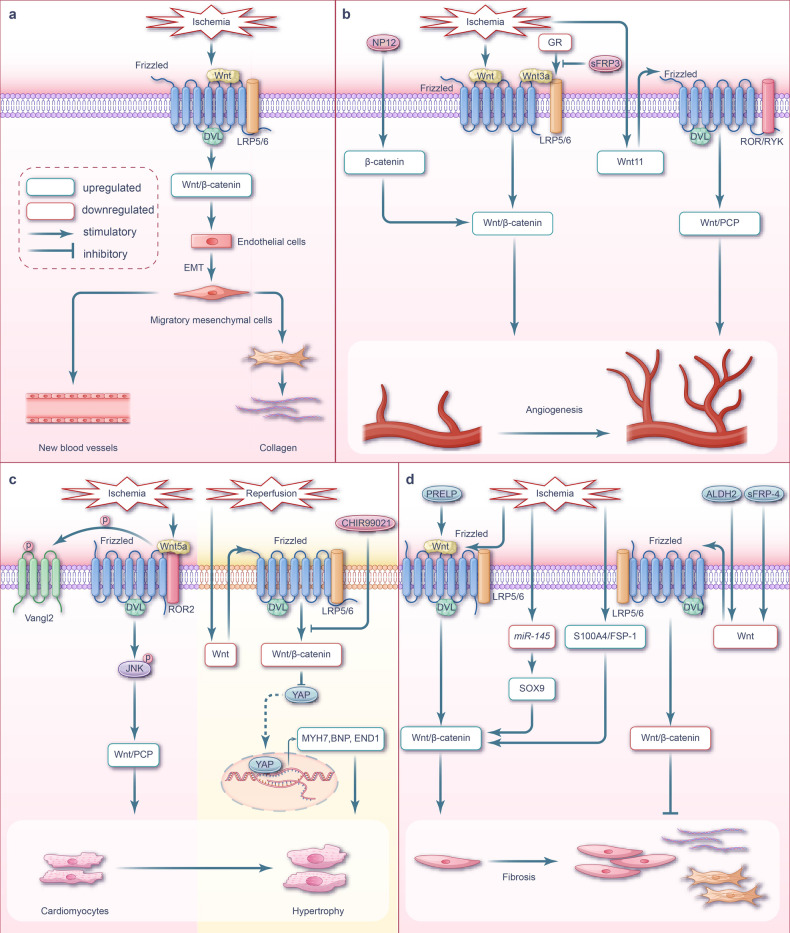
Fig. 3.
Wnt signaling pathway and targeted therapy in ECM remodeling, angiogenesis, cardiac hypertrophy, and fibrosis during myocardial I/R injury. a Wnt signaling-mediated ECM remodeling during myocardial I/R injury. During myocardial ischemic phase, there is a substantial activation of Wnt/β-catenin in endothelial cells, which leads to the promotion of EMT. b Wnt signaling-mediated angiogenesis during myocardial I/R injury. During myocardial ischemic phase, Wnt/β-catenin signaling is activated in vascular endothelial cells, which affects the proliferation and migration of these cells during the process of neovascularization. Conversely, in the infarct region of ischemic mice, the activation of the canonical Wnt pathway functions to suppress cardiac angiogenesis. NP12, an allosteric inhibitor of GSK-3β promotes cardiac angiogenesis by activating Wnt/β-catenin signaling. Additionally, the deficiency of GR activates Wnt/β-catenin signaling, leading to the upregulation of cyclin D1 and ultimately promoting cardiac angiogenesis. Moreover, Wnt/PCP activation contributes to angiogenesis during myocardial ischemia injury. c Wnt signaling pathway-mediated cardiac hypertrophy during myocardial I/R injury. During myocardial ischemic phase, Wnt5a triggers the activation of Wnt/PCP signaling through JNK phosphorylation, which subsequently induces induces cardiomyocyte hypertrophy. In the AC16 human cardiomyocyte cell line model of I/R injury, Wnt/β-catenin signaling is inhibited, and this inhibition synergistically exacerbates myocardial hypertrophy in cooperates with YAP signaling. d Wnt signaling-mediated fibrosis during myocardial I/R injury. During myocardial ischemic phase, the expression of Wnt1 is increased, resulting in the activation of Wnt/β-catenin signaling in cardiac fibroblasts. This activation leads to the proliferation of fibroblasts and ultimately contributes to cardiac repair. MiR‐145 expression was lower in myocardial ischemic phase. The down-regulation of miR-145 directly targets SOX9 in fibroblasts, leading to cardiac fibrosis by activating Wnt/β-catenin signaling. Both PRELP and S100A4/FSP1 promote myocardial fibrosis by activating Wnt/β-catenin signaling. On the other hand, the Wnt antagonist sFRP-4 reduces myocardial fibrosis by inhibiting Wnt/β-catenin signaling. Additionally, ALDH2 suppresses myocardial fibrosis by inhibiting Wnt/β-catenin signaling. Note: The pink background represents the ischemic phase, while the cream background represents the reperfusion phase. EMT endothelial-mesenchymal transition, GR glucocorticoid receptor, sFRP secretory frizzled-related protein, LRP low-density lipoprotein receptor-related protein, ROR recombinant receptor tyrosine kinase like orphan receptor, RYK receptor tyrosine kinase, YAP Yes-associated protein, MYH7 myosin heavy chain 7, BNP brain natriuretic peptide, END1 endothelin 1, SOX9 sex‐determining region Y box 9, FSP1 fibroblast-specific protein 1, PRELP proline/arginine-terminal leucine-rich repeat protein
Using a mouse ischemia model, a previous study has demonstrated that β-catenin-mediated signaling was activated in cardiac macrophages, especially in pro-inflammatory subsets.121 In vitro experiments confirmed that β-catenin activation, and its associated signal transduction pathway, exhibited pro-inflammatory activity in the mouse macrophage cell line RAW264.7 when transduced by lentivirus.121
The expression of Wnt ligands and DKK family members is significantly upregulated in macrophages following MI.122 β-Catenin activation could potentially be attributed to this increase in Wnt ligand expression, although the precise factors involved in this process are yet to be determined. The expression of receptors associated with advanced glycation end products is associated with cell migration, proliferation, inflammation, and I/R injury.123,124 Park et al. determined that RAGE was upregulated, while Wnt1 and Dvl3 were downregulated at the infarct edge of rat I/R cardiomyocytes, consequently promoting the inflammatory response and exacerbating cardiomyocyte apoptosis by inhibiting the Wnt/β-catenin pathway.125
Studies have also indicated that Wnt5a plays a role in the immune system and is upregulated in activated macrophages.101,126 Palevski et al. found that the expression of Wnt ligands, such as Wnt5a and Wnt11, was significantly increased in macrophages at infarcts during MI in mice, while the β-catenin expression levels remained unchanged during MI. The authors hypothesized that non-canonical Wnt signaling, rather than canonical Wnt signaling, is activated in macrophages at infarct sites.118 Following MI in mice, pJNK expression was increased in macrophages at the ischemic edge, the Wnt/PCP pathway was activated, and the Wnt/β-catenin pathway was downregulated, which promoted the transformation of myeloid cells toward a proinflammatory state, thereby aggravating MI.127 While Wnt5a is expressed in cardiomyocytes,128 in vitro cell culture studies have shown that macrophage-derived Wnt5a activated the non-canonical Wnt/Ca2+ signaling pathway via CaMKII and activated NFAT during sepsis.61 This signaling pathway induces the expression of pro-inflammatory factors, such as IL-1, IL-6, IL-8, and MIP-1, and enhances inflammatory macrophage activation.129
Overall, these findings suggest that targeting the upstream Wnt signaling components or Wnt itself can inhibit the inflammatory response by reversing the Wnt signaling pathway (Fig. 2b). Adeno-associated virus-mediated overexpression of Wnt Inhibitory Factor 1 inhibits the activation of non-canonical Wnt signaling, thereby lowering the expression of IL-1b and IL-6, and exerting an anti-inflammatory effect in heart tissue following acute MI.127 To inhibit the expression of RAGE induced by I/R, Park et al. used deoxycholic acid-modified polyethyleneimine as a carrier to introduce RAGE-targeting siRNA into the myocardium. Treatment of rat I/R cardiomyocytes (H9C2 cells) with this PEI-DA/siRAGE complex downregulated the expression of pro-inflammatory cytokines IL-6 and TNF-α, reduced cardiomyocyte apoptosis, and suppressed the infiltration/proliferation of non-cardiomyocytes, exerting anti-apoptotic and anti-inflammatory effects via Wnt/β-catenin activation.125
Wntless (Wls) is a conserved multi-channel transmembrane protein that promotes the release of Wnt ligands.130 In a mouse model with Wls−/− myeloid cells, Wnt signaling was blocked in cardiac macrophages during ischemia and Wnt signaling–mediated macrophage transition toward the M1 phenotype was inhibited, resulting in an accumulation of M2-like macrophages in the MI region. Wls–deficient mice have reduced cardiac remodeling and improved cardiac function following MI due to the anti-inflammatory, repair-promoting, and angiogenesis effects of M2 macrophage.118 Data on whether canonical or non-canonical Wnt signaling is activated in the cardiac macrophages of ischemic mice remain inconsistent,119–121,125 possibly attributed to the different research models used. Indeed, in vivo studies typically indicate that the activation of non-canonical Wnt signaling leads to macrophage polarization,118,127 whereas in vitro studies often implicate canonical Wnt signaling activation in macrophage polarization.119,121,125 In addition, even when in vivo models are used, the results differ depending on the pathological regions of interest. For example, in the non-infarcted area of the heart, canonical Wnt signaling is dominant, whereas, in the infarct area, non-canonical Wnt signaling is more prominent125,127; therefore, the primary signaling pathway that regulates macrophage polarization within the infarct area during I/R may be the non-canonical Wnt signaling pathway.
Oxidative stress
Cardiac ischemia leads to Wnt/β-catenin signaling inhibition, oxidative stress elevation, and additional damage to cardiomyocytes (Fig. 2c).
In the cardiomyocytes of ischemic mice, Wnt protein expression is reduced and Wnt/β-catenin signaling is inhibited. As the β-catenin in the cytoplasm is degraded, it cannot enter the nucleus, which blocks the inhibition of the transcription of cytochrome c (cyt c).78 The corresponding increase in cyt c aggravates oxidative stress within the cells and mediates an increase in intracellular reactive oxygen species (ROS).78 In turn, this excessive production of ROS increases mitochondrial membrane transport channel permeability, mitochondrial membrane potential loss, and cyt c release, ultimately inducing cardiomyocyte apoptosis.78 Guo et al. determined that the Wnt/β-catenin pathway was similarly inhibited in ischemic H9C2 cells and also leads to oxidative stress.131
These findings suggest that targeting the upstream molecules of the Wnt signaling pathway or directly addressing Wnt can reverse the effects of Wnt signaling and reduce oxidative damage (Fig. 2c). Correspondingly, exogenous Wnt3a administration has been reported to activate the Wnt/β-catenin pathway and inhibit oxidative stress.78 Additionally, a peptide derived from tuna backbone protein (APTBP), known for its antioxidant properties, can scavenge ROS.132 Under ischemia and I/R injury conditions, APTBP eliminates ROS, restores the activity of Wnt/β-catenin in a dose-dependent manner, protects mitochondria from oxidative stress, and maintains myocardial function.133 Alternatively, Guo et al. determined that supplementation of H9C2 cells with induced pluripotent stem cell–derived conditioned medium upregulated Wnt/β-catenin signaling, promoted cardiomyocyte proliferation, and inhibited oxidative stress and cell senescence.131
ECM remodeling
The Wnt/β-catenin signaling pathway is activated in the myocardium of ischemic mice and promotes ECM remodeling (Fig. 3a).
Four days following experimental MI, β-catenin was upregulated and Wnt/β-catenin signaling was significantly enhanced in mouse subepicardial ECs and mesenchymal cells expressing smooth muscle actin.122 Similarly, in mature ECs following MI, the nuclear translocation of β-catenin, upregulation of canonical Wnt signaling response promoters, and activation of canonical Wnt signaling were found to inhibit endothelial markers, induce mesenchymal phenotypes, and upregulate smooth muscle and myofibroblast markers, which are potentially involved in angiogenesis and fibrosis.122 Thus, Wnt/β-catenin signaling may be involved in cardiac tissue repair via endothelial–mesenchymal transition (EMT) during MI (Fig. 3a).
Angiogenesis
During myocardial I/R, Wnt/β-catenin pathway activation promotes angiogenesis, although some studies have reported that activation of this pathway inhibits angiogenesis. In addition to canonical Wnt signaling, activation of the non-canonical Wnt/PCP pathway is beneficial to angiogenesis (Fig. 3b).
Angiogenesis can repair the injury caused by I/R injury and mitigate cell death. Evidence of intracellular localization of β-catenin indicates that Wnt/β-catenin signaling participates in the proliferation and migration of vascular ECs during neovascularization.134 Blankesteijn et al. determined that β-catenin protein was expressed in new blood vessels and original vascular ECs within the infarcted area one-week post MI; additionally, expression of the protein upstream of the Wnt/β-catenin pathway, DVL1, was upregulated in the infarcted rat heart.134The authors speculated that the Wnt/β-catenin signaling pathway is activated in the vascular endothelial cells in the infarcted area following ischemia, influencing the proliferation and migration of vascular ECs during neovascularization.134 In AMI mice, the canonical Wnt pathway was activated at the infarct area, β-catenin accumulated in the cardiac vascular cells, the capillary density of the ischemic scar was reduced, and cardiac function damage was aggravated.135,136
Wang et al. established that after the left anterior descending coronary artery (LAD) ligation, Wnt11 expression in rat myocardial tissue significantly decreased in a time-dependent manner, while infarct size increased; following reperfusion, capillary-like tube formation and human umbilical vein endothelial cells angiogenesis were also observed. The authors postulated that the activation of atypical Wnt11/PCP pathway increases angiogenesis and improves cardiac function.50
These findings suggest that targeting the Wnt signaling pathway can promote vascular regeneration following cardiac I/R injury (Fig. 3b). The GSK-3β allosteric inhibitor NP12, which stabilizes β-catenin and activates the Wnt signaling pathway, promotes angiogenesis, and improves cardiac function during MI.136 In primary mouse aortic ECs, glucocorticoid receptor (GR) deficiency promotes angiogenesis. Under these conditions, GR deficiency activates Wnt/β-catenin signaling by facilitating the binding of LRP5/6 to Wnt3a, leading to the accumulation of β-catenin in the nucleus and resulting in the upregulation of the angiogenic regulator cyclin D1.137 Alternatively, the addition of Wnt11 activated the atypical Wnt11/PCP pathway to upregulated angiogenesis.50
Cell hypertrophy
Cardiomyocytes exit the cell cycle and begin terminal differentiation shortly after birth.138 Therefore, in the adult heart, the increase in cardiomyocyte size, rather than number, induces hypertrophy. This hypertrophy helps to reduce wall pressure and maintain cardiac function and efficiency in response to increased workload.139 However, pathological hypertrophy can occur under adverse stimulation conditions, such as myocardial ischemia, and lead to maladaptive cardiac remodeling and heart failure.140 In myocardial I/R, Wnt/β-catenin signaling is inhibited and cell hypertrophy is promoted, by the Wnt/PCP signaling pathway61 (Fig. 3c).
Dpr1 is necessary for Wnt5a signaling, which induces cardiomyocyte hypertrophy and activates Wnt/PCP signaling in cardiomyocytes.141 Localization of the Wnt/PCP transmembrane receptor Van-Gogh-like-2 (Vangl2) is predominantly within the membrane and cytoplasm; however, in cells without Dpr1, Vangl2 has been shown to significantly accumulate within vesicles in the perinuclear region.141 During ischemic injury, the Wnt5a /PCP pathway is activated by the ROR2/Vangl2/JNK axis, and Wnt/β-catenin signaling is inhibited, thus promoting post-ischemic myocardial hypertrophy.141 Following I/R injury, the cell hypertrophy of AC16 human left ventricular cardiomyocytes is exacerbated, Wnt/β-catenin signaling is inhibited, and the expression of hypertrophy markers, including myosin heavy chain 7, brain natriuretic peptide, and endothelin 1, is significantly increased.142
Overall, therapy targeting the Wnt/β-catenin signaling pathway may potentially mitigate cellular hypertrophy and reduce myocardial remodeling and heart failure. For instance, treatment with CHIR99021, a GSK-3β inhibitor, following MI activates Wnt/β-catenin signaling, which, in conjunction with the Yes-associated protein (YAP) pathway, can alleviate cardiomyocyte hypertrophy.142
Fibrosis
In the normal heart, fibroblasts remain quiescent and are predominantly involved in the daily maintenance of the ECM. These cells are activated and significantly expand following ischemic myocardial injury, thereby initiating excessive ECM remodeling and fibrosis. Interestingly, the Wnt/β-catenin signaling pathway is activated in the hearts of ischemic mice and promotes fibrosis (Fig. 3c).
Zhao et al. established a rat ischemia model via ligation of LAD ligation model. The authors established that the expression levels of Wnt1, β-catenin, and phosphorylated GSK-3β were significantly higher in this ischemic model than those in the corresponding control group; further, ischemic rats exhibited left ventricular dysfunction, pathological heart failure, and signs of cardiac remodeling. The authors attributed these changes to the corresponding activation of Wnt/β-catenin signaling after MI.143 Similarly, Qian et al. found that the expression levels of β-catenin and cardiac fibrosis markers were significantly increased in hypoxic cardiac fibroblasts cultured in vitro and ischemia mouse models, alongside observations of aggravated myocardial fibrosis, which was attributed to the activation of Wnt/β-catenin signaling.144 In addition, miR‐145 expression was lower in MI rats and hypoxic CFs, which was accompanied by cardiac dysfunction and excessive fibrosis in vivo, and activated CFs in vitro.145 Cui et al. determined that miR-145 could directly target sex-determining region Y box 9 (SOX9) in fibroblasts and reduce cardiac fibrosis by downregulating the canonical Wnt signaling pathway in an ischemic rat model.145 Further, Matsushima et al. discovered that the left ventricular wall of the rat heart decreased on thickness during ischemia, while the left ventricular cavity increased significantly; these effects were similarly accompanied by an increase in inactivated GSK-3β expression and β-catenin activity. This activation of β-catenin stimulated the proliferation of collagen-producing cells at the ischemic edge and, ultimately, promoted fibrosis.146 Following acute ischemic heart injury, Wnt1 expression is upregulated, which induces the proliferation of cardiac fibroblasts, increases the expression of pro-fibrotic genes by activating Wnt1/β-catenin signaling, and promotes cardiac repair.147 In contrast, inhibition of Wnt1/β-catenin signaling in cardiac fibroblasts can impair cardiac function and ventricular dilatation.147
These findings ultimately suggest that targeting factors upstream of Wnt and its corresponding signaling pathways can reduce myocardial fibrosis (Fig. 3d). Zhang et al. established AMI mouse and cell culture models within an oxygen-glucose deprivation environment. Proline/arginine-rich terminal leucine repeat protein was found to increase myocardial infarct size following ischemia, both in vivo and in vitro, by activating the downstream Wnt/β-catenin signaling pathway and promoting myocardial fibrosis and ventricular remodeling.148 Knockouts of S100A4, a calcium-binding protein observed in mouse cardiac fibroblasts and cardiomyocytes, showed a significant reduction in β-catenin levels and cardiac fibrosis.144 Matsushima et al. demonstrated that sFRP-4 expression was upregulated in the ischemic border region of LAD-ligated rat hearts.146 Following ischemia and reperfusion injury, administration of sFRP-4 protein to rat intracardiac muscle improved cardiac function. Histological and immunohistochemical staining of cardiac sections from the untreated (without sFRP-4) rats indicated that following I/R injury, the left ventricular wall thickness decreased, left ventricular cavity size significantly increased, deactivated GSK-3β levels increased, and β-catenin activity was upregulated. Overall, this stimulated the proliferation of collagen-producing cells at the ischemic border region and promoted fibrosis. In contrast, the size of the left ventricular cavity in sFRP-4-treated rat hearts did not increase in size; further, sFRP-4 treatment at the early stages of ischemia could inhibit cell proliferation and reduce cardiac fibrosis by inhibiting Wnt/β-catenin signaling activation.146
Wnt pathways during cerebral I/R injury
The timely identification and intervention of ischemic stroke are of utmost importance. The current recommended therapeutic strategy for ischemic stroke involves thrombolysis, via recombinant tissue plasminogen activator (rtPA) injection within 4.5 h post onset,149–152 alongside mechanical thrombectomy within 24 h post onset.150,151,153 Extending the treatment time window has been a focus of previous studies, and conflicting results on the clinical effects of bridging therapy (intravenous thrombolysis with rtPA before mechanical thrombectomy) have been reported154–157; leading to ongoing controversy.158
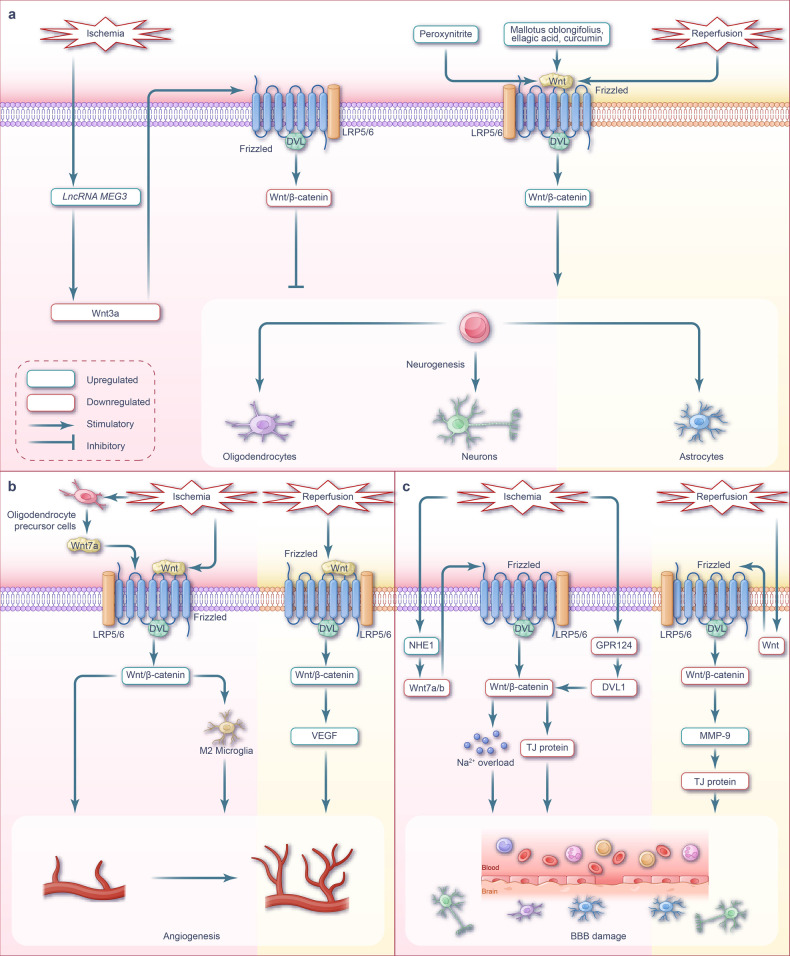
Cerebral I/R injury usually occurs during reperfusion therapy for cerebrovascular diseases such as ischemic stroke. The Wnt signaling pathway controls the proliferation, differentiation, and migration of neurons, the development of neural crests, the growth of axons and dendrites, and the maintenance of angiogenesis and the BBB within the mammalian embryonic and postnatal brain.159–166 Nonetheless, this Wnt-mediated regulation can continue into adulthood. During cerebral I/R injury, the Wnt signaling pathway transitions from an activated state to an inhibited state as ischemia time increases, and is regulated by many processes such as autophagy.10,29,149,167,168 The inhibitory Wnt signaling pathway is primarily associated with apoptosis, ferroptosis, neurogenesis, angiogenesis, BBB damage, inflammatory responses, and oxidative stress. Abnormal activation or inhibition of the Wnt signaling pathway has been observed in different cell types within the nervous system, including neurons, microglia, astrocytes, oligodendrocytes, and ECs. Therefore, targeting this pathway holds potential for mitigating cerebral ischemia and subsequent reperfusion injury.
Apoptosis
Cerebral I/R injury inhibits Wnt/β-catenin signaling, which contributes to neuronal apoptosis.169,170 However, compensatory activation of the Wnt/β-catenin signaling pathway can occur during early ischemia to counteract apoptosis.171 Previous studies have shown that during cerebral I/R injury, Wnt/PCP signaling is initially activated before being inhibited, which can antagonize Wnt/β-catenin signaling and accelerate apoptosis. This early activation of Wnt/PCP signaling may be a significant factor contributing to the inhibition of Wnt/β-catenin signaling.29 Changes in Wnt/Ca2+ signaling during cerebral I/R injury remain unclear; however, the intracellular calcium overload induced by its activation is a crucial mechanism of apoptosis172 (Fig. 4a).
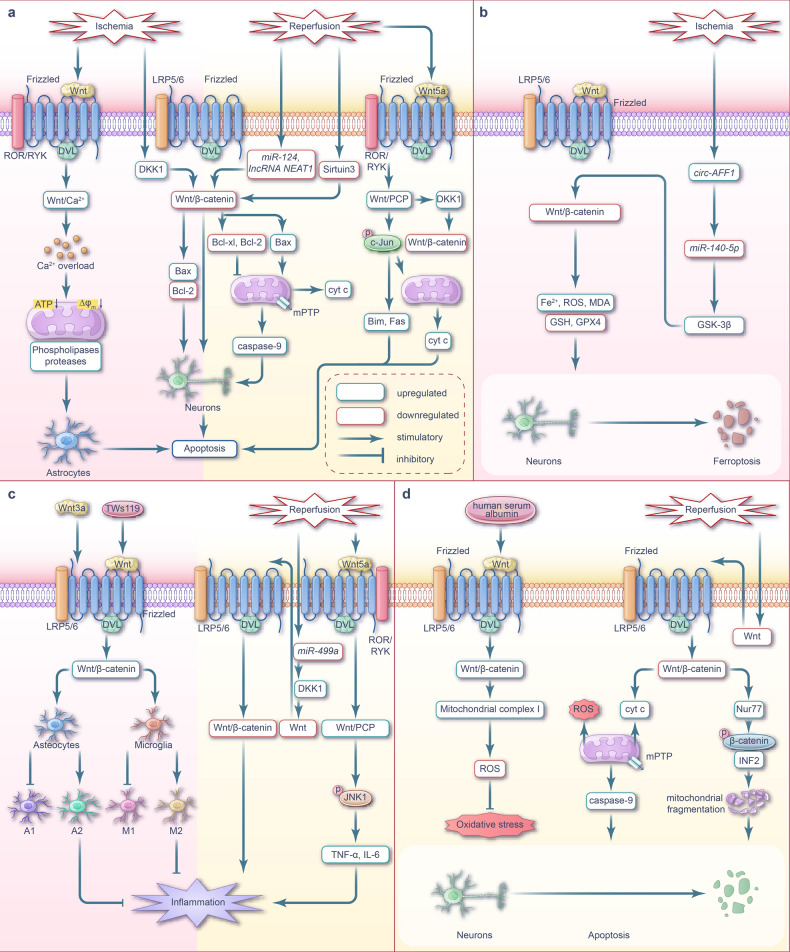
Fig. 4.
Wnt signaling pathway and targeted therapy for apoptosis, ferroptosis, inflammation, and oxidative stress during cerebral I/R injury. a Wnt signaling-mediated apoptosis during cerebral I/R injury. During cerebral ischemic phase, Wnt/Ca2+ signaling is activated, leading to intracellular calcium overload and and subsequent astrocyte apoptosis. The upregulation of DKK1 inhibits Wnt/β-catenin pathway, leading to neuronal apoptosis. During cerebral I/R phase, Wnt5a-mediated Wnt/PCP signaling is activated, promoting c-Jun phosphorylation, inducing cyt c release from mitochondria, inhibiting Wnt/β-catenin signaling, and ultimately leads to neuronal apoptosis. Downregulation of Sirtuin3, miR-124 and lncRNA NEAT1 also inhibit Wnt/β-catenin signaling. b Wnt signaling-mediated ferroptosis during cerebral I/R injury. During cerebral ischemia phase, circ-AFF1 is highly expressed and directly targets miR-140-5p to upregulate GSK-3β. The highly expressed GSK-3β inhibits Wnt/β-catenin signaling. The inhibition of Wnt/β-catenin signaling leads to excessive accumulation of Fe2+, ROS and MDA, and suppression of GSH and GPX4 expression, thereby aggravating neuronal ferroptosis. c Wnt signaling-mediated inflammation during cerebral I/R injury. During cerebral ischemic phase, Wnt/β-catenin signaling is activated, which promotes the polarization of reactive microglia to M2 phenotype, increases the number of A2 type of astrocytes, and reduces the number of A1 type of astrocytes, thereby playing a protective effect and reducing the inflammatory response caused by cerebral ischemia. During cerebral I/R phase, downregulation of miR-499a leads to inhibition of Wnt/β-catenin signaling, thereby aggravating the inflammatory response. Wnt5a-mediated Wnt/PCP signaling is activated during cerebral I/R, leading to upregulation of the pro-inflammatory cytokines, thus aggravates the inflammatory response during cerebral I/R. d Wnt signaling-mediated oxidative stress during cerebral I/R injury. During cerebral I/R phase, Wnt/β-catenin signaling is inhibited which lead to neuronal apoptosis via mitochondria dysfunction. The expression of Nur77 is stimulated in the oxidative stress environment during cerebral I/R, which leads to mitochondrial fragmentation by promoting β-catenin phosphorylation and INF2 expression. Intravenous injection of human serum albumin activates Wnt/β-catenin signaling, thereby increasing mitochondrial complex I activity, reducing ROS generation, suppressing oxidative stress, and playing a therapeutic role during cerebral I/R. Note: The pink background represents the ischemic phase, while the cream background represents the reperfusion phase. LRP low-density lipoprotein receptor-related protein, ROR recombinant receptor tyrosine kinase like orphan receptor, RYK receptor tyrosine kinase, ATP adenosine triphosphate, Δφ membrane potential, DKK1 Dickkopf-1, cyt c cytochrome-c, GSH glutathione, GPX4 glutathione peroxidase 4, GSK-3β glucogen synthase kinase 3β, JNK1 c-Jun amino-terminal kinase1, TNF-α tumor necrosis factor-α, TWS119 a GSK-3β inhibitor that activates Wnt/β-catenin signaling, Nur77 nuclear hormone receptor NUR/77, INF2 inverted formin 2, mPTP mitochondrial permeability transition pore
In the hours to days following acute cerebral ischemia or traumatic brain injury, neuronal and glial cell apoptosis is initiated, predominantly in the ischemic penumbra; while rapid cell necrosis occurs in this ischemic core.173 DKK1, a negative regulator of Wnt/β-catenin signaling, is elevated in the plasma of patients with ischemic stroke and in the neurons of cerebral ischemia animal models.174–176 Specifically, DKK1 has been reported to bind to LRP5/6, activate GSK-3β, inhibit Wnt/β-catenin signaling, and promote neuronal apoptosis by increasing the expression of the pro-apoptotic protein Bax and reducing the expression of the anti-apoptotic protein Bcl-2.177 During cerebral I/R, Wnt/β-catenin signaling is inhibited by a decrease in Sirtuin3 levels169 and downregulation of miR-124178 and lncRNA NEAT1,179 ultimately leading to neuronal apoptosis.
Previous studies using mouse and rat cerebral I/R and cellular H/R models have reported that the downregulation or inactivation of Wnt/β-catenin signaling can induce the expression of the pro-apoptotic protein Bax and downregulate the anti-apoptotic protein Bcl-2, promoting neuronal apoptosis.169,170,180,181 Specifically, Bax promotes the release of cyt c from the mitochondria, whereas Bcl-xL and Bcl-2 inhibit this process.182 During cerebral I/R injury, the inhibition of Wnt/β-catenin signaling leads to mitochondrial damage, including excessive mitochondrial fission,169 cyt c release, caspase 9 activation,181 and subsequent caspase three activation, ultimately resulting in cell apoptosis.182 Li et al. demonstrated that in vitro neuronal oxygen-glucose deprivation/reoxygenation (OGD/R) treatment activated the Wnt/β-catenin signaling pathway and resulted in apoptosis inhibition and improved neuronal survival. Correspondingly, the authors suggested that this may be an early adaptive response to cerebral I/R injury.171
The non-canonical Wnt/PCP and Wnt/Ca2+ signaling pathways also play key roles in apoptosis following cerebral I/R injury. JNK3 is a major JNK subtype activated in cerebral ischemia, while β-arrestin2 is a scaffold protein involved in the regulation of JNK3 signaling.183 Wei et al. showed that Wnt5a expression increased after cerebral I/R in rats but decreased 24 h later. Additionally, the interaction between Dvl-1, β-arrestin2, and JNK3 was enhanced 3 h post-reperfusion. Wnt5a promotes the assembly of the Dvl-1–arrestin2–JNK3 module, thereby activating JNK3 and promoting c-Jun phosphorylation.29 Whether JNK-related signals promote or inhibit apoptosis depends on various conditions. JNK3 activation promotes the release of mitochondrial cyt c and induces the transcription of pro-apoptotic proteins, such as Bim and Fas, and their corresponding receptor genes, resulting in neuronal apoptosis.184 Zhang et al. demonstrated that JNK/c-Jun pathway activation can induce the expression of DKK1, thus inhibiting the canonical Wnt pathway.185 The increased expression of Wnt5a not only activates the Wnt/PCP pathway, but also upregulates DKK1—which has an antagonistic effect on canonical Wnt signaling—jointly mediating the occurrence of apoptosis and aggravating cerebral I/R injury.29 Niu et al. reported that activation of Wnt/Ca2+ signaling during brain injury resulted in calcium overload and cell death in hippocampal astrocytes.172 Cerebral ischemia increases H+ levels in brain cells, thereby opening acid-sensitive ion channels. The acid-sensing ion channel is a dual-ligand gated channel using Ca2+ and H+; therefore, its activation leads to Ca2+ overload in brain cells during cerebral ischemia. Abnormally high levels of Ca2+ eliminate mitochondrial oxidative phosphorylation, reduce mitochondrial membrane potential and adenosine triphosphate content, activate phospholipases and proteases, and cause irreversible damage to brain cells.186
Overall, targeting the Wnt signaling pathway can potentially mitigate apoptosis caused by cerebral I/R injury (Fig. 4a). Isoflurane, an inhalational anesthetic, exhibits neuroprotective effects in ischemic and hypoxic brain injury.187 In a rat cerebral I/R injury model, isoflurane postconditioning (inhalation of 1.5% isoflurane for 60 min following reperfusion) could activate the Wnt/β-catenin signaling pathway and inhibit neuronal apoptosis.170 In addition, similar effects have been reported in animal and cell models of cerebral I/R that were treated with ginkgolide B derivative XQ-1H by gavage180 and oxymatrine via intraperitoneal injection.188
Ferroptosis
Ferroptosis is a form of cell death driven by oxidative stress and iron overload. Cerebral I/R injury-initiated ferroptosis is regulated by Wnt/β-catenin signaling. Therefore, Wnt/β-catenin signaling is a promising target for cerebral I/R injury treatment (Fig. 4b).
