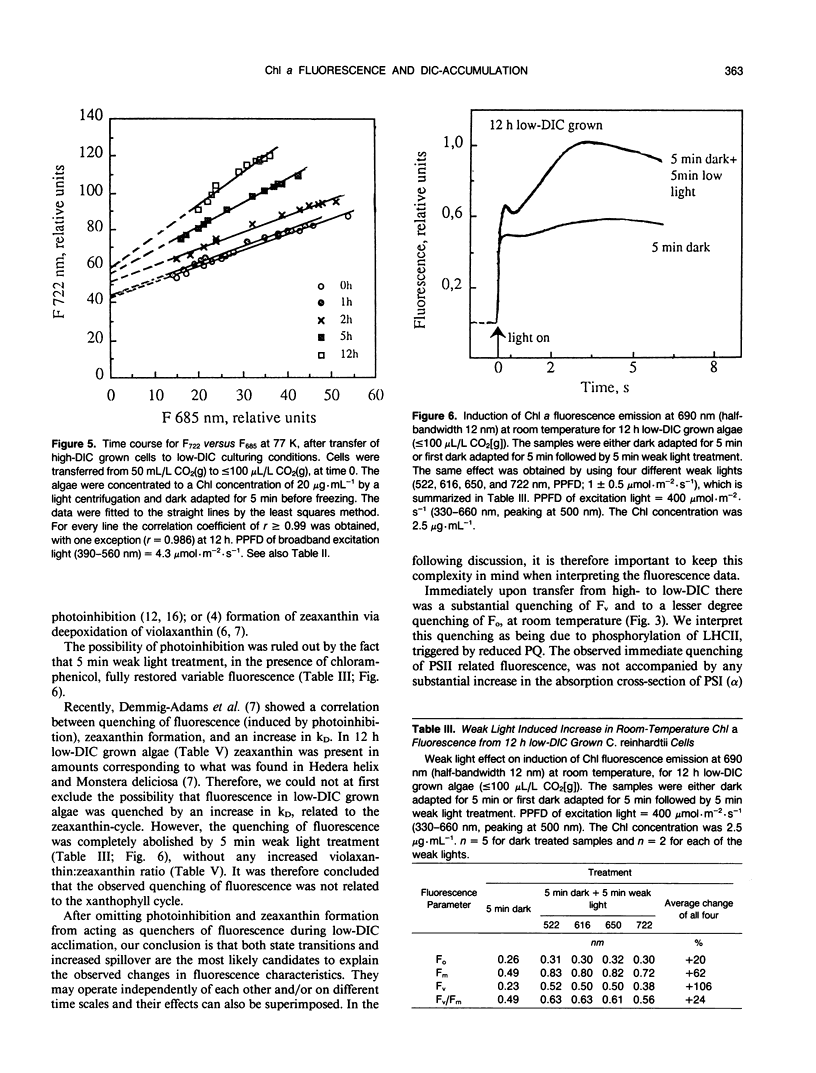
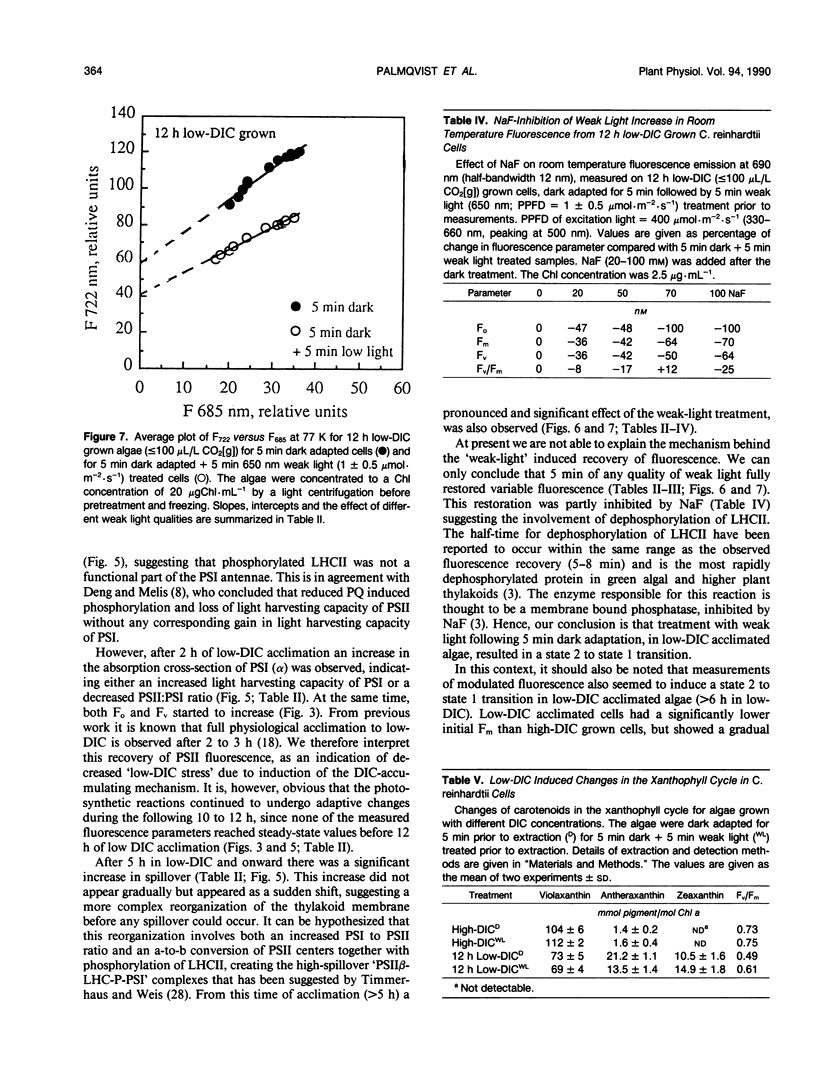
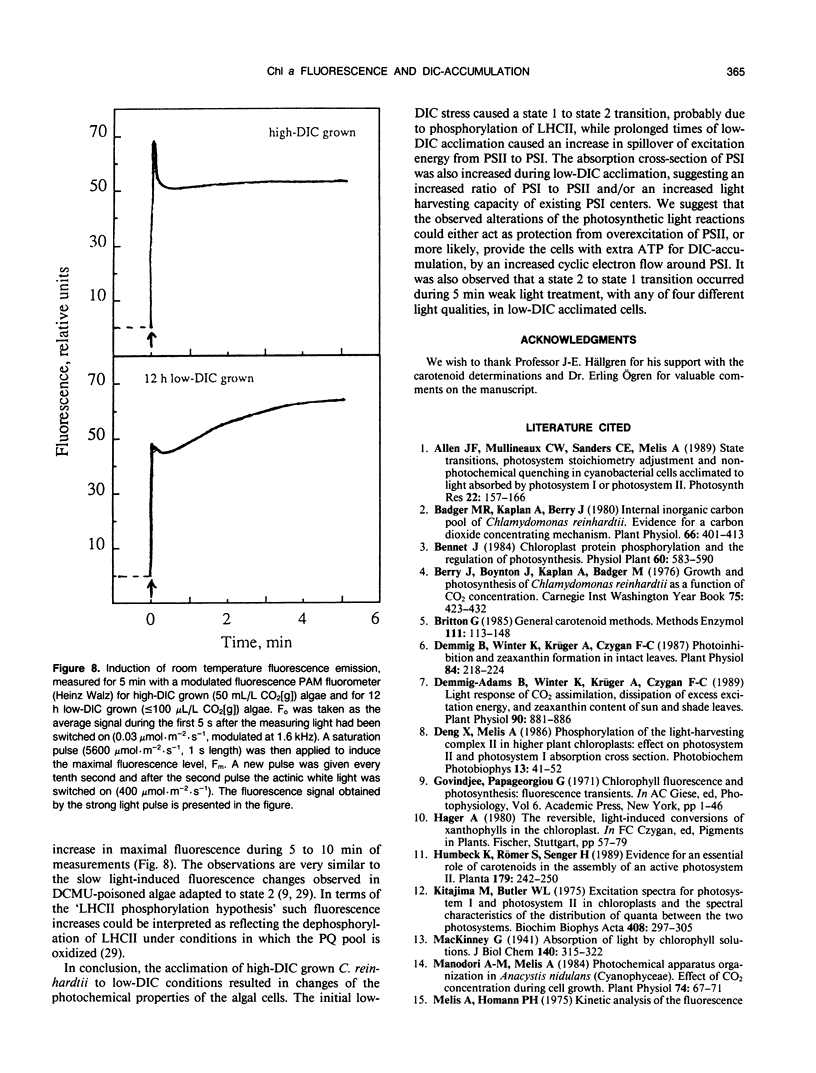
Abstract
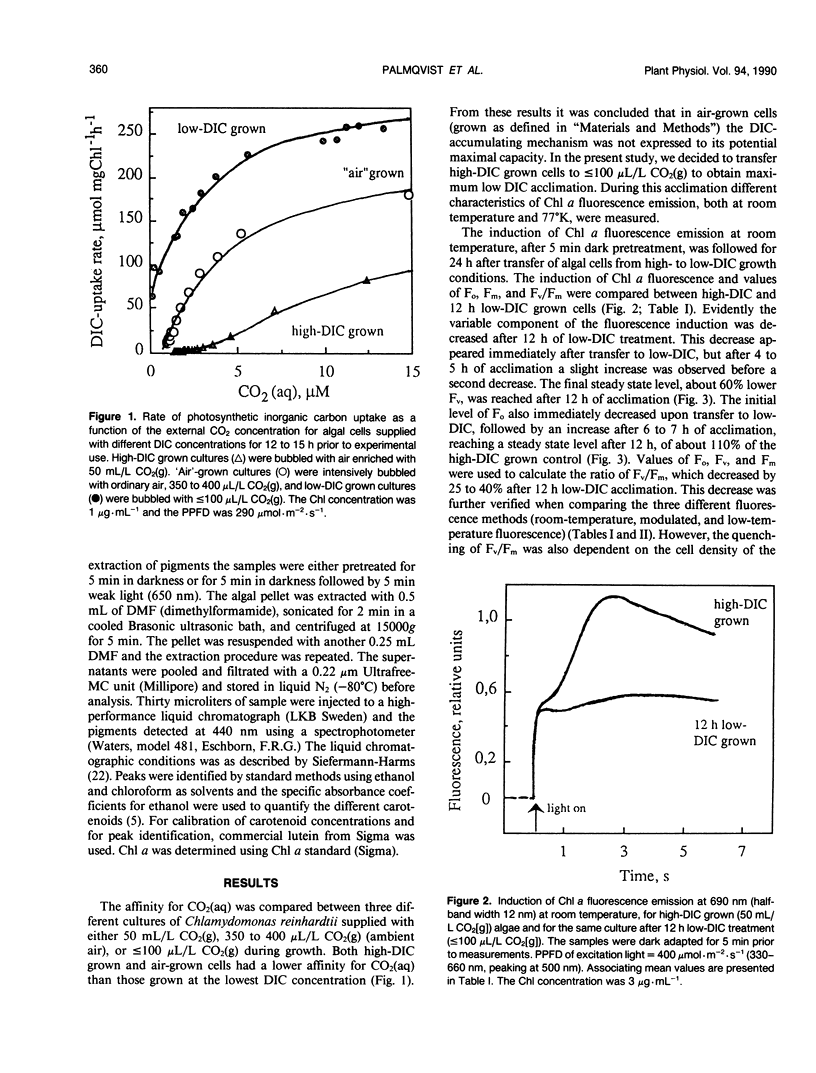
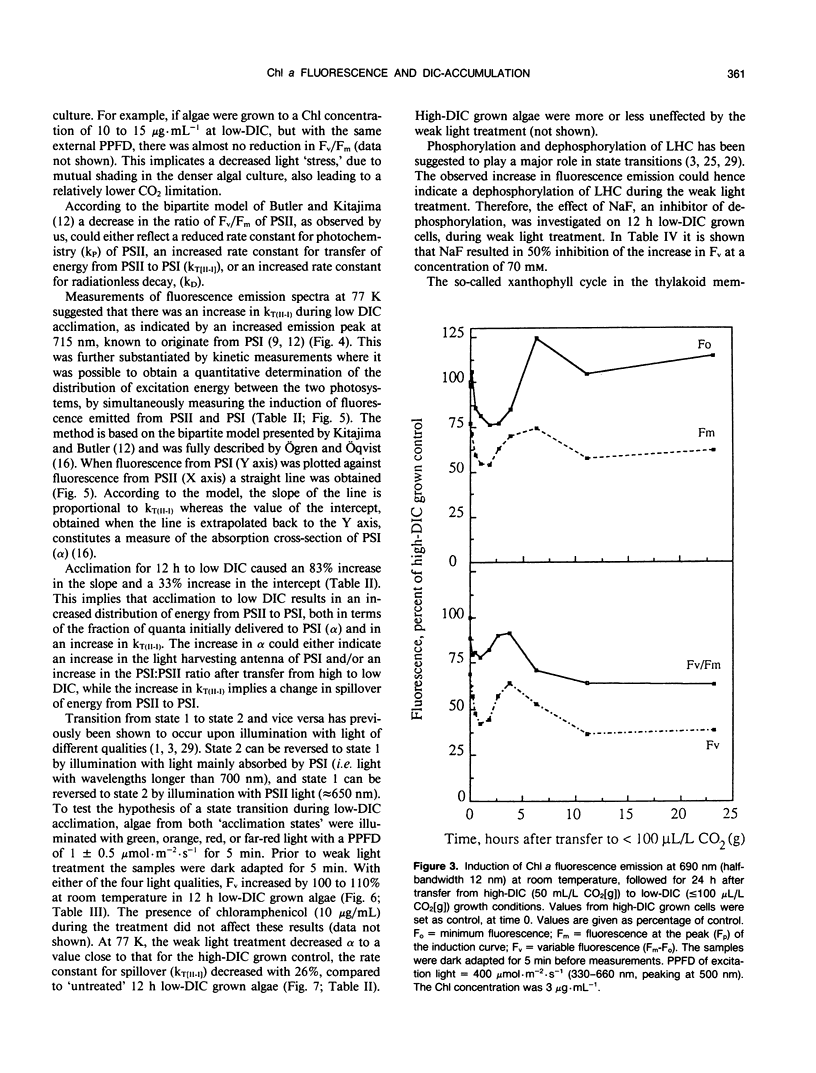
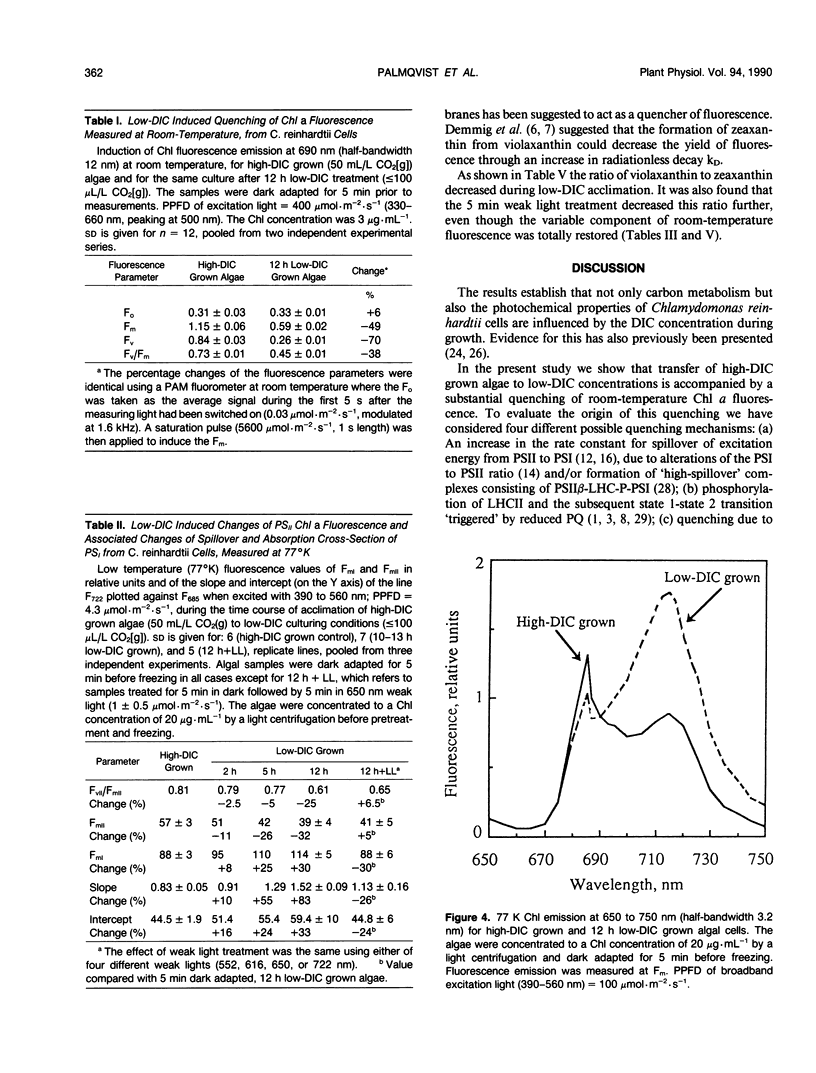
Cells of the unicellular green algae Chlamydomonas reinhardtii were grown in high dissolved inorganic carbon (DIC) concentrations (supplied