Abstract
Background & Aims
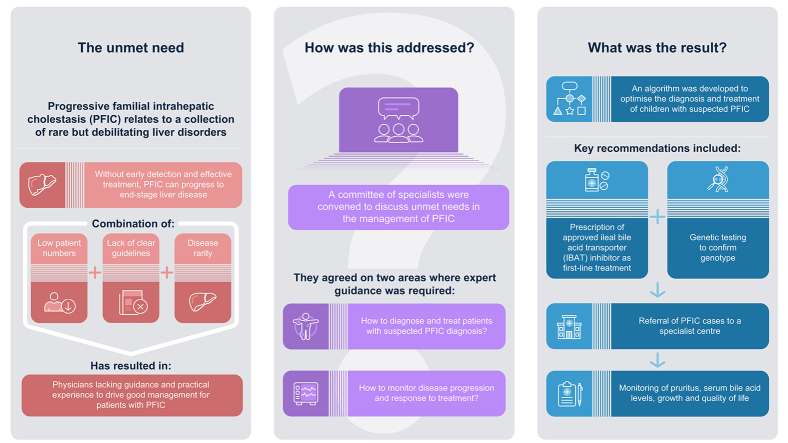
Progressive familial intrahepatic cholestasis (PFIC) relates to a group of rare, debilitating, liver disorders which typically present in early childhood, but have also been reported in adults. Without early detection and effective treatment, PFIC can result in end-stage liver disease. The aim of the paper was to put forward recommendations that promote standardisation of the management of PFIC in clinical practice.
Methods
A committee of six specialists came together to discuss the challenges faced by physicians in the management of PFIC. The committee agreed on two key areas where expert guidance is required to optimise care: (1) how to diagnose and treat patients with a clinical presentation of PFIC in the absence of clear genetic test results/whilst awaiting results, and (2) how to monitor disease progression and response to treatment. A systematic literature review was undertaken to contextualise and inform the recommendations.
Results
An algorithm was developed for the diagnosis and treatment of children with suspected PFIC. The algorithm recommends the use of licensed inhibitors of ileal bile acid transporters as the first-line treatment for patients with PFIC and suggests that genetic testing be used to confirm genotype whilst treatment is initiated in patients in whom PFIC is suspected. The authors recommend referring patients to an experienced centre, and ensuring that monitoring includes measurements of pruritus, serum bile acid levels, growth, and quality of life following diagnosis and during treatment.
Conclusions
The algorithm presented within this paper offers guidance to optimise the management of paediatric PFIC. The authors hope that these recommendations will help to standardise the management of PFIC in the absence of clear clinical guidelines.
Impact and implications
This opinion paper outlines a consistent approach to the contemporaneous diagnosis, monitoring, referral and management of children with progressive familial intrahepatic cholestasis. This should assist physicians given the recent developments in genetic diagnosis and the availability of effective drug therapy. This manuscript will also help to raise awareness of current developments and educate health planners on the place for new drug therapies in progressive familial intrahepatic cholestasis.
Keywords: PFIC, diagnosis, treatment, IBAT inhibitor
Graphical abstract
Highlights
-
•
An algorithm was created for suspected progressive familial intrahepatic cholestasis.
-
•
Licensed inhibitors of ileal bile acid transporters are recommended for first-line treatment.
-
•
Genetic testing is recommended to confirm genotype while treatment is initiated.
-
•
Patient referral to an experienced centre is also recommended.
Introduction
Progressive familial intrahepatic cholestasis (PFIC) refers to a group of rare, autosomal recessive liver disorders caused by defects in bile constitution/formation, typically presenting as intrahepatic cholestasis in early childhood.1,2 PFIC is caused by mutations in the genes coding for proteins mainly involved in hepatocellular transport and maintenance.2 Historically, PFIC subtypes have been determined by which protein is affected.2,3,4 However, these subtypes are constantly evolving as new mutations in different genes are identified, so the nomenclature has moved towards naming based on the deficiency of the respective gene product (Table 1).2,3,4 In children, the clinical presentation of PFIC can include: jaundice, pruritus, elevated serum bile acid (SBA) values, malabsorption, and failure to thrive.1 PFIC can be a debilitating condition that significantly impacts quality of life, and can result in end-stage liver disease and need for transplantation.2 As such, early detection and effective intervention are imperative for the prevention of disease progression.
Table 1.
Overview of genes associated with PFIC and their related histological and phenotypic characteristics.[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14].
| Protein deficiency | Mutated gene | Histological characteristics | Phenotypic characteristics |
|---|---|---|---|
| FIC15 | ATP8B1 |
|
|
| BSEP6 | ABCB11 |
|
|
| MDR37 | ABCB4 |
|
|
| TJP28 | TJP2 |
|
|
| FXR9 | NR1H4 |
|
|
| OSTα-OSTβ10 | SLC51A |
|
|
| USP5311 | USP53 |
|
|
| KIF1212 | KIF12 |
|
|
| MYO5B13 | MYO5B |
|
|
| LSR14 | LSR |
|
|
| WDR83OS11 | WDR83OS |
|
|
GGT, gamma-glutamyltransferase; n.a., not applicable; PFIC, progressive familial intrahepatic cholestasis.
The estimated incidence of PFIC ranges between 1 per 50,000 and 1 per 100,000 births,3 which has made it challenging to collect and share data on best practice. The historical lack of effective treatment options and differences in diagnostic and disease monitoring practices have been barriers to optimising clinical practice.