Yan et al. demonstrated that neuronal ferroptosis occurred during the reperfusion of cerebral I/R. Ferroptosis is associated with an increase in brain-derived, rather than blood-derived, thrombin and the subsequent release of arachidonic acid.189 Acyl-CoA synthetase long-chain family member 4 (ACSL4) can catalyze the formation of CoA from arachidonic acid, resulting in an accumulation of lipid peroxides and, ultimately, triggering ferroptosis.190 Timely downregulation of ACSL4 during cerebral I/R exhibits a protective role via the initiation or inhibition of thrombin elevation, which can reduce subsequent neuronal ferroptosis.189 Additionally, neuronal ferroptosis causes iron overload191–193 and decreases GPX4 expression194 in the brain tissue during cerebral ischemia. The application of iron chelators195,196 or drugs that improve iron metabolism, such as lycopene,197 to reduce brain iron levels can alleviate cerebral I/R injury. Notably, selenium can promote mitochondrial fusion by inducing Mfn1 expression, thereby improving the oxidative stress and ferroptosis caused by brain I/R in mice.194
In cerebral hemorrhage injury, downregulation of Wnt/β-catenin signaling contributes to neuronal ferroptosis.198 Nuclear erythroid 2-related factor 2 (Nrf2) is a transcription factor associated with antioxidant stress. GSK-3β reduces the expression of GPX 4 and induces ROS generation,199 which initiates a cytotoxic response caused by oxidative stress during dopaminergic neuronal death progression via the inhibition of Nrf2 signaling.200
Targeting GSK-3β to activate the Wnt/β-catenin signaling pathway is a potential therapeutic strategy to curb ferroptosis in cerebral I/R injury (Fig. 4b). For example, the knockdown of the circAFF1 gene can upregulate miR-140-5p, thereby reducing GSK-3β expression, which in turn activates the Wnt/β-catenin signaling pathway.198 The activated Wnt/β-catenin signaling pathway reduces the accumulation of Fe2+, ROS and malondialdehyde, and induces the expression of glutathione and GPX4, thus inhibits neuronal ferroptosis.198 Wang et al. demonstrated that direct knockout of GSK-3β could downregulate the expression of the divalent metal transporter 1, ferritin heavy chain, and ferritin heavy chain polypeptide 1 genes, reduce the number of intracellular free iron, and initiate anti-ferroptosis mechanisms. The authors attributed these findings to the GSK-3β knockout preventing the activation of downstream Wnt/β-catenin signaling.201
Inflammatory response
The Wnt/β-catenin signaling pathway is inhibited during cerebral I/R, which exacerbates the pro-inflammatory response. The non-canonical Wnt/PCP signaling pathway is also activated following cerebral I/R and may also aggravate inflammation via unclear mechanisms (Fig. 4c).
The inflammatory response following cerebral ischemia exhibits a dual role. On one hand, the release of inflammatory mediators initiates acute BBB disruption and neuronal damage, while on the other hand, inflammation plays a vital role in the repair process during ischemia.202 The rapid expression of multiple inflammatory factors in patients with ischemic stroke has been determined to aggravate BBB injury. This damaged BBB is the site of neutrophil and monocyte infiltration and matrix metalloproteinase 9 (MMP9) release.203 Microglia, the innate immune effector cells of the central nervous system, are activated during ischemic stroke.202,204,205 In the early stage of cerebral ischemia, microglia undergo a phenotype switch from the anti-inflammatory M2 phenotype to the pro-inflammatory M1 phenotype.206,207 The Wnt signaling pathway is involved in the toll-like receptor (TLR)-mediated central nervous system immune response.208
Wnt protein is expressed in microglia. For instance, Wnt5a expression in microglia activates the non-canonical Wnt signaling pathway, which initiates an immune response against nerve injury by increasing the genetic expression of TNF-α, IL-6, and IL-1β.209 Microglia in different activation states secrete distinct Wnt proteins; for example, M1 phenotype microglia secrete Wnt5a, whereas M2 phenotype microglia secrete Wnt7a.210 The Wnt signaling pathway has been implicated in the regulation of the neuro-inflammation caused by cerebral I/R injury. In experimental multiple sclerosis, activation of Wnt/β-catenin signaling reduced neutrophil and monocyte infiltration and limited the progression of neuro-inflammation.203 Wnt/β-catenin signaling is inhibited in ischemic stroke patients and corresponding mouse models, which contributes to the release of inflammatory factors TNF-α, IL-1, IL-6, and IL-8, and aggravates the inflammatory response.211 During cerebral I/R, miR-499a downregulation mitigates the inhibition of downstream target DKK1, which further suppresses Wnt/β-catenin signaling and exacerbates the inflammatory response.212 In H/R-treated neurons, the Wnt5a-mediated Wnt/PCP signaling pathway is activated and JNK1 is phosphorylated; consequently, an increase in the expression of pro-inflammatory cytokines TNF-α and IL-6 is observed, which amplifies the inflammatory responses.213 Alternatively, Wnt/β-catenin signaling activation inhibits the inflammatory response during I/R; however, the mechanism by which the non-canonical Wnt/PCP signaling pathway regulates neuro-inflammation during cerebral I/R injury remains unclear; therefore, further research is warranted. One possibility is that Wnt5a drives the non-canonical Wnt signaling pathway and induces inflammation by promoting microglial polarization toward the M1 phenotype.209,210
Targeting the Wnt signaling pathway or related proteins is a potential strategy to reduce the damage caused by the inflammatory response in cerebral I/R injury (Fig. 4c). TWS119 is a GSK-3β inhibitor that activates Wnt/β-catenin signaling. On days 14 and 21 following experimental ischemic stroke, TWS119 treatment promoted microglial polarization by activating Wnt/β-catenin signaling, which ultimately improved the local inflammatory microenvironment during the chronic phase of ischemic stroke. These observations are accompanied by angiogenesis surrounding the infarct area.214
Alternatively, intranasal administration of Wnt3a reduced the volume of cerebral infarction and the number of apoptotic cells 72 h post-transient middle cerebral artery occlusion (MCAO) in mouse models. Additionally, this treatment promoted the polarization of reactive microglia toward the M2 phenotype; increased the number of A2 phenotype astrocytes with neuroprotective effects; reduced the number of neurotoxic A1 phenotype astrocytes; initiated the anti-inflammatory and neuroprotective effects of microglia and astrocytes; reduced the neuroinflammation following cerebral ischemia, which may be attributed to the Wnt3a-mediated activation of the Wnt/β-catenin signaling pathway.215 Finally, treatment with curcumin has shown efficacy in reversing the inflammatory response caused by Wnt/PCP signaling activation in neuronal cells subjected to H/R.216
Oxidative stress
Oxidative stress plays a crucial role in brain injury following cerebral I/R injury, and inhibition of the Wnt/β-catenin signaling pathway is a key factor in the pathogenesis (Fig. 4d).
One study demonstrated that rat cerebral I/R injury inhibited Wnt/β-catenin signaling; this signal inhibition decreased the activity of mitochondrial complex I and caused an oxidative stress state via excessive ROS generation, which contributed to brain injury.217 During reperfusion, the rate of mitochondrial ROS production increases, resulting in the opening of mitochondrial permeability transition pores and subsequent cell death.216,218 Further, brain I/R reduces antioxidant levels and leads to excessive production of mitochondrial ROS, which damages the mitochondrial membrane, triggers the release of cyt c and expression of caspase 9, and ultimately induces neuronal apoptosis.181
The inverted formin two protein is required for excessive mitochondrial fission in mammalian cells.181,219 In the oxidative stress environment of cerebral I/R, the expression of the nuclear hormone receptor Nur77 is stimulated, promoting β-catenin phosphorylation and a subsequent increase in inverted formin two expression, which leads to mitochondrial fragmentation,181 an overactivation of mitochondrial fission and inhibition of fusion. This phenomenon mediates mitochondrial and neuronal cell damage during cerebral I/R, and consequently aggravates brain injury.181 Intravenous injection of human serum albumin can activate the Wnt/β-catenin signaling pathway and reduce early oxidative stress injury following cerebral I/R injury of rats.217
Neurogenesis
The activation of Wnt/β-catenin signaling promotes neurogenesis during the early stage of cerebral ischemia and I/R injury. However, the inhibition of Wnt/β-catenin signaling pathway hinders the protective effect. Therefore, targeting the Wnt/β-catenin signaling pathway is a potential treatment for restoring neurogenesis following cerebral I/R injury (Fig. 5a).
Fig. 5.
Wnt signaling pathway and targeted therapy for neurogenesis, angiogenesis, and BBB during cerebral I/R injury. a Wnt signaling-mediated neurogenesis during cerebral I/R injury. During cerebral ischemia phase, there is an elevation in the synthesis of lncRNA MEG and peroxynitrite. The highly expressed lncRNA MEG hampers the process of the Wnt/β-catenin signaling pathway. In contrast, increased levels of peroxynitrite activate the Wnt/β-catenin signaling pathway. In the subsequent phase of cerebral I/R phase, the activation of the Wnt/β-catenin signaling pathway plays a crucial role in promoting neurogenesis. Mallotus oblongifolius, ellagic acid, and curcumin enhance the activation of the Wnt/β-catenin signaling, leading to the promotion of neurogenesis and the exertion of therapeutic effects on cerebral ischemia or I/R injury. b Wnt signaling-mediated angiogenesis during cerebral I/R injury. Following cerebral ischemia, oligodendrocyte precursor cells within the brain secrete Wnt7a, which triggers the activation of Wnt/β-catenin signaling specifically in endothelial cells. This activation, in turn, facilitates the process of angiogenesis. Activation of the Wnt/β-catenin signaling pathway induces the conversion of microglia into the M2 phenotype, thereby facilitating angiogenesis following an ischemic stroke. Following cerebral I/R, the activation of the Wnt/β-catenin signaling pathway upregulates the expression of VEGF and VEGF receptors. This activation promotes angiogenesis and stimulates the proliferation and sprouting of vascular endothelial cells. c Wnt signaling-mediated the BBB during cerebral I/R injury. The mutation or deletion of GPR124 leads to a reduction in the recruitment of DVL1 to the cell membrane. Consequently, this weakens the transduction of Wnt/β-catenin signaling, resulting in the downregulation of TJ protein expression between microvascular endothelial cells. As a consequence, it exacerbates the damage to the BBB following cerebral ischemia. Moreover, during the cerebral ischemic phase, there is an upregulation of NHE1, which inhibits Wnt/β-catenin signaling and disrupts astrocyte function. This disruption is necessary to maintain the integrity of the BBB. In the context of cerebral ischemia, NHE1 undergoes upregulation, which subsequently inhibits Wnt/β-catenin signaling and disrupts the function of astrocytes. This disruption ultimately results in impaired BBB integrity. During the cerebral I/R phase, there is inhibition of Wnt/β-catenin signaling, leading to an increase in the expression of MMP-9. This elevated MMP-9 expression subsequently degrades the TJ proteins between brain endothelial cells, disrupting the integrity of the BBB. Note: The pink background represents the ischemic phase, while the cream background represents the reperfusion phase. LRP low-density lipoprotein receptor-related protein, VEGF vascular endothelial growth factor, BBB blood-brain barrier, GPR124 G protein-coupled receptor 124, TJ protein tight junction protein, MMP-9 matrix metalloproteinase-9, NHE1 the protein encoded by the Nhe1 gene
The Wnt signaling pathway is a key regulatory pathway of neurogenesis. In the forebrain tissue of embryonic mice on embryonic day 14.5, it promotes the self-renewal of neural stem cells, inhibits neural stem cell differentiation, and retains the pluripotency of neural stem cells, which allows the cells to differentiate into neurons, astrocytes, and oligodendrocytes.159 The canonical Wnt signaling pathway also promotes the differentiation of mice cortical neurons on embryonic day 10.5.160 Additionally, the non-canonical Wnt/PCP signaling pathway, activated by tyrosine kinase receptors, regulates the production of different subtypes of cortical interneurons located in the medial ganglion eminence during embryonic development.161 Thus, the Wnt signaling pathway is essential for embryonic neural stem cell proliferation and neuronal differentiation. In the adult brain, neurogenesis occurs in the subventricular zone (SVZ) of the lateral ventricle and subgranular zone of the dentate gyrus in the hippocampus.220,221 Wnt/β-catenin signaling is activated in these regions222,223 and is involved in neurogenesis. Jin et al. observed a significant increase in proliferation-related Ki-67 antigen-positive cells in the ischemic penumbra in the autopsy brain tissue of adult patients with ischemic stroke, alongside the expression of neuronal lineage doublecortin, tOAD/Ulip/CRMP family protein 4, and βIII tubulin.224 Another study demonstrated that cell proliferation was active in the SVZ region in elderly patients who died of ischemic stroke;225 which indicated that cerebral ischemic injury-induced adult neurogenesis is possible. Notably, the Wnt/β-catenin signaling pathway is widely activated during cerebral I/R injury, to possibly promote neurogenesis via the upregulation of various downstream molecules. Neurogenesis alleviates brain injury by combating apoptosis.
Cerebral hypoxic-ischemic injury increases peroxynitrite production and promotes neural stem cell proliferation and neuronal differentiation. This has been partially attributed to peroxynitrite activating the Wnt/β-catenin signaling pathway; however, when peroxynitrite production reaches a specific threshold, it exerts a cytotoxic effect.226 Instead, artificially activating the Wnt/β-catenin signaling pathway can promote the expression of the downstream targets, cyclin D1, Ngn2, Pax6, and NeuroD1, thereby promoting neurogenesis without the cytotoxic effects of peroxynitrite. Cyclin D1227 and Pax6228 promote neural stem cell proliferation, Neuro D1 promotes adult neurogenesis and maintains neuronal survival,229,230 and Ngn2 promotes neurogenesis.231 Additionally, Wnt/β-catenin signaling activates BDNF secretion from glial cells and protects adjacent neurons.232 Overall, this upregulation of BDNF by Wnt/β-catenin signaling contributes to nerve repair during an ischemic stroke by promoting neurogenesis and neuronal survival.233
Several substances, such as Mallotus oblongifolius,234 ellagic acid,235 and curcumin,236 have been shown to upregulate Wnt/β-catenin signaling during cerebral ischemia and cerebral I/R injury, promoting neurogenesis by activating the downstream targets cyclin D1, Ngn2, Pax6, and NeuroD1. Alternatively, long non-coding RNA MEG3 (lncRNA MEG3) expression increases during cerebral ischemia, and the downregulation of lncRNA MEG3 expression activates Wnt/β-catenin signaling and promotes neurogenesis.237
Angiogenesis
During cerebral I/R injury, artificial activation of the Wnt/β-catenin signaling pathway can promote angiogenesis (Fig. 5b). Specifically, in the mammalian embryonic forebrain, angiogenesis relies on the activation of Wnt signaling in vascular ECs, driven by Wnt7a and Wnt7b. Angiogenesis in the hindbrain is initiated by the binding of the Norrin ligand to the Frizzled 4 receptor, activating the β-catenin signaling pathway and promoting angiogenesis.238,239 Zhang et al. demonstrated that activation of Wnt/β-catenin signaling during cerebral I/R could promote the expression of vascular endothelial growth factor (VEGF),170 which plays a dual role in cerebral ischemia by transiently destroying the BBB and promoting angiogenesis.240 Wnt/β-catenin signaling promotes the proliferation and sprouting of vascular ECs and increases the expression of VEGF receptors, thereby promoting angiogenesis within the central nervous system.241 Wnt/β-catenin signaling induces the polarization of reactive microglia toward the M2 phenotype.242 M2 microglia secretes exosomes containing miRNA-26a, which targets ECs and promote angiogenesis during ischemic stroke.242 Angiogenesis in the penumbra of early cerebral ischemia in patients with ischemic stroke is significantly increased and correlated with patient survival.243 Wnt/β-catenin signaling activation during cerebral I/R is a potential strategy for enhancing angiogenesis. During cerebral ischemia, transplanted oligodendrocyte precursor cells secrete Wnt7a in a paracrine manner; Wnt7a can activate EC Wnt/β-catenin signaling and promote angiogenesis and neurological recovery.244 Alternatively, isoflurane postconditioning (inhalation of 1.5% isoflurane for 60 minutes after reperfusion) can activate the Wnt/β-catenin signaling pathway and promote the expression of target protein VEGF,170 which may promote angiogenesis.
BBB
Inhibition of Wnt/β-catenin signaling exacerbates BBB damage during cerebral I/R (Fig. 5c). Wnt/β-catenin signaling is activated in cerebral vessel ECs from the embryonic stage to postpartum BBB formation; however, its transduction is reduced as the BBB matures.165 Wnt/β-catenin signaling is silent in capillary ECs within circumventricular organs owing to the maintenance of highly permeable capillaries in this region.166 Vascular edema during ischemic stroke is a primary contributor of BBB breakdown. BBB dysfunction is characterized by increased permeability that allows blood-derived fluids and chemicals to enter the brain parenchyma, ultimately resulting in brain edema.245 Ta et al. demonstrated that two single-nucleotide polymorphisms in Wnt7a and three single-nucleotide polymorphisms in the adhesion G protein-coupled receptor GPR124 were associated with an increased risk of hemorrhagic transformation following rtPA thrombolysis in patients with acute ischemic stroke. Specifically, a GPR124 c.3587G>A mutation reduced GPR124-mediated recruitment of sufficient DVL1 from the cytoplasm to the cell membrane, thereby reducing the interaction between DVL1 and Wnt receptors and weakening the Wnt signaling pathway.246 Additionally, GPR124 gene deletion within ECs worsens BBB damage during cerebral ischemia247 by downregulating Wnt/β-catenin signaling; nonetheless, this effect gradually diminishes in transient MCAO mice through pericyte shedding and reperfusion from day 3–5.
Correspondingly, a decrease in TJ protein expression disrupts BBB integrity and increases the risk of hemorrhagic transformation following reperfusion.247 In animal models, Wnt/β-catenin signaling is reportedly attenuated during cerebral I/R, leading to increased BBB permeability and damage due to the downregulation of TJ protein in microvascular ECs.248,249 Capillary ECs are the main components of the BBB, with TJs being observed between these ECs. Specifically, ECs limit BBB permeability by inhibiting paracellular channels and non-specific transcellular transport. TJs consist of occludin, claudins, tricellulins, and other proteins250; a decrease in TJ protein level during ischemia increases BBB permeability. During cerebral I/R, the upregulation of MMP9 expression contributes to the degradation of TJ proteins between brain ECs, further damaging the BBB. Overall, this process is negatively regulated by Wnt/β-catenin signaling.10,251
NHE1 protein promotes the H+-Na+ exchange in astrocytes. During cerebral ischemia, NHE1 is activated, resulting in Na+ overload and cell swelling in astrocytes; this eliminates the corresponding BBB maintenance function of astrocytes, leading to BBB damage.252 Song et al. demonstrated that Wnt7a/7b expression was upregulated and the BBB was repaired, after knocking out the Nhe1 gene in the astrocytes of cerebral ischemic mice. Astrocytes with the NHE1 knockout can activate Wnt/β-catenin signaling, thereby exhibiting a protective role in cerebral ischemia. Furthermore, Song et al. demonstrated that the upregulation of Wnt7a/7b expression in NHE1−/− astrocytes promotes Wnt/β-catenin signaling activation and facilitates BBB repair.253
Wnt pathways during renal I/R injury
Renal I/R injury is an inevitable and serious complication following renal transplantation40 and the main factor that promotes acute kidney injury (AKI) and reduces long-term renal graft survival rates.254,255 In normal adult kidneys, the activity of Wnt/β-catenin signaling is relatively low.256 However, when the kidney undergoes damage, Wnt/β-catenin and Wnt/Ca2+ signaling is promoted, which further damages the kidney via cell senescence, renal fibrosis, oxidative stress, apoptosis, and ferroptosis pathways, thereby causing AKI or chronic kidney disease (CKD). Overall, this process involves intercellular communication between the renal tubular epithelium and interstitial fibroblasts.
Apoptosis
In renal I/R, the activation of Wnt/β-catenin signaling pathway has been suggested to promote apoptosis, while others have suggested otherwise (Fig. 6a). Liu et al. used HK-2 cells to construct a mouse renal I/R injury in vitro model and demonstrated that the expression of lnc MEG3 in HK-2 cells was significantly upregulated following I/R injury. The combination of lnc MEG3 and miR-145-5p reduced miR-145-5p content, upregulated the expression of its downstream target RTKN, activated the Wnt/β-catenin pathway and its downstream effector Myc, promoted the expression of lnc MEG3, and aggravated renal injury.257 Alternatively, silencing lnc MEG3 in HK-2 cells with I/R stress inhibited Wnt/β-catenin signaling, Myc expression, mitophagy, and apoptosis, and alleviated renal tubular injury.257 However, other evidence indicated that after 1 day of renal I/R-induced AKI, the expression of β-catenin in renal tubular cells was significantly upregulated, which reduced renal tubular cell apoptosis, thereby exerting a protective effect. In vitro experiments have confirmed that Wnt1 activates β-catenin, promotes the phosphorylation of Akt and the expression of survivin, and inhibits the expression of Bax and p53, and therefore activates the anti-apoptotic mechanism and reduces AKI following renal I/R injury.258
Fig. 6.
Wnt signaling pathway and targeted therapy during renal I/R injury. a Wnt signaling-mediated apoptosis during renal I/R injury. During the renal I/R phase, there is an upregulation of lncRNA MEG3, which leads to the activation of Wnt/β-catenin signaling. This activation, in turn, promotes mitophagy and induces apoptosis in renal cells. b Wnt signaling-mediated oxidative stress during renal I/R injury. In the renal ischemic phase, there is a downregulation of miR-144-5p, which in turn activates the Wnt/β-catenin signaling pathway. This activation leads to increased oxidative stress and apoptosis in renal cells. Additionally, circ-AKT3 further contributes to the reduction of miR-144-5p expression, thereby exacerbating renal cell apoptosis. c Wnt signaling-mediated cell senescence and renal fibrosis apoptosis during renal I/R injury. During the phase of renal I/R, the Wnt/β-catenin signaling pathway is activated, leading to the promotion of renal cell apoptosis and the development of fibrosis. Additionally, the activation of Wnt/Ca2+ signaling during this process contributes to chronic kidney injury. Note: The pink background represents the ischemic phase, while the cream background represents the reperfusion phase. LRP low-density lipoprotein receptor-related protein, ROR recombinant receptor tyrosine kinase like orphan receptor, RYK receptor tyrosine kinase, MDA malondialdehyde, SOD superoxide dismutase, CAT catalase, CaMKII calmodulin-dependent protein kinase II, AKI acute kidney injury, CKD chronic kidney disease
Taken together, the effects of activating Wnt/β-catenin signaling during renal I/R injury remain controversial, therefore further investigations are warranted. Nonetheless, we are more inclined to accept the findings of the study conducted by Zhou et al. Specifically, Liu et al. only evaluated the role of lncRNA MEG3 using cell experiments; the authors indicated that the Wnt signaling pathway might be only one downstream pathway and did not indicate that Wnt/β-catenin eventually leads to damage. In contrast, Zhou et al. used both in vitro and in vivo experiments to demonstrate the mechanism by which the Wnt/β-catenin pathway protects against early renal I/R injury.
Ferroptosis
The Wnt/β-catenin signaling pathway is associated with ferroptosis following renal I/R injury; however, the specific connection between these two mechanisms remains unclear. Ferroptosis aggravates AKI and delays graft function (DGF) following renal I/R injury.259 Utilizing LASSO analysis, Wei et al. determined that the emergency response gene activation transformation factor 3 (ATF3) was a high-risk gene for ferroptosis-associated DGF during renal I/R injury. ATF3 was reported to be highly expressed within the Wnt/β-catenin pathway and implicated in the regulation of chemokine-associated pathways.260 Overall, these results indicate that DGF-associated ferroptosis is linked to Wnt/β-catenin signaling; however, the precise mechanisms remain yet to be elucidated.
Oxidative stress
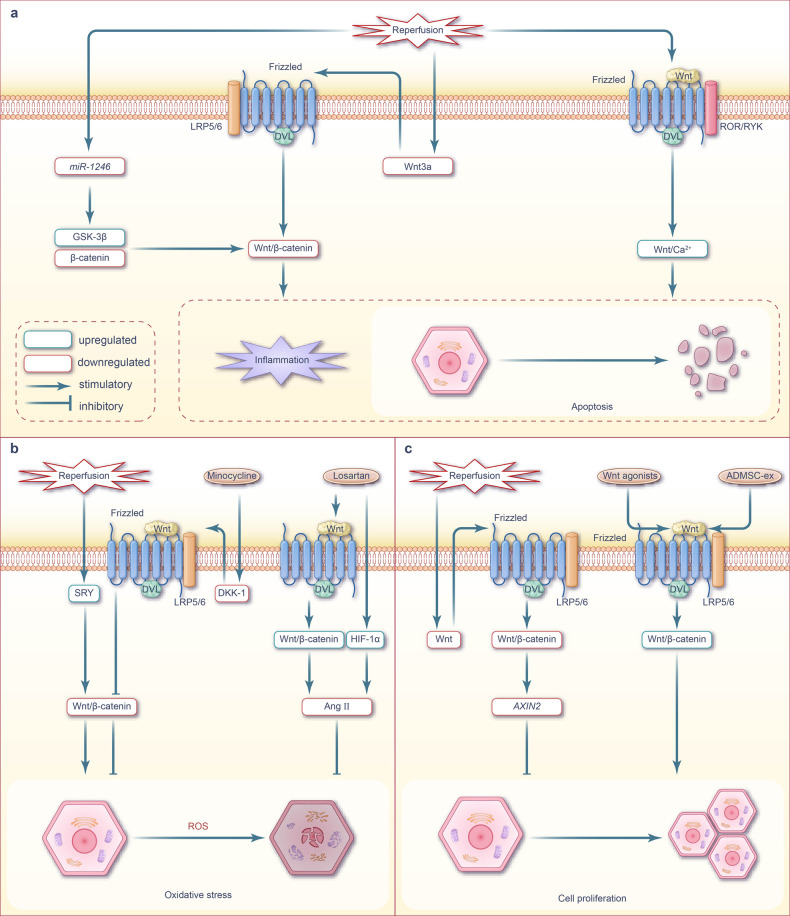
The activation of Wnt/β-catenin signaling during renal I/R promotes oxidative stress (Fig. 6b). This increase in oxidative stress and decrease in antioxidants may be the primary cause of subsequent renal injury. Studies have shown that inhibition of Wnt/β-catenin signaling can reduce the oxidative stress and inflammatory responses mediated by renal I/R injury.25
As a non-coding RNA, miR-144-5p is involved in the regulation of gene expression after transcription. Xu et al. demonstrated that in hypoxic rat HK2 cells, miR-144-5p expression was downregulated, leading to activation of Wnt/β-catenin signaling. This activation resulted in increased expression of Bax and caspase 3, along with reduced Bcl-2 expression, ultimately leading to cell injury and apoptosis. Circ-AKT3 has been shown to be an effective miR-144-5p sponge that can further reduce the expression of miR-144-5p following renal I/R injury in rats. Consequently, this miR-144-5p inhibition significantly increases the malondialdehyde and superoxide ion content, as well as reduced activity of superoxide dismutase (SOD) and catalase (CAT). Overall, this induces in oxidative stress, apoptosis, and the aggravation of renal injury.261 Therefore, circ-AKT3 causes apoptosis and aggravates renal injury by activating Wnt/β-catenin signaling and increasing oxidative stress.261
Cell senescence and renal fibrosis
The Wnt/β-catenin signaling pathway is activated after renal I/R injury and promotes senescence and renal fibrosis. Wnt/Ca2+ signaling pathway activation also results in the promotion of cell senescence and renal fibrosis (Fig. 6c). Cell growth arrest, DNA double-strand structural damage, and the accumulation of senescence-associated proteins are the main characteristics of cell senescence. The accumulation of senescence-associated proteins is predominantly mediated by p16INK4A-Rb and ARF-p53-p21 signaling.262
The Wnt signaling pathway is an important participant in renal fibrosis53 that accelerates cell senescence, especially by regulating the DNA double-stranded structure and the balance between cellular senescence proteins and anti-aging proteins, which ultimately leads to renal fibrosis. With the aggravation of renal I/R injury, sustained activation of Wnt/β-catenin signaling promotes the transcription of downstream fibrogenic genes, including SNAI1, TWIST, PAI1, and MMP7, which can induce renal fibrosis and accelerate the progression of AKI to CKD.263
Luo et al. evaluated a CKD mouse model with unilateral I/R and established that Wnt9a levels increased slightly 1 day after severe I/R injury and significantly increased 3 days later. These Wnt9a levels were positively correlated with an increase in p16INK4A and γΗ2ΑX (a selective marker of DNA double-strand break) and a decrease in the anti-aging protein Klotho (a marker of renal tubular injury and CKD).264 Increased Wnt9a ligand expression activates Wnt/β-catenin signaling, which promotes the expression of downstream profibrogenic and transcriptional targets that exacerbate renal fibrosis.264–268
In a kidney transplantation rat model, Toerne et al. observed glomerular disease, impaired tubulointerstitium and renal fibrosis in transplanted rat kidneys, similar to chronic allograft injury sites after human kidney transplantation.269 Sun et al. conducted a prospective multicenter controlled study and reported that allogeneic mesenchymal stem cells significantly reduced DGF, acute rejection, and prolong long-term survival in renal transplantation.270 Furthermore, with the progression of renal injury, the expression levels of canonical Wnt pathway–related genes Fn1, Cd44, Mmp7, and Nos2 were upregulated, and so were those of the Wnt/Ca2+ pathway–related genes Prkcb1, Prkch, Nfact1_pred, and Nfact2. Additionally, the expression of CaMKII protein in monocyte infiltration increased, and the phosphorylation of this protein increased significantly with the development of fibrosis. The authors hypothesized that this morphological change was related to the activation of canonical Wnt signaling and non-canonical Wnt/Ca2+ signaling in the transplanted kidney, contributing to chronic kidney injury following renal transplantation.269 However, the role of the Wnt/PCP pathway in cell senescence and renal fibrosis following renal transplantation is yet to be determined.
Wnt pathways during hepatic I/R injury
Hepatic I/R injury is a pathophysiological event that occurs following liver surgery or transplantation and profoundly affects the prognosis of liver function. The degree of liver I/R injury is influenced by temperature, ischemic time, and range. The injury of liver cells caused by warm ischemia is more severe than that caused by cold ischemia, whereas the injury to liver sinusoidal ECs is the opposite.271 Under physiological conditions, the Wnt signaling pathway regulates hepatocyte functions such as proliferation, survival, metabolism, regeneration, liver homeostasis, and cell–cell adhesion.272 In general, the pathological process of hepatic I/R injury involves damage to sinusoidal ECs, liver cells, hepatic stellate, and other cells. The Wnt signaling pathway participates in the regulation of apoptosis, necrosis, inflammatory responses, oxidative stress, and proliferation during hepatic I/R injury.
Inflammation and apoptosis
During hepatic I/R injury, Wnt/β-catenin signaling is inhibited, aggravating inflammation and apoptosis, while the Wnt/Ca2+ signaling pathway is activated and further promotes apoptosis (Fig. 7a). During hepatic I/R injury, sinusoidal ECs and hepatocytes release DAMPS, which trigger an inflammatory response.273 In hypoxia and H/R liver cells, both Wnt/β-catenin signaling and HIF1α signaling were inhibited,274 which synergistically aggravated liver oxidative stress and promoted apoptosis.274 Liu et al. determined that in the liver tissue of non-lethal hepatic I/R mice and H/R hepatocytes, downregulation of Wnt3a and β-catenin expression, and inhibition of Wnt/β-catenin signaling aggravated liver apoptosis, necrosis, and the inflammatory response.275 Xie et al. established that miR-1246 expression was significantly downregulated in I/R mouse liver tissues and H/R-treated LO2 cells.276 By targeting the negative regulation of GSK-3β, GSK-3β protein levels could be significantly upregulated, while β-catenin expression downregulated, which inhibited the Wnt/β-catenin pathway and upregulated hepatocyte apoptosis.276
Fig. 7.
