Abstract
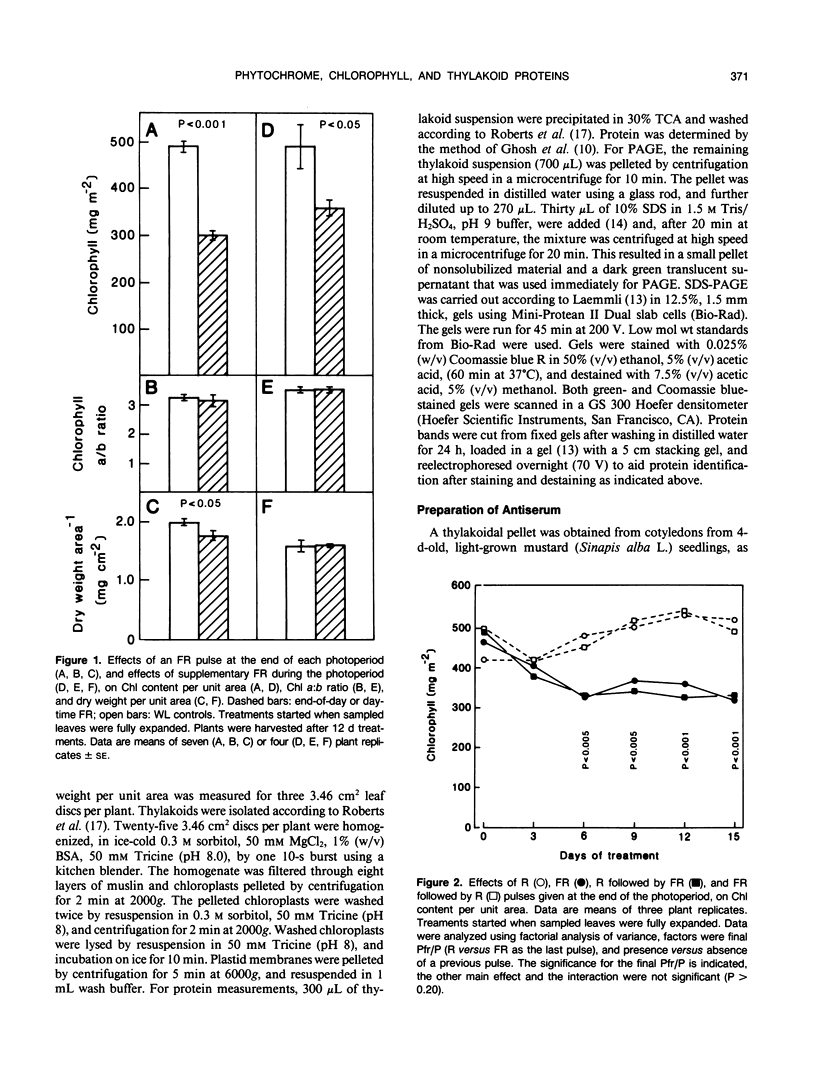
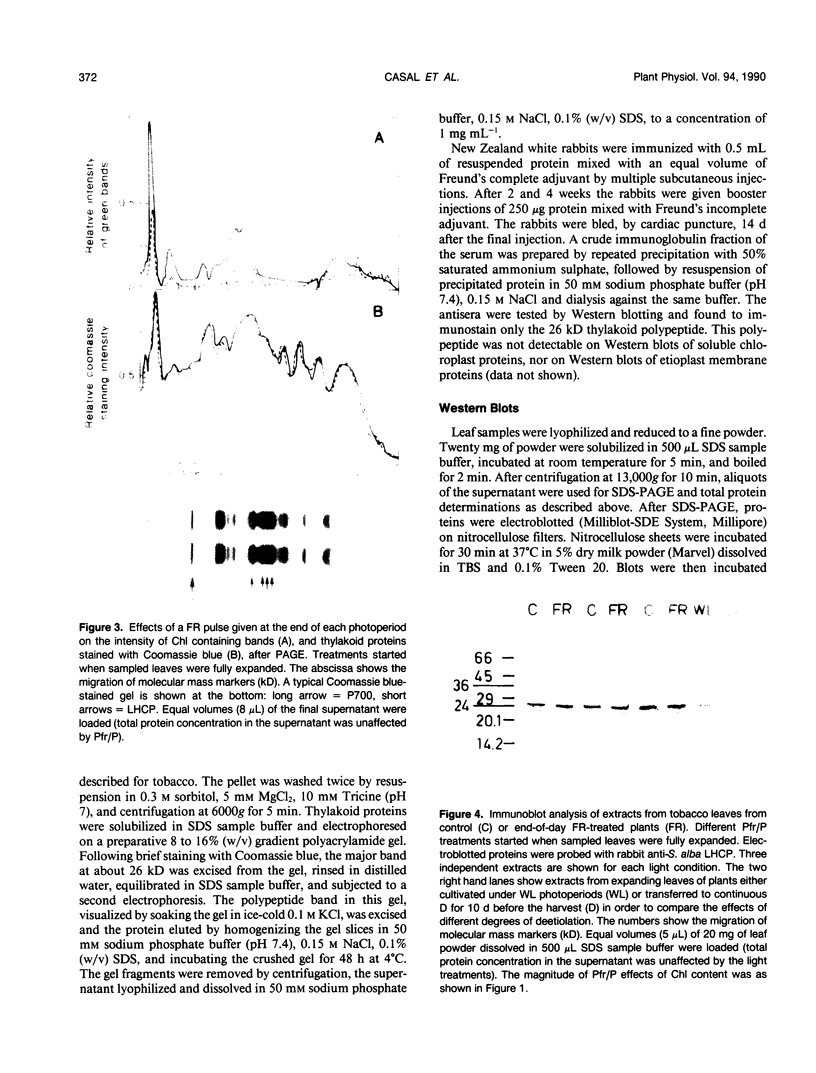
The effects of phytochrome status on chlorophyll content and on steady-state levels of thylakoid proteins were investigated in green leaves of Nicotiana tabacum L. plants grown under white light. Far-red light given either as a pulse at the end of each photoperiod, or as a supplement to white light during the photoperiod, reduced chlorophyll content per unit area and per unit dry weight. These differences were also observed after resolving chlorophyll-containing polypeptides by gel electrophoresis. Chlorophyll a:b ratio was unchanged. Both Coomassie blue-stained gels and immunochemical analyses showed that, in contrast to the observations in etiolated barley (K Apel, K Kloppstech [1980] Planta 150: 426-430) and pea (J Bennett [1981] Eur J Biochem 118: 61-70) seedlings, and in etiolated tobacco leaves (this report), in fully deetiolated tobacco plants changes in chlorophyll content were not correlated with obvious changes in the steady-state levels of thylakoid proteins (e.g. light-harvesting, chlorophyll a/b-binding proteins).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. Biosynthesis of the light-harvesting chlorophyll a/b protein. Polypeptide turnover in darkness. Eur J Biochem. 1981 Aug;118(1):61–70. doi: 10.1111/j.1432-1033.1981.tb05486.x. [DOI] [PubMed] [Google Scholar]
- Chory J., Peto C. A., Ashbaugh M., Saganich R., Pratt L., Ausubel F. Different Roles for Phytochrome in Etiolated and Green Plants Deduced from Characterization of Arabidopsis thaliana Mutants. Plant Cell. 1989 Sep;1(9):867–880. doi: 10.1105/tpc.1.9.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuming A. C., Bennett J. Biosynthesis of the light-harvesting chlorophyll a/b protein. Control of messenger RNA activity by light. Eur J Biochem. 