Diagnosis of PFIC has traditionally been made via clinical assessment (including symptoms, family history, laboratory investigation, liver ultrasound and liver biopsy); however, gene sequencing is now widely accepted as the gold standard for diagnostic confirmation.15 In addition to direct gene analysis via Sanger sequencing, three methods of next-generation sequencing have been reported in the literature for PFIC diagnosis.15,16,17,18 Targeted gene panel analysis involves sequencing several selected cholestasis-associated genes in a single analysis, and is frequently used alongside clinical diagnosis when a specific PFIC genotype is suspected.15,18 This method of next-generation sequencing offers a more time efficient and less costly method of genetic diagnosis compared with other next-generation sequencing techniques.15 The diagnostic yield of targeted gene panel sequencing varies widely in the published literature, from 28.1% to 68%, which is thought to be due to patient selection criteria and the number of genes utilised in the panel.15,16,17,18 Whole-exome and whole-genome sequencing are typically utilised in cases of complex phenotype, or when genetic diagnosis via targeted gene panel sequencing has been inconclusive.17 Despite considerable advances in genetic testing, some challenges remain. It can be difficult to predict variant pathogenicity, as genetic screening may not identify unknown pathogenic variants associated with PFIC; moreover, phenotype and genotype correlations are not always consistent, thus interpretation of molecular findings is an ongoing process.15,18,19
Medical treatment, in combination with dietary supplementation (including fat-soluble vitamins, lipids and caloric supplementation), is usually the first-line treatment choice for patients with all types of PFIC to prevent the consequences of chronic cholestasis.2 Historically, physicians have faced the challenge of the absence of an approved pharmaceutical treatment for patients with PFIC. Ursodeoxycholic acid (UDCA) is the most widely reported, off-label, medical treatment administered to patients with PFIC.1,20 UDCA is minimally effective, but is associated with few adverse events.2 Initial improvements in clinical symptoms are often not sustained, with some patients experiencing relapse and disease progression, and requiring surgical management.2,20,21,22 Rifampicin is another commonly prescribed, off-label, antipruritic drug, often used in combination with UDCA, which has demonstrated little success in terms of sustained symptomatic responses.1,23,24 Other historical treatments (mostly off-label and repurposed based on efficacy trials in other cholestatic diseases) that have been associated with limited success and which do not modify the course of disease include: sertraline, cholestyramine (licensed for cholestatic pruritus in certain countries), antihistamines, sodium 4-phenylbutyrate and naltrexone.1,20,25,26
Following failure of medical treatment, surgical management in the form of biliary diversion or liver transplantation is usually required. Biliary diversion, which disrupts the enterohepatic circulation of bile acids, can be carried out in three different ways: partial external biliary drainage, partial internal biliary drainage, or ileal exclusion.1,27 Whilst generally considered a successful treatment for patients with PFIC (lowering SBAs, attenuating pruritus intensity, and prolonging native liver survival in many cases),1,28,29,30,31 not all patients respond. A meta-analysis of 424 children with PFIC who underwent partial external biliary diversion, partial internal biliary diversion or ileal exclusion reported that pruritus resolved following biliary diversion in 59.5% of the studied cohort, but that 27% went on to require liver transplantation.27 Current literature suggests that this varied response to biliary diversion may be influenced by PFIC type and genotype; patients with PFIC2 were more likely than patients with PFIC1 or PFIC3 to develop severe liver disease, or hepatocellular carcinoma, and require liver transplantation.27 In those with PFIC2, genotype is a strong predictor of response to biliary diversion,32 with 10-year native liver survival ranging from 22% to 75%, depending on genotype.33 Reported complications associated with biliary diversion include stoma-related complications, malabsorption, diarrhoea, recurrent pruritus and progressive disease resulting in the need for liver transplantation.1,27 Transplantation is indicated for patients with end-stage liver disease or those with significant symptoms (including pruritus) who have not responded to other treatment methods.1 Liver transplant considerations specific to PFIC include the risk of antibody-induced BSEP deficiency in PFIC2, and the possibility of residual disease characterised by severe diarrhoea and progressive steatosis in PFIC1.1,34,35
A novel class of drugs, ileal bile acid transporter (IBAT) inhibitors, have been developed to combat intrahepatic cholestasis.36,37,38 IBAT inhibitors offer a non-surgical approach to achieving disruption of the enterohepatic circulation of bile acids.39 Several IBAT inhibitors are in development for a range of indications (Table 2). In 2021, odevixibat received approval for the treatment of patients over the age of 6 months with PFIC in the EU, followed shortly by approval in the US and UK for the treatment of pruritus in patients over the age of 3 months with PFIC.40 Clinical trial results with IBAT inhibitors have demonstrated normalisation of SBA values, reduction in pruritus severity, and subjectively reported improvements in quality of life for patients.36,37,38 In a randomised placebo-controlled phase III clinical trial investigating children with PFIC1 or PFIC2, odevixibat led to significant improvements in both pruritus (55% vs. 30%; p = 0.0038) and SBA response (33% vs. 0%; p = 0.003), as defined by a ≥70% reduction from baseline in fasting serum bile acids or serum bile acids ≤70 μmol/L after 24 weeks of treatment (Table 3).37 Clinically significant reductions in pruritus were also reported in a phase II study assessing the efficacy and safety of maralixibat in children diagnosed with PFIC1 or PFIC2, at week 13 compared to baseline (-0.8 vs. -1.0; p = 0.002).38
Table 2.