Wnt signaling pathway and targeted therapy during hepatic I/R injury. a Wnt signaling-mediated inflammation and apoptosis during hepatic I/R injury. During hepatic I/R injury, the Wnt/β-catenin signaling pathway is suppressed, leading to the promotion of liver inflammation and apoptosis. Concurrently, Wnt/Ca2+ signaling is activated, exacerbating cell apoptosis. b Wnt signaling-mediated oxidative stress during hepatic I/R injury. During hepatic I/R injury, the Wnt/β-catenin signaling is inhibited, resulting in the aggravation of oxidative stress. However, treatment with minocycline and losartan treatment can alleviate intracellular oxidative stress by activating the Wnt/β-catenin signaling pathway. c Wnt signaling-mediated cell proliferation during hepatic I/R injury. During hepatic I/R injury, the Wnt/β-catenin signaling is inhibited, leading to a decrease in the transcription of the downstream target gene AXIN2 and subsequent inhibition of cell proliferation. However, the activation of Wnt/β-catenin signaling through the use of Wnt agonists and ADMSCs-ex can promote cell proliferation in this context. Note: the cream background represents the reperfusion phase. LRP low-density lipoprotein receptor-related protein, ROR recombinant receptor tyrosine kinase like orphan receptor, RYK receptor tyrosine kinase, DKK-1 Dickkopf-1, HIF-1α hypoxia-inducible factor-1α, ADMSC-ex adipose-derived mesenchymal stem cell exosomes
The non-canonical Wnt/Ca2+ signaling pathway is also involved in the regulation of apoptosis during hepatic I/R injury. Sakon et al. found elevated calpain activity in allograft biopsy specimens collected from patients with poor function after liver transplantation.277 This increase in calpain activity corresponded to a similar increase in Ca2+ concentration during liver I/R.277 Hu et al. demonstrated that activation of Wnt/Ca2+ signaling promoted apoptosis in normal rat hepatocytes BRL-3A under conditions of hypoxia-reoxygenation.278 Additionally, in an I/R injury model of rat H9c2 cells, Zhou et al. found that upregulation of the Wnt5/Frizzled-2 pathway in liver tissue led to increased Ca2+ activity and apoptosis.103
Targeting the upstream elements of Wnt and its corresponding signaling pathway could alleviate hepatocyte inflammation and inhibit apoptosis following hepatic I/R (Fig. 7a). Terlipressin, a synthetic antidiuretic hormone analog, can improve the survival of patients with advanced cirrhosis.279,280 Terlipressin selectively binds to the V1 receptor in the human liver.279,280 Therefore, terlipressin treatment activates the Wnt/β-catenin/FoxO3a/AKT pathway by upregulating V1 receptor, which significantly improves I/R-induced hepatocyte apoptosis, necrosis, and inflammation, and ultimately protects the liver.275 Kohler et al. conducted a randomized, double-blind, placebo-controlled trial involving 150 patients who underwent selective large hepatectomy and determined that perioperative terlipressin did not affect the endpoint of liver-specific complications, but did significantly prevent postoperative liver function degradation.281 Similar findings were also observed by Hong.282 Alternatively, intravenous injection of human umbilical cord blood mesenchymal stem cell-derived exosomes into the portal vein of hepatic I/R mice upregulated the expression of miR-1246 and activate the Wnt/β-catenin pathway by targeting and negatively regulating GSK-3β; therefore, this treatment exerts anti-apoptotic effects and alleviates hepatic I/R injury.276 Agmatine (AGM) is an endogenous polyamine that confers a protective effect on I/R injury in the brain, kidney, heart, and other tissues and organs.283–286 Intraperitoneal injection of AGM has been observed to inhibit inflammation and apoptosis following hepatic I/R in mice via activation of the Wnt/β-catenin signaling pathway.287 Alternatively, transfection of Frizzled-2 siRNA into rat liver BRL-3A cells silenced Frizzled-2 gene expression; this reduced the intracellular Ca2+ concentration increase induced by H/R, inhibited Wnt/Ca2+ signaling, and ultimately reduced cytotoxicity and apoptosis.278
Oxidative stress
The inhibition of the Wnt/β-catenin signaling pathway during hepatic I/R contributes to oxidative stress in hepatocytes (Fig. 7b). Dong et al. established that the postoperative transaminase levels, total incidence, and incidence of liver failure in 1,267 male patients undergoing hepatectomy were significantly higher than those in 508 female patients288 and concluded that males are more prone to I/R injury than females.288 SRY, a mammalian sex determination gene,289 is significantly upregulated in hepatic I/R injury.288 Following hepatic I/R, upregulated SRY interacts with GSK-3β and β-catenin, promotes the phosphorylation and degradation of β-catenin, and inhibits Wnt/β-catenin signaling, which aggravates liver inflammation, oxidative stress, and cell necrosis.288 Furthermore, dysregulation of Wnt3a, β-catenin, and HIF-1α, as well as antioxidant enzyme activities (Mn-SOD, Cu/Zn-SOD, glutathione and CAT), synergistically promotes oxidative stress by inhibiting the Wnt/β-catenin and HIF-1α signaling pathways in the liver during I/R injury.290
Overall, these findings suggest that activation of the Wnt/β-catenin signaling pathway reduces oxidative stress following hepatic I/R (Fig. 7b). Administration of Mino, an antibiotic with anti-inflammatory, anti-apoptotic, and anti-oxidative properties,291 reduced DKK1 protein expression, increased β-catenin protein expression, and activated the Wnt/β-catenin signaling pathway, thereby protecting the liver from I/R injury.292 Ang II, the main effector peptide of the renin-angiotensin system, can stimulate hepatic stellate cells and Kupffer cells to exacerbate oxidative stress.293–296 The activity of Ang II in the liver is primarily mediated by AT 1Rs (Ang II type I receptor).293–296 Similarly, losartan, an AT 1Rs antagonist, protects against I/R injury by upregulating Wnt/β-catenin and HIF-1α signaling,297 mitigating oxidative stress and safeguarding the liver.290
Cell proliferation
Hepatic I/R inhibits hepatocyte proliferation and liver injury repair by dampening the Wnt/β-catenin signaling pathway (Fig. 7c). This signaling cascade plays a pivotal role in hepatocyte proliferation and liver regeneration.272 The downregulation of Wnt/β-catenin signaling during hepatic I/R curtails the transcription of downstream target gene Axin2, leading to a subsequent decline in hepatocyte proliferation and liver injury repair.298 Fortunately, the use of Wnt agonists can counteract this inhibition and promote the upregulation of the Wnt/β-catenin cascade, thereby stimulating hepatocyte proliferation and facilitating the repair of liver injury.298 Sun et al. demonstrated that liver regeneration mechanisms were exhibited in hepatocytes throughout the entire organ after partial hepatectomy, and the upregulation of Axin2 specifically contributed to this hepatocyte proliferation.299 Axin2 interacts with β-catenin/CTNNB1, an intracellular scaffold protein,300 although the precise dependence of Axin2 upregulation on Wnt/β-catenin signaling post-partial hepatectomy remains nebulous.299 Nonetheless, following I/R combined with hepatectomy in rats, the downregulation of Wnt2, β-catenin, and cyclin D1, as well as the inhibition of the Wnt/β-catenin pathway, hindered liver cell proliferation and regeneration.301
Collectively, these findings underscore the significance of upregulating the Wnt/β-catenin signaling pathway to drive liver cell proliferation following hepatic I/R (Fig. 7c). Intraperitoneal injection of Wnt agonists in rats undergoing hepatic I/R can effectively elevate Wnt/β-catenin signaling, resulting in a remarkable increase in hepatocyte proliferation while concurrently reducing apoptosis and necrosis.298 Additionally, intravenous injection of ADMSC-ex301 into hepatic I/R rats can activate the Wnt/β-catenin signaling pathway, upregulate the expression of the regeneration-related factors such as Cyclin D1 and VEGF, and foster the proliferation and regeneration of liver cells.301
Crosstalk between Wnt signaling and other I/R-associated pathways
Notch signaling
The Notch signaling pathway, a highly evolutionarily conserved pathway involved in embryonic development and tissue injury repair,302,303 is an important pathway in the development and prognosis of organ I/R.62,304–308 The crosstalk between the Notch and Wnt signaling pathways contributes to various cellular processes, including cell proliferation, apoptosis, fibrosis, tumorigenesis, and metastasis.309–312 Studies on cardiac and cerebral I/R have revealed that the interaction between the Notch and Wnt signaling pathways coordinates the regulation of pathological processes such as apoptosis and inflammatory responses. However, whether such crosstalk occurs during liver or kidney I/R remains to be investigated.
The Notch signaling pathway is composed of both canonical and non-canonical pathways. The canonical Notch pathway consists of five Notch ligands, namely jagged1, jagged2, and Delta-like1–4, and four receptors, namely Notch1–4. The canonical Notch signaling pathway is activated when Notch ligands bind to Notch receptors, which then release corresponding intracellular domains; these intracellular domains then translocate to the nucleus and bind to the transcription factor cardiolipin synthetic lecithin (CSL), which induces the transcription of downstream target genes.313 Meanwhile, the non-canonical Notch pathway is independent of CSL and instead regulates the transcription of target genes by synergizing with multiple signaling pathways.302
During myocardial ischemia and I/R injury, the Notch signaling pathway undergoes activation. This activation contributes to the reduction of cardiomyocyte death, the decrease in infarct volume, and the improvement of cardiac function.314,315 Zebrafish have the remarkable ability to completely regenerate damaged myocardium by enabling the proliferation of preserved cardiomyocytes. In a study using zebrafish model of endocardial injury, Zhao et al. discovered that the activated Notch signaling could inhibit Wnt signaling in the injured heart. Additionally, the authors observed that injecting the Wnt inhibitor IWR-1-endo into a Notch-deficient zebrafish heart model could partially restore the corresponding proliferation of cardiomyocytes (Fig. 8a). As a result, the authors proposed an antagonistic relationship between Notch signaling and Wnt signaling during cardiac repair.316,317 Similarly, during cerebral ischemia and I/R, the Notch signaling is activated. The activation of Notch1 signaling promotes the proliferation of neural stem cells and inhibits apoptosis,318,319 while activation of the Notch3 signaling pathway facilitates vascular EC differentiation. This leads to improved vascular adaptation to hypoxic-ischemic conditions and subsequently reduces neural injury.320,321 However, Notch signaling has also been reported to exhibit neurotoxic effects during ischemic stroke, which includes inducing apoptosis, activating microglia, and promoting inflammatory cell infiltration.21,322 In the neonatal rat cerebral ischemia model, the Notch signaling pathway was activated while the Wnt/β-catenin pathway was inhibited, and these two signals synergistically promoted apoptosis through crosstalk involving GSK-3β323 (Fig. 8a).
Fig. 8.
Interplay of Wnt, Notch, and PI3K/Akt signaling pathways during I/R Injury: Insights from Crosstalk Mechanisms. a Crosstalk between Wnt and Notch signaling pathway during I/R injury. During zebrafish myocardial I/R injury, the activated Notch signaling inhibites the Wnt signaling transduction and restored the proliferation ability of certain cardiomyocytes. In the process of cerebral ischemia phase, Wnt/β-catenin signaling is inhibited while Notch signaling is activated. These two signals crosstalk through GSK-3β to promote apoptosis. Additionally, the Wnt/Ca2+ signaling pathway is concurrently upregulated, which crosstalks with Notch signaling to enhance the Notch signaling activity. b Crosstalk between Wnt and PI3K/Akt signaling pathway during I/R injury. During myocardial I/R injury, both PI3K/Akt and Wnt/β-catenin signaling pathways are downregulated. This downregulation inhibits the crosstalk between these pathways, ultimately resulting in cardiomyocyte apoptosis and left ventricular dysfunction. Similarly, during cerebral I/R injury, both PI3K/Akt and Wnt/β-catenin signal pathways are downregulated. In this context, these pathways crosstalk through GSK-3β to promote cell apoptosis, thereby contributing to the pathophysiology of cerebral I/R injury. During hepatic I/R injury, the expression of antidiuretic hormone receptor 1 (V1R) in hepatocytes is upregulated. This upregulation plays a protective role by activating Wnt/β-catenin/FoxO3a/Akt pathway, and then conceals apoptosis induced by FoxO3a activation. c Crosstalk between Wnt and HIF-1α signaling pathway during ischemia or I/R injury. In the context of cerebral ischemia and hypoxia, the increased activity of the HIF-1α signaling pathway plays a role in enhancing the proliferation and neuronal differentiation of neural stem cells by activating the Wnt/β-catenin signaling pathway. During the early stages of the pathological process, HIF-1α disrupts TJ proteins, which subsequently leads to an elevated permeability of the BBB. As the pathological process progresses to the later stages, HIF-1α promotes angiogenesis. In the model AKI induced by H/R, the activation of Wnt/β-catenin signaling pathway has been shown to enhance the protective effect of HIF, Simultaneously, HIF increases the expression of β-catenin and its downstream target genes. This interaction and crosstalk between HIF and Wnt/β-catenin signaling pathway contribute to early renal repair after AKI. In context of hepatic hypoxia or H/R, HIF-1α has the ability to competitively bind to β-catenin, leading to enhanced HIF-1α signaling transduction. This interaction serves to reduce apoptosis and promote cell survival in hepatic cells. LRP low-density lipoprotein receptor-related protein, ROR recombinant receptor tyrosine kinase like orphan receptor, RYK receptor tyrosine kinase, CaMKII calmodulin-dependent protein kinase II, PI3K/Akt phosphoinositide-3 kinase/protein kinase B, BBB blood-brain barrier, TJ protein tight junction protein, AKI acute kidney injury, I/R ischemia–reperfusion, V1R antidiuretic hormone receptor 1, NSC neural stem cells
These findings suggest intermediate molecules in both the Wnt and Notch signaling pathways as potential targets for the effective treatment of organ I/R injury. Treatment with the GSK-3β inhibitor TWS119 showed simultaneous effects of upregulating the Wnt/β-catenin signaling pathway and inhibiting the Notch signaling pathway. This dual modulation results in synaptic proteins and reduced apoptosis in neonatal rats with cerebral ischemia.323 In a rat model of brain I/R injury, treatment with the traditional Chinese medicine (TCM) L-Borneolum during the recovery period inhibited the Wnt3a/β-catenin and Notch-1 signaling pathways. This treatment approach effectively increased cerebral blood flow, promoted the differentiation of astrocytes into neurons in the striatum region, and alleviated cerebral infarction and brain atrophy.324 Additionally, in a brain microvascular endothelial cell injury model induced by OGD/R, treatment with Zhongfenggao activated the Notch and Wnt signaling pathways. This activation resulted in a significant increase in VEGF expression and promoted angiogenesis. Based on these findings, the authors proposed that Zhongfenggao holds potential as a therapeutic candidate for the treatment of brain I/R injury.325
PI3K/Akt signaling
The PI3K/Akt signaling pathway, named after its key components, phosphatidylinositol 3-kinase (PI3K) and AKT (also known as protein kinase B, PKB), plays a crucial role in regulating cell proliferation, survival, and apoptotic processes.326 Crosstalk between the PI3K/Akt and Wnt signaling pathways influences osteoblast proliferation, differentiation, and cancer development.327 The PI3K/Akt/Wnt signaling crosstalk has been identified as a critical pathological process in I/R injury of the heart, brain, and liver, impacting apoptosis and angiogenesis. However, our current understanding regarding PI3K/Akt/Wnt crosstalk in renal I/R remains limited.
PI3K has three isoforms, namely types I–III. PI3K activation induces the conversion of phosphatidylinositol 3,4-bisphosphate to phosphatidylinositol 3,4,5-trisphosphate. Phosphatidylinositol 3,4,5-trisphosphate is a secondary messenger that promotes the translocation of the downstream protein AKT to the cell membrane. AKT is a serine/threonine protein kinase, with several isoforms: AKT1, AKT2, and AKT3. AKT phosphorylation activation results in the translocation of AKT from the cell membrane to the cytoplasm or nucleus to allow further regulation of downstream targets such as mTOR, NF-κB, and Bad.328–331
In a porcine model of chronic myocardial I/R, the inhibition of calpain activity resulted in the upregulation of both PI3K and Wnt/β-catenin pathways. As a result, there was an increase in blood vessel density observed in both the ischemic and non-ischemic myocardial tissue. Ultimately, this enhanced vascularization contributed to the survival of cardiomyocytes.332 In H/R-treated rat cardiomyocytes, overexpression of Akt1 and Wnt11 reduced cardiomyocyte apoptosis. However, this protective effect could be blocked by neutralizing antibodies against Wnt11 or inhibitors of Akt1333 (Fig. 8b).
This suggests that the synergistic mechanism of these two signaling pathways also plays a role in I/R injury of the brain and liver (Fig. 8b). The PI3K/Akt pathway acts as an inherent protective mechanism by enhancing Bcl-2 expression, which in turn exerts an anti-apoptotic effect.334 The interaction between PI3K/Akt and Wnt/β-catenin signaling occurs through GSK-3β. Activation of PI3K/Akt signaling pathway leads to phosphorylation and inactivation of GSK-3β,335 subsequently activating Wnt/β-catenin signaling336 and exerting a protective effect against cerebral I/R injury337 (Fig. 8b). FoxO3a is in the downstream of the PI3K/AKT pathway. Liu et al. conducted a study using a mouse model of hepatic I/R and found that activating the Wnt/β-catenin and Akt pathway had a mitigating effect on liver injury resulting from oxidative stress following hepatic I/R. Additionally, they observed that this activation effectively masked apoptotic events induced by elevated FoxO3a level.275 These findings highlight the potential of the Wnt/β-catenin and Akt pathway in protecting against liver damage and suppressing apoptotic processes in the context of hepatic I/R.275
As the PI3K/AKT/GSK-3β signaling pathway is positioned upstream of Wnt/β-catenin signaling; therapeutics that activate PI3K/AKT/GSK-3β, such as dexmedetomidine or eugenol, have the potential to simultaneously activate Wnt/β-catenin signaling. Therefore, these therapeutic agents are promising strategies for treating organ I/R injury.338,339 For instance, in a cerebral ischemia rat model, the administration of dexmedetomidine increased neuronal survival and reduced cerebral infarct size.338 Similarly, treatment with XQ-1H demonstrated the ability to promote angiogenesis and to restore neurological function following ischemic stroke.340 Lastly, when administered orally for 30 days, Phyllanthus emblica mitigated myocardial damage resulting from I/R in rats undergoing cardiac I/R surgery.339
HIF-1α signaling
The hypoxia-inducible factor-1α (HIF-1α) signaling pathway is responsible for conferring adaptation to hypoxic conditions and plays a crucial role in angiogenesis, oxidative stress, and cell metabolism under hypoxic conditions.341,342 Notably, there is a significant crosstalk between the HIF-1α and Wnt signaling pathways, which jointly regulate processes such as osteogenesis, angiogenesis, and the development and migration of various cancers.343–345 Recent studies have elucidated the involvement of the HIF-1α and Wnt signaling pathway crosstalk in the regulation in regulation of cell proliferation differentiation, BBB permeability, and apoptosis during I/R injury affecting the brain, liver, kidney, and other organs.
HIF-1 is a member of the HIF family and consists of the HIF-1α and HIF-1β subunits. HIF-1α is an oxygen-dependent protein that exhibits a short half-life under high oxygen conditions and is rapidly degraded by proteasomes. It is, however, stable under hypoxic conditions; under such conditions, HIF-1 translocates to the nucleus, co-polymerizes with HIF-1β to form a heterodimer, and binds to hypoxic response elements to promote the expression of the downstream transcription factors VEGF and glucose transporter-1.346
Research has shown that under conditions of cerebral ischemia and hypoxia, the HIF-1α signaling pathway is activated.347 The upregulated HIF-1α signaling pathway can induce the activation of the Wnt/β-catenin signaling pathway, promoting the proliferation of neural stem cells and neuronal differentiation226 (Fig. 8c). It can also disrupt TJ proteins, leading to increased permeability of the BBB and upregulation of the target gene VEGF, thereby exacerbating vascular leakage.347 Based on the dual role of VEGF, the elevation of VEGF promotes angiogenesis during the late stage of ischemia and hypoxia240 (Fig. 8c). In contrast, a study on cerebral ischemia revealed that the HIF-1α/VEGF signaling pathway was downregulated during the treatment period; this suppression, in turn, activated the Wnt/β-catenin signaling pathway, thereby improving brain microenvironment in rats with MCAO.348 The conflicting outcomes of these findings can be attributed to the intricate interplay and complexity of mechanisms between these signaling pathways.
Similarly, the crosstalk between the HIF-1α and Wnt/β-catenin signaling pathways in liver I/R injury synergistically reduces oxidative stress following hepatic I/R290(Fig. 8c). After hepatic ischemia or reperfusion, the expression level of β-catenin regulates the activity of the HIF-1α signal,274 and the lack of β-catenin leads to the inhibition of HIF-1α signal transduction after hepatocyte hypoxia.274 Under hypoxia or H/R conditions, HIF-1α can competitively inhibit the interaction between TCF4 and β-catenin, which enhances HIF-1α signal transduction, reduces apoptosis, and promotes cell survival.274,349 Furthermore, Crosstalk between HIF and Wnt/β-catenin signaling pathway has also been reported in renal I/R. Xu et al. demonstrated that in a cellular model of H/R injury, activated Wnt/β-catenin and HIF signaling pathways promoted each other and enhanced the expression of downstream target genes of Wnt/β-catenin signaling pathway, synergistically promoted early renal repair following AKI350 (Fig. 8c).
Collective evidence suggests that that the crosstalk between the HIF-1α and Wnt/β-catenin signaling pathways plays a crucial role in mitigating organ damage caused by organ I/R injury. This beneficial effect has been observed in various organs, including the brain, kidneys, and liver. In a hypoxic environment, peroxynitrite production activates HIF-1, which is associated with the Wnt/β-catenin signaling pathway, leading to the promotion of neural stem cell proliferation, self-renewal, and neuronal differentiation.226 Moreover, HIF activation promotes cell proliferation and inhibits apoptosis in renal IR-induced AKI, and these effects can be reversed by treatment with the β-catenin inhibitor IWR-1-endo, indicating interaction between HIF and the Wnt/β-catenin signaling pathway in providing renal protection.350 Additionally, the angiotensin II type 1 receptor antagonist losartan enhances HIF-1α and Wnt/β-catenin signaling, thereby alleviating liver I/R damage290 by restoring HIF-1α and β-catenin content. These findings highlight the therapeutic potential of modulating the crosstalk between HIF-1α and Wnt/β-catenin signaling pathways as a strategy to protect organs from I/R injury and promote their recovery.
TGF-β signaling
The TGF-β signaling pathway, comprising both Smad-dependent and Smad-independent pathways, plays a crucial regulatory role in early embryonic development as well as in disease states such as fibrosis and cancer.351 Notably, crosstalk has been observed between the TGF-β and Wnt signaling pathways, where Smad3 forms a complex with β-catenin, inhibiting its degradation and facilitating its nuclear translocation, thus activating the Wnt/β-catenin signaling pathway.352 This crosstalk has been implicated in heart, brain, liver, and kidney I/R injury, contributing to pathological processes such as apoptosis and fibrosis.
TβR-I–III are the three types of TGF-β receptors. Smad family proteins are downstream of the TGF-β signaling pathway, and include receptor-regulated Smads, common pathway Smads, and inhibitory Smads. In the Smad-dependent pathway, the binding of TGF-β to its receptors leads to phosphorylation of receptor-regulated Smads. Phosphorylated R-mads then forms complexes with Co-Smads and translocates to the nucleus to further regulate the transcription of downstream target genes. Alternatively, the Smad-independent pathway utilizes TGF-β to activate a variety of downstream cascades, such as mitogen-activated protein kinase (MAPK), PI3K/AKT, Rho-like, and JNK signaling pathways,351,353 but not Smad signaling.
TGF-β can directly activate the Wnt/β-catenin signaling, while the Wnt/β-catenin signaling pathway helps to stabilize the TGF-β/Smad signaling. Therefore, these two pathways work synergistically to facilitate their respective functions.354 Following MI, the crosstalk between the TGF-β and Wnt/β-catenin signaling pathways promotes the progression of myocardial fibrosis355 (Fig. 9a).
Fig. 9.
Interplay between Wnt, TGF-β, NF-κB, Hippo-YAP, BMP, NMDAR-Ca2+-ActA, TLR4/TRIF and HGF/c-Met signaling pathways during ischemia or I/R injury. a Crosstalk between Wnt and TGF-β signaling pathway during ischemia injury. After myocardial ischemia, there is an increased expression of TGF-β1, which subsequently activates the Wnt/β-catenin signaling pathway. These two pathways work synergistically to promote the process of myocardial fibrosis. During cerebral ischemia and I/R injury, the TGF-β/Smad signaling pathway is activated and collaborates with the Wnt/β-catenin signaling pathway to reduce the apoptosis in cortical neuronal caused by cerebral ischemia. Similarly, in renal I/R injury, the TGF-β signaling pathway is activated and interacts with the Wnt/β-catenin signaling pathway, contributing to the progression of fibrosis. During this process, β-catenin functions as a transcription cofactor of Smad3, promoting the transcription of downstream target genes and facilitating EMT. b Crosstalk between Wnt and NF-κB signaling pathway during ischemia or I/R injury. During myocardial ischemia, the upregulated Wnt/β-catenin signaling pathway facilitates the activation of NF-κB signaling pathway by promoting nuclear translocation of p65, this activation induces the migration of cardiac fibroblasts. Additionally, the activated Wnt/β-catenin signaling promotes the degradation of phosphorylated IκB mediated through β-TrCP, subsequently promoting the nuclear translocation of NF-κB, leading to myocardial fibrosis and apoptosis. In contrast, In the process of liver I/R injury, the Wnt/β-catenin signaling pathway is inhibited, while NF-κB signaling pathway is activated. The transcription of NF-κB is positively regulated by GSK-3β, which promoted inflammation. the Wnt/β-catenin signaling pathway is inhibited, while the NF-κB signaling pathway is activated. The nuclear translocation of NF-κB is positively regulated by GSK-3β, thereby promoting inflammation. c Crosstalk between Wnt and Hippo-YAP signaling pathway during I/R injury. Following myocardial I/R injury, the Wnt/β-catenin signaling is downregulated, leading to the inhibition of YAP1 transcription. Consequently, the activity of the Hippo-YAP signaling pathway is suppressed. This cooperative effect between the two signaling pathways contributes to the promotion of myocardial hypertrophy. d Crosstalk between Wnt and BMP signaling pathway during ischemia injury. During cerebral hypoxia, there is an upregulation in the expression of BMP4, while the Wnt/β-catenin signaling is inhibited. Inhibition of Wnt/β-catenin downregulates BMP2 protein expression; thus, the suppressed Wnt/β-catenin signaling and BMP signaling synergistically inhibit the differentiation of neural stem cells into neurons and oligodendrocytes, thereby aggravating cerebral ischemic injury. e Crosstalk between Wnt and NMDAR-Ca2+-ActA signaling pathway during I/R injury. During cerebral ischemia, ActA expression is upregulated, which leads to the Ca2+ influx during synaptic transmission and can also activate the Wnt/β-catenin signaling pathway, synergistically regulating synaptic plasticity. However, Ca2+ influx mediated by NMDAR activation can also trigger the activation of calpain. This activation of calpain subsequently induces cleavage of β-catenin, resulting in a decrease in synaptic stability. f Crosstalk between Wnt and TLR4/TRIF signaling pathway during I/R injury. During hepatic I/R injury, upregulated WISP1 expression activates the TLR4/TRIF signaling pathway, promoting liver injury. g Crosstalk between Wnt and HGF/c-Met signaling pathway during I/R injury. During renal I/R injury, activated HGF promotes the phosphorylation of LRP5/6 and plays an anti-apoptotic effect by activating the Wnt/β-catenin signaling pathway. AKI induces an elevation in Wnt protein levels within renal tubular epithelial cells, consequently activating the Wnt/β-catenin signaling pathway. The activated Wnt/β-catenin signaling inhibits the secretion of HGF and HGF/c-Met in renal interstitial fibroblasts. Through this coordinated regulation, both the Wnt/β-catenin signaling pathway and the HGF/c-Met signaling pathway play a role in the modulation of apoptosis processes in the context of AKI. LRP low-density lipoprotein receptor-related protein, TGF-β transforming growth factor-β, NF-κB nuclear factor-κB, YAP Yes-associated protein, TLR Toll-like receptor, HGF/c-Met hepatocyte growth factor receptor/mesenchymal-epithelial transition factor, BMP bone morphogenetic protein, NMDAR N-Methyl-D-Aspartate Receptor, ActA Activin A; I/R ischemia–reperfusion
Similarly, in cases of cerebral ischemia and I/R, activation of TGF-β signaling leads to the activation of Wnt signaling. The combined action of the TGF-β/Smad and Wnt/β-catenin signaling pathways helps to decrease cortical neuron apoptosis resulting from cerebral ischemia, thus reducing brain injury170,356 (Fig. 9a). In case of renal I/R injury, the Wnt/β-catenin/TGF-β signaling crosstalk promotes renal fibrosis (Fig. 9a). Chen et al. demonstrated that inhibition of Wnt/β-catenin signaling, TGF-β signaling, and the interaction between these signaling pathways reduced renal fibrosis in renal I/R model in rats.357 EMT of renal tubular epithelial cells is an important mechanism underlying renal fibrosis. As a major participant in the EMT process, the TGF-β signaling pathway is activated after renal I/R, and the crosstalk with the Wnt/β-catenin signaling pathway exacerbates this fibrotic process.
The fibrotic effects of TGF-β1 rely on the binding of β-catenin and Smad3 in the nucleus. β-catenin can act as a transcriptional co-factor for Smad3, activating the transcription of downstream target genes and promoting EMT.358 Additionally, TGF-β1 can activate the Wnt/β-catenin signaling pathway by inhibiting DKK1 expression, a negative regulator of Wnt signaling.359 In pathological conditions such as CKD, increased levels of Wnt9a lead to renal tubular cell senescence and the initiation of TGF-β1 production. TGF-β1 promotes the proliferation of mesenchymal fibroblasts and their transformation into myofibroblasts, while also inducing activated fibroblasts to produce Wnt9a,268 thus establishing an intercellular communication loop through different signaling pathways that perpetuates renal fibrosis. The interplay between Wnt and TGF-β signaling pathways has been established in liver fibrosis, but as for its relevance to liver I/R in terms of TGF-β-related treatment approaches, Zhang et al. observed an upregulation of Wnt3a, β-catenin, VEGF, and Cyclin D1, as well as a downregulation of GSK-3β and caspase 3 in a rat model of MCAO following isoflurane injection. This was accompanied by a reduction in infarct size and neuronal apoptosis.170 Treatment with TGF-β1 inhibitor LY2157299 before inducing MCAO significantly reduced β-catenin expression in a rat model. Conversely, the Wnt inhibitor DKK-1 did not impact the expression levels of TGF-β1 and Smad3.170 These findings suggest that the TGF-β1/Smad3 signaling pathway may have a protective effect by promoting β-catenin expression and reducing apoptosis.170 Treatment with Melatonin and Poria acid A may protect the kidneys by inhibiting Smad3 phosphorylation, interfering with β-catenin signaling transduction, suppressing downstream fibrotic targets of the β-catenin pathway, disrupting the interaction between Smad3 and β-catenin, and counteracting the pro-fibrotic effects resulting from the crosstalk between TGF-β/Smad and Wnt/β-catenin signaling pathways in the transition from AKI to CKD.357 These findings offer promising therapeutic approaches for attenuating kidney fibrosis.357
NF-κB signaling
NF-κB was initially established as an important transcription factor in the induction of various immune and inflammatory responses.
Nonetheless, the interplay between Wnt and NF-κB signaling pathways has attracted attention due to its regulatory role in inflammation-associated events such as cell proliferation, apoptosis, tumor differentiation, and migration.79–81 Moreover, the NF-κB pathway is intricately linked to the progression and prognosis of organ I/R injury.360–363 Recent studies have shed light on the crosstalk between Wnt and NF-κB signaling in processes like apoptosis, inflammation, oxidative stress, and fibrosis following ischemic heart injury and liver I/R injury.
The NF-κB family comprises five protein monomers, including p65/RelA, RelB, cRel, p50, and p52, which form homodimers or heterodimers that bind DNA. NF-κB signaling is regulated by two pathways: (1) the NEMO-dependent canonical pathway, where NF-κB acts as a critical modulator of NEMO, and (2) the NEMO-independent non-canonical pathway.364–366 In the canonical pathway, inflammatory cytokines, pathogen-associated molecular patterns, or antigen/antibody stimulation trigger IKK phosphorylation, which activates a specific serine on the N-terminus of IκB protein, thereby causing ubiquitination and subsequent proteasomal degradation of IκB.364–366 Following the release of IκB, the NF-κB subunit undergoes various post-translational modifications that enable it to bind to specific sites on DNA.364–366 The non-canonical pathway, on the other hand, relies on NF-κB-inducing-kinase and IKKα for its activation.364–366
Serum Wnt2 and Wnt4 are elevated in patients with acute ischemia.367 The upregulation of these Wnt ligands activates Wnt/β-catenin signaling, resulting in p65 nuclear translocation, NF-κB signaling activation, fibroblast migration, and ultimately myocardial fibrosis.367 In the inflammatory heart tissue of patients with AMI and obese rats, increased expression of β-catenin induced NF-κB activation and nuclear localization, resulting in myocardial fibrosis and apoptosis368,369 (Fig. 9b). The intermediate protein β-transducing repeat-containing protein (βTrCP) plays a crucial role in the crosstalk mechanism between Wnt/β-catenin and NF-κB pathway 370. In MI, the activation of Wnt/β-catenin signaling promotes NF-κB nuclear translocation through βTrCP-mediated degradation of phosphorylated IκB.370–373 However, there may be antagonism between the Wnt/β-catenin and NF-κB signaling pathways during liver I/R301 (Fig. 9b).
