Abstract
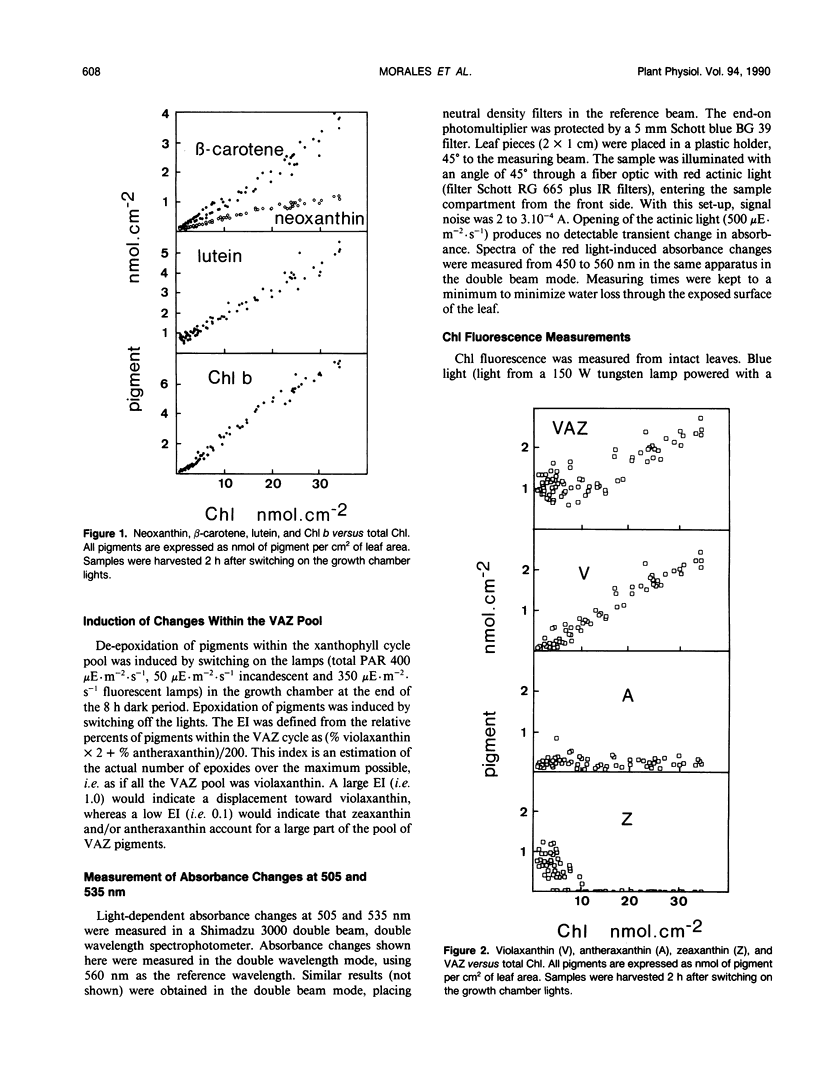
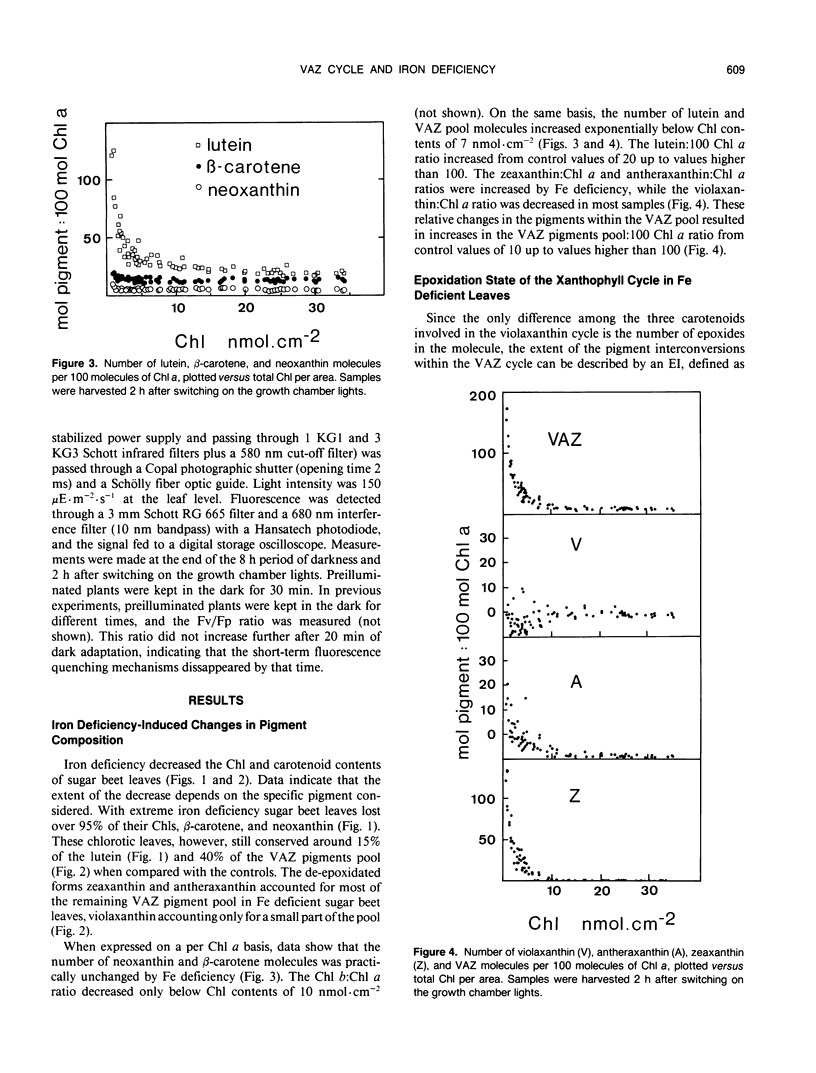
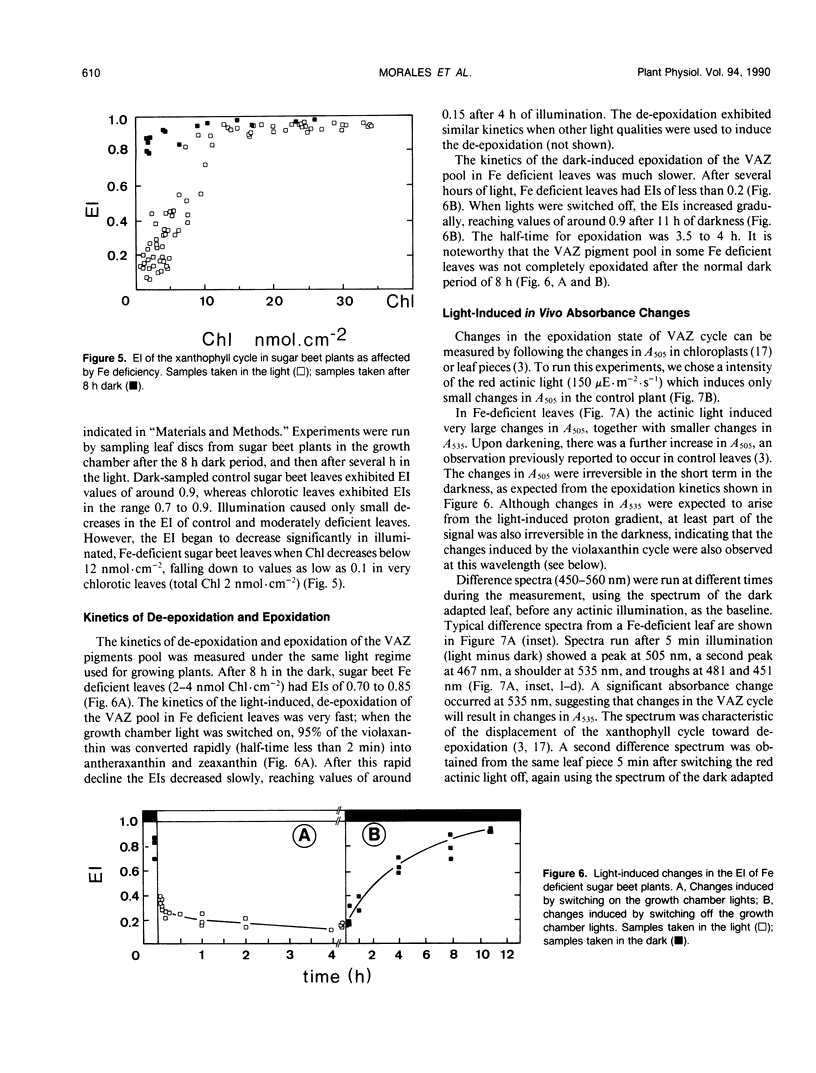
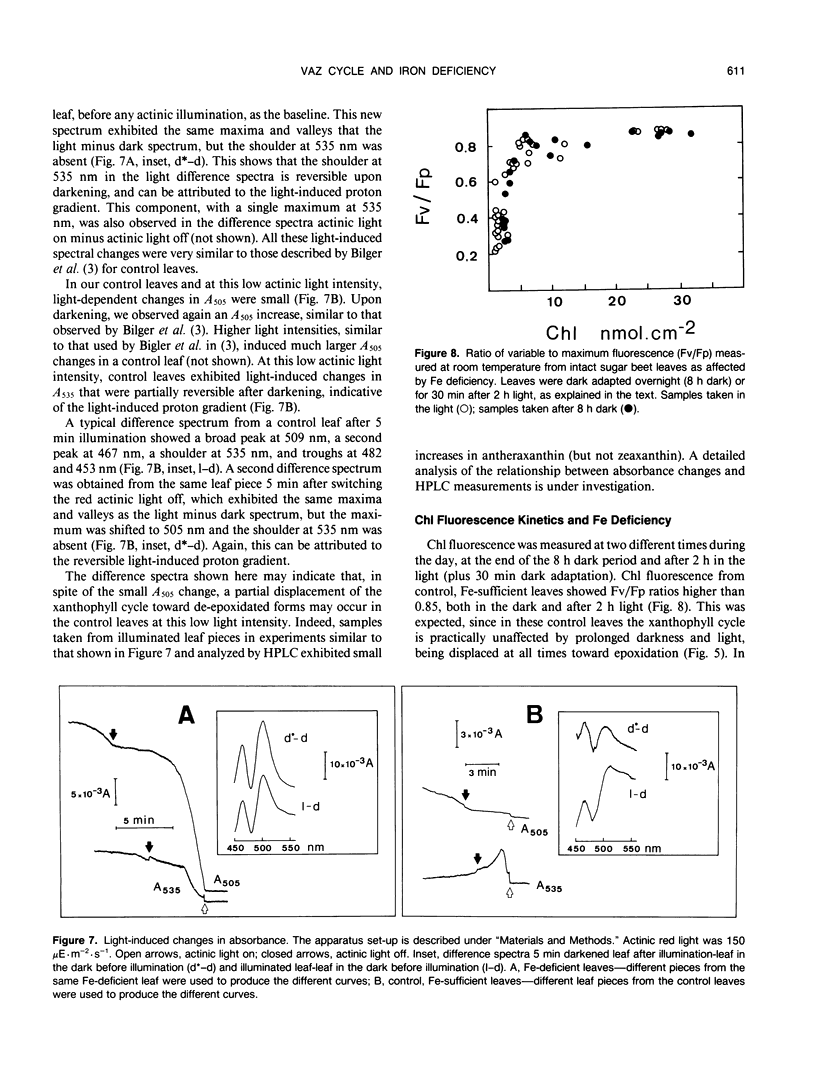
In this work we characterize the changes induced by iron deficiency in the pigment composition of sugar beet (Beta vulgaris L.) leaves. When sugar beet plants were grown hydroponically under limited iron supply, neoxanthin and β-carotene decreased concomitantly with chlorophyll a, whereas lutein and the carotenoids within the xanthophyll cycle were less affected. Iron deficiency caused major increases in the lutein/chlorophyll a and xanthophyll cycle pigments/chlorophyll a molar ratios. Xanthophyll cycle carotenoids in Fe-deficient plants underwent epoxidations and de-epoxidations in response to ambient light conditions. In dark adapted Fe-deficient plants most of the xanthophyll cycle pigment pool was in the epoxidated form violaxanthin. We show, both by HPLC and by in vivo 505 nanometers absorbance changes, that in Fe deficient plants and in response to light, the de-epoxidated forms antheraxanthin and zeaxanthin were rapidly formed at the expense of violaxanthin. Several hours after returning to dark, the xanthophyll cycle was shifted again toward violaxanthin. The