IBAT inhibitors in development.[40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52]
| IBAT inhibitor | Approved indication | Target indication(s) |
|---|---|---|
| Odevixibat | PFIC (EU and US)40 Alagille syndrome (US)41 |
Biliary atresia42 |
| Maralixibat | Alagille syndrome (EU and US)43 | PFIC44 Biliary atresia45 |
| Elobixibat | Chronic constipation (Japan)46 | Non-alcoholic steatohepatitis47 Non-alcoholic fatty liver disease47 |
| Linerixibat | n.a. | Type 2 diabetes48 Cholestasis49 Primary biliary cholangitis50 |
| Volixibat | n.a. | Non-alcoholic steatohepatitis51 Primary sclerosing cholangitis52 |
IBAT, ileal bile acid transporter; PFIC, progressive familial intrahepatic cholestasis; n.a., not applicable.
Table 3.
Clinical results from the phase III odevixibat trial.37
| Placebo (n = 20) | Odevixibat 40 μg/kg/day (n = 23) | Odevixibat 120 μg/kg/day (n = 19) | Odevixibat, all doses (n = 42) | |
|---|---|---|---|---|
| Serum bile acid response (%) | 0% | 43% | 21% | 33% |
| Proportion of positive pruritus assessments (%) | 30% | 58% | 52% | 55% |
| Mean change from baseline to week 24 in ObsRO scratching score | -0.25 | NR | NR | -1.11 |
| Most common adverse events (%) | Pyrexia (25%) | Diarrhoea/frequent bowel movements (29%) | Pyrexia (26%) and upper respiratory tract infection (26%) | Diarrhoea/frequent bowel movements (31%) |
ObsRO, observer reported outcome; NR, not reported. The phase III randomised-controlled trial recruited 62 patients with either PFIC1 or PFIC2 (median age, 3.2 years) who received placebo (n = 20), or odevixibat at either 40 μg/kg/day (n = 23) or 120 μg/kg/day (n = 19) for 24 weeks.
Results from the Natural Course and Prognosis of PFIC and Effect of Biliary Diversion (NAPPED) Consortium have shown that just one protein-truncating mutation in BSEP is associated with a severe disease course, low responsiveness to biliary diversion, and reduced response to IBAT inhibitors.33 This is corroborated by the results of the maralixibat phase II study which showed that no patients with a protein-truncating mutation within the ABCB11 gene achieved a SBA response following maralixibat treatment.38 Patients with biallelic truncating mutations in ABCB11 were excluded from the odevixibat phase III trial.37
Progression of PFIC can vary widely between individuals,2 and several biomarkers have been reported as surrogate indicators of disease progression.28,30,31,32,36,37,38,53,54 Notably, a decrease in SBA levels has been shown to correlate with reduction in pruritus severity.55 In addition, elevated circulating values have also been linked with poor native liver survival in children.28 In patients with PFIC1, native liver survival at 15 years was shown to be threefold greater in patients with SBA values below 194 μmol/L than in those with elevated values above this threshold.28 Both SBA concentrations and pruritus intensity have been used as primary efficacy outcome measures to assess treatment response in clinical trials,36,37,38 with response to treatment being defined as a normalisation of SBAs and the absence of, or improvement in, pruritus intensity.
Sleep disturbance, growth retardation, total and conjugated bilirubin levels, and poor quality of life have all been associated with PFIC, and lend themselves to use as markers of disease progression.29,30,36,38,53,56,57 Other biomarkers cited in the literature include markers of fibrosis, including APRI (aspartate aminotransferase-to-platelet ratio index) and FIB-4 (fibrosis-4 index).53 Validated tools to monitor some of these markers have been developed, such as ItchRO(Observer) and PRUCISION for pruritus and sleep disturbance.53,58 In addition, a liver histology scoring system for BSEP deficiency has also been described for monitoring microscopic changes in clinical practice and as a surrogate endpoint in clinical trials.54
The authors identified a clear unmet need to optimise the treatment pathway for patients with PFIC, given the historical lack of consensus regarding diagnosis, treatment, and monitoring. As such, the authors put forward their expert opinions to promote the standardisation of the management of PFIC in clinical practice, based on their own experience and the published literature.
Materials and methods
The following recommendations were developed by six European specialists with extensive experience in the management of PFIC in paediatric and adult settings. The committee met twice (on the 29th September 2022 and 6th December 2022) to discuss the core challenges faced by physicians in the diagnosis and management of PFIC. During the first meeting, the committee agreed on two key areas where expert guidance is required to optimise the care of patients with PFIC, these were:
-
○
How to diagnose and treat patients with a clinical presentation of PFIC in the absence of clear genetic test results/whilst awaiting genetic testing results.
-
○
How to monitor disease progression in patients with PFIC.
At the second meeting, the committee discussed how these concerns could be addressed using their own clinical expertise.
Literature searches were conducted on 3rd January 2023, using the PubMed database, to contextualise and inform any expert recommendations and elucidate the current understanding in the field. Three search strategies (Table S1) were defined and agreed by the committee to answer the following questions:
-
1.
When does genetic testing for PFIC currently take place?
-
2.