The Wnt2/β-catenin signaling pathway associated with liver regeneration is inhibited during liver I/R injury, whereas inflammation-associated NF-κB signaling is activated. However, the crosstalk mechanism between these two pathways during liver I/R injury requires further exploration.301 NF-κB is positively regulated by GSK-3β at the transcriptional level, while GSK-3β acts as a negative regulator of the Wnt/β-catenin signaling pathway, potentially explaining this antagonistic relationship.374
Based on the crosstalk mechanism of Wnt and NF-κB signaling, Wnt signaling pathway inhibitors, such as Huoxin pill, may simultaneously inhibit NF-κB signaling to alleviate MI.371 A similar approach utilizing the Wnt inhibitor DKK1 has been explored in breast cancer treatment to inhibit Wnt/Ca2+-CaMKII-NF-κB signaling crosstalk.375 In mesenteric I/R-induced liver injury, Mangiferin has been shown to regulate oxidative stress, inflammation, and apoptosis through the Wnt/β-catenin/NF-κB signaling pathway, which involves upregulation of β-catenin and downregulation of NF-κB.376 Vitamin D deficiency is a risk factor and potential therapeutic target for AKI, caused by pathological mechanisms such as renal I/R injury.377 In a rat model of renal I/R injury, combination therapy with pioglitazone and vitamin D exerted an anti-inflammatory effect by inhibiting the NF-κB signaling pathway.378 Moreover, vitamin D has been shown to activate the Wnt4/β-catenin signaling pathway during the early stage of renal I/R and mitigate excessive Wnt4/β-catenin signaling in the later stage to induce renal fibrosis.378 However, the precise molecular mechanism of this targeted crosstalk is not yet fully understood. Another approach involves the administration of exosomes derived from fatty mesenchymal stem cells, which inhibit NF-κB phosphorylation while activating the Wnt2/β-catenin signaling pathway.301 This intervention has shown promise in reversing the inflammation and pyroptosis caused by hepatic I/R injury, and promoting liver regeneration in hepatic I/R injury.301
Hippo-YAP signaling
The Hippo-YAP signaling pathway, involved in heart development and disease,379–381 engages in bidirectional crosstalk with the Wnt signaling pathway, jointly regulating myocardial development and injury under physiological and stress conditions.379–381 The Hippo-YAP signaling pathway is an important factor of I/R injury in the heart, brain, kidney, liver, and other organs.
This signaling pathway governs essential processes such as cell proliferation, inflammation, and BBB function following I/R injury.23,382–385 Recently, crosstalk between the Hippo-YAP and Wnt signaling pathways has been reported in myocardial I/R injury, in which this crosstalk mechanism regulates myocardial hypertrophy, fibrosis, and inflammation. However, crosstalk between these two pathways has rarely been reported in other organ I/R injuries.
The mammalian Hippo-YAP signaling pathway constitutes a kinase cascade involving core components such as mammalian Ste20-like kinases 1/2, Salvador, large tumor suppressor homolog 1/2, and scaffolding protein MOB domain kinase activator 1A/B.386,387 The transcription coactivators YAP and PDZ binding motif (TAZ) serve as pivotal downstream effectors of this pathway.386,387 Hippo signaling lacks specialized receptors or extracellular ligands upstream and relies on other signaling pathways to regulate its activation.386,387 Nonetheless, upon activation, the Hippo-YAP signaling pathway inhibits downstream YAP nuclear translocation and transcriptional activity, leading to YAP degradation.386,387
After myocardial I/R injury, the activation of Wnt/β-catenin signaling can promote YAP1 transcription, thereby inhibiting Hippo-YAP signaling and subsequently suppressing cell hypertrophy induced by myocardial I/R injury142 (Fig. 9c). In addition, inhibition of the Hippo-YAP signaling pathway can also attenuate myocardial fibrosis, initiating heart regeneration and restoration of MI-induced heart damage.379,388 In post-MI neonatal hearts, YAP activates the non-canonical Wnt signaling pathway in cardiomyocytes via the downstream target gene Wls, and inhibits the expression of NFAT, Col1a1, Postn, and Fn1, thereby suppressing cardiac fibroblast proliferation, collagen formation, and the inflammatory response.388 Additionally, YAP/TAZ participates in the composition of destruction complexes within the canonical Wnt signaling pathway, modulating the accumulation or degradation of β-catenin in response to Wnt signaling activation or inhibition, respectively.389 Overall, this provides insight into the mechanism underlying Wnt/Hippo-YAP signal crosstalk.
Targeting the crosstalk between the Hippo/YAP and Wnt/β-catenin signaling pathways is a potential therapeutic approach for myocardial and cerebral I/R injury. For instance, exogenous melatonin has been shown to regulate the expression of miR-143-3p, activating downstream target genes Yap and Ctnnd1. Upregulated Ctnnd1 may activate the Wnt/β-catenin signaling pathway, promoting the formation of β-catenin and Yap complexes and enhancing cardiomyocytes proliferation post-MI.390 Further, crosstalk between YAP and the non-canonical Wnt signaling pathway (Wnt/ROR1/2) can delay the process of cardiac fibrosis during MI.388 Amani et al. designed and synthesized anti-transferrin receptor monoclonal antibody (OX26)-polyethylene glycolated selenium nanoparticles that promoted Wnt3a/β-catenin activation. When combined with YAP1, the nanoparticles can enhance FoxO1 expression, and provide neuroprotection against oxidative stress, promoting neuronal survival after stroke.391
BMP signaling
BMP, a member of the TGF-β family, is a crucial player in embryonic development392,393 and interacts with the Wnt signaling pathway.394 In brain I/R, the crosstalk between BMP and Wnt signaling regulates neurogenesis and neuronal differentiation, which is an essential process for brain injury repair. However, crosstalk between these two pathways remains unexplored in other organs.
The BMP signaling pathway can be categorized into either canonical or non-canonical pathways. In mammals, this pathway comprises over 20 ligands, 4 type I receptors, and 3 type II receptors.395 In the canonical pathway, BMP type II receptors bind to ligands and phosphorylate type I receptors. Phosphorylated type I receptors recruit and phosphorylate Smad1/5/8 receptors, which bind Smad4 to form complexes that translocate to the nucleus to regulate the expression of multiple downstream target genes. In the non-canonical pathway, type I receptors activate the downstream MAPK pathway, resulting in the translocation and phosphorylation of MAPK signaling proteins (p38, ERK1/2, and JNK). This phosphorylation triggers the activation of ATF2, c-JUN, and c-FOS, which controls the transcription of downstream target genes.395,396
The Wnt signaling pathway can promote neurogenesis and induce BMP production in differentiated neurons, facilitating astrocyte differentiation and inhibiting oligodendrocyte differentiation.34 BMP2, in conjunction with Wnt1 or Wnt3, helps to maintain the undifferentiated state of mouse trunk neural crest cells and promotes the formation of neural crest–derived stem cells.397 Neural crest cells in an undifferentiated state possess pluripotent capabilities and can differentiate into neurons, glial cells, or smooth muscle cells. Changes in the BMP signaling pathway have been observed in hypoxic-ischemic brain damage, with upregulated BMP4 signaling in perinatal hypoxic brains398 and neuroprotective effects of BMP7 following cerebral ischemic injury.399 In a rat model of hypoxic-ischemic encephalopathy, Wnt signaling promoted the differentiation of neural stem cells into neurons and oligodendrocytes by upregulating BMP2 protein expression, aiding in the repair of cerebral ischemic injury.400 However, activation of the Wnt/β-catenin signaling pathway can downregulate BMP4 expression, promoting striatal neurogenesis during cerebral ischemic injury.401 These findings suggest synergistic and antagonistic effects between the Wnt/β-catenin and BMP signaling pathways during cerebral ischemia or reperfusion (Fig. 9d).
Endoglin, a co-receptor of the TGF-β family, is indispensable during early hematopoiesis402–404 and can regulate BMP/Smad1 and Wnt/β-catenin signaling pathways and target Jdp2 to promote the integration of hematopoietic and cardiac progenitor cells in the heart and hematopoietic myeloid system.405 Furthermore, inhibition of GSK-3β by MLT and T63 activates the BMP/Smad and Wnt/β-catenin signaling pathways, initiating osteogenesis.406
NMDAR-Ca2+-ActA signaling
NMDAR is an important ion channel for excitatory synaptic transmission, and ActA regulates synaptic plasticity through NMDAR phosphorylation activation and Ca2+ influx.407 Recent studies have highlighted the interplay between the NMDAR-Ca2+-ActA and Wnt/β-catenin signaling pathways in regulating synaptic plasticity during cerebral ischemia.407
NMDAR containing the GluN2A subunit exhibits a neuroprotective effect, while GluN2B-containing NMDAR induces excitatory neurotoxicity following ischemic I/R injury, contributing to intracellular calcium overload.408 In a rat model of chronic cerebral ischemia, the NMDAR-Ca2+-ActA and Wnt/β-catenin signaling pathways exhibited crosstalk407 (Fig. 9e). Furthermore, NMDAR activation-mediated calcium influx can trigger calpain activation.409 Activated calpain then cleaves β-catenin, allowing the resulting fragmented β-catenin to evade–degradation mediated by damaged complex and translocate into the nucleus to promote downstream TCF gene transcription.409
However, this cleaved β-catenin cannot bind to cadherin, resulting in decreased synaptic stability.409 In summary, the activation of the Wnt/β-catenin signaling pathway by the NMDAR-Ca2+-ActA signaling pathway influences synaptic transmission in two aspects, its beneficial effects on cerebral I/R remains to be determined.
Previous research has targeted NMDAR as a key receptor for the treatment of cerebral I/R injury in animal models.410–412 Enhancing neuronal NMDAR activity exerts a neuroprotective effect on cerebral ischemia,411 reducing the content of bound NMDAR and preserving the physiological function of free NMDAR. Consequently, NMDAR treatment can reduce the infarct volume of ischemic I/R injury.412 Taken together, the crosstalk between NMDAR-Ca2+-ActA and Wnt/β-catenin pathways presents a promising therapeutic approach for I/R injury.
TLR4/TRIF signaling
The TLR family plays an integral role in the human immune system,413 and its pathway has been implicated in the progression of organ I/R injury.414 The crosstalk between the Wnt and TLR signaling pathways influences inflammation-associated cell proliferation, lung cancer cell proliferation, invasion, and metastasis.415,416 Recent findings have reported the presence of Wnt signaling during liver I/R injury, demonstrating crosstalk between Wnt and TLR signaling pathways.
The TLR family consists of 10 members and is involved in inflammation. The TLR signaling pathway comprises MyD88-dependent and MyD88-independent pathways, specific to TLR3 and TLR4, respectively. In MyD88-dependent pathway, MyD88 recruits IL-1 receptor-associated kinase to its C-terminal TIR structural domain, leading to phosphorylation of IL-1 receptor-associated kinase and activation of JNK and NF-κB.413
Tong et al. established that when receiving liver I/R, the serum transaminase levels of C1 wild-type mice were significantly elevated when treated with recombinant WISP1 protein. However, in WISP1-treated TLR4 knockout or junction-induced interferon β (TRIF) knockout mice, these levels were not elevated. The authors concluded that WISP1 causes liver I/R damage in mice through TLR4/TRIF signaling, and both factors play a synergistic role in hepatic IRI414 (Fig. 9f).
Furthermore, Mark et al. identified the expression of Wnt5a within human and mouse atherosclerotic lesions using apolipoprotein e-deficient mice and concluded that activation of the TLR-4 signaling cascade induces Wnt5a expression, and the crosstalk between TLR-4 and atypical Wnt family members, including Wnt5a, synergistically contributes to atherosclerosis.417 Additionally, a TLR4/AKT pathway that mediates Wnt5a expression has also been identified in human dental pulp stem cells.418
Although the therapeutic role of targeting TLR4/TRIF crosstalk through the Wnt signaling pathway has been explored in cardiac injury and neurological disorders, research regarding this crosstalk mechanism in I/R injury remains limited. Salwa et al. determined that the flavonoid baicalin reduced cardiac TLR4 overexpression, downregulated NF-κB expression, inhibited inflammation, attenuated cardiac fibrosis, and exerted cardioprotective effects in a doxorubicin-induced cardiotoxicity mouse model of heart injury.419 In addition, baicalin ameliorated dobiezosin-induced cardiomyopathy by significantly reducing cardiac levels of the secretory protein DKK1, upregulating Wnt/β-catenin activity, and attenuating cardiac inflammation and oxidative stress. The Wnt/β-catenin pathway has been suggested to play an antagonistic role with TLR4 in cardiac injury.419 Alternatively, REM sleep deprivation activated the TLR4/NF-κB pathway and inhibited the Wnt/β-catenin pathway in rats, resulting in neuronal damage and cognitive dysfunction in the CA1 region of the hippocampus and cerebral cortex. Nonetheless, oral administration of asparagine reversed this effect in rats and ameliorated the associated sleep disturbance and cognitive dysfunction induced by REM sleep deprivation.420
HGF/c-Met signaling
The HGF/c-Met signaling pathway is associated with cell proliferation, survival, apoptosis, migration, and embryogenesis421 and regulates cell proliferation and fibrotic processes during renal I/R through GSK3-mediated crosstalk with the Wnt/β-catenin signaling pathway. However, data on whether this crosstalk mechanism is involved in heart, brain, or liver I/R injury remain limited.
HGF consists of α and β chains that are bound via disulfide bonds, while c-Met is a MET family RYK. The activity of c-Met is initiated when the β chain of HGF binds to the Sema region of c-Met. Activated c-Met undergoes dimerization and autophosphorylation, leading to the recruitment of intracellular growth factor receptor binding protein 2 and PI3K, as well as activation of downstream signaling pathways.422,423
Crosstalk occurs between the HGF/c-Met and Wnt/β-catenin signaling pathways during renal I/R (Fig. 9g). HGF binds to the c-Met receptor and activates downstream Akt, which promotes GSK-3β amino-terminal Ser9 phosphorylation, inhibits GSK-3β activity, and activates the Wnt/β-catenin signaling pathway. Combined treatment with anti-TNF-α and HGF has been demonstrated to attenuate renal fibrosis caused by renal I/R injury in mice.424 After renal I/R injury, the remaining renal tubular epithelial cells are crucial for repairing the injured renal units.425 Koraishy et al. reported that, in early renal ischemia, activated HGF promotes LRP5/6 phosphorylation in dedifferentiated tubular epithelial cells.426 The phosphorylated LRP5/6 disrupts the destruction complex, leading to the accumulation and nuclear translocation of β-catenin, thereby activating the Wnt/β-catenin signaling pathway and exerting anti-apoptotic effects. This phosphorylation of LRP5/6 depends on the activation of the c-Met receptor, which recruits active GSK3 to LRP5/6, rather than being stimulated by Wnt protein.426 Further, β-catenin regulates HGF secretion, and the crosstalk between Wnt/β-catenin and HGF/c-Met signaling pathways enhances intercellular communication. Renal tubular epithelial cell–derived Wnt proteins communicate with mesenchymal fibroblasts via paracrine secretion.427 AKI increases renal tubular epithelial cell–derived Wnt proteins, which target mesenchymal fibroblasts and activate the Wnt/β-catenin signaling pathway. Inhibition of Wnt/β-catenin signaling induces HGF secretion from mesenchymal fibroblasts following renal I/R injury. HGF activates the HGF/c-Met signaling pathway, promoting renal tubular cell survival and proliferation.428 This suggests a negative regulatory effect of β-catenin on HGF, and the crosstalk between these two signaling pathways facilitates communication between renal tubular cells and mesenchymal fibroblasts, ultimately exacerbating AKI following renal I/R injury.
The HGF/c-Met signaling pathway also plays an important role in the treatment of cerebral ischemia.426 In a mouse stroke model, intrastriatal injection of HGF solution promoted cell proliferation and inactivate MMP activity, maintaining BBB integrity.429 It has also been found to protect against apoptosis and autophagy in rats with transient MCAO430 and stimulate neurogenesis in neural stem cells of the SVZ when directly injected into the cerebral parenchyma.431 Furthermore, BB3, a small molecule with HGF-like activity, can cross the BBB and improve neurological function after ischemic stroke by stimulating the HGF pathway.432 In renal I/R injury experiments, knockdown of β-catenin in fibroblasts activated the HGF/c-Met signaling pathway and promoted renal tubular cell proliferation.428 Based on these findings and the identified crosstalk mechanisms between HGF/c-Met and Wnt signaling, targeting crosstalk signaling pathways may hold promise as an effective therapeutic approach for organ I/R injury.
Therapeutic strategies
I/R injury is a leading cause of death in ischemic diseases, posing a significant challenge for clinicians in developing effective treatment strategies. This complication can arise in both surgical and non-surgical scenarios, and despite the development of various therapeutic approaches such as antiplatelet and antithrombotic agents, their effectiveness in reducing I/R injury remains limited. Therefore, there is a pressing need for novel treatment strategies to address ischemic diseases more effectively. The Wnt signaling pathway, along with its interplay with other signaling pathways, emerges as a critical regulator of the occurrence and progression of I/R injury. Consequently, targeting this signaling network holds promise as an innovative therapeutic strategy for this condition.
Potential therapeutic strategies targeting Wnt signaling in I/R injury
Several therapeutic approaches focusing on Wnt signaling, including cell and exosome therapy, gene therapy, protein therapy, and drug therapy, have shown promising prospects for clinical application. Table 1 summarizes the therapeutic strategies targeting Wnt signaling for the treatment of I/R injury.
Table 1.
Therapeutic strategies targeting Wnt signaling for the treatment of I/R injury
| Therapeutic strategy | Target pathways | Strategy/Molecular/ Drugs | Organ | Effects | References |
|---|---|---|---|---|---|
| Cell therapy | Active Wnt/β-catenin pathway | Exosomes isolated from adipose-derived mesenchymal stem cells, ADMSCs-EX | Heart | Up-regulate Wnt3a; Inhibiting apoptosis | 95 |
| Oligodendrocyte precursor cell transplantation | Brain | Promoting angiogenesis; Repair BBB integrity | 239 | ||
| Transplanted adipose stem cell exosomes (ADSCs-Exo) | Liver | Inhibit NF-κB pathway; Reducing pyroptosis of damaged liver | 296 | ||
| Gene therapy | Active Wnt/β-catenin pathway | Down-regulated miR-148b | Heart | Up-regulate Wnt1; Inhibiting apoptosis and oxidative damage | 93 |
| Up-regulation of LncRNA AZIN1-AS1 | Heart | LncRNA AZIN1-AS1/miR-6838-5p active Wnt3a /β-catenin; Inhibiting apoptosis | 94 | ||
| Nur77 gene ablation | Brain | Inhibiting mitochondrial fragmentation | 176 | ||
| Overexpression of LncRNA NEAT1 | Brain | Stable Wnt3a; Inhibiting apoptosis | 174 | ||
| Down-regulation of lncRNA MEG | Brain | Promoting neurogenesis | 232,252 | ||
| Molecular therapy | Inhibit Wnt non-canonical pathways | Up-regulation of Sfrp5 | Heart | Inhibit Wnt5a/JNK and Wnt/PCP; Inhibiting apoptosis and inflammation | 96–99,102 |
| Medication | Active Wnt/β-catenin pathway | A polypeptide of tuna stem protein, APTBP | Heart | Inhibiting apoptosis | 127,128 |
| Phyllanthus emblica (P. emblica) | Heart | Active PI3K/Akt/GSK3β/β-catenin; Inhibiting apoptosis and collagen fibrosis | 334 | ||
| CHIR99021 (GSK3β inhibitor) | Heart | Active Hippo pathway; Inhibiting apoptosis and cell hypertrophy | 137 | ||
| Isoflurane | Brain | Inhibiting apoptosis | 165 | ||
| XQ-1H; gastrodin | Brain | Inhibiting apoptosis; Promoting neurogenesis | 175,252 | ||
| Peroxynitrite; Mallotus oblongifolius;ellagic acid | Brain | Promoting neurogenesis | 221,229,230 | ||
| Curcumin | Brain | Promoting neurogenesis; Inhibiting apoptosis; Relieving inflammation | 208,211,231 | ||
| TWS119 | Brain | Repairing BBB; Reducing neuroinflammation | 209 | ||
| TWS119 | Brain | Inhibit Notch; Inhibiting apoptosis | 318 | ||
| Quercetin | Brain | Repairing BBB | 246,254 | ||
| Human serum albumin | Brain | Reducing oxidative stress | 212 | ||
| Galangin | Brain | nhibit HIF-1α/VEGF; Improving the neurovascular unit microenvironment | 343 | ||
| Dexmedetomidine | Brain | Active PI3K/AKT; Reducing cerebral infarct volume; Promoting neuronal survival | 333,334 | ||
| Wnt agonist (a synthetic pyrimidine) | Kidney | Inhibiting inflammatory response and oxidative stress | 293 | ||
| Minocycline | Liver | Reducing oxidative stress; Inhibiting the release of proinflammatory cytokines | 287 | ||
| Agmatine | Liver | Promoting cell proliferation; Reducing inflammation and apoptosis | 282 | ||
| Losartan | Liver | Up-regulate HIF-1α and Wnt/β-catenin signaling pathways; Up-regulate IL-6, IFN-γ, and Wnt3a; Reducing liver blood flow; Reducing liver congestion, vacuolization and necrosis | 285 | ||
| Inhibit Wnt/β-catenin pathway | Dexamethasone, Dex | Heart | MiR-208b-3p/Med13/Wnt/β-catenin; LncRNA CCAT1/miR-8063/Wnt/β-catenin; Inhibit Wnt3a and Wnt5a; Inhibitingapoptosis | 333,334 | |
| Baicalin | Heart | Reducing oxidative damage to cardiomyocytes | 414 | ||
| Huoxin pill | Heart | Inhibit NF-κB; Inhibiting inflammation | 107,366 | ||
| Melatonin | Kidney | Improving renal fibrosis | 385 | ||
| Inhibit Wnt non- canonical pathways | Curcumin | Brain | Inhibit Wnt/PCP; Promoting neurogenesis; Inhibiting apoptosis; Relieving inflammation | 211,231 |
Preclinical Studies on therapeutic strategies targeting Wnt in I/R injury
A recent clinical trial utilizing the GSK-3 inhibitor Tideglusib has demonstrated the feasibility of targeting GSK-3 in human diseases.433,434 Furthermore, lithium, another GSK-3 inhibitor used for bipolar disorder, has shown no significant adverse effects on the heart.435,436 Wen-Bin Fu et al. have extensively reviewed the therapeutic effects of Wnt pathway inhibitor in the treatment of MI.437 Several inhibitors, including pyrvinium,438 UM206,439 ICG-001,440 Wnt-974,128 CGX1321,441 and GNF-623191 have proven to be safe in clinical trials and exhibit potential for MI treatment. Additionally, Novel Wnt pathway inhibitors like Cardionogen442 and IWR1,443 have also been developed. Moreover, Wnt pathway inhibitors have recently garnered attention as potential anti-tumor medicine and are currently being investigated in ongoing clinical trials. These advancements have sparked further interest in exploring the effects of Wnt pathway inhibitors61 on organ I/R injury437; however, the development of novel Wnt pathway inhibitors with minimal clinical toxicity and unique effects on heart remains of utmost importance.
Enhancing therapeutic strategies by targeting the Wnt/crosstalk signaling pathway in I/R injury
In the pursuit of overcoming I/R injuries, clinicians are currently utilizing preconditioning and postconditioning approaches. These strategies have shown promising therapeutic effects by specifically targeting the intricate network of signaling pathways implicated in I/R pathology.
Preconditioning
Preconditioning methods have been employed to mitigate I/R injury encompass (IPC), remote ischemic preconditioning (RIPC), and pharmacological preconditioning.444 IPC treatment effectively triggers the production and release of various endogenous ligands, including adenosine,445 bradykinin,446,447 opioids,448 norepinephrine,449 and acetylcholine.450 These pharmacological pretreatment strategies have demonstrated efficacy in preventing I/R injury.444 Upon administration of IPC, the respective ligand binds to its receptor431 subsequently initiating downstream signaling cascades.443,444 The Preconditioning treatment has been shown to activate various potential pathways, including the Wnt/β-catenin,451–458 PI3K,459 Akt,460 PKC, eNOS,461 GSK-3β phosphorylation, ERK1/2, p38, MAPK,462 and JAK-STAT3 signaling. Previous research suggested that the cardioprotective effects can be achieved through targeting of GSK-3β via Wnt signaling pathway.463,464 Correa-Costa et al. postulated that Wnt signaling might play a crucial role in protection against the renal I/R injury when the ischemic IPC treatment strategy is applied.465
Currently, RIPC is considered a safe and highly appealing conditioning technique for minimizing additional damage caused by ischemic466 and has been shown to upregulate VEGF expression, followed by activation of endothelial transcription factor Id1, Wnt2, and β-catenin expression.467 This suggests that RIPC exerts its protective effects on organs, at least in part, by modulating the Wnt signaling pathway. Furthermore, RIPC decreases myocardial I/R injury by activating the JAK/STAT pathway through the involvement unacylated ghrelin.468 However, a drawback of both IPC and RIPC treatment strategies is that they must be administered prior to the onset of an ischemic event, which can be unpredictable in clinical scenarios. Consequently, researchers have developed postconditioning treatment strategies to overcome this limitation.
Postconditioning
Postconditioning encompasses different techniques, namely ischemic postconditioning (IPOSTC), remote ischemic postconditioning (RIPOSTC), and pharmacological postconditioning (PPC). IPOSTC, a relatively recent method, can be applied during reperfusion initiation to reduce infarct size.469–471 The organ protection effects of IPOSTC are mediated by the activation of network transduction pathways, including the PI3K/Akt, PI3K/Akt/eNOS/NO, MAPK, NF-κB, Gluk2/PSD95/MLK3/MKK7/JNK3, JAK2/STAT3, eNOS, MEK1/2/Erk1/2, GSK-3β, β-catenin, reperfusion injury salvage kinase, and Akt/pkB pathways.472–482 RIPOSTC is a technique that entails subjecting a distant organ to brief I/R at the onset of reperfusion in the affected organ.483 RIPOSTC is more applicable in clinical settings as it can be performed on non-vital organs, minimizing the risk of damage to the affected organ caused by reperfusion therapy following ischemia. This clinical approach is also suitable for long-term rehabilitation.484,485 RIPOSTC exerts its organ protection effects through the activation of network signaling pathways, including the eNOS, PI3K, Akt, GSK-3β, and T-LAK-cell-originated protein kinase pathways. It also enhances endogenous antioxidant enzyme activity, and inhibit δ-PKC.486–490 PPC is a therapeutic strategy applied after a severe ischemic event or at the onset of reperfusion. Various medications, including morphine, propofol, and sufentanil, have been used in clinical practice as part of PPC to prevent I/R injury. Previous studies have shown that morphine can activate Wnt/β-catenin signaling491–493; propofol has a therapeutic effect against esophageal cancer,494 gastric cancer,495 hepatocellular carcinoma,496 and colon cancer497 by blocking Wnt/β-catenin signaling; Sufentanil inhibits the proliferation of lung cancer cells by suppressing Wnt/β-catenin signaling.498 During PPC, Wnt/β-catenin signaling may be one of the molecular pathways involved in protecting organs from further damage.
Preconditioning and postconditioning strategies have demonstrated efficacy in preventing I/R in injury in clinical settings. However, the clinical feasibility of preconditioning is limited, while postconditioning holds more promise for application in clinical practice. Therefore, future research should prioritize investigating the molecular mechanisms underlying postconditioning to translate these findings into effective clinical strategies.
Discussion and perspectives
The Wnt signaling pathway encompasses various signaling branches, among which Wnt/β-catenin, Wnt/PCP, and Wnt/Ca2+ are the principal pathways implicated in organ I/R injury. Through a comprehensive analysis of available literature, it has been established that both canonical and non-canonical Wnt signaling pathways exhibit consistent patterns during the process of ischemia and reperfusion. Specifically, the canonical Wnt/β-catenin pathway is activated during the ischemic phase. This activation of the canonical pathway plays a beneficial role in injured organs through various processes such as inflammation, ECM remodeling, angiogenesis, fibrosis, and nerve regeneration. Within different organs,49,120–122,134,135,146,499–501 it serves as a compensatory protective response aimed at mitigating damage caused by ischemia and promoting the repair of resulting injuries. However, when these mechanisms become decompensated, corresponding pathological processes occur. In vitro experiments showed that neuronal OGD/R treatment can activate the Wnt/β-catenin signaling pathway to inhibit apoptosis and improve neuronal survival,171 further confirm the idea that activation of Wnt/β-catenin function to organ protect. Studies have demonstrated that as ischemia progresses, that Wnt/β-catenin signaling is inhibited in the heart, brain, and liver during the reperfusion phase. This inhibition leads to detrimental processes including apoptosis, ferroptosis, inflammation, inhibition of nerve regeneration and angiogenesis, and disruption of BBB.174–176,178,198,214,215,233,244,247,253,348,400 These pathological events contribute to organ damage promotion. In summary, the activation of the canonical Wnt/β-catenin pathway during ischemia serves as a protective mechanism, while its inhibition during reperfusion contributes to organ damage. On the other hand, activation of the non-canonical Wnt pathway contributes to organ damage during both ischemia and reperfusion.151,172 In the case of myocardial ischemia, the Wnt/PCP signaling pathway is activated, while during cerebral ischemia, the Wnt/Ca2+ signaling pathway is activated.151,172
Conflicting reports exist regarding the role of Wnt/β-catenin signaling during ischemia and reperfusion phases. Some studies showed that Wnt/β-catenin was inhibited in the heart during ischemia phase, contrary to its activation,78,502 while other studies reported that Wnt/β-catenin was activated during the reperfusion phase in renal I/R injury, instead of being inhibited.263–268 These contradictory findings may be attributed to the varying effects of Wnt/β-catenin within different organs or different cell types within the same organ. Further investigation is required to gain a comprehensive understanding of the role of Wnt/β-catenin in different contexts. In summary, our review supports the notion that activation of the canonical Wnt/β-catenin pathway serves as a protective factor, while non-canonical pathways act as mechanisms that promote organ damage. However, the precise role of Wnt/β-catenin signaling during ischemia and reperfusion requires further investigation to reconcile the conflicting reports and establish a clearer understanding of its implications in I/R injury. Detailed information on the effects of Wnt signaling pathways during I/R injury in four different organs are listed in Table 2.
Table 2.
Effects of Wnt signaling pathways during I/R injury in four different organs
| Phase | Wnt signaling pathway | Activity | Organ | Effect | References |
|---|---|---|---|---|---|
| Ischemia | Wnt/β-catenin | Activated | Heart | Promoting inflammation, ECM remodeling, angiogenesis, fibrosis | 115–117,129,130,138–141 |
| Brain | Promoting neurogenesis (in vitro) | 221 | |||
| Kidney | Promoting apoptosis and oxidative stress | 256 | |||
| Inhibited | Heart | Promoting oxidative stress | 78,126 | ||
| Brain | Promoting apoptosis, ferroptosis, inflammation, inhibiting neurogenesis, inhibiting angiogenesis, destroying BBB | 169–171,173,193,209,210,228,239,242,248,343,395 | |||
| Wnt/PCP | Activated | Heart | Promoting inflammation, cell hypertrophy | 146 | |
| Wnt/Ca2+ | Activated | Brain | Promoting apoptosis (in vitro) | 167 | |
| Reperfusion | Wnt/β-catenin | Inhibited | Heart | Promoting apoptosis inflammation, cell hypertrophy | 93–95,120,137 |
| Brain | Promoting apoptosis, inflammation, oxidative stress, inhibiting neurogenesis, inhibiting angiogenesis, destroying BBB | 10,164,165,174,175,207,232,244,246 | |||
| Liver | Promoting apoptosis, oxidative stress, inflammation, inhibiting cell proliferation | 267–271,283,285,293,296 | |||
| Activated | Brain | Inhibiting apoptosis (in vitro) | 166 | ||
| Kidney | Inhibiting apoptosis, promoting apoptosis (in vitro), mitophagy, cell autophagy, cell aging and renal fibrosis | 25,252,253,258–263 | |||
| Wnt/PCP | Activated | Heart | Promoting apoptosis, inflammation, angiogenesis | 50,102 | |
| Brain | Promote apoptosis, inflammation | 29,208 | |||
| Wnt/Ca2+ | Activated | Heart | Promoting apoptosis | 103 | |
| Kidney | Promoting renal fibrosis | 264 | |||
| Liver | Promoting apoptosis | 103,272,273 |
The Wnt signaling pathways exhibit crosstalk with a various key signaling pathways, forming a network that play a broad role in the regulation of I/R injury. Besides the mentioned crosstalk signaling pathways, we hypothesize that other signaling pathways may also be involved in this mechanism, among them, the Rho/Rho-associated protein kinase,503,504 MAPK/ERK,505 JAK/STAT,468,506 Nrf2,507 and AMPK508 signaling pathways deserve further investigation.
Co-targeting Wnt signaling pathways and their crosstalk signaling pathways presents a promising therapeutic strategy for I/R injury. In recent years, TCM has emerged as a research focus for treating organ I/R injury due to its potential therapeutic effects, minimal side effects, and promising outcomes in clinical rehabilitation. Given the diverse components of TCM, its therapeutic targets often involve multiple signaling pathways. Studies by Zhao et al.509 and Li et al.510 reported the beneficial effects of TCM components such as Astragalus,511,512 Salvia Miltiorrhiza,513,514 Angelica Sinensis,515,516 Harpagide,517 Icariin,518 pachymic acid.519 Zhao et al.509 and Li et al.510 have shown that treatment with Astragalus,511,512 Salvia Miltiorrhiza,513,514 Angelica Sinensis,515,516 Harpagide,517 Icariin,518 pachymic acid,519 and Lycopene197,520,521 confer therapeutic effects against I/R injury in the heart, brain, and other organs. Moreover, Zhao et al.509 reported that TCM like Tricin,522 Platycodin D,523 Baicalein,524 Lupeol,525 Paeoniflorin,526 and Bauhinia Championii527 could activate the PI3K/Akt signaling pathway and mitigate brain I/R injury. Additionally, these TCM treatments have also been found to modulate the activity of the Wnt/β-catenin signaling pathway in different developmental or pathological contexts.528–537 Moreover, TCM have shown activation of PI3K/Akt,522–526 NF-κB,538–540 HIF-1α,541 and Notch signaling pathways542 during organ injury treatment, indicating their potential to alleviate I/R injury by targeting multiple signaling pathways. Exercise-based cardiac rehabilitation has demonstrated numerous benefits for patients with cardiac disease, including a reduced risk of MI.543 Our previous research has shown that programmed exercise can inhibit pathological ventricular hypertrophy and myocardial fibrosis gene expression through the suppression of PKC-α/NFAT signaling in a mouse model.544 Furthermore, in an arrhythmogenic cardiomyopathy mouse model, treadmill exercise restored transcriptional levels of most differentially expressed genes and improved dysfunctional biological pathways associated with EMT, inflammation, and Wnt signaling, indicating a connection between exercise and signaling modulation.545 Therefore, we propose that a combined therapy involving targeting related network signaling pathways and exercise intervention may benefit the recovery of patients with cardiac or other organ I/R injury.
