Abstract
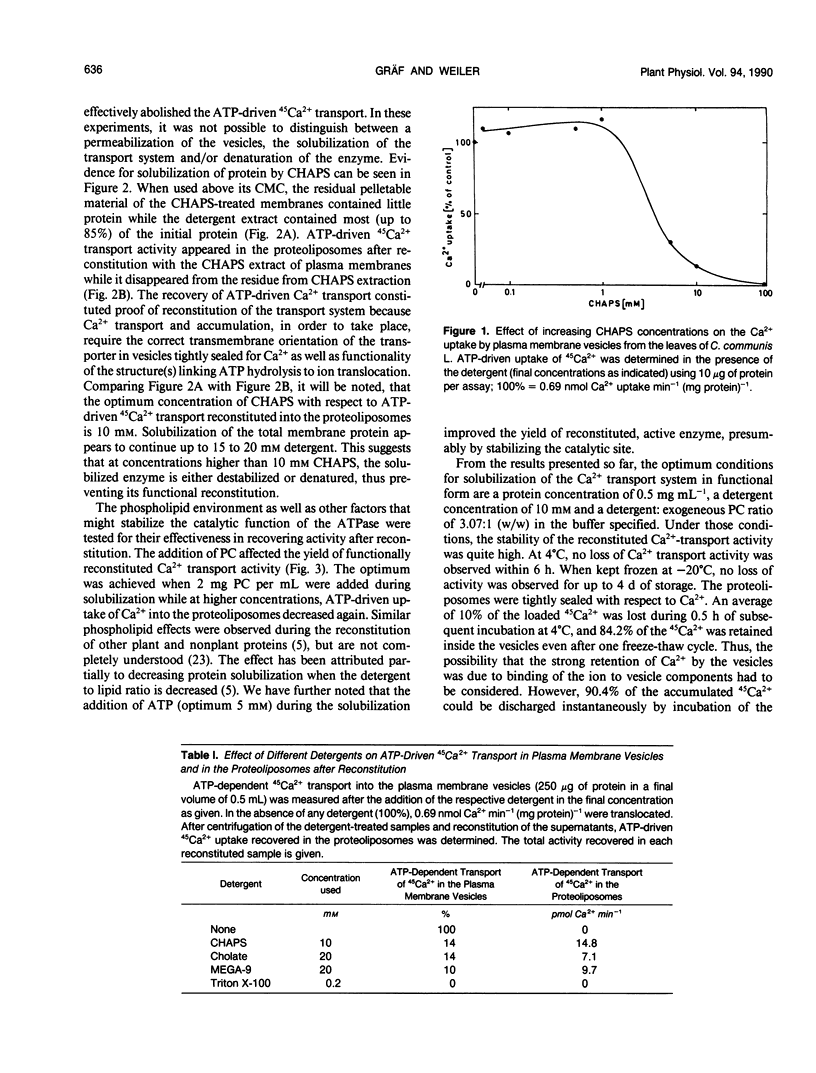
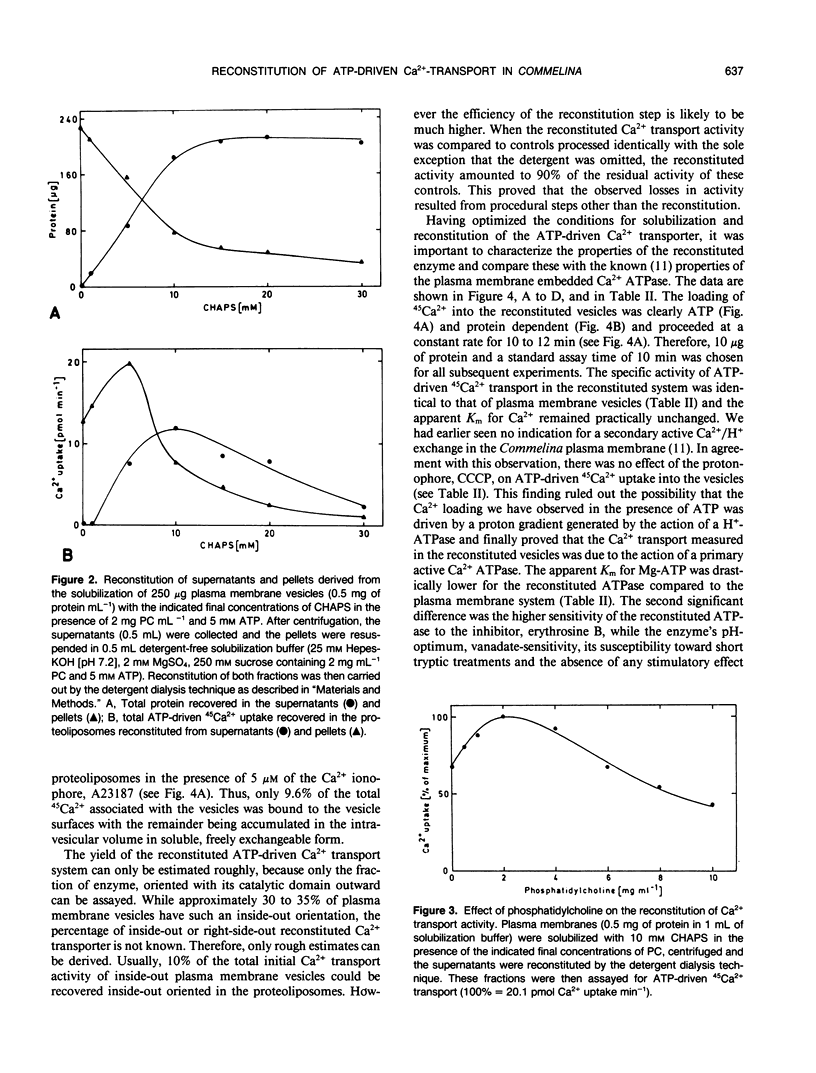
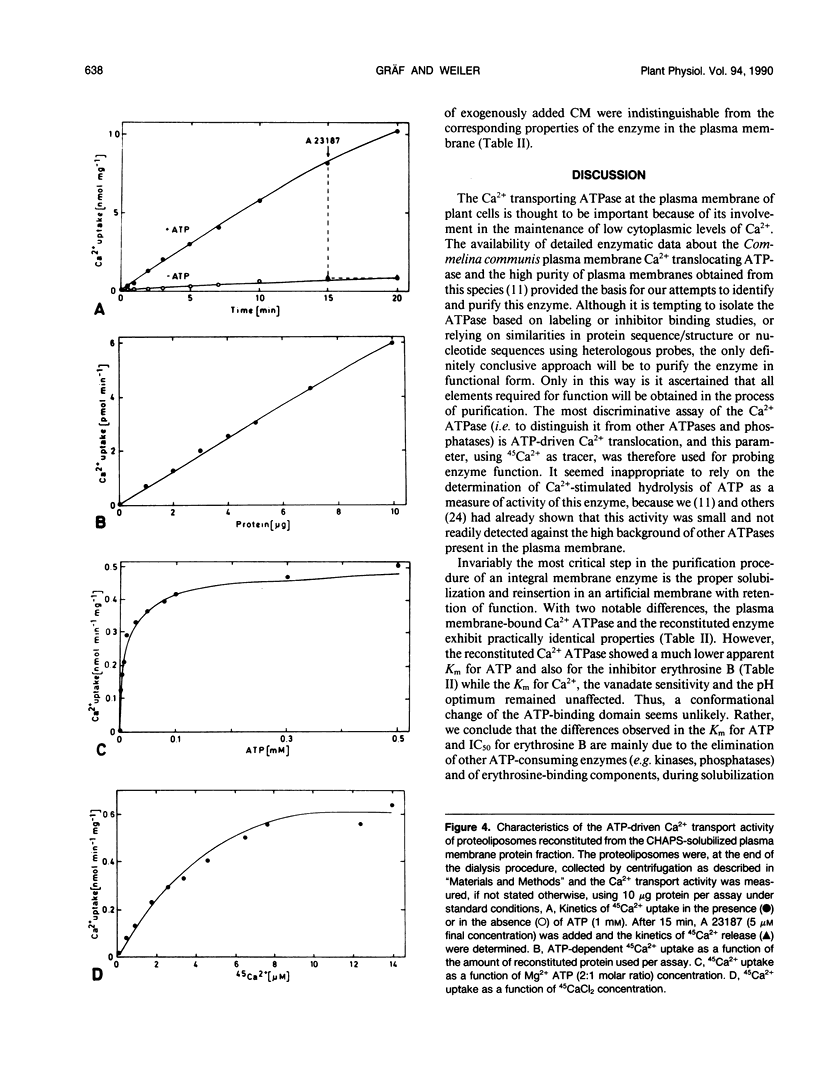
The protein(s) that constitute(s) the ATP-driven Ca2+-translocator of plasma membrane enriched vesicles obtained by aqueous two-phase partitioning from leaves of Commelina communis L. has/have been solubilized and reincorporated into tightly sealed liposomes. The reconstituted Ca2+-transport system was studied using ATP-driven 45Ca2+ import into the proteoliposomes as a measure of activity. The detergent, 3-[(3-cholamidopropyl) dimethylammonio]-1-propane-sulfonate proved to be the most suitable and was used at 10 millimolar concentration, i.e. just above its critical micellar concentration. The presence of additional phospholipid (2 milligrams phosphatidylcholine per milliliter) and ATP (5 millimolar) improved the solubilization and/or reconstitution. The characteristics of the reconstituted system were similar to those of the plasma membrane-bound activity, including the apparent Km for Ca2+ (5.2 micromolar), inhibition by relatively high levels of vanadate (IC50 = 500 micromolar) and lacking response to added calmodulin. The reconstituted transport system was very strongly inhibited by erythrosine B (IC50 = 0.01 micromolar) and had a low apparent Km for ATP (11.4 micromolar). As in the plasma membrane vesicles, the protonophore carbonylcyanide m-chlorophenyl hydrazone did not affect Ca2+-transport detectably in the reconstituted system. However, low levels of the Ca2+-ionophore A 23187 instantaneously discharged 90% of the Ca2+ associated with the vesicles, proving that it had been accumulated in the intravesicular volume in soluble, freely exchangeable form. Ca2+-transport in the reconstituted system was thus primary active, through a Ca2+-translocating ATPase. The system reported here may serve as a valuable tool for purifying the Ca2+-ATPase and for studying structural and functional aspects of the purified enzyme.