What are the diagnostic clinical biomarkers that indicate disease progression for PFIC?
-
3.
What is the current treatment pathway for PFIC?
Titles and abstracts identified through the searches were reviewed independently by two reviewers (CC and LD). Studies were included if they were: 1) related to PFIC and addressed the question, 2) a clinical study, 3) published in the past 5 years. Studies were excluded if they were: 1) a non-peer-reviewed paper, 2) related to other intrahepatic conditions other than PFIC, 3) not primary research, 4) not written in English. Data extraction was then carried out by one reviewer (CC).
Results
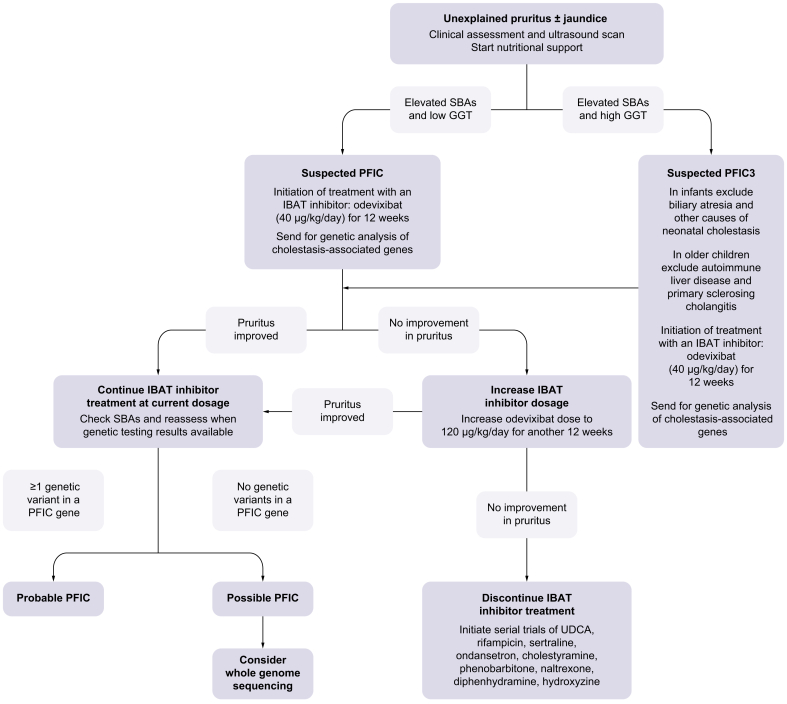
The authors proposed a diagnostic and treatment algorithm for children with suspected PFIC (Fig. 1) to help to guide clinical practice.
Fig. 1.
Proposed diagnosis and treatment algorithm for patients with unexplained pruritus and/or jaundice in conjunction with elevated SBAs.
GGT, gamma-glutamytransferase; IBAT, ileal bile acid transporter; PFIC, progressive familial intrahepatic cholestasis; SBA, serum bile acids; UDCA, ursodeoxycholic acid; USS, ultrasound scan.
In neonates and infants with unexplained jaundice and predominant conjugated hyperbilirubinaemia, structured investigations according to an established protocol should be undertaken to exclude biliary atresia or other causes of neonatal jaundice.59 Patients with unexplained pruritus (with or without jaundice) are advised to undergo: 1) clinical assessment to quantify bilirubin, SBAs, gamma-glutamyltransferase (GGT) and fat-soluble vitamin levels, and 2) an abdominal ultrasound scan to exclude obstructive causes of cholestasis, such as a mass or stones.
The panel agreed that a diagnosis of PFIC should be suspected in cholestatic patients with elevated SBAs and low GGT values (Fig. 1). For such patients with clinical and biochemical factors highly suggestive of a PFIC diagnosis, the authors propose that empirical treatment with an IBAT inhibitor can be initiated as confirmatory genetic testing via targeted gene panels is requested. Treatment with an IBAT inhibitor should also be considered if PFIC3 is suspected in cholestatic patients with elevated GGT (provided differential diagnoses with urgent treatment requirements are excluded). Presently, odevixibat is the only approved IBAT inhibitor for patients with PFIC and can be initiated at a recommended dose of 40 μg/kg/day for an initial 12-week period. If the patient shows a clinical and/or biochemical improvement after treatment initiation, the authors recommend continuing odevixibat treatment. The authors suggest that an adequate response to IBAT inhibitor therapy is defined as a >70% fall in SBAs, or a ≥1 reduction in pruritus score. The authors note that there are several tools available to monitor pruritus,57,58 for example the PRUCISION tool, and recommend that the pruritus score is quantified ahead of treatment initiation so that response to treatment can be monitored.
If genetic screening, via gene panel diagnostics, identifies one or more genetic variants associated with cholestasis, then this would be supportive of a PFIC diagnosis. If no indicative mutations are observed yet the index of suspicion remains high, whole-exome sequencing should be considered whilst continuing to treat the patient with odevixibat. The authors note that IBAT inhibitor treatment may not be available to all physicians. If it is not possible to access treatment through tertiary or quaternary centre referral, then alternative therapies (pharmacological or surgical) may be explored in conjunction with a specialist liver unit.