Overall, this comprehensive review of the Wnt/crosstalk signaling pathways network implicated in organ I/R injury underscores the need for novel treatment strategies in I/R injury.
Currently, most therapeutic interventions target individual signaling pathways, neglecting the complexity of the network. Therefore, future research efforts should be directed toward developing approaches that modulate this network signaling system as a cohesive unit. Such a comprehensive approach holds immense clinical potential and has the capacity to significantly enhance patient survival rates and improve their quality of life. Understanding and targeting the interconnected signaling pathways will help to facilitate the development of effective and holistic therapeutic interventions in the management of organ I/R injury.
Supplementary information
Acknowledgements
The authors would like to acknowledge the support of the research team at Jining Medical University working on the molecular mechanisms and intervention of MI. The authors would like to acknowledge Dr. Yuanchao Ye (UW Medicine Diabetes Institute, Department of Medicine, University of Washington, Seattle, WA, 98109, USA., yuancye@uw.edu), Dr. Huaping Xie (Animal Nutrition and Human Health Laboratory, School of Life Sciences, Hunan Normal University, Changsha, Hunan, 410081, China; Hunan International Joint Laboratory of Animal Intestinal Ecology and Health, Laboratory of Animal Nutrition and Human Health, School of Life Sciences, Hunan Normal University, Changsha, Hunan, 410081, China, hpxie@hunnu.edu.cn), Dr. Liwei Jia (Department of pathology, UT Southwestern Medical Center, Dallas, USA, wccjia@gmail.com), Qianxue Yu (Jining Medical University, Jining, Shandong, 272067, China, yu0203182022@163.com), Wenjie Qin (Jining Medical University, Jining, Shandong, 272067, China, qinwenjie2023@163.com), Dr. Jin Li (Division of Meyabolism, Endocrinology & Diabetes and Department of Internal Medicine, University of Michigan, Ann Arbor, MI, 48105, USA., jinlix@umich.edu), and Dr. Erge Zhang (Department of cardiac surgery, University of California, Los Angeles, USA., ErgeZhang@mednet.ucla.edu) for for their critical reading and suggestions on the manuscript. The authors would also like to express their gratitude to Editage (https://www.editage.com/) for the expert linguistic services. Thanks to “freescience” for providing guidance in drawing.
Author contributions
J.Y., R.T., and S.W.: conceptualization and resources, original draft preparation. M.Z., Q.L., and H.M.: original draft preparation, data collection, and analysis, review, and editing. H.D. and X.L.: review and editing. J.W.: data collection and revision. F.G.: project design and supervision. All authors have read and approved the final version of the manuscript.
Funding
The authors would like to acknowledge the Research Start up Fund of Jining Medical University (Reference: 600791001, J.Y.); the National Natural Science Foundation of China (81700055, R.T.), the Outstanding Talent Research Funding of Xuzhou Medical University (D2016021, R.T.), the Natural Science Foundation of Jiangsu Province (BK20160229, R.T.); the National Nature Science Foundation of China (82170255, S.W.), Shanghai Pujiang Program (21PJD013, S.W.); Shandong Provincial Higher Education Science and Technology Plan Project (J18KA177, M.Z.), Shandong Provincial University Youth Innovation Team, China (2022KJ102, M.Z.); the National Natural Science Foundation of China (82170389, J.W.), Laboratory Animal Science Foundation of Shanghai Committee of Science and Technology grant (21140904400, J.W.).
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Meng Zhang, Qian Liu, Hui Meng
Contributor Information
Shijun Wang, Email: shijun_w@126.com.
Rubin Tan, Email: tanrubin11@126.com.
Jinxiang Yuan, Email: yuanjinxiang18@163.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41392-023-01688-x.
References
- 1.Frangogiannis NG. Pathophysiology of myocardial infarction. Compr. Physiol. 2015;5:1841–1875. doi: 10.1002/cphy.c150006. [DOI] [PubMed] [Google Scholar]
- 2.Zhao, Y., Zhang, X., Chen, X. & Wei, Y. Neuronal injuries in cerebral infarction and ischemic stroke: from mechanisms to treatment (Review). Int. J. Mol. Med. 49, 15 (2022). [DOI] [PMC free article] [PubMed]
- 3.Yapca OE, Borekci B, Suleyman H. Ischemia-reperfusion damage. Eurasia. J. Med. 2013;45:126–127. doi: 10.5152/eajm.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eltzschig HK, Eckle T. Ischemia and reperfusion–from mechanism to translation. Nat. Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu MY, et al. Current mechanistic concepts in ischemia and reperfusion injury. Cell. Physiol. Biochem. 2018;46:1650–1667. doi: 10.1159/000489241. [DOI] [PubMed] [Google Scholar]
- 6.Hosohata, K., Harnsirikarn, T. & Chokesuwattanaskul, S. Ferroptosis: a potential therapeutic target in acute kidney injury. Int. J. Mol. Sci. 23, 6583 (2022). [DOI] [PMC free article] [PubMed]
- 7.Tan H, Chen L, Ma J. Penehyclidine hydrochloride post-conditioning reduces ischemia/reperfusion-induced cardiomyocyte apoptosis in rats. Exp. Ther. Med. 2017;14:4272–4278. doi: 10.3892/etm.2017.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H, et al. Inhibition of Brd4 alleviates renal ischemia/reperfusion injury-induced apoptosis and endoplasmic reticulum stress by blocking FoxO4-mediated oxidative stress. Redox Biol. 2019;24:101195. doi: 10.1016/j.redox.2019.101195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo Z, et al. NLRP3 is involved in ischemia/reperfusion injury. CNS Neurol. Disord. Drug Targets. 2016;15:699–712. doi: 10.2174/1871527315666160321111829. [DOI] [PubMed] [Google Scholar]
- 10.Ji YB, et al. Lithium alleviates blood-brain barrier breakdown after cerebral ischemia and reperfusion by upregulating endothelial Wnt/β-catenin signaling in mice. Neuropharmacology. 2021;186:108474. doi: 10.1016/j.neuropharm.2021.108474. [DOI] [PubMed] [Google Scholar]
- 11.Burke RM, Burgos Villar KN, Small EM. Fibroblast contributions to ischemic cardiac remodeling. Cell Signal. 2021;77:109824. doi: 10.1016/j.cellsig.2020.109824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X, Reboll MR, Korf-Klingebiel M, Wollert KC. Angiogenesis after acute myocardial infarction. Cardiovasc. Res. 2021;117:1257–1273. doi: 10.1093/cvr/cvaa287. [DOI] [PubMed] [Google Scholar]
- 13.Smiley D, et al. Increased fibrosis and progression to heart failure in MRL mice following ischemia/reperfusion injury. Cardiovasc. Pathol. 2014;23:327–334. doi: 10.1016/j.carpath.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Salminen A, Liu PK, Hsu CY. Alteration of transcription factor binding activities in the ischemic rat brain. Biochem. Biophys. Res. Commun. 1995;212:939–944. doi: 10.1006/bbrc.1995.2060. [DOI] [PubMed] [Google Scholar]
- 15.Werling LL, et al. Increased activation of L-type voltage-dependent calcium channels is associated with glycine enhancement of N-methyl-D-aspartate-stimulated dopamine release in global cerebral ischemia/reperfusion. J. Neurochem. 1994;63:215–221. doi: 10.1046/j.1471-4159.1994.63010215.x. [DOI] [PubMed] [Google Scholar]
- 16.Lefer AM. Mechanisms of the protective effects of transforming growth factor-beta in reperfusion injury. Biochem. Pharm. 1991;42:1323–1327. doi: 10.1016/0006-2952(91)90441-7. [DOI] [PubMed] [Google Scholar]
- 17.Tacchini L, Radice L, Bernelli-Zazzera A. Differential activation of some transcription factors during rat liver ischemia, reperfusion, and heat shock. J. Cell Physiol. 1999;180:255–262. doi: 10.1002/(SICI)1097-4652(199908)180:2<255::AID-JCP13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 18.Vukicevic S, et al. Osteogenic protein-1 (bone morphogenetic protein-7) reduces the severity of injury after ischemic acute renal failure in rat. J. Clin. Investig. 1998;102:202–214. doi: 10.1172/JCI2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mockridge JW, Marber MS, Heads RJ. Activation of Akt during simulated ischemia/reperfusion in cardiac myocytes. Biochem. Biophys. Res. Commun. 2000;270:947–952. doi: 10.1006/bbrc.2000.2522. [DOI] [PubMed] [Google Scholar]
- 20.Sakakura Y, et al. Recombinant human hepatocyte growth factor protects the liver against hepatic ischemia and reperfusion injury in rats. J. Surg. Res. 2000;92:261–266. doi: 10.1006/jsre.2000.5913. [DOI] [PubMed] [Google Scholar]
- 21.Arumugam TV, et al. Gamma secretase-mediated Notch signaling worsens brain damage and functional outcomes in ischemic stroke. Nat. Med. 2006;12:621–623. doi: 10.1038/nm1403. [DOI] [PubMed] [Google Scholar]
- 22.Terada Y, et al. Expression and function of the developmental gene Wnt-4 during experimental acute renal failure in rats. J. Am. Soc. Nephrol. 2003;14:1223–1233. doi: 10.1097/01.ASN.0000060577.94532.06. [DOI] [PubMed] [Google Scholar]
- 23.Shao D, et al. A functional interaction between Hippo-YAP signaling and FoxO1 mediates the oxidative stress response. Nat. Commun. 2014;5:3315. doi: 10.1038/ncomms4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pulskens WP, et al. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PLoS One. 2008;3:e3596. doi: 10.1371/journal.pone.0003596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong Q, et al. Wnt/β-catenin signaling pathway promotes renal ischemia-reperfusion injury by inducing oxidative stress and inflammation response. J. Recept. Signal Transduct. Res. 2021;41:15–18. doi: 10.1080/10799893.2020.1783555. [DOI] [PubMed] [Google Scholar]
- 26.Meyer, I. S. et al. Blockade of Wnt Secretion Attenuates Myocardial Ischemia-Reperfusion Injury by Modulating the Inflammatory Response. Int. J. Mol. Sci. 23, 12252 (2022). [DOI] [PMC free article] [PubMed]
- 27.Liu J, Zheng X, Zhang C, Zhang C, Bu P. Lcz696 alleviates myocardial fibrosis after myocardial infarction through the sFRP-1/Wnt/β-catenin signaling pathway. Front. Pharm. 2021;12:724147. doi: 10.3389/fphar.2021.724147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuping Z, et al. Tao-Hong-Si-Wu decoction reduces ischemia reperfusion rat myoblast cells calcium overloading and inflammation through the Wnt/IP3R/CAMKII pathway. J. Cell. Biochem. 2019;120:13095–13106. doi: 10.1002/jcb.28582. [DOI] [PubMed] [Google Scholar]
- 29.Wei X, et al. Targeting the Dvl-1/β-arrestin2/JNK3 interaction disrupts Wnt5a-JNK3 signaling and protects hippocampal CA1 neurons during cerebral ischemia reperfusion. Neuropharmacology. 2018;135:11–21. doi: 10.1016/j.neuropharm.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Gao C, Chen YG. Dishevelled: the hub of Wnt signaling. Cell. Signal. 2010;22:717–727. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Ghedalia-Peled, N. & Vago, R. Wnt Signaling in the Development of Bone Metastasis. Cells. 11, 3934 (2022). [DOI] [PMC free article] [PubMed]
- 32.Carmon KS, Gong X, Lin Q, Thomas A, Liu Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc. Natl Acad. Sci. USA. 2011;108:11452–11457. doi: 10.1073/pnas.1106083108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Lau W, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–297. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- 34.Kasai M, Satoh K, Akiyama T. Wnt signaling regulates the sequential onset of neurogenesis and gliogenesis via induction of BMPs. Genes Cells. 2005;10:777–783. doi: 10.1111/j.1365-2443.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- 35.Trifan G, Biller J, Testai FD. Mechanical thrombectomy vs bridging therapy for anterior circulation large vessel occlusion stroke: systematic review and meta-analysis. Neurology. 2022;98:e1361–e1373. doi: 10.1212/WNL.0000000000200029. [DOI] [PubMed] [Google Scholar]
- 36.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Ischemia/reperfusion. Compr. Physiol. 2016;7:113–170. doi: 10.1002/cphy.c160006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng TI, Jou MJ. Oxidative stress caused by mitochondrial calcium overload. Ann. N. Y. Acad. Sci. 2010;1201:183–188. doi: 10.1111/j.1749-6632.2010.05634.x. [DOI] [PubMed] [Google Scholar]
- 38.Salvadori M, Rosso G, Bertoni E. Update on ischemia-reperfusion injury in kidney transplantation: pathogenesis and treatment. World J. Transpl. 2015;5:52–67. doi: 10.5500/wjt.v5.i2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malis CD, Bonventre JV. Mechanism of calcium potentiation of oxygen free radical injury to renal mitochondria. a model for post-ischemic and toxic mitochondrial damage. J. Biol. Chem. 1986;261:14201–14208. doi: 10.1016/S0021-9258(18)67004-8. [DOI] [PubMed] [Google Scholar]
- 40.Nieuwenhuijs-Moeke, G. J. et al. Ischemia and reperfusion injury in kidney transplantation: relevant mechanisms in injury and repair. J Clin Med. 9, 253 (2020). [DOI] [PMC free article] [PubMed]
- 41.Gujral JS, Bucci TJ, Farhood A, Jaeschke H. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis? Hepatology. 2001;33:397–405. doi: 10.1053/jhep.2001.22002. [DOI] [PubMed] [Google Scholar]
- 42.Glinka A, et al. LGR4 and LGR5 are R-spondin receptors mediating Wnt/β-catenin and Wnt/PCP signalling. EMBO Rep. 2011;12:1055–1061. doi: 10.1038/embor.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Lau W, Peng WC, Gros P, Clevers H. The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev. 2014;28:305–316. doi: 10.1101/gad.235473.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molenaar M, et al. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/S0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 45.Pell VR, et al. Ischemic preconditioning protects against cardiac ischemia reperfusion injury without affecting succinate accumulation or oxidation. J. Mol. Cell Cardiol. 2018;123:88–91. doi: 10.1016/j.yjmcc.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee SM, Hutchinson M, Staikopoulos V, Saint DA. Amitriptyline pharmacologically preconditions rat hearts against cardiac ischemic-reperfusion injury. Int. J. Cardiol. 2015;190:353–359. doi: 10.1016/j.ijcard.2015.04.120. [DOI] [PubMed] [Google Scholar]
- 47.Li Y, Cai M, Xu Y, Swartz HM, He G. Late phase ischemic preconditioning preserves mitochondrial oxygen metabolism and attenuates post-ischemic myocardial tissue hyper oxygenation. Life Sci. 2011;88:57–64. doi: 10.1016/j.lfs.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sárközy M, et al. Ischemic preconditioning protects the heart against ischemia-reperfusion injury in chronic kidney disease in both males and females. Biol. Sex. Differ. 2021;12:49. doi: 10.1186/s13293-021-00392-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 50.Wang, J. et al. WNT11-conditioned medium promotes angiogenesis through the activation of non-canonical WNT-PKC-JNK signaling pathway. Genes11, 1277 (2020). [DOI] [PMC free article] [PubMed]
- 51.Gajos-Michniewicz, A. & Czyz, M. WNT Signaling in Melanoma. Int. J. Mol. Sci. 21, 4852 (2020). [DOI] [PMC free article] [PubMed]
- 52.Rim EY, Clevers H, Nusse R. The Wnt pathway: from signaling mechanisms to synthetic modulators. Annu. Rev. Biochem. 2022;91:571–598. doi: 10.1146/annurev-biochem-040320-103615. [DOI] [PubMed] [Google Scholar]
- 53.Wang H, et al. The Wnt signaling pathway in diabetic nephropathy. Front. Cell. Dev. Biol. 2021;9:701547. doi: 10.3389/fcell.2021.701547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malik, S. A., Modarage, K. & Goggolidou, P. The Role of Wnt Signalling in Chronic Kidney Disease (CKD). Genes11, 496 (2020). [DOI] [PMC free article] [PubMed]
- 55.Wang HY, Liu T, Malbon CC. Structure-function analysis of Frizzleds. Cell. Signal. 2006;18:934–941. doi: 10.1016/j.cellsig.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 56.Joiner DM, Ke J, Zhong Z, Xu HE, Williams BO. LRP5 and LRP6 in development and disease. Trends Endocrinol. Metab. 2013;24:31–39. doi: 10.1016/j.tem.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Menck, K., Heinrichs, S., Baden, C. & Bleckmann, A. The WNT/ROR Pathway in Cancer: From Signaling to Therapeutic Intervention. Cells. 10, 142 (2021). [DOI] [PMC free article] [PubMed]
- 58.Jung YS, Park JI. Wnt signaling in cancer: therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Exp. Mol. Med. 2020;52:183–191. doi: 10.1038/s12276-020-0380-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chae WJ, Bothwell ALM. Canonical and non-canonical Wnt signaling in immune cells. Trends Immunol. 2018;39:830–847. doi: 10.1016/j.it.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tran FH, Zheng JJ. Modulating the wnt signaling pathway with small molecules. Protein Sci. 2017;26:650–661. doi: 10.1002/pro.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akoumianakis I, Polkinghorne M, Antoniades C. Non-canonical WNT signaling in cardiovascular disease: mechanisms and therapeutic implications. Nat. Rev. Cardiol. 2022;19:783–797. doi: 10.1038/s41569-022-00718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jin Z, et al. Neuroprotective effects of irisin against cerebral ischemia/ reperfusion injury via Notch signaling pathway. Biomed. Pharmacother. 2019;120:109452. doi: 10.1016/j.biopha.2019.109452. [DOI] [PubMed] [Google Scholar]
- 63.Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J. Biol. Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 64.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev. Cell. 2003;5:367–377. doi: 10.1016/S1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 65.Kikuchi A, Yamamoto H. Tumor formation due to abnormalities in the beta-catenin-independent pathway of Wnt signaling. Cancer Sci. 2008;99:202–208. doi: 10.1111/j.1349-7006.2007.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi DL. Decoding dishevelled-mediated Wnt signaling in vertebrate early development. Front. Cell Dev. Biol. 2020;8:588370. doi: 10.3389/fcell.2020.588370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lerner UH, Ohlsson C. The WNT system: background and its role in bone. J. Intern. Med. 2015;277:630–649. doi: 10.1111/joim.12368. [DOI] [PubMed] [Google Scholar]
- 68.VanderVorst K, et al. Wnt/PCP signaling contribution to carcinoma collective cell migration and metastasis. Cancer Res. 2019;79:1719–1729. doi: 10.1158/0008-5472.CAN-18-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frenquelli M, Tonon G. WNT signaling in hematological malignancies. Front. Oncol. 2020;10:615190. doi: 10.3389/fonc.2020.615190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cho SJ, et al. Wip1 directly dephosphorylates NLK and increases Wnt activity during germ cell development. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:1013–1022. doi: 10.1016/j.bbadis.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 71.Xiao Q, Chen Z, Jin X, Mao R, Chen Z. The many postures of noncanonical Wnt signaling in development and diseases. Biomed. Pharmacother. 2017;93:359–369. doi: 10.1016/j.biopha.2017.06.061. [DOI] [PubMed] [Google Scholar]
- 72.Ma L, Wang HY. Mitogen-activated protein kinase p38 regulates the Wnt/cyclic GMP/Ca2+ non-canonical pathway. J. Biol. Chem. 2007;282:28980–28990. doi: 10.1074/jbc.M702840200. [DOI] [PubMed] [Google Scholar]
- 73.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J. Clin. Investig. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vaage J, Valen G. Pathophysiology and mediators of ischemia-reperfusion injury with special reference to cardiac surgery. A review. Scand. J. Thorac. Cardiovasc Surg. Suppl. 1993;41:1–18. doi: 10.3109/14017439309100154. [DOI] [PubMed] [Google Scholar]
- 75.Tanaka M, et al. Cardiomyocyte-specific Bcl-2 overexpression attenuates ischemia-reperfusion injury, immune response during acute rejection, and graft coronary artery disease. Blood. 2004;104:3789–3796. doi: 10.1182/blood-2004-02-0666. [DOI] [PubMed] [Google Scholar]
- 76.Kleinbongard P, Heusch G, Schulz R. TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharm. Ther. 2010;127:295–314. doi: 10.1016/j.pharmthera.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 77.Frohlich GM, Meier P, White SK, Yellon DM, Hausenloy DJ. Myocardial reperfusion injury: looking beyond primary PCI. Eur. Heart J. 2013;34:1714–1722. doi: 10.1093/eurheartj/eht090. [DOI] [PubMed] [Google Scholar]
- 78.Shen J, et al. Wnt 3a protects myocardial injury in elderly acute myocardial infarction by inhibiting serum cystatin C/ROS-induced mitochondrial damage. Front. Physiol. 2022;13:950960. doi: 10.3389/fphys.2022.950960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Piper HM, García-Dorado D, Ovize M. A fresh look at reperfusion injury. Cardiovasc. Res. 1998;38:291–300. doi: 10.1016/S0008-6363(98)00033-9. [DOI] [PubMed] [Google Scholar]
- 80.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N. Engl. J. Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 81.Logue SE, Gustafsson AB, Samali A, Gottlieb RA. Ischemia/reperfusion injury at the intersection with cell death. J. Mol. Cell Cardiol. 2005;38:21–33. doi: 10.1016/j.yjmcc.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 82.Gottlieb RA. Cell death pathways in acute ischemia/reperfusion injury. J. Cardiovasc. Pharm. Ther. 2011;16:233–238. doi: 10.1177/1074248411409581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Y, et al. Ferroptosis: a novel therapeutic target for ischemia-reperfusion injury. Front. Cell Dev. Biol. 2021;9:688605. doi: 10.3389/fcell.2021.688605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hamacher-Brady A, Brady NR, Gottlieb RA. The interplay between pro-death and pro-survival signaling pathways in myocardial ischemia/reperfusion injury: apoptosis meets autophagy. Cardiovasc Drugs Ther. 2006;20:445–462. doi: 10.1007/s10557-006-0583-7. [DOI] [PubMed] [Google Scholar]
- 85.Deb A. Cell-cell interaction in the heart via Wnt/beta-catenin pathway after cardiac injury. Cardiovasc. Res. 2014;102:214–223. doi: 10.1093/cvr/cvu054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lorenzon A, et al. Wnt/beta-catenin pathway in arrhythmogenic cardiomyopathy. Oncotarget. 2017;8:60640–60655. doi: 10.18632/oncotarget.17457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bergmann MW. WNT signaling in adult cardiac hypertrophy and remodeling: lessons learned from cardiac development. Circ. Res. 2010;107:1198–1208. doi: 10.1161/CIRCRESAHA.110.223768. [DOI] [PubMed] [Google Scholar]
- 88.Oerlemans MI, et al. Active Wnt signaling in response to cardiac injury. Basic Res. Cardiol. 2010;105:631–641. doi: 10.1007/s00395-010-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haybar H, Khodadi E, Shahrabi S. Wnt/β-catenin in ischemic myocardium: interactions and signaling pathways as a therapeutic target. Heart Fail. Rev. 2019;24:411–419. doi: 10.1007/s10741-018-9759-z. [DOI] [PubMed] [Google Scholar]
- 90.Litvinukova M, et al. Cells of the adult human heart. Nature. 2020;588:466–472. doi: 10.1038/s41586-020-2797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bastakoty, D. et al. Temporary, Systemic inhibition of the WNT/beta-catenin pathway promotes regenerative cardiac repair following myocardial infarct. Cell Stem Cells Regen. Med. 2, 16966 (2016). [DOI] [PMC free article] [PubMed]
- 92.Haybar H, Khodadi E, Shahrabi S. Wnt/beta-catenin in ischemic myocardium: interactions and signaling pathways as a therapeutic target. Heart Fail Rev. 2019;24:411–419. doi: 10.1007/s10741-018-9759-z. [DOI] [PubMed] [Google Scholar]
- 93.Yang M, Kong DY, Chen JC. Inhibition of miR-148b ameliorates myocardial ischemia/reperfusion injury via regulation of Wnt/beta-catenin signaling pathway. J. Cell Physiol. 2019;234:17757–17766. doi: 10.1002/jcp.28401. [DOI] [PubMed] [Google Scholar]
- 94.Zhang G, et al. LncRNA AZIN1-AS1 ameliorates myocardial ischemia-reperfusion injury by targeting miR-6838-5p/WNT3A axis to activate Wnt-beta/catenin signaling pathway. Vitr. Cell Dev. Biol. Anim. 2022;58:54–68. doi: 10.1007/s11626-022-00646-1. [DOI] [PubMed] [Google Scholar]
- 95.Cui X, et al. Exosomes from adipose-derived mesenchymal stem cells protect the myocardium against ischemia/reperfusion injury through Wnt/beta-catenin signaling pathway. J. Cardiovasc. Pharm. 2017;70:225–231. doi: 10.1097/FJC.0000000000000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Finch PW, et al. Purification and molecular cloning of a secreted, Frizzled-related antagonist of Wnt action. Proc. Natl Acad. Sci. USA. 1997;94:6770–6775. doi: 10.1073/pnas.94.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J. Cell Sci. 2008;121:737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- 98.Ouchi N, et al. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 2010;329:454–457. doi: 10.1126/science.1188280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li Y, et al. Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev. 2008;22:3050–3063. doi: 10.1101/gad.1687308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kikuchi R, et al. An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat. Med. 2014;20:1464–1471. doi: 10.1038/nm.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fuster JJ, et al. Noncanonical Wnt signaling promotes obesity-induced adipose tissue inflammation and metabolic dysfunction independent of adipose tissue expansion. Diabetes. 2015;64:1235–1248. doi: 10.2337/db14-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nakamura K, et al. Secreted Frizzled-related protein 5 diminishes cardiac inflammation and protects the heart from ischemia/reperfusion injury. J. Biol. Chem. 2016;291:2566–2575. doi: 10.1074/jbc.M115.693937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou SS, He F, Chen AH, Hao PY, Song XD. Suppression of rat Frizzled-2 attenuates hypoxia/reoxygenation-induced Ca2+ accumulation in rat H9c2 cells. Exp. Cell Res. 2012;318:1480–1491. doi: 10.1016/j.yexcr.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 104.Zhang L, et al. Inhibition of Rac1 reduces store overload-induced calcium release and protects against ventricular arrhythmia. J. Cell Mol. Med. 2016;20:1513–1522. doi: 10.1111/jcmm.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Belevych AE, et al. Redox modification of ryanodine receptors underlies calcium alternans in a canine model of sudden cardiac death. Cardiovasc Res. 2009;84:387–395. doi: 10.1093/cvr/cvp246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fauconnier J, et al. Ryanodine receptor leak mediated by caspase-8 activation leads to left ventricular injury after myocardial ischemia-reperfusion. Proc. Natl Acad. Sci. USA. 2011;108:13258–13263. doi: 10.1073/pnas.1100286108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.He J, et al. Huoxin pill prevents excessive inflammation and cardiac dysfunction following myocardial infarction by inhibiting adverse Wnt/betacatenin signaling activation. Phytomedicine. 2022;104:154293. doi: 10.1016/j.phymed.2022.154293. [DOI] [PubMed] [Google Scholar]
- 108.Hu Y, et al. Class A scavenger receptor attenuates myocardial infarction-induced cardiomyocyte necrosis through suppressing M1 macrophage subset polarization. Basic Res. Cardiol. 2011;106:1311–1328. doi: 10.1007/s00395-011-0204-x. [DOI] [PubMed] [Google Scholar]
- 109.Cutolo M, Campitiello R, Gotelli E, Soldano S. The role of M1/M2 macrophage polarization in rheumatoid arthritis synovitis. Front. Immunol. 2022;13:867260. doi: 10.3389/fimmu.2022.867260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang RYK, Cochran BJ, Thomas SR, Rye KA. Impact of reperfusion on temporal immune cell dynamics after myocardial infarction. J. Am. Heart Assoc. 2023;12:e027600. doi: 10.1161/JAHA.122.027600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ. Res. 2016;119:91–112. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nahrendorf M, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cheng B, Chen HC, Chou IW, Tang TW, Hsieh PC. Harnessing the early post-injury inflammatory responses for cardiac regeneration. J. Biomed. Sci. 2017;24:7. doi: 10.1186/s12929-017-0315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang CK, et al. Lgr4 governs a pro-inflammatory program in macrophages to antagonize post-infarction cardiac repair. Circ. Res. 2020;127:953–973. doi: 10.1161/CIRCRESAHA.119.315807. [DOI] [PubMed] [Google Scholar]
- 115.Zhao G, et al. CXCR6 deficiency ameliorated myocardial ischemia/reperfusion injury by inhibiting infiltration of monocytes and IFN-γ-dependent autophagy. Int. J. Cardiol. 2013;168:853–862. doi: 10.1016/j.ijcard.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 116.Gombozhapova A, et al. Macrophage activation and polarization in post-infarction cardiac remodeling. J. Biomed. Sci. 2017;24:13. doi: 10.1186/s12929-017-0322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yuan C, et al. Modulation of Wnt/β-catenin signaling in IL-17A-mediated macrophage polarization of RAW264.7 cells. Braz. J. Med. Biol. Res. 2020;53:e9488. doi: 10.1590/1414-431x20209488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Palevski, D. et al. Loss of macrophage Wnt secretion improves remodeling and function after myocardial infarction in mice. J. Am. Heart Assoc. 6, e004387 (2017). [DOI] [PMC free article] [PubMed]
- 119.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodeling. Nat. Rev. Cardiol. 2014;11:255–265. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun S, Wu Y, Maimaitijiang A, Huang Q, Chen Q. Ferroptotic cardiomyocyte-derived exosomes promote cardiac macrophage M1 polarization during myocardial infarction. PeerJ. 2022;10:e13717. doi: 10.7717/peerj.13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang L, Xiang M, Ye P, Zhou W, Chen M. Beta-catenin promotes macrophage-mediated acute inflammatory response after myocardial infarction. Immunol. Cell. Biol. 2018;96:100–113. doi: 10.1111/imcb.1019. [DOI] [PubMed] [Google Scholar]
- 122.Aisagbonhi O, et al. Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition. Dis. Model Mech. 2011;4:469–483. doi: 10.1242/dmm.006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Aleshin A, et al. RAGE modulates myocardial injury consequent to LAD infarction via impact on JNK and STAT signaling in a murine model. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H1823–H1832. doi: 10.1152/ajpheart.01210.2007. [DOI] [PubMed] [Google Scholar]
- 124.Bucciarelli LG, et al. Receptor for advanced-glycation end products: key modulator of myocardial ischemic injury. Circulation. 2006;113:1226–1234. doi: 10.1161/CIRCULATIONAHA.105.575993. [DOI] [PubMed] [Google Scholar]
- 125.Park H, et al. RAGE siRNA-mediated gene silencing provides cardioprotection against ventricular arrhythmias in acute ischemia and reperfusion. J. Control Release. 2015;217:315–326. doi: 10.1016/j.jconrel.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 126.Rauner M, et al. WNT5A is induced by inflammatory mediators in bone marrow stromal cells and regulates cytokine and chemokine production. J. Bone Min. Res. 2012;27:575–585. doi: 10.1002/jbmr.1488. [DOI] [PubMed] [Google Scholar]
- 127.Meyer IS, et al. The cardiac microenvironment uses non-canonical WNT signaling to activate monocytes after myocardial infarction. EMBO Mol. Med. 2017;9:1279–1293. doi: 10.15252/emmm.201707565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Moon J, et al. Blockade to pathological remodeling of infarcted heart tissue using a porcupine antagonist. Proc. Natl Acad. Sci. USA. 2017;114:1649–1654. doi: 10.1073/pnas.1621346114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler. Thromb. Vasc. Biol. 2008;28:504–510. doi: 10.1161/ATVBAHA.107.157438. [DOI] [PubMed] [Google Scholar]
- 130.Port F, et al. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat. Cell Biol. 2008;10:178–185. doi: 10.1038/ncb1687. [DOI] [PubMed] [Google Scholar]
- 131.Guo X, et al. Induced pluripotent stem cell-conditional medium inhibits H9C2 cardiomyocytes apoptosis via autophagy flux and Wnt/beta-catenin pathway. J. Cell. Mol. Med. 2019;23:4358–4374. doi: 10.1111/jcmm.14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Je, J. Y., Qian, Z. J., Byun, H. G. & Kim, S. K. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 42, 840–846 (2007).
