Abstract
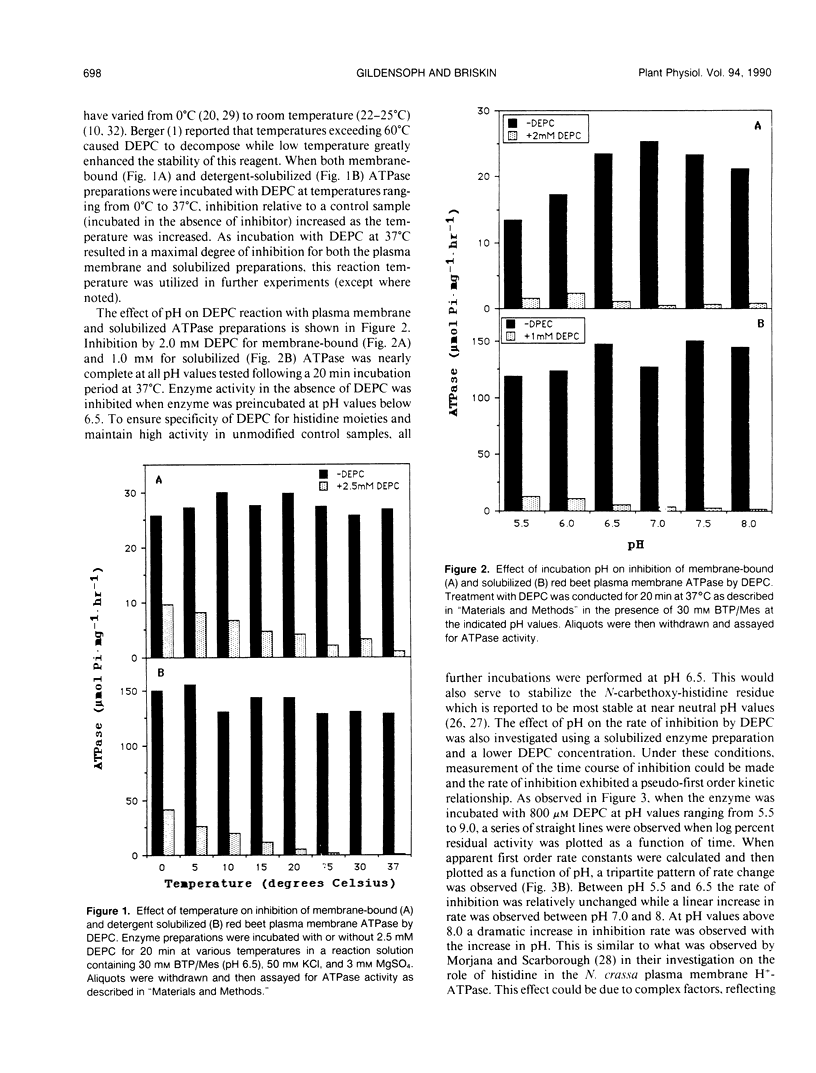