For any patients who experience a partial improvement or no improvement in pruritus 12 weeks after initiation of odevixibat treatment, the odevixibat dose can be increased to 120 μg/kg/day for a further 12 weeks, following agreement with the patient and their parent or caregiver. If the increased dosage does not improve pruritus, then treatment should be discontinued. In this case, treatment with serial trials of UDCA, rifampicin, sertraline, ondansetron, cholestyramine, phenobarbitone, naltrexone, diphenhydramine and hydroxyzine should be considered. Surgical biliary diversion should be considered if the patient fails to respond to pharmacological intervention. Such therapeutic decisions need to be decided by an expert team or medical centre, in agreement with the caregiver.
The committee agreed that diagnosis of PFIC is just the start of a patient’s journey and emphasised that monitoring for wellbeing and disease progression is critically important. Monitoring treatment effectiveness and attenuating progression towards end-stage liver disease are the main goals of these assessments. Assessing liver function, pruritus, SBAs, fat-soluble vitamins, liver stiffness, growth, and quality of life is recommended for patients with suspected PFIC, based on synthesis of the literature and the authors’ own clinical experience. Clinical assessment, including liver biochemistry, fat-soluble vitamin levels and SBA levels, as well as monitoring of patient growth, is recommended, with an arbitrary frequency recommendation of every 3–6 months. Pruritus and quality of life surveys should be conducted by the patient/caregiver. An ultrasound scan of the liver, and assessment of liver stiffness as a surrogate marker of fibrosis/cirrhosis should be considered every 6–12 months. For those with established cirrhosis, surveillance ultrasound scans and alpha-fetoprotein levels should be checked every 6 months, in line with guidelines for hepatocellular carcinoma.60 If, after treatment and dose escalation, the patient does not present any clinical improvement, the authors recommend considering other medical treatment options on a case-by-case basis, such as a liver transplantation (based on the patient’s clinical condition, e.g. end-stage liver disease or intractable pruritus).
Discussion
Based on the published literature,15 and their own clinical expertise, the authors agree that genetic testing is the gold standard for PFIC diagnosis. However, they also understand from clinical experience that there can be barriers to genetic testing, such as cost implications, delays in result turnaround time, patients without genetic confirmation of PFIC, and complex cases where genotype and phenotype do not correspond.15,18 To meet these potential challenges, the proposed algorithm suggests that initially patients should be assessed clinically, with treatment initiated in patients with clinical and biochemical factors suggestive of a PFIC diagnosis. Genetic testing is recommended during treatment initiation to genetically confirm diagnosis, without delaying the start of treatment. The decision-making process on whether to start treatment with an IBAT inhibitor or not should consider the clinical conditions of the patient, the severity of the symptoms and the turnaround time of genetic or confirmatory testing.
The proposed algorithm recommends the initiation of treatment with the IBAT inhibitor odevixibat40 following clinical diagnosis. It should be remembered that the treatment landscape is evolving, with additional IBAT inhibitors in the pipeline,[40], [41], [42], [43] and research into other treatments such as gene therapy is ongoing.28 As such, the algorithm will need to be dynamic, with reassessment as new data become available. However, the authors hope that the algorithm will offer physicians the confidence to start their patients on the most appropriate treatment as early as possible, without relying on off-label treatments, which often have limited efficacy.1,20,21,22,23,24,25,26
With a progressive disease such as PFIC, it is important to continually monitor patients following treatment initiation to determine if an adequate response has been achieved. The authors suggest that the most appropriate markers to use are pruritus, SBAs, growth, and quality of life. In addition, liver synthesis and development of fibrosis/cirrhosis with associated complications should be monitored. The literature review highlighted a number of diagnostic tools that may enable effective monitoring of a patient’s quality of life and levels of pruritus, including PRUCISION and ItchRO(Observer)53,58 which have been validated and could be used within clinical practice. New biomarkers are emerging (such as the potential to monitor disease progression by assessing markers of fibrosis54); however, further research into their clinical utility is required. Given these complexities, we recommend that children with suspected PFIC should be referred to, and discussed with, a specialist centre.
Whilst this paper focusses on paediatric presentations of PFIC, the authors note that further research and guidance is required for adult patients with idiopathic cholestasis. The authors also recognised that transitional care from paediatric to adult services, whilst also beyond the scope of this paper, is an important area for further expert guidance. Transitional care is a difficult, often stressful, time for patients, in which it is essential for paediatric physicians to be transparent and set realistic expectations of the process to both patients and parents/carers. This is an area that needs further focus and clear guidance to standardise the approach and improve patient experience.
In summary, it is hoped that the content of this paper can be used as a supporting reference for the management of PFIC that will help to standardise the care of patients in clinical practice.
Financial support
Two meetings of the expert committee (including travel expenses) were organised and funded by Albireo Pharma, an Ipsen company. Albireo did not influence the content of the manuscript and was not involved in the publication process. Langland provided editorial and medical writing assistance for the preparation of the manuscript; this was funded by Albireo.