- 133.Zhang L, et al. The restoration of Wnt/β-catenin signaling activity by a tuna backbone-derived peptide ameliorates hypoxia-induced cardiomyocyte injury. Am. J. Transl. Res. 2020;12:5221–5236. [PMC free article] [PubMed] [Google Scholar]
- 134.Blankesteijn WM, van Gijn ME, Essers-Janssen YP, Daemen MJ, Smits JF. Beta-catenin, an inducer of uncontrolled cell proliferation and migration in malignancies, is localized in the cytoplasm of vascular endothelium during neovascularization after myocardial infarction. Am. J. Pathol. 2000;157:877–883. doi: 10.1016/S0002-9440(10)64601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Barandon L, et al. Reduction of infarct size and prevention of cardiac rupture in transgenic mice overexpressing FrzA. Circulation. 2003;108:2282–2289. doi: 10.1161/01.CIR.0000093186.22847.4C. [DOI] [PubMed] [Google Scholar]
- 136.Baruah J, et al. The allosteric glycogen synthase kinase-3 inhibitor NP12 limits myocardial remodeling and promotes angiogenesis in an acute myocardial infarction model. J. Biol. Chem. 2017;292:20785–20798. doi: 10.1074/jbc.M117.814376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu B, et al. Loss of endothelial glucocorticoid receptor promotes angiogenesis via upregulation of Wnt/beta-catenin pathway. Angiogenesis. 2021;24:631–645. doi: 10.1007/s10456-021-09773-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.MacLellan WR, Schneider MD. Genetic dissection of cardiac growth control pathways. Annu. Rev. Physiol. 2000;62:289–319. doi: 10.1146/annurev.physiol.62.1.289. [DOI] [PubMed] [Google Scholar]
- 139.Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018;15:387–407. doi: 10.1038/s41569-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 140.Shimizu I, Minamino T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell Cardiol. 2016;97:245–262. doi: 10.1016/j.yjmcc.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 141.Hagenmueller M, et al. Dapper-1 is essential for Wnt5a induced cardiomyocyte hypertrophy by regulating the Wnt/PCP pathway. FEBS Lett. 2014;588:2230–2237. doi: 10.1016/j.febslet.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 142.Khan K, Makhoul G, Yu B, Schwertani A, Cecere R. The cytoprotective impact of yes-associated protein 1 after ischemia-reperfusion injury in AC16 human cardiomyocytes. Exp. Biol. Med. 2019;244:802–812. doi: 10.1177/1535370219851243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhao X, et al. Aldehyde dehydrogenase-2 protects against myocardial infarction-related cardiac fibrosis through modulation of the Wnt/beta-catenin signaling pathway. Ther. Clin. Risk Manag. 2015;11:1371–1381. doi: 10.2147/TCRM.S88297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Qian L, et al. Downregulation of S100A4 Alleviates Cardiac Fibrosis via Wnt/beta -Catenin Pathway in Mice. Cell Physiol. Biochem. 2018;46:2551–2560. doi: 10.1159/000489683. [DOI] [PubMed] [Google Scholar]
- 145.Cui S, et al. miR-145 attenuates cardiac fibrosis through the AKT/GSK-3beta/beta-catenin signaling pathway by directly targeting SOX9 in fibroblasts. J. Cell Biochem. 2021;122:209–221. doi: 10.1002/jcb.29843. [DOI] [PubMed] [Google Scholar]
- 146.Matsushima K, et al. Secreted frizzled related protein 4 reduces fibrosis scar size and ameliorates cardiac function after ischemic injury. Tissue Eng. Part A. 2010;16:3329–3341. doi: 10.1089/ten.tea.2009.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Duan J, et al. Wnt1/betacatenin injury response activates the epicardium and cardiac fibroblasts to promote cardiac repair. EMBO J. 2012;31:429–442. doi: 10.1038/emboj.2011.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang Y, et al. PRELP promotes myocardial fibrosis and ventricular remodeling after acute myocardial infarction by the wnt/β-catenin signalling pathway. Cardiovasc J. Afr. 2022;33:228–233. doi: 10.5830/CVJA-2022-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jean LeBlanc N, et al. Canonical Wnt pathway maintains blood-brain barrier integrity upon ischemic stroke and its activation ameliorates tissue plasminogen activator therapy. Mol. Neurobiol. 2019;56:6521–6538. doi: 10.1007/s12035-019-1539-9. [DOI] [PubMed] [Google Scholar]
- 150.Abuelazm M, et al. The efficacy and safety of tenecteplase versus alteplase for acute ischemic stroke: an updated systematic review, pairwise, and network meta-analysis of randomized controlled trials. J. Thromb. Thrombolysis. 2023;55:322–338. doi: 10.1007/s11239-022-02730-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Berge, E. et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 6, I–lxii (2021). [DOI] [PMC free article] [PubMed]
- 152.Emberson J, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomized trials. Lancet. 2014;384:1929–1935. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xiong Y, Wakhloo AK, Fisher M. Advances in Acute Ischemic Stroke Therapy. Circ. Res. 2022;130:1230–1251. doi: 10.1161/CIRCRESAHA.121.319948. [DOI] [PubMed] [Google Scholar]
- 154.Katsanos AH, et al. Intravenous thrombolysis prior to mechanical thrombectomy in large vessel occlusions. Ann. Neurol. 2019;86:395–406. doi: 10.1002/ana.25544. [DOI] [PubMed] [Google Scholar]
- 155.Fischer U, et al. Primary thrombectomy in tPA (Tissue-Type Plasminogen Activator) eligible stroke patients with proximal intracranial occlusions. Stroke. 2018;49:265–269. doi: 10.1161/STROKEAHA.117.018564. [DOI] [PubMed] [Google Scholar]
- 156.Rai AT, et al. Intravenous thrombolysis before endovascular therapy for large vessel strokes can lead to significantly higher hospital costs without improving outcomes. J. Neurointerv. Surg. 2018;10:17–21. doi: 10.1136/neurintsurg-2016-012830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Goyal N, et al. Impact of pretreatment with intravenous thrombolysis on reperfusion status in acute strokes treated with mechanical thrombectomy. J. Neurointerv Surg. 2019;11:1073–1079. doi: 10.1136/neurintsurg-2019-014746. [DOI] [PubMed] [Google Scholar]
- 158.Rossi R, et al. Does prior administration of rtPA influence acute ischemic stroke clot composition? Findings from the analysis of clots retrieved with mechanical thrombectomy from the RESTORE registry. J. Neurol. 2022;269:1913–1920. doi: 10.1007/s00415-021-10758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Muroyama Y, Kondoh H, Takada S. Wnt proteins promote neuronal differentiation in neural stem cell culture. Biochem. Biophys. Res. Commun. 2004;313:915–921. doi: 10.1016/j.bbrc.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 160.Maretto S, et al. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc. Natl Acad. Sci. USA. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McKenzie MG, et al. Non-canonical Wnt signaling through Ryk regulates the generation of somatostatin- and parvalbumin-expressing cortical interneurons. Neuron. 2019;103:853–864.e854. doi: 10.1016/j.neuron.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lewis JL, et al. Reiterated Wnt signaling during zebrafish neural crest development. Development. 2004;131:1299–1308. doi: 10.1242/dev.01007. [DOI] [PubMed] [Google Scholar]
- 163.Hutchins BI, Li L, Kalil K. Wnt-induced calcium signaling mediates axon growth and guidance in the developing corpus callosum. Sci. Signal. 2012;5:pt1. doi: 10.1126/scisignal.2002523. [DOI] [PubMed] [Google Scholar]
- 164.Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac, and JNK regulates dendritic development. Nat. Neurosci. 2005;8:34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- 165.Liebner S, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J. Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Benz, F. et al. Low wnt/β-catenin signaling determines leaky vessels in the subfornical organ and affects water homeostasis in mice. Elife8, e43818 (2019). [DOI] [PMC free article] [PubMed]
- 167.Shi ZY, et al. Protective effect of autophagy in neural ischemia and hypoxia: negative regulation of the Wnt/β-catenin pathway. Int J. Mol. Med. 2017;40:1699–1708. doi: 10.3892/ijmm.2017.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ji YB, Wang TX, Gao Q, Huang XW, Chang J. Normalization of non-canonical Wnt signalings does not compromise blood-brain barrier protection conferred by upregulating endothelial Wnt/β-catenin signaling following ischemic stroke. CNS Neurosci. Ther. 2021;27:1085–1096. doi: 10.1111/cns.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhao H, et al. Sirt3 inhibits cerebral ischemia-reperfusion injury through normalizing Wnt/β-catenin pathway and blocking mitochondrial fission. Cell Stress Chaperones. 2018;23:1079–1092. doi: 10.1007/s12192-018-0917-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang G, et al. Wnt/β-catenin signaling pathway contributes to isoflurane postconditioning against cerebral ischemia-reperfusion injury and is possibly related to the transforming growth factorβ1/Smad3 signaling pathway. Biomed. Pharmacother. 2019;110:420–430. doi: 10.1016/j.biopha.2018.11.143. [DOI] [PubMed] [Google Scholar]
- 171.Li T, et al. DIXDC1 prevents oxygen-glucose deprivation/reoxygenation-induced injury in hippocampal neurons in vitro by promoting Wnt/β-catenin signaling. Eur. Rev. Med. Pharm. Sci. 2018;22:5678–5687. doi: 10.26355/eurrev_201809_15835. [DOI] [PubMed] [Google Scholar]
- 172.Niu LJ, Xu RX, Zhang P, Du MX, Jiang XD. Suppression of Frizzled-2-mediated Wnt/Ca²+ signaling significantly attenuates intracellular calcium accumulation in vitro and in a rat model of traumatic brain injury. Neuroscience. 2012;213:19–28. doi: 10.1016/j.neuroscience.2012.03.057. [DOI] [PubMed] [Google Scholar]
- 173.Kunz A, Dirnagl U, Mergenthaler P. Acute pathophysiological processes after ischaemic and traumatic brain injury. Best. Pr. Res. Clin. Anaesthesiol. 2010;24:495–509. doi: 10.1016/j.bpa.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 174.Seifert-Held T, et al. Circulating Dickkopf-1 in acute ischemic stroke and clinically stable cerebrovascular disease. Atherosclerosis. 2011;218:233–237. doi: 10.1016/j.atherosclerosis.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 175.Cappuccio I, et al. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is required for the development of ischemic neuronal death. J. Neurosci. 2005;25:2647–2657. doi: 10.1523/JNEUROSCI.5230-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mastroiacovo F, et al. Induction of the Wnt antagonist, Dickkopf-1, contributes to the development of neuronal death in models of brain focal ischemia. J. Cereb. Blood Flow. Metab. 2009;29:264–276. doi: 10.1038/jcbfm.2008.111. [DOI] [PubMed] [Google Scholar]
- 177.Scali C, et al. Inhibition of Wnt signaling, modulation of Tau phosphorylation and induction of neuronal cell death by DKK1. Neurobiol. Dis. 2006;24:254–265. doi: 10.1016/j.nbd.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 178.Che QQ, Huang T, Zhang YD, Qian XJ. Effect of miR-124 on neuronal apoptosis in rats with cerebral infarction through Wnt/β-catenin signaling pathway. Eur. Rev. Med. Pharm. Sci. 2019;23:6657–6664. doi: 10.26355/eurrev_201908_18556. [DOI] [PubMed] [Google Scholar]
- 179.Zhou Z, Ren X, Zheng L, Li A, Zhou W. LncRNA NEAT1 stabilized Wnt3a via U2AF2 and activated Wnt/β-catenin pathway to alleviate ischemia stroke induced injury. Brain Res. 2022;1788:147921. doi: 10.1016/j.brainres.2022.147921. [DOI] [PubMed] [Google Scholar]
- 180.Xu D, et al. XQ-1H alleviates cerebral ischemia in mice through inhibition of apoptosis and promotion of neurogenesis in a Wnt/β-catenin signaling dependent way. Life Sci. 2019;235:116844. doi: 10.1016/j.lfs.2019.116844. [DOI] [PubMed] [Google Scholar]
- 181.Zhao H, Pan W, Chen L, Luo Y, Xu R. Nur77 promotes cerebral ischemia-reperfusion injury via activating INF2-mediated mitochondrial fragmentation. J. Mol. Histol. 2018;49:599–613. doi: 10.1007/s10735-018-9798-8. [DOI] [PubMed] [Google Scholar]
- 182.Chong ZZ, Maiese K. Targeting WNT, protein kinase B, and mitochondrial membrane integrity to foster cellular survival in the nervous system. Histol. Histopathol. 2004;19:495–504. doi: 10.14670/hh-19.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Guo C, Whitmarsh AJ. The beta-arrestin-2 scaffold protein promotes c-Jun N-terminal kinase-3 activation by binding to its nonconserved N terminus. J. Biol. Chem. 2008;283:15903–15911. doi: 10.1074/jbc.M710006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kuan CY, et al. A critical role of neural-specific JNK3 for ischemic apoptosis. Proc. Natl Acad. Sci. USA. 2003;100:15184–15189. doi: 10.1073/pnas.2336254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang QG, Wang R, Khan M, Mahesh V, Brann DW. Role of Dickkopf-1, an antagonist of the Wnt/beta-catenin signaling pathway, in estrogen-induced neuroprotection and attenuation of tau phosphorylation. J. Neurosci. 2008;28:8430–8441. doi: 10.1523/JNEUROSCI.2752-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cheng YL, et al. Evidence that collaboration between HIF-1α and Notch-1 promotes neuronal cell death in ischemic stroke. Neurobiol. Dis. 2014;62:286–295. doi: 10.1016/j.nbd.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Burchell SR, Dixon BJ, Tang J, Zhang JH. Isoflurane provides neuroprotection in neonatal hypoxic ischemic brain injury. J. Investig. Med. 2013;61:1078–1083. doi: 10.2310/JIM.0b013e3182a07921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lan XB, et al. Neuroprotective effects of oxymatrine on hypoxic-ischemic brain damage in neonatal rats by activating the Wnt/β-catenin pathway. Biomed. Pharmacother. 2023;159:114266. doi: 10.1016/j.biopha.2023.114266. [DOI] [PubMed] [Google Scholar]
- 189.Yan HF, Tuo QZ, Yin QZ, Lei P. The pathological role of ferroptosis in ischemia/reperfusion-related injury. Zool. Res. 2020;41:220–230. doi: 10.24272/j.issn.2095-8137.2020.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Li D, Li Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct. Target Ther. 2020;5:108. doi: 10.1038/s41392-020-00216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Li L, Li YW, Zhao JY, Liu YZ, Holscher C. Quantitative analysis of iron concentration and expression of ferroportin 1 in the cortex and hippocampus of rats induced by cerebral ischemia. J. Clin. Neurosci. 2009;16:1466–1472. doi: 10.1016/j.jocn.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 192.Won SM, et al. Iron mediates endothelial cell damage and blood-brain barrier opening in the hippocampus after transient forebrain ischemia in rats. Exp. Mol. Med. 2011;43:121–128. doi: 10.3858/emm.2011.43.2.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hällgren R, Terent A, Wide L, Bergström K, Birgegård G. Cerebrospinal fluid ferritin in patients with cerebral infarction or bleeding. Acta Neurol. Scand. 1980;61:384–392. doi: 10.1111/j.1600-0404.1980.tb01508.x. [DOI] [PubMed] [Google Scholar]
- 194.Shi Y, et al. Selenium alleviates cerebral ischemia/reperfusion injury by regulating oxidative stress, mitochondrial fusion and ferroptosis. Neurochem. Res. 2022;47:2992–3002. doi: 10.1007/s11064-022-03643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Groenendaal F, Shadid M, McGowan JE, Mishra OP, van Bel F. Effects of deferoxamine, a chelator of free iron, on NA(+), K(+)-ATPase activity of cortical brain cell membrane during early reperfusion after hypoxia-ischemia in newborn lambs. Pediatr. Res. 2000;48:560–564. doi: 10.1203/00006450-200010000-00023. [DOI] [PubMed] [Google Scholar]
- 196.Shadid M, et al. Effect of deferoxamine and allopurinol on non-protein-bound iron concentrations in plasma and cortical brain tissue of newborn lambs following hypoxia-ischemia. Neurosci. Lett. 1998;248:5–8. doi: 10.1016/S0304-3940(98)00303-6. [DOI] [PubMed] [Google Scholar]
- 197.Zhao Y, et al. Nano-liposomes of lycopene reduces ischemic brain damage in rodents by regulating iron metabolism. Free Radic. Biol. Med. 2018;124:1–11. doi: 10.1016/j.freeradbiomed.2018.05.082. [DOI] [PubMed] [Google Scholar]
- 198.Yin M, et al. circAFF1 enhances intracerebral hemorrhage induced neuronal ferroptosis by targeting miR-140-5p to regulate GSK-3β mediated Wnt/β-catenin signal pathway. Brain Res. Bull. 2022;189:11–21. doi: 10.1016/j.brainresbull.2022.08.005. [DOI] [PubMed] [Google Scholar]
- 199.Wu X, et al. Regulation of GSK3β/Nrf2 signaling pathway modulated erastin-induced ferroptosis in breast cancer. Mol. Cell Biochem. 2020;473:217–228. doi: 10.1007/s11010-020-03821-8. [DOI] [PubMed] [Google Scholar]
- 200.Armagan, G. et al. Regulation of the Nrf2 Pathway by Glycogen Synthase Kinase-3β in MPP+-Induced Cell Damage. Molecules. 24, 1377 (2019). [DOI] [PMC free article] [PubMed]
- 201.Wang L, Ouyang S, Li B, Wu H, Wang F. GSK-3β manipulates ferroptosis sensitivity by dominating iron homeostasis. Cell Death Discov. 2021;7:334. doi: 10.1038/s41420-021-00726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Candelario-Jalil E, Dijkhuizen RM, Magnus T. Neuroinflammation, stroke, blood-brain barrier dysfunction, and imaging modalities. Stroke. 2022;53:1473–1486. doi: 10.1161/STROKEAHA.122.036946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lengfeld JE, et al. Endothelial Wnt/β-catenin signaling reduces immune cell infiltration in multiple sclerosis. Proc. Natl Acad. Sci. USA. 2017;114:E1168–e1177. doi: 10.1073/pnas.1609905114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Liu Z, Chopp M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog. Neurobiol. 2016;144:103–120. doi: 10.1016/j.pneurobio.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ma, Y., Wang, J., Wang, Y. & Yang, G. Y. The biphasic function of microglia in ischemic stroke. Prog. Neurobiol.157, 247–272 (2017). [DOI] [PubMed]
- 206.Kanazawa, M., Ninomiya, I., Hatakeyama, M., Takahashi, T. & Shimohata, T. Microglia and Monocytes/Macrophages Polarization Reveal Novel Therapeutic Mechanism against Stroke. Int. J. Mol. Sci. 18, 2135 (2017). [DOI] [PMC free article] [PubMed]
- 207.Wang Y, et al. Antioxidants & Redox Signaling. Antioxid. Redox Signal. 2020;32:213–214. doi: 10.1089/ars.2017.7003.correx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zolezzi JM, Inestrosa NC. Wnt/TLR Dialog in Neuroinflammation, Relevance in Alzheimer’s Disease. Front. Immunol. 2017;8:187. doi: 10.3389/fimmu.2017.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yeh H, Woodbury ME, Ingraham Dixie KL, Ikezu T, Ikezu S. Microglial WNT5A supports dendritic spines maturation and neuronal firing. Brain Behav. Immun. 2023;107:403–413. doi: 10.1016/j.bbi.2022.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mecha M, et al. Involvement of Wnt7a in the role of M2c microglia in neural stem cell oligodendrogenesis. J. Neuroinflamm. 2020;17:88. doi: 10.1186/s12974-020-01734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Xie K, Cai Y, Yang P, Du F, Wu K. Upregulating microRNA-874-3p inhibits CXCL12 expression to promote angiogenesis and suppress inflammatory response in ischemic stroke. Am. J. Physiol. Cell Physiol. 2020;319:C579–c588. doi: 10.1152/ajpcell.00001.2020. [DOI] [PubMed] [Google Scholar]
- 212.Zhao J, Li L, Fang G. Salvianolic acid A attenuates cerebral ischemia/reperfusion injury induced rat brain damage, inflammation, and apoptosis by regulating miR-499a/DDK1. Am. J. Transl. Res. 2020;12:3288–3301. [PMC free article] [PubMed] [Google Scholar]
- 213.Zhou J, Wu N, Lin L. Curcumin suppresses apoptosis and inflammation in hypoxia/reperfusion-exposed neurons via Wnt Signaling pathway. Med Sci. Monit. 2020;26:e920445. doi: 10.12659/MSM.920445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Song D, et al. Wnt canonical pathway activator TWS119 drives microglial anti-inflammatory activation and facilitates neurological recovery following experimental stroke. J. Neuroinflamm. 2019;16:256. doi: 10.1186/s12974-019-1660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhao B, Wang P, Yu J, Zhang Y. MicroRNA-376b-5p targets SOX7 to alleviate ischemic brain injury in a mouse model through activating Wnt/β-catenin signaling pathway. Life Sci. 2021;270:119072. doi: 10.1016/j.lfs.2021.119072. [DOI] [PubMed] [Google Scholar]
- 216.Kalogeris T, Bao Y, Korthuis RJ. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014;2:702–714. doi: 10.1016/j.redox.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tang Y, Shen J, Zhang F, Yang FY, Liu M. Human serum albumin attenuates global cerebral ischemia/reperfusion-induced brain injury in a Wnt/β-Catenin/ROS signaling-dependent manner in rats. Biomed. Pharmacother. 2019;115:108871. doi: 10.1016/j.biopha.2019.108871. [DOI] [PubMed] [Google Scholar]
- 218.Ten VS, Starkov A. Hypoxic-ischemic injury in the developing brain: the role of reactive oxygen species originating in mitochondria. Neurol. Res. Int. 2012;2012:542976. doi: 10.1155/2012/542976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Korobova F, Ramabhadran V, Higgs HN. An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science. 2013;339:464–467. doi: 10.1126/science.1228360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J. Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/S0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 222.Adachi K, et al. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- 223.Lie DC, et al. Wnt signaling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 224.Jin K, et al. Evidence for stroke-induced neurogenesis in the human brain. Proc. Natl Acad. Sci. USA. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Martí-Fàbregas J, et al. Proliferation in the human ipsilateral subventricular zone after ischemic stroke. Neurology. 2010;74:357–365. doi: 10.1212/WNL.0b013e3181cbccec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chen X, et al. Peroxynitrite enhances self-renewal, proliferation, and neuronal differentiation of neural stem/progenitor cells through activating HIF-1α and Wnt/β-catenin signaling pathway. Free Radic. Biol. Med. 2018;117:158–167. doi: 10.1016/j.freeradbiomed.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 227.Tiwari SK, et al. Inhibitory effects of Bisphenol-A on neural stem cells proliferation and differentiation in the rat brain are dependent on Wnt/β-catenin pathway. Mol. Neurobiol. 2015;52:1735–1757. doi: 10.1007/s12035-014-8940-1. [DOI] [PubMed] [Google Scholar]
- 228.Gan Q, et al. Pax6 mediates ß-catenin signaling for self-renewal and neurogenesis by neocortical radial glial stem cells. Stem Cells. 2014;32:45–58. doi: 10.1002/stem.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kuwabara T, et al. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat. Neurosci. 2009;12:1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gao Z, et al. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat. Neurosci. 2009;12:1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Joksimovic M, Awatramani R. Wnt/β-catenin signaling in midbrain dopaminergic neuron specification and neurogenesis. J. Mol. Cell Biol. 2014;6:27–33. doi: 10.1093/jmcb/mjt043. [DOI] [PubMed] [Google Scholar]
- 232.Yi H, Hu J, Qian J, Hackam AS. Expression of brain-derived neurotrophic factor is regulated by the Wnt signaling pathway. Neuroreport. 2012;23:189–194. doi: 10.1097/WNR.0b013e32834fab06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wei ZZ, et al. Neuroprotective and regenerative roles of intranasal Wnt-3a administration after focal ischemic stroke in mice. J. Cereb. Blood Flow. Metab. 2018;38:404–421. doi: 10.1177/0271678X17702669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Li SR, et al. Mallotus oblongifolius extracts ameliorate ischemic nerve damage by increasing endogenous neural stem cell proliferation through the Wnt/β-catenin signaling pathway. Food Funct. 2020;11:1027–1036. doi: 10.1039/C9FO01790A. [DOI] [PubMed] [Google Scholar]
- 235.Liu, Q. et al. Ellagic acid improves endogenous neural stem cells proliferation and neurorestoration through Wnt/β-catenin signaling in vivo and in vitro. Mol. Nutr. Food Res. 61, 1600587 (2017). [DOI] [PubMed]
- 236.Yang X, et al. Curcumin promotes neurogenesis of hippocampal dentate gyrus via Wnt/β-catenin signal pathway following cerebral ischemia in mice. Brain Res. 2021;1751:147197. doi: 10.1016/j.brainres.2020.147197. [DOI] [PubMed] [Google Scholar]
- 237.You D, You H. Repression of long non-coding RNA MEG3 restores nerve growth and alleviates neurological impairment after cerebral ischemia-reperfusion injury in a rat model. Biomed. Pharmacother. 2019;111:1447–1457. doi: 10.1016/j.biopha.2018.12.067. [DOI] [PubMed] [Google Scholar]
- 238.Stenman JM, et al. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- 239.Zhou Y, Nathans J. Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical wnt signaling. Dev. Cell. 2014;31:248–256. doi: 10.1016/j.devcel.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hu Y, Zheng Y, Wang T, Jiao L, Luo Y. VEGF, a Key Factor for Blood Brain Barrier Injury After Cerebral Ischemic Stroke. Aging Dis. 2022;13:647–654. doi: 10.14336/AD.2021.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Green, D. R. Caspases and Their Substrates. Cold Spring Harb. Perspect. Biol. 14, a041012 (2022). [DOI] [PMC free article] [PubMed]
- 242.Tian Y, et al. IL-4-polarized BV2 microglia cells promote angiogenesis by secreting exosomes. Adv. Clin. Exp. Med. 2019;28:421–430. doi: 10.17219/acem/91826. [DOI] [PubMed] [Google Scholar]
- 243.Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. doi: 10.1161/01.STR.25.9.1794. [DOI] [PubMed] [Google Scholar]
- 244.Wang LP, et al. Oligodendrocyte precursor cell transplantation promotes angiogenesis and remyelination via Wnt/β-catenin pathway in a mouse model of middle cerebral artery occlusion. J. Cereb. Blood Flow. Metab. 2022;42:757–770. doi: 10.1177/0271678X211065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Jiang X, et al. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog. Neurobiol. 2018;163-164:144–171. doi: 10.1016/j.pneurobio.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ta S, et al. Variants of WNT7A and GPR124 are associated with hemorrhagic transformation following intravenous thrombolysis in ischemic stroke. CNS Neurosci. Ther. 2021;27:71–81. doi: 10.1111/cns.13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Chang J, et al. Gpr124 is essential for blood-brain barrier integrity in central nervous system disease. Nat. Med. 2017;23:450–460. doi: 10.1038/nm.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Hussain B, et al. Endothelial β-catenin deficiency causes blood-brain barrier breakdown via enhancing the paracellular and transcellular permeability. Front. Mol. Neurosci. 2022;15:895429. doi: 10.3389/fnmol.2022.895429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chen XY, et al. Inhibition of the immunoproteasome LMP2 ameliorates ischemia/hypoxia-induced blood-brain barrier injury through the Wnt/β-catenin signaling pathway. Mil. Med. Res. 2021;8:62. doi: 10.1186/s40779-021-00356-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Langen UH, Ayloo S, Gu C. Development and cell biology of the blood-brain barrier. Annu. Rev. Cell Dev. Biol. 2019;35:591–613. doi: 10.1146/annurev-cellbio-100617-062608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Jin Z, Ke J, Guo P, Wang Y, Wu H. Quercetin improves blood-brain barrier dysfunction in rats with cerebral ischemia reperfusion via Wnt signaling pathway. Am. J. Transl. Res. 2019;11:4683–4695. [PMC free article] [PubMed] [Google Scholar]
- 252.Kintner DB, et al. Increased tolerance to oxygen and glucose deprivation in astrocytes from Na(+)/H(+) exchanger isoform 1 null mice. Am. J. Physiol. Cell Physiol. 2004;287:C12–C21. doi: 10.1152/ajpcell.00560.2003. [DOI] [PubMed] [Google Scholar]
- 253.Song S, et al. Activation of endothelial Wnt/β-catenin signaling by protective astrocytes repairs BBB damage in ischemic stroke. Prog. Neurobiol. 2021;199:101963. doi: 10.1016/j.pneurobio.2020.101963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zhao H, Alam A, Soo AP, George AJT, Ma D. Ischemia-reperfusion injury reduces long term renal graft survival: mechanism and beyond. EBioMedicine. 2018;28:31–42. doi: 10.1016/j.ebiom.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wang W, Sai WL, Yang B. [The role of macrophage polarization and interaction with renal tubular epithelial cells in ischemia-reperfusion induced acute kidney injury] Sheng Li Xue Bao. 2022;74:28–38. [PubMed] [Google Scholar]
- 256.He W, et al. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J. Am. Soc. Nephrol. 2009;20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Liu D, Liu Y, Zheng X, Liu N. c-MYC-induced long noncoding RNA MEG3 aggravates kidney ischemia-reperfusion injury through activating mitophagy by upregulation of RTKN to trigger the Wnt/β-catenin pathway. Cell Death Dis. 2021;12:191. doi: 10.1038/s41419-021-03466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Zhou D, et al. Tubule-specific ablation of endogenous β-catenin aggravates acute kidney injury in mice. Kidney Int. 2012;82:537–547. doi: 10.1038/ki.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wang Y, et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 2021;28:231–243. doi: 10.1016/j.jare.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wei X, et al. Identification of subtypes and a delayed graft function predictive signature based on ferroptosis in renal ischemia-reperfusion injury. Front. Cell Dev. Biol. 2022;10:800650. doi: 10.3389/fcell.2022.800650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Xu Y, et al. circ-AKT3 aggravates renal ischaemia-reperfusion injury via regulating miR-144-5p /Wnt/β-catenin pathway and oxidative stress. J. Cell Mol. Med. 2022;26:1766–1775. doi: 10.1111/jcmm.16072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Sturmlechner I, Durik M, Sieben CJ, Baker DJ, van Deursen JM. Cellular senescence in renal aging and disease. Nat. Rev. Nephrol. 2017;13:77–89. doi: 10.1038/nrneph.2016.183. [DOI] [PubMed] [Google Scholar]
- 263.Xiao L, et al. Sustained Activation of Wnt/β-Catenin Signaling Drives AKI to CKD Progression. J. Am. Soc. Nephrol. 2016;27:1727–1740. doi: 10.1681/ASN.2015040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zhou L, et al. Multiple genes of the renin-angiotensin system are novel targets of Wnt/β-catenin signaling. J. Am. Soc. Nephrol. 2015;26:107–120. doi: 10.1681/ASN.2014010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Zhou D, et al. Matrix metalloproteinase-7 is an urinary biomarker and pathogenic mediator of kidney fibrosis. J. Am. Soc. Nephrol. 2017;28:598–611. doi: 10.1681/ASN.2016030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.He W, et al. Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/beta-catenin signaling. J. Biol. Chem. 2010;285:24665–24675. doi: 10.1074/jbc.M109.091256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Simon-Tillaux N, Hertig A. Snail and kidney fibrosis. Nephrol. Dial. Transpl. 2017;32:224–233. doi: 10.1093/ndt/gfw333. [DOI] [PubMed] [Google Scholar]
- 268.Luo C, et al. Wnt9a promotes renal fibrosis by accelerating cellular senescence in tubular epithelial cells. J. Am. Soc. Nephrol. 2018;29:1238–1256. doi: 10.1681/ASN.2017050574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.von Toerne C, et al. Wnt pathway regulation in chronic renal allograft damage. Am. J. Transpl. 2009;9:2223–2239. doi: 10.1111/j.1600-6143.2009.02762.x. [DOI] [PubMed] [Google Scholar]
- 270.Sun Q, et al. Allogeneic mesenchymal stem cells as induction therapy are safe and feasible in renal allografts: pilot results of a multicenter randomized controlled trial. J. Transl. Med. 2018;16:52. doi: 10.1186/s12967-018-1422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Peralta C, Jiménez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J. Hepatol. 2013;59:1094–1106. doi: 10.1016/j.jhep.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 272.Russell JO, Monga SP. Wnt/β-catenin signaling in liver development, homeostasis, and pathobiology. Annu. Rev. Pathol. 2018;13:351–378. doi: 10.1146/annurev-pathol-020117-044010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Dar WA, Sullivan E, Bynon JS, Eltzschig H, Ju C. Ischaemia reperfusion injury in liver transplantation: cellular and molecular mechanisms. Liver Int. 2019;39:788–801. doi: 10.1111/liv.14091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Lehwald N, et al. Wnt-β-catenin signaling protects against hepatic ischemia and reperfusion injury in mice. Gastroenterology. 2011;141:707–718. doi: 10.1053/j.gastro.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Liu X, et al. Signaling through hepatocyte vasopressin receptor 1 protects mouse liver from ischemia-reperfusion injury. Oncotarget. 2016;7:69276–69290. doi: 10.18632/oncotarget.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Xie K, Liu L, Chen J, Liu F. Exosomes derived from human umbilical cord blood mesenchymal stem cells improve hepatic ischemia reperfusion injury via delivering miR-1246. Cell Cycle. 2019;18:3491–3501. doi: 10.1080/15384101.2019.1689480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Sakon M, Ariyoshi H, Umeshita K, Monden M. Ischemia-reperfusion injury of the liver with special reference to calcium-dependent mechanisms. Surg. Today. 2002;32:1–12. doi: 10.1007/s595-002-8105-8. [DOI] [PubMed] [Google Scholar]