with 50 milliliters per liter CO2[g]) and transferred to low DIC concentrations (supplied with ≤ 100 microliters per liter CO2[g]). Immediately after transfer from high to low DIC the emission of photosystem II related chlorophyll a fluorescence was substantially quenched. It is hypothesized that the suddenly induced inorganic carbon limitation of photosynthesis resulted in a phosphorylation of LHCII, leading to the subsequent state 1 to state 2 transition. After 2 hours of low-DIC acclimation, 77 K fluorescence measurements revealed an increase in the fluorescence emitted from photosystem I, due to direct excitation, suggesting a change in photosystem II/photosystem I stoichiometry or an increased light harvesting capacity of photosystem I. After 5 to 6 hours of acclimation a considerable increase in spillover from photosystem II to photosystem I was observed. These adjustments of the photosynthetic light reactions reached steady-state after about 12 hours of low DIC treatment. The quencher of fluorescence could be removed by 5 minutes of dark treatment followed by 5 minutes of weak light treatment, of any of four different light qualities. It is hypothesized that this restoration of fluorescence was due to a state 2 to state 1 transition in low-DIC acclimated cells. A decreased ratio of violaxanthin to zeaxanthin was also observed in 12 hour low DIC treated cells, compared with high DIC grown cells. This ratio was not coupled to the level of fluorescence quenching. The role of different processes during the induction of a DIC accumulating mechanism is discussed.