Authors’ contributions
Each author participated in two expert committee meetings, reviewed the results of the systematic literature review, established the expert opinion recommendations, and revised and approved the manuscript. The views and opinions expressed in this manuscript are those of the authors alone.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Conflict of interest
PM: consultant for Albireo Pharma. JQB: consultant for Albireo Pharma, Mirum, Orphalan, Astra-Zeneca and Intercept Pharmaceuticals. MG: consultant for Albireo Pharma, Mirum and Orphalan. GI: consultant for Albireo Pharma, Mirum and Kedrion Pharma. EL: speaker agreements with Albireo Pharma, Mirum, Nutiricia and Takeda. PT: consultant for Albireo Pharma, GSK, Dr Falk Pharma, Gilead Medical, Advanz / Intercept Pharmaceuticals, Pliant Pharma, Cymabay. Grant Support from BMS, GSK, Dr Falk Pharma, Gilead Medical, Advanz / Intercept Pharmaceuticals, Regeneron, the Wellcome Trust, the Medical Research Foundation, LifeArc, Innovate UK and NIHR.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
The authors thank Chelsea Creak PhD and Leanne Darling, of Langland, for providing medical writing support, which was funded by Albireo Pharma, an Ipsen company.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100949.
Supplementary data
The following are the supplementary data to this article:
:
References
- 1.Henkel S.A., Squires J.H., Ayers M., et al. Expanding etiology of progressive familial intrahepatic cholestasis. World J Hepatol. 2019;11(5):450–463. doi: 10.4254/wjh.v11.i5.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones-Hughes T., Campbell J., Crathorne L. Epidemiology and burden of progressive familial intrahepatic cholestasis: a systematic review. Orphanet J Rare Dis. 2021;16(1):255. doi: 10.1186/s13023-021-01884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felzen A., Verkade H.J. The spectrum of progressive familial intrahepatic cholestasis disease: update on pathophysiology and emerging treatments. Eur J Med Genet. 2021;64(11) doi: 10.1016/j.ejmg.2021.104317. [DOI] [PubMed] [Google Scholar]
- 4.Vitale G., Mattiaccio A., Conti A., et al. Genetics in familial intrahepatic cholestasis: clinical patterns and development of liver and biliary cancers: a review of the literature. Cancers (Basel) 2022;14(14):3421. doi: 10.3390/cancers14143421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clayton R.J., Iber F.L., Ruebner B.H., et al. Byler disease. Fatal familial intrahepatic cholestasis in an Amish kindred. Am J Dis Child. 1969;117:112–124. [PubMed] [Google Scholar]
- 6.Strautnieks S.S., Bull L.N., Knisely A.S., et al. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet. 1998;20:233–238. doi: 10.1038/3034. [DOI] [PubMed] [Google Scholar]
- 7.de Vree J.M., Jacquemin E., Sturm E., et al. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci USA. 1998;95:282–287. doi: 10.1073/pnas.95.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sambrotta M., Strautnieks S., Papouli E., et al. Mutations in TJP2 cause progressive cholestatic liver disease. Nat Genet. 2014;46:326–328. doi: 10.1038/ng.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez-Ospina N., Potter C.J., Xiao R., et al. Mutations in the nuclear bile acid receptor FXR cause progressive familial intrahepatic cholestasis. Nat Commun. 2016;7 doi: 10.1038/ncomms10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao E., Cheema H., Waheed N., et al. Organic solute transporter alpha deficiency: a disorder with cholestasis, liver fibrosis, and congenital diarrhea. Hepatology. 2020;71:1879–1882. doi: 10.1002/hep.31087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maddirevula S., Alhebbi H., Alqahtani A., et al. Identification of novel loci for pediatric cholestatic liver disease defined by KIF12, PPM1F, USP53, LSR, and WDR83OS pathogenic variants. Genet Med. 2019;21:1164–1172. doi: 10.1038/s41436-018-0288-x. [DOI] [PubMed] [Google Scholar]
- 12.Unlusoy Aksu A., Das S.K., Nelson-Williams C., et al. Recessive mutations in KIF12 cause high gamma-glutamyltransferase cholestasis. Hepatol Commun. 2019;3:471–477. doi: 10.1002/hep4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzales E., Taylor S.A., Davit-Spraul A., et al. MYO5B mutations cause cholestasis with normal serum gamma-glutamyl transferase activity in children without microvillous inclusion disease. Hepatology. 2017;65:164–173. doi: 10.1002/hep.28779. [DOI] [PubMed] [Google Scholar]
- 14.Uehara T., Yamada M., Umetsu S., et al. Biallelic mutations in the LSR gene cause a novel type of infantile intrahepatic cholestasis. J Pediatr. 2020;221:251–254. doi: 10.1016/j.jpeds.2020.01.064. [DOI] [PubMed] [Google Scholar]
- 15.Almes M., Spraul A., Ruiz M., et al. Targeted-capture next-generation sequencing in diagnosis approach of pediatric cholestasis. Diagnostics (BASL) 2022;12:1169. doi: 10.3390/diagnostics12051169. Diagnostics (Basel). 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H.L., Li H.Y., Wu J.F., et al. Panel-based next-generation sequencing for the diagnosis of cholestatic genetic liver diseases: clinical utility and challenges. J Pediatr. 2019;205:153–159. doi: 10.1016/j.jpeds.2018.09.028. [DOI] [PubMed] [Google Scholar]