- 278.Hu X, et al. Inhibition of Frizzled-2 by small interfering RNA protects rat hepatic BRL-3A cells against cytotoxicity and apoptosis induced by Hypoxia/Reoxygenation. Gastroenterol. Hepatol. 2020;43:107–116. doi: 10.1016/j.gastrohep.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 279.Yim SY, et al. Risk factors for developing hyponatremia during terlipressin treatment: a retrospective analyses in variceal bleeding. J. Clin. Gastroenterol. 2015;49:607–612. doi: 10.1097/MCG.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 280.Koshimizu TA, et al. Vasopressin V1a and V1b receptors: from molecules to physiological systems. Physiol. Rev. 2012;92:1813–1864. doi: 10.1152/physrev.00035.2011. [DOI] [PubMed] [Google Scholar]
- 281.Kohler A, Perrodin S, De Gottardi A, Candinas D, Beldi G. Effectiveness of terlipressin for prevention of complications after major liver resection-A randomized placebo-controlled trial. HPB. 2020;22:884–891. doi: 10.1016/j.hpb.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 282.Hong SH, et al. Perioperative assessment of terlipressin infusion during living donor liver transplantation. J. Int. Med. Res. 2012;40:225–236. doi: 10.1177/147323001204000123. [DOI] [PubMed] [Google Scholar]
- 283.Reis DJ, Regunathan S. Is agmatine a novel neurotransmitter in brain? Trends Pharm. Sci. 2000;21:187–193. doi: 10.1016/S0165-6147(00)01460-7. [DOI] [PubMed] [Google Scholar]
- 284.Kim DJ, et al. Protective effect of agmatine on a reperfusion model after transient cerebral ischemia: Temporal evolution on perfusion MR imaging and histopathologic findings. AJNR Am. J. Neuroradiol. 2006;27:780–785. [PMC free article] [PubMed] [Google Scholar]
- 285.Sugiura T, et al. Protective effect of agmatine on ischemia/reperfusion-induced renal injury in rats. J. Cardiovasc. Pharm. 2008;51:223–230. doi: 10.1097/FJC.0b013e318161d758. [DOI] [PubMed] [Google Scholar]
- 286.Greenberg S, et al. The effect of agmatine administration on ischemic-reperfused isolated rat heart. J. Cardiovasc. Pharm. Ther. 2001;6:37–45. doi: 10.1177/107424840100600105. [DOI] [PubMed] [Google Scholar]
- 287.Han Z, et al. Agmatine attenuates liver ischemia reperfusion injury by activating Wnt/β-catenin signaling in mice. Transplantation. 2020;104:1906–1916. doi: 10.1097/TP.0000000000003161. [DOI] [PubMed] [Google Scholar]
- 288.Dong J, et al. SRY is a Key Mediator of Sexual Dimorphism in Hepatic Ischemia/Reperfusion Injury. Ann. Surg. 2022;276:345–356. doi: 10.1097/SLA.0000000000004422. [DOI] [PubMed] [Google Scholar]
- 289.O’Neill MJ, O’Neill RJ. Whatever happened to SRY? Cell Mol. Life Sci. 1999;56:883–893. doi: 10.1007/s000180050481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Yang YY, et al. Involvement of the HIF-1α and Wnt/β-catenin pathways in the protective effects of losartan on fatty liver graft with ischaemia/reperfusion injury. Clin. Sci. 2014;126:163–174. doi: 10.1042/CS20130025. [DOI] [PubMed] [Google Scholar]
- 291.Griffin MO, Ceballos G, Villarreal FJ. Tetracycline compounds with non-antimicrobial organ protective properties: possible mechanisms of action. Pharm. Res. 2011;63:102–107. doi: 10.1016/j.phrs.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Li Y, Li T, Qi H, Yuan F. Minocycline protects against hepatic ischemia/reperfusion injury in a rat model. Biomed. Rep. 2015;3:19–24. doi: 10.3892/br.2014.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Bataller R, et al. Prolonged infusion of angiotensin II into normal rats induces stellate cell activation and proinflammatory events in liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G642–G651. doi: 10.1152/ajpgi.00037.2003. [DOI] [PubMed] [Google Scholar]
- 294.Kanno K, Tazuma S, Nishioka T, Hyogo H, Chayama K. Angiotensin II participates in hepatic inflammation and fibrosis through MCP-1 expression. Dig. Dis. Sci. 2005;50:942–948. doi: 10.1007/s10620-005-2669-7. [DOI] [PubMed] [Google Scholar]
- 295.Bataller R, et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J. Clin. Investig. 2003;112:1383–1394. doi: 10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Harrison DG, Cai H, Landmesser U, Griendling KK. Interactions of angiotensin II with NAD(P)H oxidase, oxidant stress and cardiovascular disease. J. Renin Angiotensin Aldosterone Syst. 2003;4:51–61. doi: 10.3317/jraas.2003.014. [DOI] [PubMed] [Google Scholar]
- 297.Guo L, et al. Role of the renin-angiotensin system in hepatic ischemia reperfusion injury in rats. Hepatology. 2004;40:583–589. doi: 10.1002/hep.20369. [DOI] [PubMed] [Google Scholar]
- 298.Kuncewitch M, et al. Wnt agonist attenuates liver injury and improves survival after hepatic ischemia/reperfusion. Shock. 2013;39:3–10. doi: 10.1097/SHK.0b013e3182764fe8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Sun T, et al. AXIN2(+) Pericentral hepatocytes have limited contributions to liver homeostasis and regeneration. Cell Stem Cell. 2020;26:97–107.e106. doi: 10.1016/j.stem.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 300.Katoh M. Multi‑layered prevention and treatment of chronic inflammation, organ fibrosis and cancer associated with canonical WNT/β‑catenin signaling activation (Review) Int J. Mol. Med. 2018;42:713–725. doi: 10.3892/ijmm.2018.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Piao, C. et al. Effects of Exosomes Derived from Adipose-Derived Mesenchymal Stem Cells on Pyroptosis and Regeneration of Injured Liver. Int. J. Mol. Sci. 23, 12065 (2022). [DOI] [PMC free article] [PubMed]
- 302.Zhou B, et al. Notch signaling pathway: architecture, disease, and therapeutics. Signal Transduct. Target. Ther. 2022;7:95. doi: 10.1038/s41392-022-00934-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Chen G, et al. The canonical Notch signaling was involved in the regulation of intestinal epithelial cells apoptosis after intestinal ischemia/reperfusion injury. Int. J. Mol. Sci. 2014;15:7883–7896. doi: 10.3390/ijms15057883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Guo P, et al. Dexmedetomidine alleviates myocardial ischemia-reperfusion injury by down-regulating miR-34b-3p to activate the Jagged1/Notch signaling pathway. Int. Immunopharmacol. 2023;116:109766. doi: 10.1016/j.intimp.2023.109766. [DOI] [PubMed] [Google Scholar]
- 306.Li H, et al. Botch protects neurons from ischemic insult by antagonizing Notch-mediated neuroinflammation. Exp. Neurol. 2019;321:113028. doi: 10.1016/j.expneurol.2019.113028. [DOI] [PubMed] [Google Scholar]
- 307.Pei H, et al. Notch1 cardioprotection in myocardial ischemia/reperfusion involves reduction of oxidative/nitrative stress. Basic Res. Cardiol. 2013;108:373. doi: 10.1007/s00395-013-0373-x. [DOI] [PubMed] [Google Scholar]
- 308.Yu HC, et al. Canonical notch pathway protects hepatocytes from ischemia/reperfusion injury in mice by repressing reactive oxygen species production through JAK2/STAT3 signaling. Hepatology. 2011;54:979–988. doi: 10.1002/hep.24469. [DOI] [PubMed] [Google Scholar]
- 309.Chatterjee S, Sil PC. Targeting the crosstalks of Wnt pathway with Hedgehog and Notch for cancer therapy. Pharm. Res. 2019;142:251–261. doi: 10.1016/j.phrs.2019.02.027. [DOI] [PubMed] [Google Scholar]
- 310.Patni AP, et al. Comprehending the crosstalk between Notch, Wnt, and Hedgehog signaling pathways in oral squamous cell carcinoma - clinical implications. Cell Oncol. 2021;44:473–494. doi: 10.1007/s13402-021-00591-3. [DOI] [PubMed] [Google Scholar]
- 311.Kim HA, et al. Notch1 counteracts WNT/β-catenin signaling through chromatin modification in colorectal cancer. J. Clin. Investig. 2012;122:3248–3259. doi: 10.1172/JCI61216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Kim W, et al. Hippo signaling interactions with Wnt/β-catenin and Notch signaling repress liver tumorigenesis. J. Clin. Investig. 2017;127:137–152. doi: 10.1172/JCI88486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Sprinzak D, Blacklow SC. Biophysics of Notch Signaling. Annu. Rev. Biophys. 2021;50:157–189. doi: 10.1146/annurev-biophys-101920-082204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Gude NA, et al. Activation of Notch-mediated protective signaling in the myocardium. Circ. Res. 2008;102:1025–1035. doi: 10.1161/CIRCRESAHA.107.164749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Ashton KJ, Willems L, Holmgren K, Ferreira L, Headrick JP. Age-associated shifts in cardiac gene transcription and transcriptional responses to ischemic stress. Exp. Gerontol. 2006;41:189–204. doi: 10.1016/j.exger.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 316.Zhao L, et al. Notch signaling regulates cardiomyocyte proliferation during zebrafish heart regeneration. Proc. Natl Acad. Sci. USA. 2014;111:1403–1408. doi: 10.1073/pnas.1311705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Zhao L, Ben-Yair R, Burns CE, Burns CG. Endocardial Notch signaling promotes cardiomyocyte proliferation in the regenerating zebrafish heart through wnt pathway antagonism. Cell Rep. 2019;26:546–554.e545. doi: 10.1016/j.celrep.2018.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Zhang HP, et al. The neuroprotective effects of isoflurane preconditioning in a murine transient global cerebral ischemia-reperfusion model: the role of the Notch signaling pathway. Neuromolecular Med. 2014;16:191–204. doi: 10.1007/s12017-013-8273-7. [DOI] [PubMed] [Google Scholar]
- 319.Yang Q, et al. Activation of canonical notch signaling pathway is involved in the ischemic tolerance induced by sevoflurane preconditioning in mice. Anesthesiology. 2012;117:996–1005. doi: 10.1097/ALN.0b013e31826cb469. [DOI] [PubMed] [Google Scholar]
- 320.Zhang H, et al. [Expressions of Notch3, Notch4, Frizzled2, and Tead1 in rats with focal cerebral ischemia-reperfusion] Zhonghua Yi Xue Za Zhi. 2015;95:3766–3769. [PubMed] [Google Scholar]
- 321.Arboleda-Velasquez JF, et al. Linking Notch signaling to ischemic stroke. Proc. Natl Acad. Sci. USA. 2008;105:4856–4861. doi: 10.1073/pnas.0709867105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Arumugam TV, et al. Notch signaling and neuronal death in stroke. Prog. Neurobiol. 2018;165-167:103–116. doi: 10.1016/j.pneurobio.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Gao L, Yang L, Cui H. GSK-3β inhibitor TWS119 alleviates hypoxic-ischemic brain damage via a crosstalk with Wnt and Notch signaling pathways in neonatal rats. Brain Res. 2021;1768:147588. doi: 10.1016/j.brainres.2021.147588. [DOI] [PubMed] [Google Scholar]
- 324.Ma R, et al. l-Borneol and d-Borneol promote transdifferentiation of astrocytes into neurons in rats by regulating Wnt/Notch pathway to exert neuroprotective effect during recovery from cerebral ischemia. Phytomedicine. 2023;109:154583. doi: 10.1016/j.phymed.2022.154583. [DOI] [PubMed] [Google Scholar]
- 325.Huang S, et al. Zhongfenggao protects brain microvascular endothelial cells from oxygen-glucose deprivation/reoxygenation-induced injury by angiogenesis. Biol. Pharm. Bull. 2019;42:222–230. doi: 10.1248/bpb.b18-00650. [DOI] [PubMed] [Google Scholar]
- 326.Zhang Z, Yao L, Yang J, Wang Z, Du G. PI3K/Akt and HIF‑1 signaling pathway in hypoxia‑ischemia (Review) Mol. Med. Rep. 2018;18:3547–3554. doi: 10.3892/mmr.2018.9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Dong J, Xu X, Zhang Q, Yuan Z, Tan B. The PI3K/AKT pathway promotes fracture healing through its crosstalk with Wnt/β-catenin. Exp. Cell Res. 2020;394:112137. doi: 10.1016/j.yexcr.2020.112137. [DOI] [PubMed] [Google Scholar]
- 328.Deng, S. et al. PI3K/AKT signaling tips the balance of cytoskeletal forces for cancer progression. Cancers14, 1652 (2022). [DOI] [PMC free article] [PubMed]
- 329.Papadimitrakopoulou V. Development of PI3K/AKT/mTOR pathway inhibitors and their application in personalized therapy for non-small-cell lung cancer. J. Thorac. Oncol. 2012;7:1315–1326. doi: 10.1097/JTO.0b013e31825493eb. [DOI] [PubMed] [Google Scholar]
- 330.Tewari D, Patni P, Bishayee A, Sah AN, Bishayee A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. Semin. Cancer Biol. 2022;80:1–17. doi: 10.1016/j.semcancer.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 331.Xiao CL, et al. The role of PI3K/Akt signaling pathway in spinal cord injury. Biomed. Pharmacother. 2022;156:113881. doi: 10.1016/j.biopha.2022.113881. [DOI] [PubMed] [Google Scholar]
- 332.Potz BA, et al. Calpain inhibition modulates glycogen synthase kinase 3β pathways in ischemic myocardium: a proteomic and mechanistic analysis. J. Thorac. Cardiovasc Surg. 2017;153:342–357. doi: 10.1016/j.jtcvs.2016.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Chen B, et al. Co-expression of Akt1 and Wnt11 promotes the proliferation and cardiac differentiation of mesenchymal stem cells and attenuates hypoxia/reoxygenation-induced cardiomyocyte apoptosis. Biomed. Pharmacother. 2018;108:508–514. doi: 10.1016/j.biopha.2018.09.047. [DOI] [PubMed] [Google Scholar]
- 334.Zhuang Q, et al. Stimulated CB1 cannabinoid receptor inducing ischemic tolerance and protecting neurons from cerebral ischemia. Cent. Nerv. Syst. Agents Med. Chem. 2017;17:141–150. doi: 10.2174/1871524916666160504104624. [DOI] [PubMed] [Google Scholar]
- 335.Blankesteijn WM, van de Schans VA, ter Horst P, Smits JF. The Wnt/frizzled/GSK-3 beta pathway: a novel therapeutic target for cardiac hypertrophy. Trends Pharm. Sci. 2008;29:175–180. doi: 10.1016/j.tips.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 336.Hur EM, Zhou FQ. GSK3 signaling in neural development. Nat. Rev. Neurosci. 2010;11:539–551. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Xing XS, Liu F, He ZY. Akt regulates β-catenin in a rat model of focal cerebral ischemia-reperfusion injury. Mol. Med. Rep. 2015;11:3122–3128. doi: 10.3892/mmr.2014.3000. [DOI] [PubMed] [Google Scholar]
- 338.Li P, Zhang Y, Liu H. The role of Wnt/β-catenin pathway in the protection process by dexmedetomidine against cerebral ischemia/reperfusion injury in rats. Life Sci. 2019;236:116921. doi: 10.1016/j.lfs.2019.116921. [DOI] [PubMed] [Google Scholar]
- 339.Thirunavukkarasu M, et al. Protective effects of Phyllanthus emblica against myocardial ischemia-reperfusion injury: the role of PI3-kinase/glycogen synthase kinase 3β/β-catenin pathway. J. Physiol. Biochem. 2015;71:623–633. doi: 10.1007/s13105-015-0426-8. [DOI] [PubMed] [Google Scholar]
- 340.Fei Y, Zhao B, Zhu J, Fang W, Li Y. XQ-1H promotes cerebral angiogenesis via activating PI3K/Akt/GSK3β/β-catenin/VEGF signal in mice exposed to cerebral ischemic injury. Life Sci. 2021;272:119234. doi: 10.1016/j.lfs.2021.119234. [DOI] [PubMed] [Google Scholar]
- 341.Martínez-Sánchez G, Giuliani A. Cellular redox status regulates hypoxia inducible factor-1 activity. Role in tumor development. J. Exp. Clin. Cancer Res. 2007;26:39–50. [PubMed] [Google Scholar]
- 342.Semenza GL. Hypoxia-inducible factor 1 and cardiovascular disease. Annu. Rev. Physiol. 2014;76:39–56. doi: 10.1146/annurev-physiol-021113-170322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Tan Z, et al. Lithium and copper induce the osteogenesis-angiogenesis coupling of bone marrow mesenchymal stem cells via crosstalk between canonical Wnt and HIF-1α signaling pathways. Stem Cells Int. 2021;2021:6662164. doi: 10.1155/2021/6662164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Tang K, et al. HIF-1α stimulates the progression of oesophageal squamous cell carcinoma by activating the Wnt/β-catenin signaling pathway. Br. J. Cancer. 2022;127:474–487. doi: 10.1038/s41416-022-01825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Zhang Q, et al. Wnt/β-catenin signaling enhances hypoxia-induced epithelial-mesenchymal transition in hepatocellular carcinoma via crosstalk with hif-1α signaling. Carcinogenesis. 2013;34:962–973. doi: 10.1093/carcin/bgt027. [DOI] [PubMed] [Google Scholar]
- 346.DeFrates KG, Franco D, Heber-Katz E, Messersmith PB. Unlocking mammalian regeneration through hypoxia inducible factor one alpha signaling. Biomaterials. 2021;269:120646. doi: 10.1016/j.biomaterials.2020.120646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Engelhardt S, Al-Ahmad AJ, Gassmann M, Ogunshola OO. Hypoxia selectively disrupts brain microvascular endothelial tight junction complexes through a hypoxia-inducible factor-1 (HIF-1) dependent mechanism. J. Cell Physiol. 2014;229:1096–1105. doi: 10.1002/jcp.24544. [DOI] [PubMed] [Google Scholar]
- 348.Wu C, et al. Wnt/β-catenin coupled with HIF-1α/VEGF signaling pathways involved in galangin neurovascular unit protection from focal cerebral ischemia. Sci. Rep. 2015;5:16151. doi: 10.1038/srep16151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Kaidi A, Williams AC, Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat. Cell Biol. 2007;9:210–217. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]
- 350.Xu ZH, et al. Hypoxia-inducible factor protects against acute kidney injury via the Wnt/β-catenin signaling pathway. Am. J. Physiol. Ren. Physiol. 2022;322:F611–f624. doi: 10.1152/ajprenal.00023.2022. [DOI] [PubMed] [Google Scholar]
- 351.Peng D, Fu M, Wang M, Wei Y, Wei X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol. Cancer. 2022;21:104. doi: 10.1186/s12943-022-01569-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Li TF, et al. Transforming growth factor-beta stimulates cyclin D1 expression through activation of beta-catenin signaling in chondrocytes. J. Biol. Chem. 2006;281:21296–21304. doi: 10.1074/jbc.M600514200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Liu J, Jin J, Liang T, Feng XH. To Ub or not to Ub: a regulatory question in TGF-β signaling. Trends Biochem. Sci. 2022;47:1059–1072. doi: 10.1016/j.tibs.2022.06.001. [DOI] [PubMed] [Google Scholar]
- 354.Działo E, Tkacz K, Błyszczuk P. Crosstalk between the TGF-β and WNT signaling pathways during cardiac fibrogenesis. Acta Biochim. Pol. 2018;65:341–349. doi: 10.18388/abp.2018_2635. [DOI] [PubMed] [Google Scholar]
- 355.Eid RA, et al. Exendin-4 Attenuates Remodeling in the Remote Myocardium of Rats After an Acute Myocardial Infarction by Activating β-Arrestin-2, Protein Phosphatase 2A, and Glycogen Synthase Kinase-3 and Inhibiting β-Catenin. Cardiovasc. Drugs Ther. 2021;35:1095–1110. doi: 10.1007/s10557-020-07006-9. [DOI] [PubMed] [Google Scholar]
- 356.Wang S, et al. Transforming growth-beta 1 contributes to isoflurane postconditioning against cerebral ischemia-reperfusion injury by regulating the c-Jun N-terminal kinase signaling pathway. Biomed. Pharmacother. 2016;78:280–290. doi: 10.1016/j.biopha.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 357.Chen DQ, et al. Combined melatonin and poricoic acid A inhibits renal fibrosis through modulating the interaction of Smad3 and β-catenin pathway in AKI-to-CKD continuum. Ther. Adv. Chronic Dis. 2019;10:2040622319869116. doi: 10.1177/2040622319869116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Tian X, et al. Association of β-catenin with P-Smad3 but not LEF-1 dissociates in vitro profibrotic from anti-inflammatory effects of TGF-β1. J. Cell Sci. 2013;126:67–76. doi: 10.1242/jcs.103036. [DOI] [PubMed] [Google Scholar]
- 359.Vallée A, Lecarpentier Y. TGF-β in fibrosis by acting as a conductor for contractile properties of myofibroblasts. Cell Biosci. 2019;9:98. doi: 10.1186/s13578-019-0362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Huber N, et al. Age-related decrease in proteasome expression contributes to defective nuclear factor-kappaB activation during hepatic ischemia/reperfusion. Hepatology. 2009;49:1718–1728. doi: 10.1002/hep.22840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Liang W, et al. Preactivation of Notch1 in remote ischemic preconditioning reduces cerebral ischemia-reperfusion injury through crosstalk with the NF-κB pathway. J. Neuroinflamm. 2019;16:181. doi: 10.1186/s12974-019-1570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Ling H, et al. Ca2+/Calmodulin-dependent protein kinase II δ mediates myocardial ischemia/reperfusion injury through nuclear factor-κB. Circ. Res. 2013;112:935–944. doi: 10.1161/CIRCRESAHA.112.276915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Sakai N, et al. Receptor activator of nuclear factor-κB ligand (RANKL) protects against hepatic ischemia/reperfusion injury in mice. Hepatology. 2012;55:888–897. doi: 10.1002/hep.24756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat. Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 365.Mitchell S, Vargas J, Hoffmann A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016;8:227–241. doi: 10.1002/wsbm.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Yu H, Lin L, Zhang Z, Zhang H, Hu H. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct. Target Ther. 2020;5:209. doi: 10.1038/s41392-020-00312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Yin C, et al. Elevated Wnt2 and Wnt4 activate NF-κB signaling to promote cardiac fibrosis by cooperation of Fzd4/2 and LRP6 following myocardial infarction. EBioMedicine. 2021;74:103745. doi: 10.1016/j.ebiom.2021.103745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Lin JC, et al. Enhancement of beta-catenin in cardiomyocytes suppresses survival protein expression but promotes apoptosis and fibrosis. Cardiol. J. 2017;24:195–205. doi: 10.5603/CJ.a2016.0087. [DOI] [PubMed] [Google Scholar]
- 369.Lin JC, et al. β-Catenin overexpression causes an increase in inflammatory cytokines and NF-κB activation in cardiomyocytes. Cell. Mol. Biol. 2016;63:17–22. doi: 10.14715/cmb/2017.63.1.4. [DOI] [PubMed] [Google Scholar]
- 370.Spiegelman VS, et al. Wnt/beta-catenin signaling induces the expression and activity of betaTrCP ubiquitin ligase receptor. Mol. Cell. 2000;5:877–882. doi: 10.1016/S1097-2765(00)80327-5. [DOI] [PubMed] [Google Scholar]
- 371.He J, et al. Huoxin pill prevents excessive inflammation and cardiac dysfunction following myocardial infarction by inhibiting adverse Wnt/β‑catenin signaling activation. Phytomedicine. 2022;104:154293. doi: 10.1016/j.phymed.2022.154293. [DOI] [PubMed] [Google Scholar]
- 372.Winston JT, et al. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev. 1999;13:270–283. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Noubissi FK, et al. CRD-BP mediates stabilization of betaTrCP1 and c-myc mRNA in response to beta-catenin signaling. Nature. 2006;441:898–901. doi: 10.1038/nature04839. [DOI] [PubMed] [Google Scholar]
- 374.Hoeflich KP, et al. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 375.Zhuang X, et al. Differential effects on lung and bone metastasis of breast cancer by Wnt signaling inhibitor DKK1. Nat. Cell Biol. 2017;19:1274–1285. doi: 10.1038/ncb3613. [DOI] [PubMed] [Google Scholar]
- 376.El-Sayyad SM, Soubh AA, Awad AS, El-Abhar HS. Mangiferin protects against intestinal ischemia/reperfusion-induced liver injury: Involvement of PPAR-γ, GSK-3β and Wnt/β-catenin pathway. Eur. J. Pharm. 2017;809:80–86. doi: 10.1016/j.ejphar.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 377.Jiang S, Huang L, Zhang W, Zhang H. Vitamin D/VDR in acute kidney injury: a potential therapeutic target. Curr. Med. Chem. 2021;28:3865–3876. doi: 10.2174/0929867327666201118155625. [DOI] [PubMed] [Google Scholar]
- 378.Ali RM, Al-Shorbagy MY, Helmy MW, El-Abhar HS. Role of Wnt4/β-catenin, Ang II/TGFβ, ACE2, NF-κB, and IL-18 in attenuating renal ischemia/reperfusion-induced injury in rats treated with Vit D and pioglitazone. Eur. J. Pharm. 2018;831:68–76. doi: 10.1016/j.ejphar.2018.04.032. [DOI] [PubMed] [Google Scholar]
- 379.Wang J, Liu S, Heallen T, Martin JF. The Hippo pathway in the heart: pivotal roles in development, disease, and regeneration. Nat. Rev. Cardiol. 2018;15:672–684. doi: 10.1038/s41569-018-0063-3. [DOI] [PubMed] [Google Scholar]
- 380.Zhou Q, Li L, Zhao B, Guan KL. The hippo pathway in heart development, regeneration, and diseases. Circ. Res. 2015;116:1431–1447. doi: 10.1161/CIRCRESAHA.116.303311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Heallen T, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Gong P, et al. Hippo/YAP signaling pathway mitigates blood-brain barrier disruption after cerebral ischemia/reperfusion injury. Behav. Brain Res. 2019;356:8–17. doi: 10.1016/j.bbr.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Yu H, et al. RRM2 improves cardiomyocyte proliferation after myocardial ischemia reperfusion injury through the hippo-YAP pathway. Dis. Mark. 2021;2021:5089872. doi: 10.1155/2021/5089872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Zheng Z, et al. Hippo-YAP/MCP-1 mediated tubular maladaptive repair promote inflammation in renal failed recovery after ischemic AKI. Cell Death Dis. 2021;12:754. doi: 10.1038/s41419-021-04041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Zhou J, et al. TNFAIP3 interacting protein 3 is an activator of Hippo-YAP signaling protecting against hepatic ischemia/reperfusion injury. Hepatology. 2021;74:2133–2153. doi: 10.1002/hep.32015. [DOI] [PubMed] [Google Scholar]
- 386.Zheng A, Chen Q, Zhang L. The Hippo-YAP pathway in various cardiovascular diseases: focusing on the inflammatory response. Front. Immunol. 2022;13:971416. doi: 10.3389/fimmu.2022.971416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Nishina H. Physiological and pathological roles of the Hippo-YAP/TAZ signaling pathway in liver formation, homeostasis, and tumorigenesis. Cancer Sci. 2022;113:1900–1908. doi: 10.1111/cas.15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Liu S, et al. Yap promotes noncanonical Wnt signals from cardiomyocytes for heart regeneration. Circ. Res. 2021;129:782–797. doi: 10.1161/CIRCRESAHA.121.318966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Azzolin L, et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 390.Ma WY, et al. Melatonin promotes cardiomyocyte proliferation and heart repair in mice with myocardial infarction via miR-143-3p/Yap/Ctnnd1 signaling pathway. Acta Pharm. Sin. 2021;42:921–931. doi: 10.1038/s41401-020-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Amani H, et al. Selenium nanoparticles for targeted stroke therapy through modulation of inflammatory and metabolic signaling. Sci. Rep. 2019;9:6044. doi: 10.1038/s41598-019-42633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Kanzler B, Foreman RK, Labosky PA, Mallo M. BMP signaling is essential for development of skeletogenic and neurogenic cranial neural crest. Development. 2000;127:1095–1104. doi: 10.1242/dev.127.5.1095. [DOI] [PubMed] [Google Scholar]
- 393.Mabie PC, Mehler MF, Kessler JA. Multiple roles of bone morphogenetic protein signaling in the regulation of cortical cell number and phenotype. J. Neurosci. 1999;19:7077–7088. doi: 10.1523/JNEUROSCI.19-16-07077.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Baker JC, Beddington RS, Harland RM. Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development. Genes Dev. 1999;13:3149–3159. doi: 10.1101/gad.13.23.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Antebi YE, et al. Combinatorial signal perception in the BMP pathway. Cell. 2017;170:1184–1196.e1124. doi: 10.1016/j.cell.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Zhang Y, Que J. BMP signaling in development, stem cells, and diseases of the gastrointestinal tract. Annu. Rev. Physiol. 2020;82:251–273. doi: 10.1146/annurev-physiol-021119-034500. [DOI] [PubMed] [Google Scholar]
- 397.Fujita K, Ogawa R, Kawawaki S, Ito K. Roles of chromatin remodelers in maintenance mechanisms of multipotency of mouse trunk neural crest cells in the formation of neural crest-derived stem cells. Mech. Dev. 2014;133:126–145. doi: 10.1016/j.mod.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 398.Dizon ML, Maa T, Kessler JA. The bone morphogenetic protein antagonist noggin protects white matter after perinatal hypoxia-ischemia. Neurobiol. Dis. 2011;42:318–326. doi: 10.1016/j.nbd.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Guan J, et al. Bone morphogenetic protein-7 (BMP-7) mediates ischemic preconditioning-induced ischemic tolerance via attenuating apoptosis in rat brain. Biochem. Biophys. Res. Commun. 2013;441:560–566. doi: 10.1016/j.bbrc.2013.10.121. [DOI] [PubMed] [Google Scholar]
- 400.Chen C, Yang Y, Yao Y. HBO promotes the differentiation of neural stem cells via interactions between the Wnt3/β-catenin and BMP2 signaling pathways. Cell Transpl. 2019;28:1686–1699. doi: 10.1177/0963689719883578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Lei ZN, Liu F, Zhang LM, Huang YL, Sun FY. Bcl-2 increases stroke-induced striatal neurogenesis in adult brains by inhibiting BMP-4 function via activation of β-catenin signaling. Neurochem. Int. 2012;61:34–42. doi: 10.1016/j.neuint.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 402.Baik J, Borges L, Magli A, Thatava T, Perlingeiro RC. Effect of endoglin overexpression during embryoid body development. Exp. Hematol. 2012;40:837–846. doi: 10.1016/j.exphem.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Zhang L, et al. Modulation of TGF-β signaling by endoglin in murine hemangioblast development and primitive hematopoiesis. Blood. 2011;118:88–97. doi: 10.1182/blood-2010-12-325019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Borges L, et al. A critical role for endoglin in the emergence of blood during embryonic development. Blood. 2012;119:5417–5428. doi: 10.1182/blood-2011-11-391896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Baik J, et al. Endoglin integrates BMP and Wnt signalling to induce haematopoiesis through JDP2. Nat. Commun. 2016;7:13101. doi: 10.1038/ncomms13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Ahmadi A, et al. Recent advances on small molecules in osteogenic differentiation of stem cells and the underlying signaling pathways. Stem Cell Res. Ther. 2022;13:518. doi: 10.1186/s13287-022-03204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Zhang X, Shi X, Wang J, Xu Z, He J. Enriched environment remedies cognitive dysfunctions and synaptic plasticity through NMDAR-Ca(2+)-Activin A circuit in chronic cerebral hypoperfusion rats. Aging (Albany NY) 2021;13:20748–20761. doi: 10.18632/aging.203462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Liu S, et al. Icaritin alleviates cerebral ischemia‒reperfusion injury by regulating NMDA receptors through ERK signaling. Eur. J. Pharm. 2023;941:175492. doi: 10.1016/j.ejphar.2023.175492. [DOI] [PubMed] [Google Scholar]
- 409.Abe K, Takeichi M. NMDA-receptor activation induces calpain-mediated beta-catenin cleavages for triggering gene expression. Neuron. 2007;53:387–397. doi: 10.1016/j.neuron.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 410.Villmann C, Becker CM. On the hypes and falls in neuroprotection: targeting the NMDA receptor. Neuroscientist. 2007;13:594–615. doi: 10.1177/1073858406296259. [DOI] [PubMed] [Google Scholar]
- 411.Jolly S, et al. G protein-coupled receptor 37-like 1 modulates astrocyte glutamate transporters and neuronal NMDA receptors and is neuroprotective in ischemia. Glia. 2018;66:47–61. doi: 10.1002/glia.23198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Luo Y, et al. Focal cerebral ischemia and reperfusion induce brain injury through α2δ-1-Bound NMDA receptors. Stroke. 2018;49:2464–2472. doi: 10.1161/STROKEAHA.118.022330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Kawai T, Akira S. TLR signaling. Semin. Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 414.Tong Y, et al. WISP1 mediates hepatic warm ischemia reperfusion injury via TLR4 signaling in mice. Sci. Rep. 2016;6:20141. doi: 10.1038/srep20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Undi RB, Sarvothaman S, Narasaiah K, Gutti U, Gutti RK. Toll-like receptor 2 signalings: significance in megakaryocyte development through wnt signalling cross-talk and cytokine induction. Cytokine. 2016;83:245–249. doi: 10.1016/j.cyto.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 416.Martín-Medina, A. et al. TLR/WNT: A Novel Relationship in Immunomodulation of Lung Cancer. Int. J Mol Sci. 23, 6539 (2022). [DOI] [PMC free article] [PubMed]