ratio of variable to maximum chlorophyll fluorescence from intact leaves was decreased by iron deficiency. However, in iron deficient leaves this ratio was little affected by light conditions which displace the xanthophyll cycle toward epoxidation or de-epoxidation. This suggests that the functioning of the xanthophyll cycle is not necessarily linked to protection against excess light input.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilger W., Björkman O., Thayer S. S. Light-induced spectral absorbance changes in relation to photosynthesis and the epoxidation state of xanthophyll cycle components in cotton leaves. Plant Physiol. 1989 Oct;91(2):542–551. doi: 10.1104/pp.91.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B., Winter K., Krüger A., Czygan F. C. Zeaxanthin Synthesis, Energy Dissipation, and Photoprotection of Photosystem II at Chilling Temperatures. Plant Physiol. 1989 Jul;90(3):894–898. doi: 10.1104/pp.90.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B., Winter K., Krüger A., Czygan F. C. Zeaxanthin and the Induction and Relaxation Kinetics of the Dissipation of Excess Excitation Energy in Leaves in 2% O(2), 0% CO(2). Plant Physiol. 1989 Jul;90(3):887–893. doi: 10.1104/pp.90.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig B., Winter K., Krüger A., Czygan F. C. Photoinhibition and zeaxanthin formation in intact leaves : a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol. 1987 Jun;84(2):218–224. doi: 10.1104/pp.84.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig B., Winter K., Krüger A., Czygan F. C. Zeaxanthin and the Heat Dissipation of Excess Light Energy in Nerium oleander Exposed to a Combination of High Light and Water Stress. Plant Physiol. 1988 May;87(1):17–24. doi: 10.1104/pp.87.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N. Limiting Factors in Photosynthesis: I. USE OF IRON STRESS TO CONTROL PHOTOCHEMICAL CAPACITY IN VIVO. Plant Physiol. 1980 Jan;65(1):114–120. doi: 10.1104/pp.65.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H. Y., Kamite L., Wang Y. Y. An Ascorbate-induced Absorbance Change in Chloroplasts from Violaxanthin De-epoxidation. Plant Physiol. 1972 Feb;49(2):224–228. doi: 10.1104/pp.49.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Las Rivas J., Abadía A., Abadía J. A New Reversed Phase-HPLC Method Resolving All Major Higher Plant Photosynthetic Pigments. Plant Physiol. 1989 Sep;91(1):190–192. doi: 10.1104/pp.91.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]


