Abstract
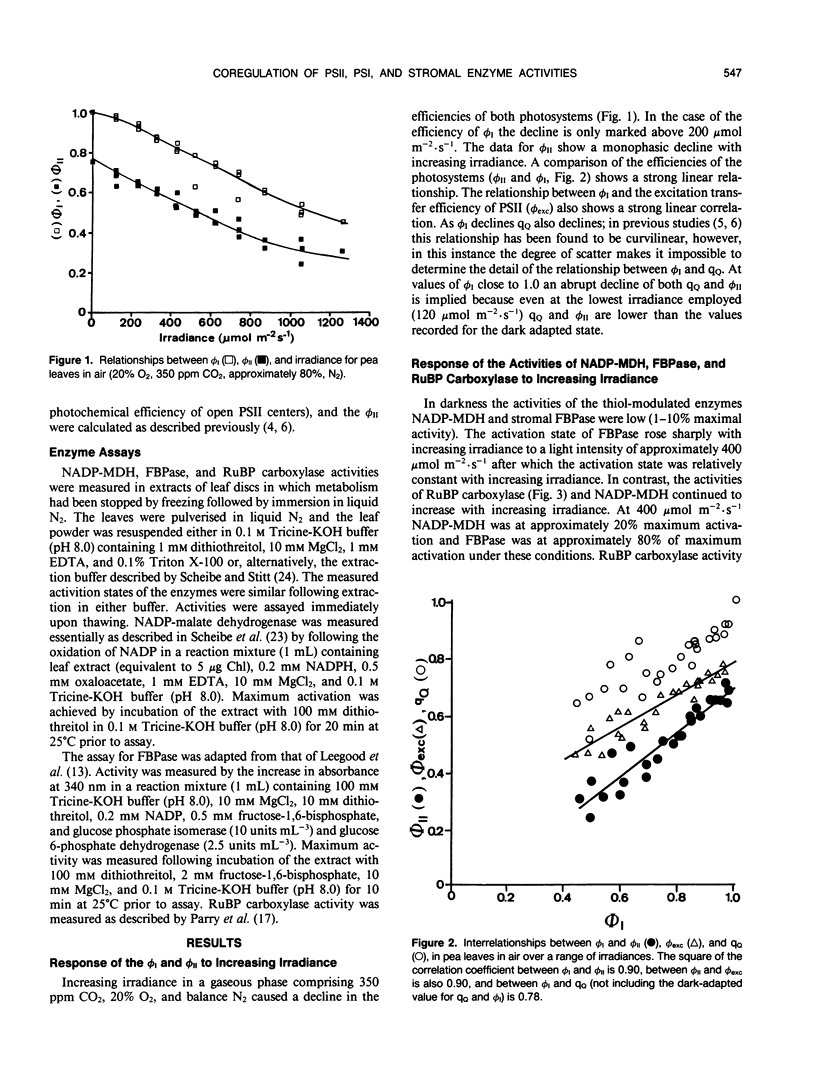
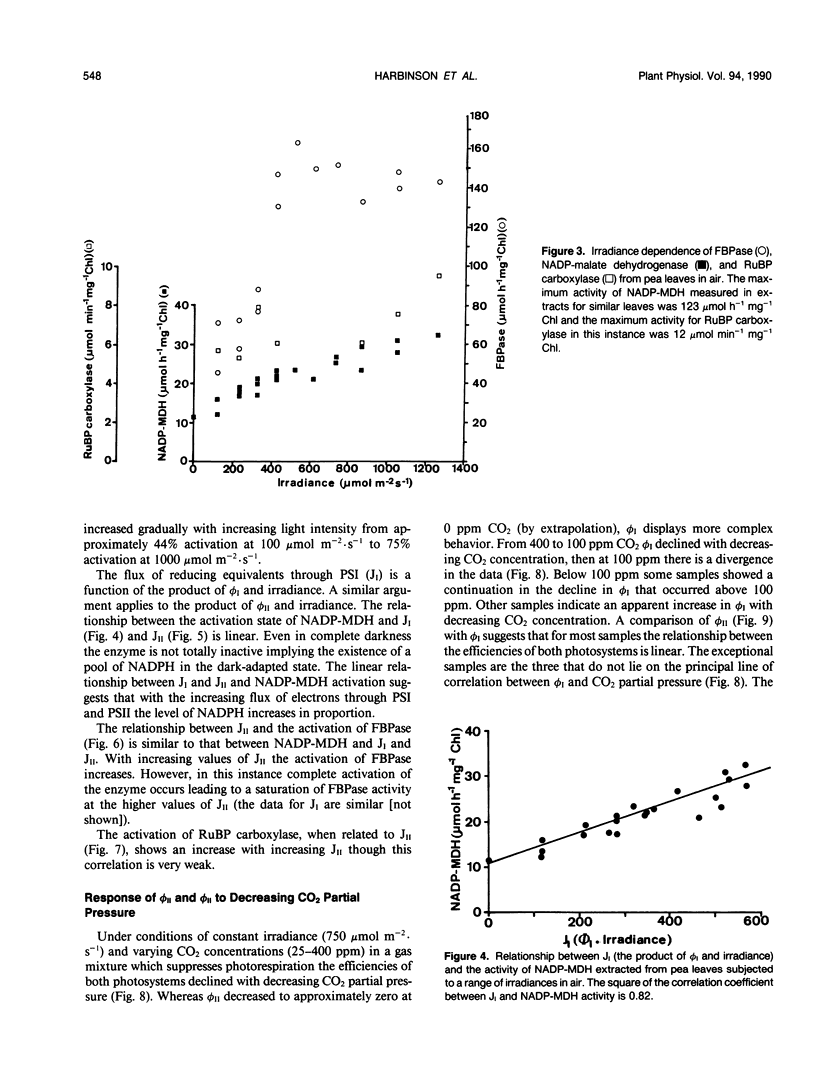
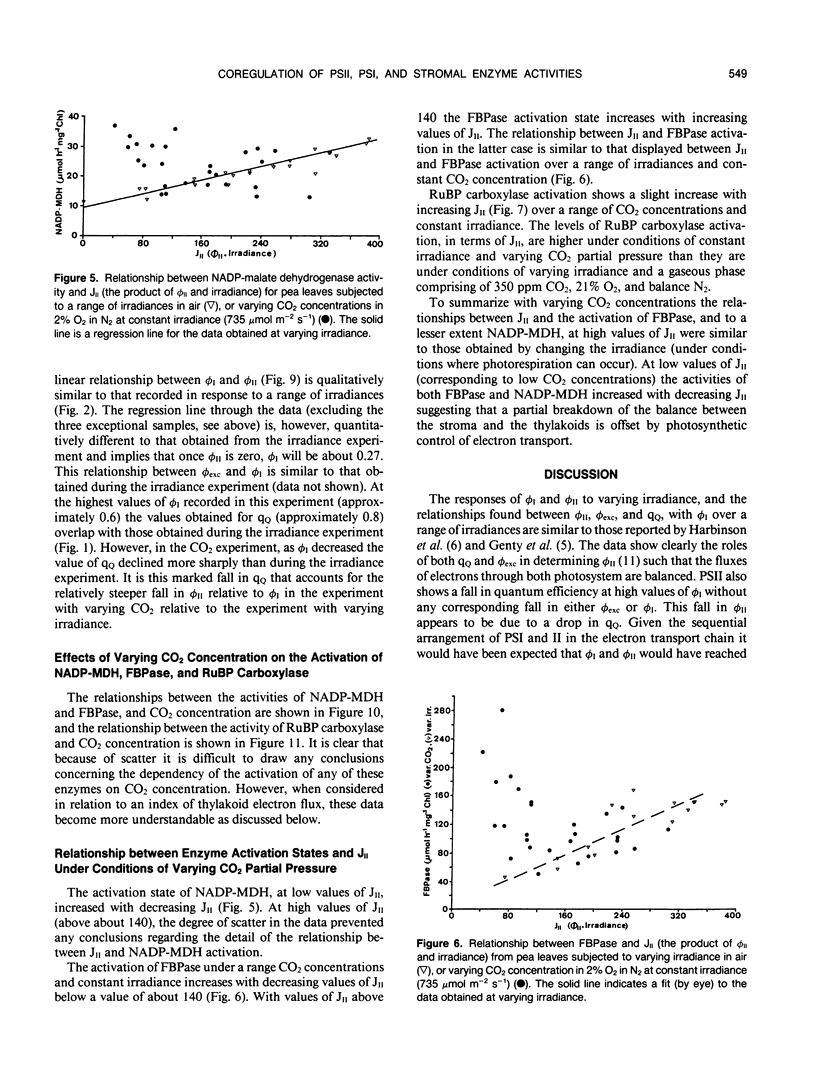
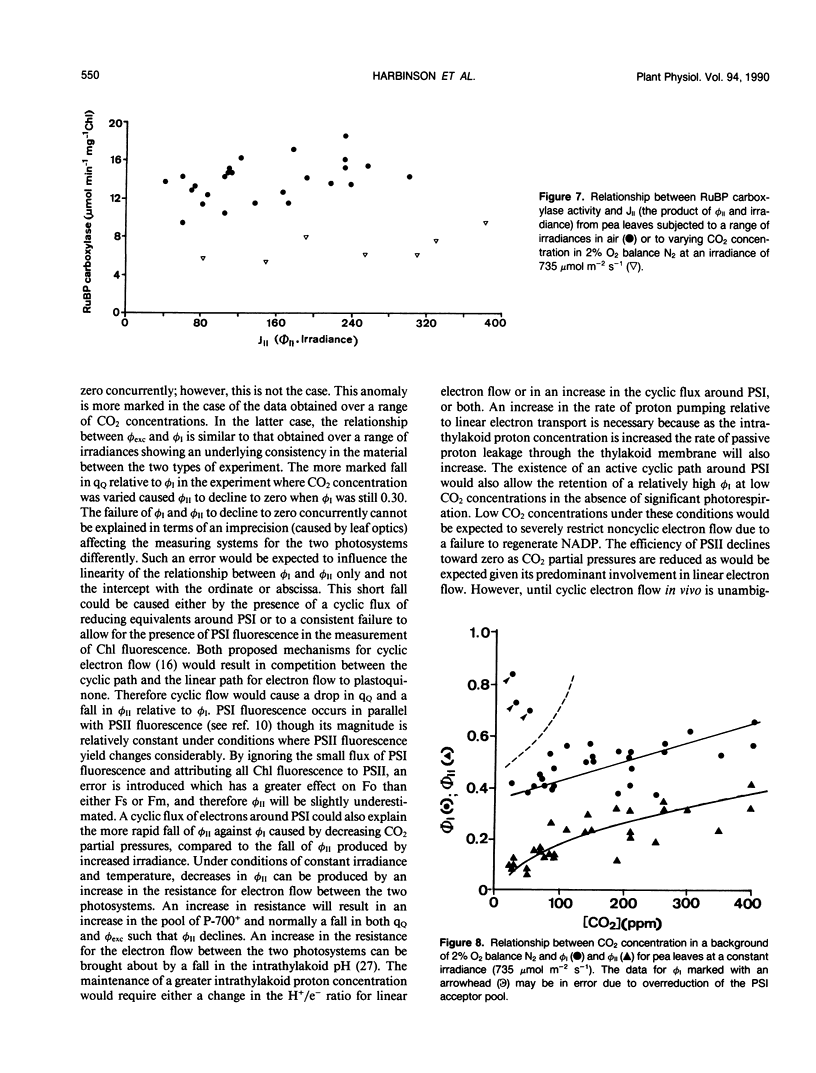
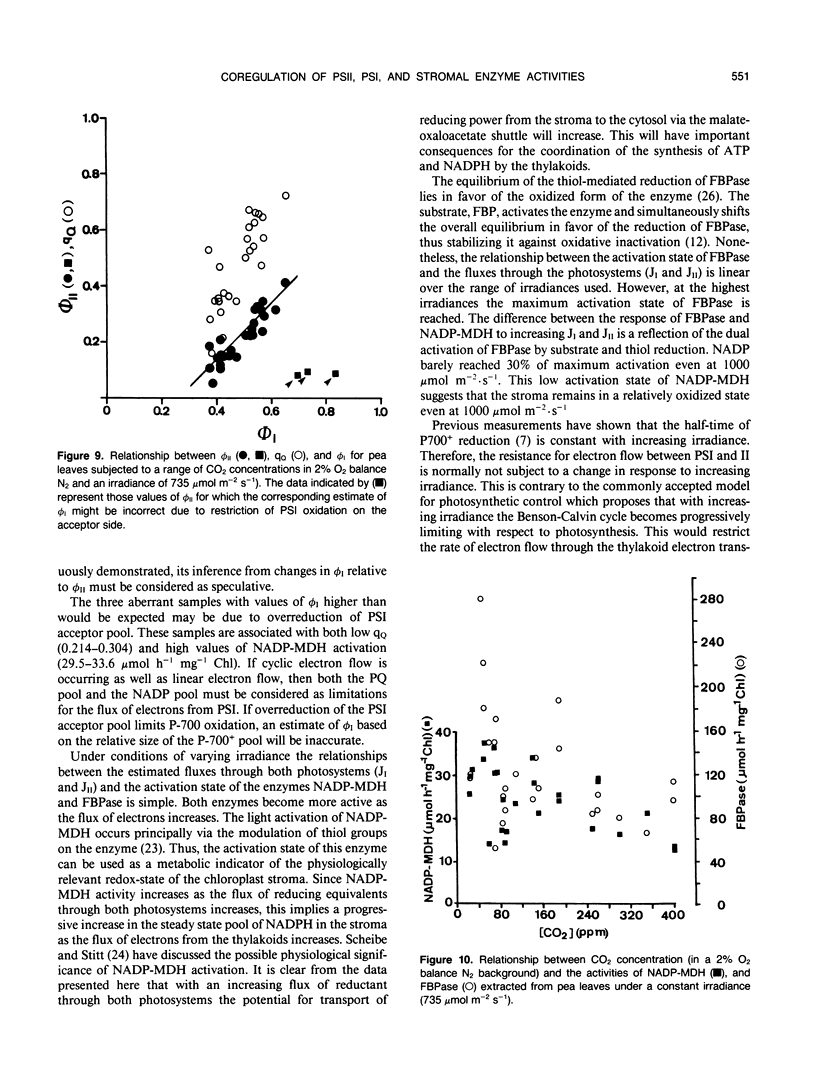
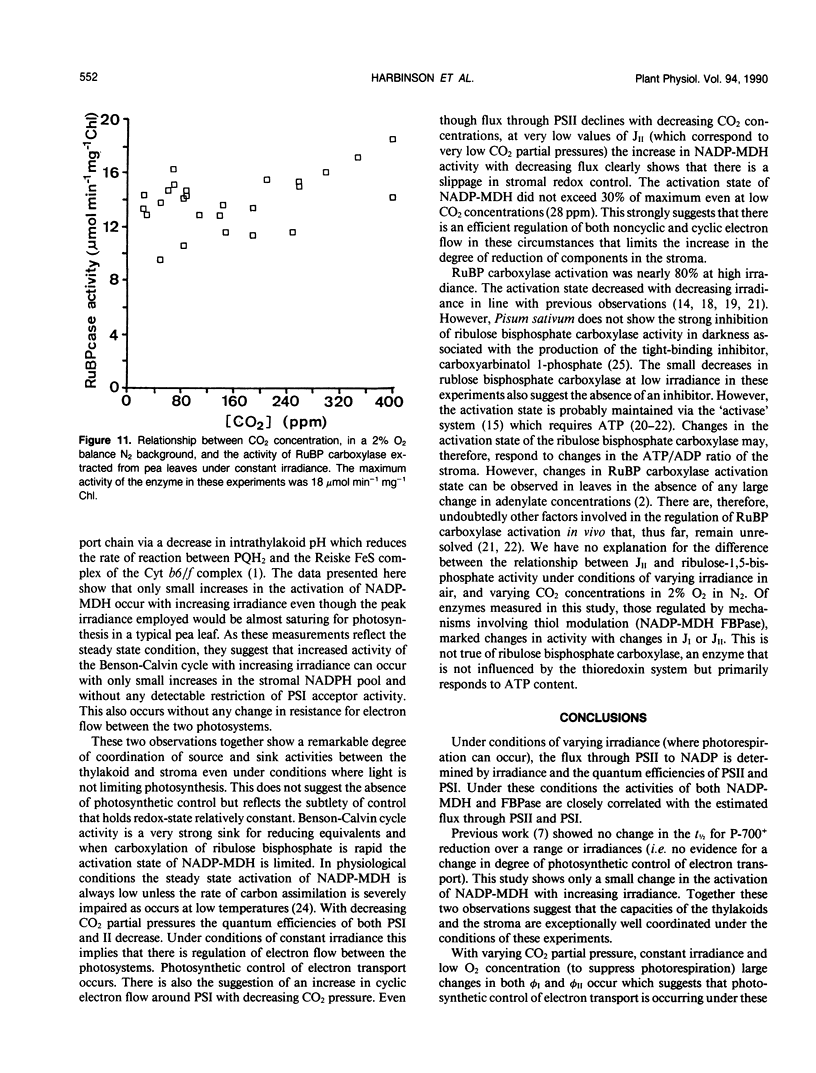
The responses of the quantum efficiencies of photosystem (PS) II and PSI measured in vivo simultaneously with estimations of the activities and activation states of NADP-malate dehydrogenase, chloroplast fructose-1,6-bisphosphatase, and ribulose-1,5-bisphosphate carboxylase were used to study the relationship between electron transport and carbon metabolism. The effects of varying irradiance and CO2 partial pressure on the relationship between the quantum efficiencies of PSI and II, and the activity of these enzymes shows that the interrelationships vary according to the limitations placed on the system. The relationship between the quantum efficiencies of PSII and PSI was linear in most situations. In response to