1981 Aug;118(1):71–80. doi: 10.1111/j.1432-1033.1981.tb05487.x. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Gepstein S., Heikkila J. J., Dumbroff E. B. Use of a scanning densitometer or an ELISA plate reader for measurement of nanogram amounts of protein in crude extracts from biological tissues. Anal Biochem. 1988 Mar;169(2):227–233. doi: 10.1016/0003-2697(88)90278-3. [DOI] [PubMed] [Google Scholar]
- Inskeep W. P., Bloom P. R. Extinction coefficients of chlorophyll a and B in n,n-dimethylformamide and 80% acetone. Plant Physiol. 1985 Feb;77(2):483–485. doi: 10.1104/pp.77.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasperbauer M. J., Peaslee D. E. Morphology and Photosynthetic Efficiency of Tobacco Leaves That Received End-of-Day Red and Far Red Light during Development. Plant Physiol. 1973 Nov;52(5):440–442. doi: 10.1104/pp.52.5.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Remy R., Hoarau J., Leclerc J. C. Electrophoretic and spectrophotometric studies of chlorophyll-protein complexes from tobacco chloroplasts. Isolation of a light harvesting pigment protein complex with a molecular weight of 70,000. Photochem Photobiol. 1977 Aug;26(2):151–158. doi: 10.1111/j.1751-1097.1977.tb07466.x. [DOI] [PubMed] [Google Scholar]
- Sagar A. D., Horwitz B. A., Elliott R. C., Thompson W. F., Briggs W. R. Light effects on several chloroplast components in norflurazon-treated pea seedlings. Plant Physiol. 1988 Oct;88(2):340–347. doi: 10.1104/pp.88.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satter R. L., Wetherell D. F. Photomorphogenesis in Sinningia speciosa, cv. Queen Victoria II. Stem Elongation: Interaction of a Phytochrome Controlled Process and a Red-requiring, Energy Dependent Reaction. Plant Physiol. 1968 Jun;43(6):961–967. doi: 10.1104/pp.43.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]