- 417.Christman MA, 2nd, et al. Wnt5a is expressed in murine and human atherosclerotic lesions. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H2864–H2870. doi: 10.1152/ajpheart.00982.2007. [DOI] [PubMed] [Google Scholar]
- 418.He W, et al. Lipopolysaccharide enhances Wnt5a expression through toll-like receptor 4, myeloid differentiating factor 88, phosphatidylinositol 3-OH kinase/AKT and nuclear factor kappa B pathways in human dental pulp stem cells. J. Endod. 2014;40:69–75. doi: 10.1016/j.joen.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 419.El-Ela SRA, Zaghloul RA, Eissa LA. Promising cardioprotective effect of baicalin in doxorubicin-induced cardiotoxicity through targeting toll-like receptor 4/nuclear factor-κB and Wnt/β-catenin pathways. Nutrition. 2022;102:111732. doi: 10.1016/j.nut.2022.111732. [DOI] [PubMed] [Google Scholar]
- 420.Liu, B., Li, F., Xu, Y., Wu, Q. & Shi, J. Gastrodin improves cognitive dysfunction in REM Sleep-deprived rats by regulating TLR4/NF-κB and Wnt/β-catenin signaling pathways. Brain Sci. 13, 179 (2023). [DOI] [PMC free article] [PubMed]
- 421.Tanaka, R., Terai, M., Londin, E. & Sato, T. The role of HGF/MET signaling in metastatic uveal melanoma. Cancers13, 5457 (2021). [DOI] [PMC free article] [PubMed]
- 422.Demkova L, Kucerova L. Role of the HGF/c-MET tyrosine kinase inhibitors in metastasic melanoma. Mol. Cancer. 2018;17:26. doi: 10.1186/s12943-018-0795-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Zhang Y, et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol. Cancer. 2018;17:45. doi: 10.1186/s12943-018-0796-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Liu S, et al. A self-assembling peptide hydrogel-based drug co-delivery platform to improve tissue repair after ischemia-reperfusion injury. Acta Biomater. 2020;103:102–114. doi: 10.1016/j.actbio.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 425.Humphreys BD, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 426.Koraishy FM, Silva C, Mason S, Wu D, Cantley LG. Hepatocyte growth factor (Hgf) stimulates low density lipoprotein receptor-related protein (Lrp) 5/6 phosphorylation and promotes canonical Wnt signaling. J. Biol. Chem. 2014;289:14341–14350. doi: 10.1074/jbc.M114.563213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Maarouf OH, et al. Paracrine Wnt1 drives interstitial fibrosis without inflammation by tubulointerstitial cross-talk. J. Am. Soc. Nephrol. 2016;27:781–790. doi: 10.1681/ASN.2014121188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Zhou D, et al. Fibroblast-specific β-catenin signaling dictates the outcome of AKI. J. Am. Soc. Nephrol. 2018;29:1257–1271. doi: 10.1681/ASN.2017080903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Doeppner TR, et al. Acute hepatocyte growth factor treatment induces long-term neuroprotection and stroke recovery via mechanisms involving neural precursor cell proliferation and differentiation. J. Cereb. Blood Flow. Metab. 2011;31:1251–1262. doi: 10.1038/jcbfm.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Shang J, et al. Antiapoptotic and anti autophagic effects of glial cell line-derived neurotrophic factor and hepatocyte growth factor after transient middle cerebral artery occlusion in rats. J. Neurosci. Res. 2010;88:2197–2206. doi: 10.1002/jnr.22373. [DOI] [PubMed] [Google Scholar]
- 431.Nakaguchi K, et al. Growth factors released from gelatin hydrogel microspheres increase new neurons in the adult mouse brain. Stem Cells Int. 2012;2012:915160. doi: 10.1155/2012/915160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Chaparro RE, et al. Sustained functional improvement by hepatocyte growth factor-like small molecule BB3 after focal cerebral ischemia in rats and mice. J. Cereb. Blood Flow. Metab. 2015;35:1044–1053. doi: 10.1038/jcbfm.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Matsunaga S, Fujishiro H, Takechi H. Efficacy and Safety of Glycogen Synthase Kinase 3 Inhibitors for Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2019;69:1031–1039. doi: 10.3233/JAD-190256. [DOI] [PubMed] [Google Scholar]
- 434.del Ser T, et al. Treatment of Alzheimer’s disease with the GSK-3 inhibitor tideglusib: a pilot study. J. Alzheimers Dis. 2013;33:205–215. doi: 10.3233/JAD-2012-120805. [DOI] [PubMed] [Google Scholar]
- 435.O’Leary O, Nolan Y. Glycogen synthase kinase-3 as a therapeutic target for cognitive dysfunction in neuropsychiatric disorders. CNS Drugs. 2015;29:1–15. doi: 10.1007/s40263-014-0213-z. [DOI] [PubMed] [Google Scholar]
- 436.Singh AP, et al. Inhibition of GSK-3 to induce cardiomyocyte proliferation: a recipe for in situ cardiac regeneration. Cardiovasc. Res. 2019;115:20–30. doi: 10.1093/cvr/cvy255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Fu WB, Wang WE, Zeng CY. Wnt signaling pathways in myocardial infarction and the therapeutic effects of Wnt pathway inhibitors. Acta Pharm. Sin. 2019;40:9–12. doi: 10.1038/s41401-018-0060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Saraswati S, et al. Pyrvinium, a potent small molecule Wnt inhibitor, promotes wound repair and post-MI cardiac remodeling. PLoS One. 2010;5:e15521. doi: 10.1371/journal.pone.0015521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Laeremans H, et al. Blocking of frizzled signaling with a homologous peptide fragment of wnt3a/wnt5a reduces infarct expansion and prevents the development of heart failure after myocardial infarction. Circulation. 2011;124:1626–1635. doi: 10.1161/CIRCULATIONAHA.110.976969. [DOI] [PubMed] [Google Scholar]
- 440.Sasaki T, Hwang H, Nguyen C, Kloner RA, Kahn M. The small molecule Wnt signaling modulator ICG-001 improves contractile function in chronically infarcted rat myocardium. PLoS One. 2013;8:e75010. doi: 10.1371/journal.pone.0075010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Jiang J, et al. A novel porcupine inhibitor blocks WNT pathways and attenuates cardiac hypertrophy. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:3459–3467. doi: 10.1016/j.bbadis.2018.07.035. [DOI] [PubMed] [Google Scholar]
- 442.Xie S, et al. Discovering small molecules as Wnt inhibitors that promote heart regeneration and injury repair. J. Mol. Cell Biol. 2020;12:42–54. doi: 10.1093/jmcb/mjz023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Ni TT, et al. Discovering small molecules that promote cardiomyocyte generation by modulating Wnt signaling. Chem. Biol. 2011;18:1658–1668. doi: 10.1016/j.chembiol.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Kumar K, Singh N, Jaggi AS, Maslov L. Clinical applicability of conditioning techniques in ischemia-reperfusion injury: a review of the literature. Curr. Cardiol. Rev. 2021;17:306–318. doi: 10.2174/1573403X16999200817170619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Liu GS, et al. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991;84:350–356. doi: 10.1161/01.CIR.84.1.350. [DOI] [PubMed] [Google Scholar]
- 446.Goto M, et al. Role of bradykinin in protection of ischemic preconditioning in rabbit hearts. Circ. Res. 1995;77:611–621. doi: 10.1161/01.RES.77.3.611. [DOI] [PubMed] [Google Scholar]
- 447.Cohen MV, et al. Preconditioning-mimetics bradykinin and DADLE activate PI3-kinase through divergent pathways. J. Mol. Cell Cardiol. 2007;42:842–851. doi: 10.1016/j.yjmcc.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Schultz JE, Rose E, Yao Z, Gross GJ. Evidence for involvement of opioid receptors in ischemic preconditioning in rat hearts. Am. J. Physiol. 1995;268:H2157–H2161. doi: 10.1152/ajpheart.1995.268.5.H2157. [DOI] [PubMed] [Google Scholar]
- 449.Banerjee A, et al. Preconditioning against myocardial dysfunction after ischemia and reperfusion by an alpha 1-adrenergic mechanism. Circ. Res. 1993;73:656–670. doi: 10.1161/01.RES.73.4.656. [DOI] [PubMed] [Google Scholar]
- 450.Yao Z, Gross GJ. Role of nitric oxide, muscarinic receptors, and the ATP-sensitive K+ channel in mediating the effects of acetylcholine to mimic preconditioning in dogs. Circ. Res. 1993;73:1193–1201. doi: 10.1161/01.RES.73.6.1193. [DOI] [PubMed] [Google Scholar]
- 451.Kim, J. et al. Adenosine and Cordycepin Accelerate Tissue Remodeling Process through Adenosine Receptor Mediated Wnt/β-Catenin Pathway Stimulation by Regulating GSK3b Activity. Int J Mol Sci. 22 (2021). [DOI] [PMC free article] [PubMed]
- 452.Kim, J., Shin, J. Y., Choi, Y. H., Kang, N. G. & Lee, S. Anti-Hair Loss Effect of Adenosine Is Exerted by cAMP Mediated Wnt/β-catenin Pathway Stimulation via Modulation of Gsk3β Activity in Cultured Human Dermal Papilla Cells. Molecules27, 2184 (2022). [DOI] [PMC free article] [PubMed]
- 453.Borhani S, Corciulo C, Larranaga-Vera A, Cronstein BN. Adenosine A(2A) receptor (A2AR) activation triggers Akt signaling and enhances nuclear localization of β-catenin in osteoblasts. FASEB J. 2019;33:7555–7562. doi: 10.1096/fj.201900014R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Yan L, Yao X, Bachvarov D, Saifudeen Z, El-Dahr SS. Genome-wide analysis of gestational gene-environment interactions in the developing kidney. Physiol. Genom. 2014;46:655–670. doi: 10.1152/physiolgenomics.00035.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Liu Y, et al. Wnt/β-catenin signaling plays an essential role in α7 nicotinic receptor-mediated neuroprotection of dopaminergic neurons in a mouse Parkinson’s disease model. Biochem. Pharm. 2017;140:115–123. doi: 10.1016/j.bcp.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 456.Li Y, et al. Propoxyphene mediates oxyhemoglobin-induced injury in rat cortical neurons through up-regulation of active-β-catenin. Front. Pharm. 2019;10:1616. doi: 10.3389/fphar.2019.01616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Wang J, et al. Pentazocine Protects SN4741 Cells Against MPP(+)-Induced Cell Damage via Up-Regulation of the Canonical Wnt/β-Catenin Signaling Pathway. Front. Aging Neurosci. 2017;9:196. doi: 10.3389/fnagi.2017.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Guan M, Huang Y, Lin X. Sufentanil inhibits the proliferation and epithelial mesenchymal transition of lung cancer cells through Wnt/beta-catenin signaling pathway. Bioengineered. 2022;13:10857–10865. doi: 10.1080/21655979.2022.2066045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Mocanu MM, Bell RM, Yellon DM. PI3 kinase and not p42/p44 appears to be implicated in the protection conferred by ischemic preconditioning. J. Mol. Cell Cardiol. 2002;34:661–668. doi: 10.1006/jmcc.2002.2006. [DOI] [PubMed] [Google Scholar]
- 460.Jonassen AK, Mjøs OD, Sack MN. p70s6 kinase is a functional target of insulin activated Akt cell-survival signaling. Biochem. Biophys. Res. Commun. 2004;315:160–165. doi: 10.1016/j.bbrc.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 461.Tong H, Chen W, Steenbergen C, Murphy E. Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein kinase C. Circ. Res. 2000;87:309–315. doi: 10.1161/01.RES.87.4.309. [DOI] [PubMed] [Google Scholar]
- 462.Juhaszova M, et al. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J. Clin. Investig. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Barandon L, et al. Involvement of FrzA/sFRP-1 and the Wnt/frizzled pathway in ischemic preconditioning. Circ. Res. 2005;96:1299–1306. doi: 10.1161/01.RES.0000171895.06914.2c. [DOI] [PubMed] [Google Scholar]
- 464.Vigneron F, et al. GSK-3β at the crossroads in the signalling of heart preconditioning: implication of mTOR and Wnt pathways. Cardiovasc. Res. 2011;90:49–56. doi: 10.1093/cvr/cvr002. [DOI] [PubMed] [Google Scholar]
- 465.Correa-Costa M, et al. Transcriptome analysis of renal ischemia/reperfusion injury and its modulation by ischemic pre-conditioning or hemin treatment. PLoS One. 2012;7:e49569. doi: 10.1371/journal.pone.0049569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–899. doi: 10.1161/01.CIR.87.3.893. [DOI] [PubMed] [Google Scholar]
- 467.Kambakamba P, et al. Novel benefits of remote ischemic preconditioning through VEGF-dependent protection from resection-induced liver failure in the mouse. Ann. Surg. 2018;268:885–893. doi: 10.1097/SLA.0000000000002891. [DOI] [PubMed] [Google Scholar]
- 468.Sawashita Y, et al. Remote ischemic preconditioning reduces myocardial ischemia-reperfusion injury through unacylated ghrelin-induced activation of the JAK/STAT pathway. Basic Res. Cardiol. 2020;115:50. doi: 10.1007/s00395-020-0809-z. [DOI] [PubMed] [Google Scholar]
- 469.Sörensson P, et al. Effect of postconditioning on infarct size in patients with ST elevation myocardial infarction. Heart. 2010;96:1710–1715. doi: 10.1136/hrt.2010.199430. [DOI] [PubMed] [Google Scholar]
- 470.Woo JS, et al. Cardioprotective effects of exenatide in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of exenatide myocardial protection in revascularization study. Arterioscler. Thromb. Vasc. Biol. 2013;33:2252–2260. doi: 10.1161/ATVBAHA.113.301586. [DOI] [PubMed] [Google Scholar]
- 471.Koyama T, et al. Impact of postconditioning with lactate-enriched blood on in-hospital outcomes of patients with ST-segment elevation myocardial infarction. Int. J. Cardiol. 2016;220:146–148. doi: 10.1016/j.ijcard.2016.06.176. [DOI] [PubMed] [Google Scholar]
- 472.Zhu M, et al. Ischemic postconditioning protects remodeled myocardium via the PI3K-PKB/Akt reperfusion injury salvage kinase pathway. Cardiovasc. Res. 2006;72:152–162. doi: 10.1016/j.cardiores.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 473.Wagner C, Tillack D, Simonis G, Strasser RH, Weinbrenner C. Ischemic post-conditioning reduces infarct size of the in vivo rat heart: role of PI3-K, mTOR, GSK-3beta, and apoptosis. Mol. Cell. Biochem. 2010;339:135–147. doi: 10.1007/s11010-009-0377-x. [DOI] [PubMed] [Google Scholar]
- 474.Guo JY, et al. Ischemic postconditioning attenuates liver warm ischemia-reperfusion injury through Akt-eNOS-NO-HIF pathway. J. Biomed. Sci. 2011;18:79. doi: 10.1186/1423-0127-18-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Darling CE, et al. Postconditioning via stuttering reperfusion limits myocardial infarct size in rabbit hearts: role of ERK1/2. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H1618–H1626. doi: 10.1152/ajpheart.00055.2005. [DOI] [PubMed] [Google Scholar]
- 476.Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ. Res. 2004;95:230–232. doi: 10.1161/01.RES.0000138303.76488.fe. [DOI] [PubMed] [Google Scholar]
- 477.Hausenloy DJ, Tsang A, Yellon DM. The reperfusion injury salvage kinase pathway: a common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc. Med. 2005;15:69–75. doi: 10.1016/j.tcm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 478.Díaz-Ruíz JL, et al. Redox signaling in ischemic postconditioning protection involves PKCε and Erk1/2 pathways and converges indirectly in Nrf2 activation. Cell Signal. 2019;64:109417. doi: 10.1016/j.cellsig.2019.109417. [DOI] [PubMed] [Google Scholar]
- 479.He N, et al. Remote ischemic perconditioning prevents liver transplantation-induced ischemia/reperfusion injury in rats: role of ROS/RNS and eNOS. World J. Gastroenterol. 2017;23:830–841. doi: 10.3748/wjg.v23.i5.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Qiu, Y. et al. Hyperglycemia-Induced Overexpression of PH Domain Leucine-Rich Repeat Protein Phosphatase 1 (PHLPP1) Compromises the Cardioprotective Effect of Ischemic Postconditioning Via Modulation of the Akt/Mst1 Pathway Signaling. Cardiovasc. Drugs Ther. 10.1007/s10557-022-07349-5 (2022). [DOI] [PubMed]
- 481.Chen H, Shen J, Zhao H. Ischemic postconditioning for stroke treatment: current experimental advances and future directions. Cond. Med. 2020;3:104–115. [PMC free article] [PubMed] [Google Scholar]
- 482.Xue R, et al. Selective inhibition of PTEN preserves ischaemic post-conditioning cardioprotection in STZ-induced Type 1 diabetic rats: role of the PI3K/Akt and JAK2/STAT3 pathways. Clin. Sci. 2016;130:377–392. doi: 10.1042/CS20150496. [DOI] [PubMed] [Google Scholar]
- 483.Kerendi F, et al. Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res. Cardiol. 2005;100:404–412. doi: 10.1007/s00395-005-0539-2. [DOI] [PubMed] [Google Scholar]
- 484.Sun J, et al. Protective effect of delayed remote limb ischemic postconditioning: role of mitochondrial K(ATP) channels in a rat model of focal cerebral ischemic reperfusion injury. J. Cereb. Blood Flow. Metab. 2012;32:851–859. doi: 10.1038/jcbfm.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Hu X, Lv T, Yang SF, Zhang XH, Miao YF. Limb remote ischemic post‑conditioning reduces injury and improves long‑term behavioral recovery in rats following subarachnoid hemorrhage: possible involvement of the autophagic process. Mol. Med. Rep. 2018;17:21–30. doi: 10.3892/mmr.2017.7858. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 486.Peng B, et al. Remote ischemic postconditioning protects the brain from global cerebral ischemia/reperfusion injury by up-regulating endothelial nitric oxide synthase through the PI3K/Akt pathway. Brain Res. 2012;1445:92–102. doi: 10.1016/j.brainres.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 487.Qi ZF, et al. AKT/GSK3β-dependent autophagy contributes to the neuroprotection of limb remote ischemic postconditioning in the transient cerebral ischemic rat model. CNS Neurosci. Ther. 2012;18:965–973. doi: 10.1111/cns.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Gao S, et al. Remote ischemic postconditioning protects against renal ischemia/reperfusion injury by activation of T-LAK-cell-originated protein kinase (TOPK)/PTEN/Akt signaling pathway mediated anti-oxidation and anti-inflammation. Int. Immunopharmacol. 2016;38:395–401. doi: 10.1016/j.intimp.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 489.Danielisová V, Némethová M, Gottlieb M, Burda J. The changes in endogenous antioxidant enzyme activity after postconditioning. Cell. Mol. Neurobiol. 2006;26:1181–1191. doi: 10.1007/s10571-006-9034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Wang Q, et al. Limb remote postconditioning alleviates cerebral reperfusion injury through reactive oxygen species-mediated inhibition of delta protein kinase C in rats. Anesth. Analg. 2011;113:1180–1187. doi: 10.1213/ANE.0b013e31822b885f. [DOI] [PubMed] [Google Scholar]
- 491.Niu DG, et al. Morphine promotes cancer stem cell properties, contributing to chemoresistance in breast cancer. Oncotarget. 2015;6:3963–3976. doi: 10.18632/oncotarget.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Wang KP, Bai Y, Wang J, Zhang JZ. Morphine protects SH-SY5Y human neuroblastoma cells against Dickkopf1-induced apoptosis. Mol. Med. Rep. 2015;11:1174–1180. doi: 10.3892/mmr.2014.2832. [DOI] [PubMed] [Google Scholar]
- 493.Zhou Z, Liu T, Zhang J. Morphine activates blast-phase chronic myeloid leukemia cells and alleviates the effects of tyrosine kinase inhibitors. Biochem. Biophys. Res. Commun. 2019;520:560–565. doi: 10.1016/j.bbrc.2019.10.067. [DOI] [PubMed] [Google Scholar]
- 494.Xue JJ, et al. Protective effect of propofol on hydrogen peroxide-induced human esophageal carcinoma via blocking the Wnt/β-catenin signaling pathway. Iran. J. Basic Med. Sci. 2018;21:1297–1304. doi: 10.22038/ijbms.2018.29141.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Zhan K, Song X, Zhang Q, Yang J, Lu S. Propofol-Induced miR-493-3p Inhibits Growth and Invasion of Gastric Cancer through Suppression of DKK1-Mediated Wnt/β-Catenin Signaling Activation. Dis. Mark. 2023;2023:7698706. doi: 10.1155/2023/7698706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Gong T, et al. Propofol-induced miR-219-5p inhibits growth and invasion of hepatocellular carcinoma through suppression of GPC3-mediated Wnt/β-catenin signalling activation. J. Cell Biochem. 2019;120:16934–16945. doi: 10.1002/jcb.28952. [DOI] [PubMed] [Google Scholar]
- 497.Zhang YF, Li CS, Zhou Y, Lu XH. Effects of propofol on colon cancer metastasis through STAT3/HOTAIR axis by activating WIF-1 and suppressing Wnt pathway. Cancer Med. 2020;9:1842–1854. doi: 10.1002/cam4.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Lin XH, et al. Norepinephrine-stimulated HSCs secrete sFRP1 to promote HCC progression following chronic stress via augmentation of a Wnt16B/β-catenin positive feedback loop. J. Exp. Clin. Cancer Res. 2020;39:64. doi: 10.1186/s13046-020-01568-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Zhao X, et al. Aldehyde dehydrogenase-2 protects against myocardial infarction-related cardiac fibrosis through modulation of the Wnt/β-catenin signaling pathway. Ther. Clin. Risk Manag. 2015;11:1371–1381. doi: 10.2147/TCRM.S88297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Qian L, et al. Downregulation of S100A4 alleviates cardiac fibrosis via Wnt/β -catenin pathway in mice. Cell. Physiol. Biochem. 2018;46:2551–2560. doi: 10.1159/000489683. [DOI] [PubMed] [Google Scholar]
- 501.Cui S, et al. miR-145 attenuates cardiac fibrosis through the AKT/GSK-3β/β-catenin signaling pathway by directly targeting SOX9 in fibroblasts. J. Cell. Biochem. 2021;122:209–221. doi: 10.1002/jcb.29843. [DOI] [PubMed] [Google Scholar]
- 502.Guo X, et al. Induced pluripotent stem cell-conditional medium inhibits H9C2 cardiomyocytes apoptosis via autophagy flux and Wnt/β-catenin pathway. J. Cell. Mol. Med. 2019;23:4358–4374. doi: 10.1111/jcmm.14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Liu C, Li Y. Propofol relieves inflammation in MIRI rats by inhibiting Rho/Rock signaling pathway. Eur. Rev. Med. Pharm. Sci. 2021;25:976–984. doi: 10.26355/eurrev_202101_24667. [DOI] [PubMed] [Google Scholar]
- 504.Chen F, et al. Activation of EphA4 induced by EphrinA1 exacerbates disruption of the blood-brain barrier following cerebral ischemia-reperfusion via the Rho/ROCK signaling pathway. Exp. Ther. Med. 2018;16:2651–2658. doi: 10.3892/etm.2018.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Zhou D, Zhang M, Min L, Jiang K, Jiang Y. Cerebral ischemia-reperfusion is modulated by macrophage-stimulating 1 through the MAPK-ERK signaling pathway. J. Cell Physiol. 2020;235:7067–7080. doi: 10.1002/jcp.29603. [DOI] [PubMed] [Google Scholar]
- 506.Zhang F, Cao X, Zhao C, Chen L, Chen X. Empagliflozin activates JAK2/STAT3 signaling and protects cardiomyocytes from hypoxia/reoxygenation injury under high glucose conditions. J. Thromb. Thrombolysis. 2023;55:116–125. doi: 10.1007/s11239-022-02719-0. [DOI] [PubMed] [Google Scholar]
- 507.Li Z, et al. Theaflavin ameliorates renal ischemia/reperfusion injury by activating the Nrf2 signaling pathway in vivo and in vitro. Biomed. Pharmacother. 2021;134:111097. doi: 10.1016/j.biopha.2020.111097. [DOI] [PubMed] [Google Scholar]
- 508.Wu JW, Hu H, Hua JS, Ma LK. ATPase inhibitory factor 1 protects the heart from acute myocardial ischemia/reperfusion injury through activating AMPK signaling pathway. Int J. Biol. Sci. 2022;18:731–741. doi: 10.7150/ijbs.64956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Zhao, N., Gao, Y., Jia, H. & Jiang, X. Anti-apoptosis effect of traditional Chinese medicine in the treatment of cerebral ischemia-reperfusion injury. Apoptosis. 28, 702–729 (2023). [DOI] [PubMed]
- 510.Dong L, et al. Research Progress of Chinese Medicine in the Treatment of Myocardial Ischemia-Reperfusion Injury. Am. J. Chin. Med. 2023;51:1–17. doi: 10.1142/S0192415X23500015. [DOI] [PubMed] [Google Scholar]
- 511.Yin B, Hou XW, Lu ML. Astragaloside IV attenuates myocardial ischemia/reperfusion injury in rats via inhibition of calcium-sensing receptor-mediated apoptotic signaling pathways. Acta Pharm. Sin. 2019;40:599–607. doi: 10.1038/s41401-018-0082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Jiang M, et al. Astragaloside IV Attenuates Myocardial Ischemia-Reperfusion Injury from Oxidative Stress by Regulating Succinate, Lysophospholipid Metabolism, and ROS Scavenging System. Oxid. Med. Cell. Longev. 2019;2019:9137654. doi: 10.1155/2019/9137654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Song M, Huang L, Zhao G, Song Y. Beneficial effects of a polysaccharide from Salvia miltiorrhiza on myocardial ischemia-reperfusion injury in rats. Carbohydr. Polym. 2013;98:1631–1636. doi: 10.1016/j.carbpol.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 514.Zeng H, et al. Activated PKB/GSK-3β synergizes with PKC-δ signaling in attenuating myocardial ischemia/reperfusion injury via potentiation of NRF2 activity: Therapeutic efficacy of dihydrotanshinone-I. Acta Pharm. Sin. B. 2021;11:71–88. doi: 10.1016/j.apsb.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Huang CY, et al. Protective effect of Danggui (Radix Angelicae Sinensis) on angiotensin II-induced apoptosis in H9c2 cardiomyoblast cells. BMC Complement Alter. Med. 2014;14:358. doi: 10.1186/1472-6882-14-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Zhang S, et al. Extraction, chemical analysis of Angelica sinensis polysaccharides and antioxidant activity of the polysaccharides in ischemia-reperfusion rats. Int. J. Biol. Macromol. 2010;47:546–550. doi: 10.1016/j.ijbiomac.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 517.Wang K, Lou Y, Xu H, Zhong X, Huang Z. Harpagide from Scrophularia protects rat cortical neurons from oxygen-glucose deprivation and reoxygenation-induced injury by decreasing endoplasmic reticulum stress. J. Ethnopharmacol. 2020;253:112614. doi: 10.1016/j.jep.2020.112614. [DOI] [PubMed] [Google Scholar]
- 518.Mo ZT, Liao YL, Zheng J, Li WN. Icariin protects neurons from endoplasmic reticulum stress-induced apoptosis after OGD/R injury via suppressing IRE1α-XBP1 signaling pathway. Life Sci. 2020;255:117847. doi: 10.1016/j.lfs.2020.117847. [DOI] [PubMed] [Google Scholar]
- 519.Pang Y, Zhu S, Pei H. Pachymic acid protects against cerebral ischemia/reperfusion injury by the PI3K/Akt signaling pathway. Metab. Brain Dis. 2020;35:673–680. doi: 10.1007/s11011-020-00540-3. [DOI] [PubMed] [Google Scholar]
- 520.Tong C, et al. Intravenous administration of lycopene, a tomato extract, protects against myocardial ischemia-reperfusion injury. Nutrients. 2016;8:138. doi: 10.3390/nu8030138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Wu S, et al. Effects of lycopene attenuating injuries in ischemia and reperfusion. Oxid. Med. Cell. Longev. 2022;2022:9309327. doi: 10.1155/2022/9309327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Liu Y, Qu X, Yan M, Li D, Zou R. Tricin attenuates cerebral ischemia/reperfusion injury through inhibiting nerve cell autophagy, apoptosis and inflammation by regulating the PI3K/Akt pathway. Hum. Exp. Toxicol. 2022;41:9603271221125928. doi: 10.1177/09603271221125928. [DOI] [PubMed] [Google Scholar]
- 523.Wang G, Guo H, Wang X. Platycodin D protects cortical neurons against oxygen-glucose deprivation/reperfusion in neonatal hypoxic-ischemic encephalopathy. J. Cell. Biochem. 2019;120:14028–14034. doi: 10.1002/jcb.28677. [DOI] [PubMed] [Google Scholar]
- 524.Yang S, et al. Baicalein administered in the subacute phase ameliorates ischemia-reperfusion-induced brain injury by reducing neuroinflammation and neuronal damage. Biomed. Pharmacother. 2019;117:109102. doi: 10.1016/j.biopha.2019.109102. [DOI] [PubMed] [Google Scholar]
- 525.Wang Z, et al. Lupeol alleviates cerebral ischemia-reperfusion injury in correlation with modulation of PI3K/Akt pathway. Neuropsychiatr. Dis. Treat. 2020;16:1381–1390. doi: 10.2147/NDT.S237406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Wang PC, et al. Combination of paeoniflorin and calycosin-7-glucoside alleviates ischaemic stroke injury via the PI3K/AKT signaling pathway. Pharm. Biol. 2022;60:1469–1477. doi: 10.1080/13880209.2022.2102656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Jian J, Xuan F, Qin F, Huang R. Bauhinia championii flavone inhibits apoptosis and autophagy via the PI3K/Akt pathway in myocardial ischemia/reperfusion injury in rats. Drug Des. Dev. Ther. 2015;9:5933–5945. doi: 10.2147/DDDT.S92549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Zhang H, Li H. Tricin enhances osteoblastogenesis through the regulation of Wnt/β-catenin signaling in human mesenchymal stem cells. Mech. Dev. 2018;152:38–43. doi: 10.1016/j.mod.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 529.Lee H, Bae S, Kim YS, Yoon Y. WNT/β-catenin pathway mediates the anti-adipogenic effect of platycodin D, a natural compound found in Platycodon grandiflorum. Life Sci. 2011;89:388–394. doi: 10.1016/j.lfs.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 530.Xia X, et al. Baicalein blocked cervical carcinoma cell proliferation by targeting CCND1 via Wnt/β-catenin signaling pathway. Artif. Cells Nanomed. Biotechnol. 2019;47:2729–2736. doi: 10.1080/21691401.2019.1636055. [DOI] [PubMed] [Google Scholar]
- 531.Tarapore RS, Siddiqui IA, Adhami VM, Spiegelman VS, Mukhtar H. The dietary terpene lupeol targets colorectal cancer cells with constitutively active Wnt/β-catenin signaling. Mol. Nutr. Food Res. 2013;57:1950–1958. doi: 10.1002/mnfr.201300155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Wang Y, et al. Construing the biochemical and molecular mechanism underlying the in vivo and in vitro chemotherapeutic efficacy of ruthenium-baicalein complex in colon cancer. Int. J. Biol. Sci. 2019;15:1052–1071. doi: 10.7150/ijbs.31143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Tarapore RS, et al. Specific targeting of Wnt/β-catenin signaling in human melanoma cells by a dietary triterpene lupeol. Carcinogenesis. 2010;31:1844–1853. doi: 10.1093/carcin/bgq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Zhang L, Tu Y, He W, Peng Y, Qiu Z. A novel mechanism of hepatocellular carcinoma cell apoptosis induced by lupeol via Brain-derived neurotrophic factor inhibition and glycogen synthase kinase 3 beta reactivation. Eur. J. Pharm. 2015;762:55–62. doi: 10.1016/j.ejphar.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 535.Wu XT, et al. The enhanced effect of lupeol on the destruction of gastric cancer cells by NK cells. Int. Immunopharmacol. 2013;16:332–340. doi: 10.1016/j.intimp.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 536.Zhou Y, et al. Paeoniflorin Affects Hepatocellular Carcinoma Progression by Inhibiting Wnt/β-Catenin Pathway through Downregulation of 5-HT1D. Curr. Pharm. Biotechnol. 2021;22:1246–1253. doi: 10.2174/1389201021666201009153808. [DOI] [PubMed] [Google Scholar]
- 537.Li H, et al. Bauhinia championi (Benth.) Benth. polysaccharides upregulate Wnt/β-catenin signaling in chondrocytes. Int. J. Mol. Med. 2013;32:1329–1336. doi: 10.3892/ijmm.2013.1527. [DOI] [PubMed] [Google Scholar]
- 538.Zhang C, et al. Asiaticoside alleviates cerebral ischemia-reperfusion injury via NOD2/Mitogen-Activated Protein Kinase (MAPK)/Nuclear Factor kappa B (NF-κB) Signaling Pathway. Med. Sci. Monit. 2020;26:e920325. doi: 10.12659/MSM.920325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Ye B, et al. Emodin alleviates myocardial ischemia/reperfusion injury by inhibiting gasdermin D-mediated pyroptosis in cardiomyocytes. Drug Des. Dev. Ther. 2019;13:975–990. doi: 10.2147/DDDT.S195412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Pan, J. et al. Ginkgetin attenuates cerebral ischemia-reperfusion induced autophagy and cell death via modulation of the NF-κB/p53 signaling pathway. Biosci. Rep. 39, BSR20191452 (2019). [DOI] [PMC free article] [PubMed]
- 541.Cao W, Feng SJ, Kan MC. Naringin Targets NFKB1 to Alleviate Oxygen-Glucose Deprivation/Reoxygenation-Induced Injury in PC12 Cells Via Modulating HIF-1α/AKT/mTOR-Signaling Pathway. J. Mol. Neurosci. 2021;71:101–111. doi: 10.1007/s12031-020-01630-8. [DOI] [PubMed] [Google Scholar]
- 542.An B, et al. Crocin regulates the proliferation and migration of neural stem cells after cerebral ischemia by activating the Notch1 pathway. Folia Neuropathol. 2020;58:201–212. doi: 10.5114/fn.2020.100063. [DOI] [PubMed] [Google Scholar]
- 543.Dibben G, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. 2021;11:Cd001800. doi: 10.1002/14651858.CD001800.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Wang H, et al. Programmed exercise attenuates familial hypertrophic cardiomyopathy in transgenic E22K mice via inhibition of PKC-α/NFAT pathway. Front. Cardiovasc. Med. 2022;9:808163. doi: 10.3389/fcvm.2022.808163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Cheedipudi SM, et al. Exercise restores dysregulated gene expression in a mouse model of arrhythmogenic cardiomyopathy. Cardiovasc. Res. 2020;116:1199–1213. doi: 10.1093/cvr/cvz199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.