increasing irradiance, the activity of all three enzymes increased. In the case of NADP-malate dehydrogenase this increase was well correlated with the estimated flux of electrons through PSI and PSII. The other two enzymes showed a more complex relationship with the estimated flux of electrons through both photosystems. These relationships are consistent with the known interactions between these stromal enzymes and the thylakoids. The response to varying CO2 partial pressure is more complex. The efficiencies of PSI and II declined with decreasing CO2 partial pressure and the activity of each enzyme varied uniquely. However, there are clear correlations between the activities of the enzymes and the flux of electrons through the photosystems. In contrast to the data obtained under conditions of varying irradiance, there is clear evidence of photosynthetic control of electron transport when the CO2 concentration is varied.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks A., Portis A. R., Sharkey T. D. Effects of Irradiance and Methyl Viologen Treatment on ATP, ADP, and Activation of Ribulose Bisphosphate Carboxylase in Spinach Leaves. Plant Physiol. 1988 Nov;88(3):850–853. doi: 10.1104/pp.88.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford N. A., Droux M., Kosower N. S., Buchanan B. B. Evidence for function of the ferredoxin/thioredoxin system in the reductive activation of target enzymes of isolated intact chloroplasts. Arch Biochem Biophys. 1989 May 15;271(1):223–239. doi: 10.1016/0003-9861(89)90273-7. [DOI] [PubMed] [Google Scholar]
- Harbinson J., Genty B., Baker N. R. Relationship between the Quantum Efficiencies of Photosystems I and II in Pea Leaves. Plant Physiol. 1989 Jul;90(3):1029–1034. doi: 10.1104/pp.90.3.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry M. A., Keys A. J., Foyer C. H., Furbank R. T., Walker D. A. Regulation of ribulose-1,5-bisphosphate carboxylase activity by the activase system in lysed spinach chloroplasts. Plant Physiol. 