- 17.Nicastro E., Di Giorgio A., Marchetti D., et al. Diagnostic yield of an algorithm for neonatal and infantile cholestasis integrating next-generation sequencing. J Pediatr. 2019;211:54–62. doi: 10.1016/j.jpeds.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 18.Lipiński P., Ciara E., Jurkiewicz D., et al. Targeted next-generation sequencing in diagnostic approach to monogenic cholestatic liver disorders–single-center experience. Front Pediatr. 2020;8:414. doi: 10.3389/fped.2020.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeyaraj R., Bounford K.M., Ruth N., et al. The genetics of inherited cholestatic disorders in neonates and infants: evolving challenges. Genes (Basel) 2021;12(11):1837. doi: 10.3390/genes12111837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker A., Kerkar N., Todorova L., et al. Systematic review of progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol. 2019;43(1):20–36. doi: 10.1016/j.clinre.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Mínguez Rodríguez B., Molera Busoms C., Martorell Sampol L., et al. Heterozygous mutations of ATP8B1, ABCB11 and ABCB4 cause mild forms of Progressive Familial Intrahepatic Cholestasis in a pediatric cohort. Gastroenterol Hepatol. 2022;45(8):585–592. doi: 10.1016/j.gastrohep.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Jankowska I., Pawłowska J., Szymczak M., et al. A report of 2 infant siblings with progressive intrahepatic familial cholestasis type 1 and a novel homozygous mutation in the ATP8B1 gene treated with partial external biliary diversion and liver transplant. Am J Case Rep. 2021;22 doi: 10.12659/AJCR.932374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y., Luo E.P., Li M., et al. Two novel ATP8B1 mutations involved in progressive familial intrahepatic cholestasis type 1 that is ameliorated by rifampicin: a case report. J Dig Dis. 2022;23(2):124–129. doi: 10.1111/1751-2980.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porta G., Rigo P.S.M., Porta A., et al. Progressive familial intrahepatic cholestasis associated with USP53 gene mutation in a Brazilian child. J Pediatr Gastroenterol Nutr. 2021;72(5):674–676. doi: 10.1097/MPG.0000000000003110. [DOI] [PubMed] [Google Scholar]
- 25.Alzebaidi S., Alghamdi Y., Alghamdi A., et al. Progressive familial intrahepatic cholestasis type 1 associated with cherry-red spots in an infant: a first case report. Cureus. 2020;12(12) doi: 10.7759/cureus.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakano S., Osaka S., Sabu Y., et al. Effect of food on the pharmacokinetics and therapeutic efficacy of 4-phenylbutyrate in progressive familial intrahepatic cholestasis. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-53628-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolia R., Goel A.D., Sharma V., et al. Biliary diversion in progressive familial intrahepatic cholestasis: a systematic review and meta-analysis. Expert Rev Gastroenterol Hepatol. 2022;16(2):163–172. doi: 10.1080/17474124.2022.2032660. [DOI] [PubMed] [Google Scholar]
- 28.van Wessel D.B.E., Thompson R.J., Gonzales E., et al. Natural Course and Prognosis of PFIC and Effect of Biliary Diversion Consortium. Impact of genotype, serum bile acids, and surgical biliary diversion on native liver survival in FIC1 deficiency. Hepatology. 2021;74(2):892–906. doi: 10.1002/hep.31787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varol F.İ., Selimoğlu M.A., Güngör Ş., et al. Single-center experience in management of progressive familial intrahepatic cholestasis. Arab J Gastroenterol. 2021;22(4):310–315. doi: 10.1016/j.ajg.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 30.Verkade H.J., Thompson R.J., Arnell H., et al. Systematic review and meta-analysis: partial external biliary diversion in progressive familial intrahepatic cholestasis. J Pediatr Gastroenterol Nutr. 2020;71(2):176–183. doi: 10.1097/MPG.0000000000002789. [DOI] [PubMed] [Google Scholar]
- 31.Bjørnland K., Hukkinen M., Gatzinsky V., et al. Partial biliary diversion may promote long-term relief of pruritus and native liver survival in children with cholestatic liver diseases. Eur J Pediatr Surg. 2021;31(4):341–346. doi: 10.1055/s-0040-1714657. [DOI] [PubMed] [Google Scholar]
- 32.van Wessel D.B.E., Thompson R.J., Gonzales E., et al. NAtural course and Prognosis of PFIC and Effect of biliary Diversion (NAPPED) consortium. Genotype correlates with the natural history of severe bile salt export pump deficiency. J Hepatol. 2020;73(1):84–93. doi: 10.1016/j.jhep.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Felzen A., van Wessel D.B.E., Gonzales E., et al. NAtural course and Prognosis of PFIC and Effect of biliary Diversion (NAPPED) Consortium. Genotype-phenotype relationships of truncating mutations, p.E297G and p.D482G in bile salt export pump deficiency. JHEP Rep. 2022;5(2) doi: 10.1016/j.jhepr.2022.100626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nikeghbalian S., Malekhosseini S.A., Kazemi K., et al. The largest single center report on paediatric liver transplantation: experiences and lessons learned. Ann Surg. 2021;273(2):e70–e72. doi: 10.1097/SLA.0000000000004047. [DOI] [PubMed] [Google Scholar]
- 35.Bull L.N., Pawlikowska L., Strautnieks S., et al. Outcomes of surgical management of familial intrahepatic cholestasis 1 and bile salt export protein deficiencies. Hepatol Commun. 2018;2(5):515–528. doi: 10.1002/hep4.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baumann U., Sturm E., Lacaille F., et al. Effects of odevixibat on pruritus and bile acids in children with cholestatic liver disease: phase 2 study. Clin Res Hepatol Gastroenterol. 2021;45(5) doi: 10.1016/j.clinre.2021.101751. [DOI] [PubMed] [Google Scholar]