Full text
PDF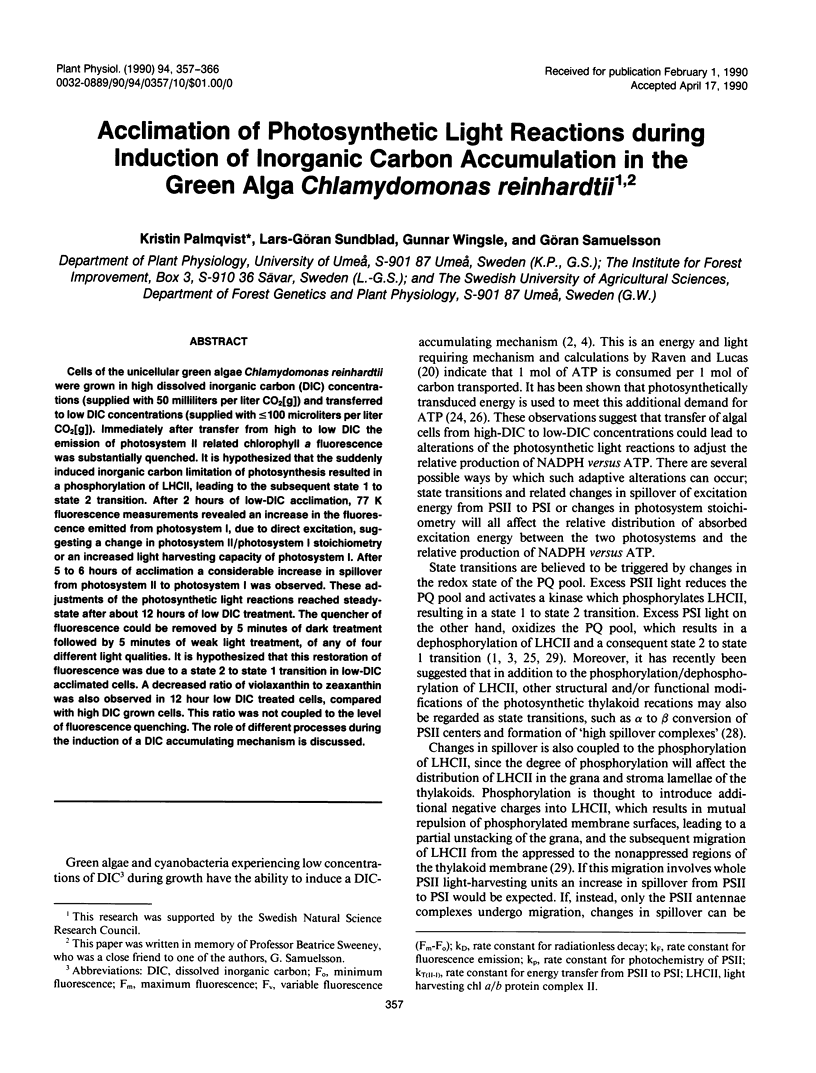



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Kaplan A., Berry J. A. Internal Inorganic Carbon Pool of Chlamydomonas reinhardtii: EVIDENCE FOR A CARBON DIOXIDE-CONCENTRATING MECHANISM. Plant Physiol. 1980 Sep;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton G. General carotenoid methods. Methods Enzymol. 1985;111:113–149. doi: 10.1016/s0076-6879(85)11007-4. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B., Winter K., Krüger A., Czygan F. C. Light Response of CO(2) Assimilation, Dissipation of Excess Excitation Energy, and Zeaxanthin Content of Sun and Shade Leaves. Plant Physiol. 1989 Jul;90(3):881–886. doi: 10.1104/pp.90.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig B., Winter K., Krüger A., Czygan F. C. Photoinhibition and zeaxanthin formation in intact leaves : a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol. 1987 Jun;84(2):218–224. doi: 10.1104/pp.84.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Butler W. L. Excitation spectra for photosystem I and photosystem II in chloroplasts and the spectral characteristics of the distributions of quanta between the two photosystems. Biochim Biophys Acta. 1975 Dec 11;408(3):297–305. doi: 10.1016/0005-2728(75)90131-0. [DOI] [PubMed] [Google Scholar]
- Manodori A., Melis A. Photochemical Apparatus Organization in Anacystis nidulans (Cyanophyceae) : Effect of CO(2) Concentration during Cell Growth. Plant Physiol. 1984 Jan;74(1):67–71. doi: 10.1104/pp.74.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist K., Sjöberg S., Samuelsson G. Induction of Inorganic Carbon Accumulation in the Unicellular Green Algae Scenedesmus obliquus and Chlamydomonas reinhardtii. Plant Physiol. 1988 Jun;87(2):437–442. doi: 10.1104/pp.87.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumley F. G., Schmidt G. W. Reconstitution of chlorophyll a/b light-harvesting complexes: Xanthophyll-dependent assembly and energy transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):146–150. doi: 10.1073/pnas.84.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]