1988 Jul;87(3):558–561. doi: 10.1104/pp.87.3.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchorowicz J. T., Jensen R. G. Photosynthesis and Activation of Ribulose Bisphosphate Carboxylase in Wheat Seedlings : Regulation by CO(2) and O(2). Plant Physiol. 1983 Apr;71(4):955–960. doi: 10.1104/pp.71.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchorowicz J. T., Raynes D. A., Jensen R. G. Light limitation of photosynthesis and activation of ribulose bisphosphate carboxylase in wheat seedlings. Proc Natl Acad Sci U S A. 1981 May;78(5):2985–2989. doi: 10.1073/pnas.78.5.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Portis A. R. Involvement of stromal ATP in the light activation of ribulose-1,5-bisphosphate carboxylase/oxygenase in intact isolated chloroplasts. Plant Physiol. 1988 Jan;86(1):293–298. doi: 10.1104/pp.86.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci M. E., Portis A. R., Ogren W. L. Light and CO(2) Response of Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase Activation in Arabidopsis Leaves. Plant Physiol. 1986 Mar;80(3):655–659. doi: 10.1104/pp.80.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C., Parry M. A., Gutteridge S., Keys A. J. Species variation in the predawn inhibition of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Physiol. 1986 Dec;82(4):1161–1163. doi: 10.1104/pp.82.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulié J. M., Buc J., Meunier J. C., Pradel J., Ricard J. Molecular properties of chloroplastic thioredoxin f and the photoregulation of the activity of fructose 1,6-bisphosphatase. Eur J Biochem. 1981 Oct;119(3):497–502. doi: 10.1111/j.1432-1033.1981.tb05635.x. [DOI] [PubMed] [Google Scholar]
- Woodrow I. E., Murphy D. J., Latzko E. Regulation of stromal sedoheptulose 1,7-bisphosphatase activity by pH and Mg2+ concentration. J Biol Chem. 1984 Mar 25;259(6):3791–3795. [PubMed] [Google Scholar]