- 37.Thompson R.J., Arnell H., Artan R., et al. Odevixibat treatment in progressive familial intrahepatic cholestasis: a randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol. 2022;7(9):830–842. doi: 10.1016/S2468-1253(22)00093-0. [DOI] [PubMed] [Google Scholar]
- 38.Loomes K.M., Squires R.H., Kelly D., et al. Maralixibat for the treatment of PFIC: long-term, IBAT inhibition in an open-label, Phase 2 study. Hepatol Commun. 2022;6(9):2379–2390. doi: 10.1002/hep4.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slavetinsky C., Sturm E. Odevixibat and partial external biliary diversion showed equal improvement of cholestasis in a patient with progressive familial intrahepatic cholestasis. BMJ Case Rep. 2020;13(6) doi: 10.1136/bcr-2019-234185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deeks E.D. Odevixibat: first approval. Drugs. 2021;81:1781–1786. doi: 10.1007/s40265-021-01594-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bylvay US Prescribing Information; 2023. [Google Scholar]
- 42.Efficacy and safety of odevixibat in children with Biliary Atresia who have undergone a Kasai HPE (BOLD) NCT04336722. 2022. https://clinicaltrials.gov/ct2/show/NCT04336722 [Google Scholar]
- 43.Shriley M. Maralixibat: first approval. Drugs. 2022;82:71–76. doi: 10.1007/s40265-021-01649-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.A study to evaluate the efficacy and safety of maralixibat in subjects with progressive familial cholestasis (MARCH-PFIC) NCT03905330. 2022. https://clinicaltrials.gov/ct2/show/NCT03905330
- 45.MRX-800 A long-term safety study of maralixibat in the treatment of cholestatic liver disease in subjects who previously participated in a maralixibat study (MERGE) NCT04168385. 2023. https://www.clinicaltrials.gov/ct2/show/NCT04168385
- 46.Study of elobixibat A3309) in patients with chronic idiopathic constipation (ACCESS) NCT01007123. 2017. https://clinicaltrials.gov/ct2/show/NCT01007123
- 47.A Phase 2 study of elobixibat in adults with NAFLD or NASH NCT04006145. 2021. https://clinicaltrials.gov/ct2/show/NCT04006145 [Google Scholar]
- 48.A study investigating safety, tolerability, pharmacokinetics and pharmacodynamics of GSK2330672 administered with Metformin to type 2 diabetes patients NCT02202161. 2017. https://clinicaltrials.gov/ct2/show/NCT02202161
- 49.Linerixibat long-term safety and tolerability study (LLSAT) NCT04167358. 2023. https://clinicaltrials.gov/ct2/show/NCT04167358
- 50.Dose response study of GSK2330672 for the treatment of pruritus in participants with primary biliary cholangitis NCT02966834. 2021. https://clinicaltrials.gov/ct2/show/NCT02966834
- 51.Volixibat (SHP626) in the treatment of adults with non-alcoholic steatohepatitis (NASH) NCT02787304. https://clinicaltrials.gov/ct2/show/NCT02787304
- 52.A study to evaluate efficacy and safety of an investigational drug named volixibat in patients with itching caused by primary sclerosing cholangitis (PSC) (VISTAS) NCT04663308. 2023. https://clinicaltrials.gov/ct2/show/NCT04663308
- 53.Zhao X., Zhang W., Vig P., et al. Serum bile acid profiling and mixed model analysis reveal biomarkers associated with pruritus reduction in maralixibat-treated patients with BSEP deficiency. Metabolites. 2022;12(10):952. doi: 10.3390/metabo12100952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shiau H., Guffey D., Loomes K.M., et al. Biopsy validated study of Biomarkers for liver fibrosis and transplant prediction in inherited cholestasis. Hepatol Commun. 2020;4(10):1516–1526. doi: 10.1002/hep4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bosonnet L. Pruritus: scratching the surface. Eur J Cancer Care (Engl) 2003;12:162–165. doi: 10.1046/j.1365-2354.2003.00395.x. [DOI] [PubMed] [Google Scholar]
- 56.Mighiu C., O'Hara S., Ferri Grazzi E., et al. Impact of progressive familial intrahepatic cholestasis on caregivers: caregiver-reported outcomes from the multinational PICTURE study. Orphanet J Rare Dis. 2022;17(1):32. doi: 10.1186/s13023-022-02177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masahata K., Ueno T., Bessho K., et al. Clinical outcomes of surgical management for rare types of progressive familial intrahepatic cholestasis: a case series. Surg Case Rep. 2022;8(1):10. doi: 10.1186/s40792-022-01365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gwaltney C., Ivanescu C., Karlsson L., et al. Validation of the PRUCISION instruments in pediatric patients with progressive familial intrahepatic cholestasis. Adv Ther. 2022;39(11):5105–5125. doi: 10.1007/s12325-022-02262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fawaz R., Baumann U., Ekong U., et al. Guideline for the evaluation of cholestatic jaundice in infants: joint recommendations of the north American society for pediatric gastroenterology, Hepatology, and nutrition and the European society for pediatric gastroenterology, Hepatology, and nutrition. J Pediatr Gastroenterol Nutr. 2017;64(1):154–168. doi: 10.1097/MPG.0000000000001334. [DOI] [PubMed] [Google Scholar]
- 60.Marrero J.A., Kulik L.M., Sirlin C.B., et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatol. 2018;68(2):723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
:
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.