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carafoli E., Zurini M. The Ca2+-pumping ATPase of plasma membranes. Purification, reconstitution and properties. Biochim Biophys Acta. 1982 Dec 31;683(3-4):279–301. doi: 10.1016/0304-4173(82)90004-0. [DOI] [PubMed] [Google Scholar]
- Collinge M., Trewavas A. J. The location of calmodulin in the pea plasma membrane. J Biol Chem. 1989 May 25;264(15):8865–8872. [PubMed] [Google Scholar]
- Cook N. J., Zeilinger C., Koch K. W., Kaupp U. B. Solubilization and functional reconstitution of the cGMP-dependent cation channel from bovine rod outer segments. J Biol Chem. 1986 Dec 25;261(36):17033–17039. [PubMed] [Google Scholar]
- Dieter P., Marmé D. Calmodulin activation of plant microsomal Ca uptake. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7311–7314. doi: 10.1073/pnas.77.12.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini J. L., Ruiz-Cristin J., Briskin D. P. Calcium Transport in Sealed Vesicles from Red Beet (Beta vulgaris L.) Storage Tissue : II. Characterization of Ca Uptake into Plasma Membrane Vesicles. Plant Physiol. 1987 Dec;85(4):1137–1142. doi: 10.1104/pp.85.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S., Hughes W. A., Trewavas A. J. A Comparison between Quin-2 and Aequorin as Indicators of Cytoplasmic Calcium Levels in Higher Plant Cell Protoplasts. Plant Physiol. 1989 Jun;90(2):482–491. doi: 10.1104/pp.90.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmeland L. M. A nondenaturing zwitterionic detergent for membrane biochemistry: design and synthesis. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6368–6370. doi: 10.1073/pnas.77.11.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmeland L. M., Chrambach A. Solubilization of functional membrane proteins. Methods Enzymol. 1984;104:305–318. doi: 10.1016/s0076-6879(84)04097-0. [DOI] [PubMed] [Google Scholar]
- Kasamo K., Nouchi I. The Role of Phospholipids in Plasma Membrane ATPase Activity in Vigna radiata L. (Mung Bean) Roots and Hypocotyls. Plant Physiol. 1987 Feb;83(2):323–328. doi: 10.1104/pp.83.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penniston J. T., Filoteo A. G., McDonough C. S., Carafoli E. Purification, reconstitution, and regulation of plasma membrane Ca2+-pumps. Methods Enzymol. 1988;157:340–351. doi: 10.1016/0076-6879(88)57089-1. [DOI] [PubMed] [Google Scholar]
- Racker E. Reconstitution of membrane processes. Methods Enzymol. 1979;55:699–711. doi: 10.1016/0076-6879(79)55078-2. [DOI] [PubMed] [Google Scholar]
- Rasi-Caldogno F., Pugliarello M. C., De Michelis M. I. The Ca-Transport ATPase of Plant Plasma Membrane Catalyzes a nH/Ca Exchange. Plant Physiol. 1987 Apr;83(4):994–1000. doi: 10.1104/pp.83.4.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivnay B., Metzger H. Reconstitution of the receptor for immunoglobulin E into liposomes. Conditions for incorporation of the receptor into vesicles. J Biol Chem. 1982 Nov 10;257(21):12800–12808. [PubMed] [Google Scholar]
- Serrano R. H+-ATPase from plasma membranes of Saccharomyces cerevisiae and Avena sativa roots: purification and reconstitution. Methods Enzymol. 1988;157:533–544. doi: 10.1016/0076-6879(88)57102-1. [DOI] [PubMed] [Google Scholar]