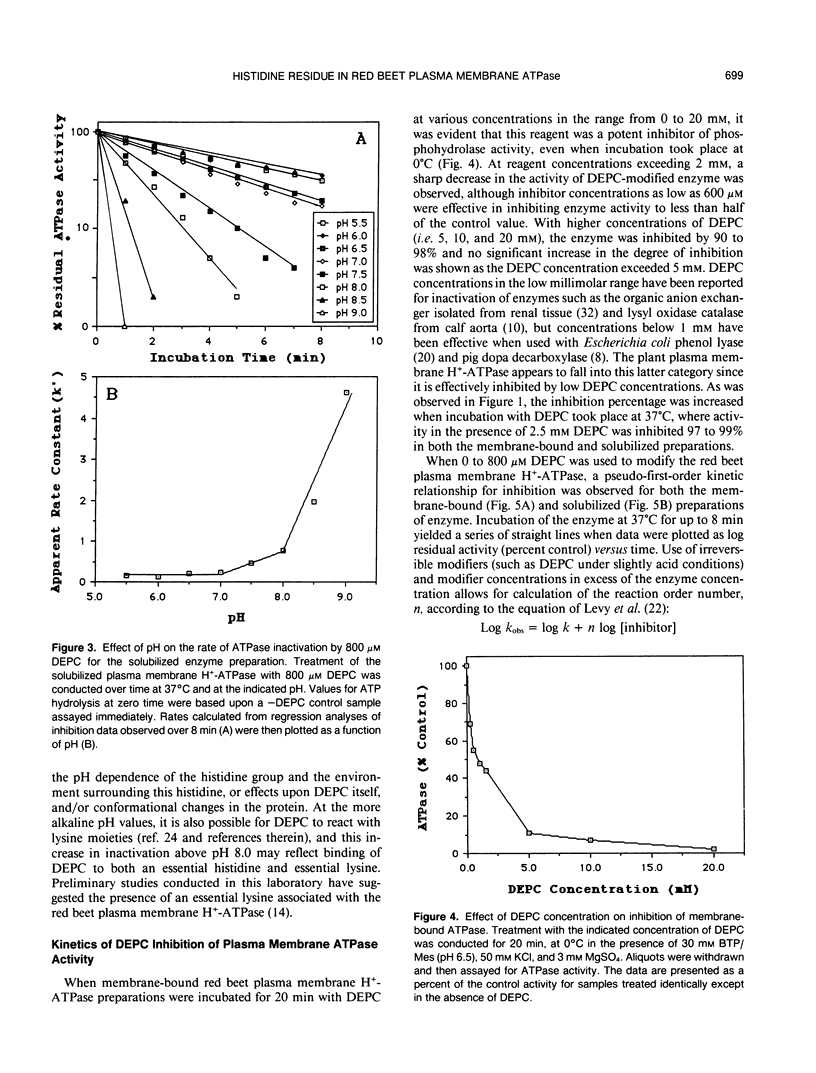
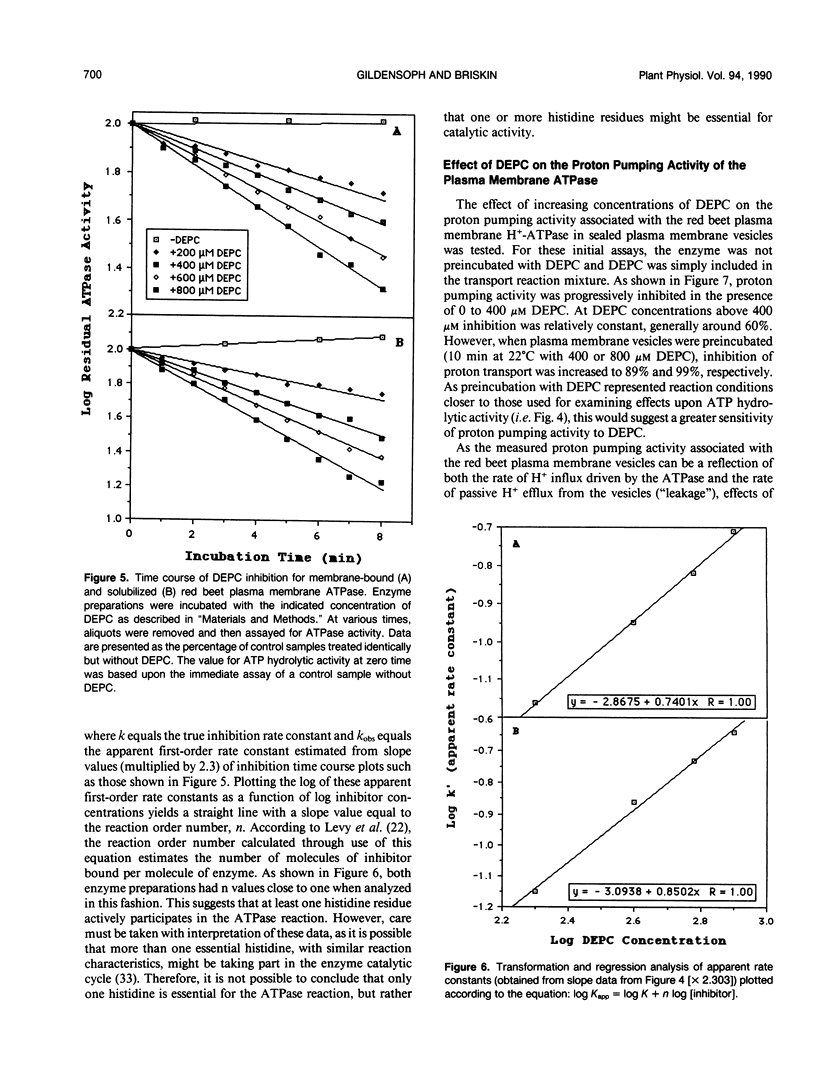
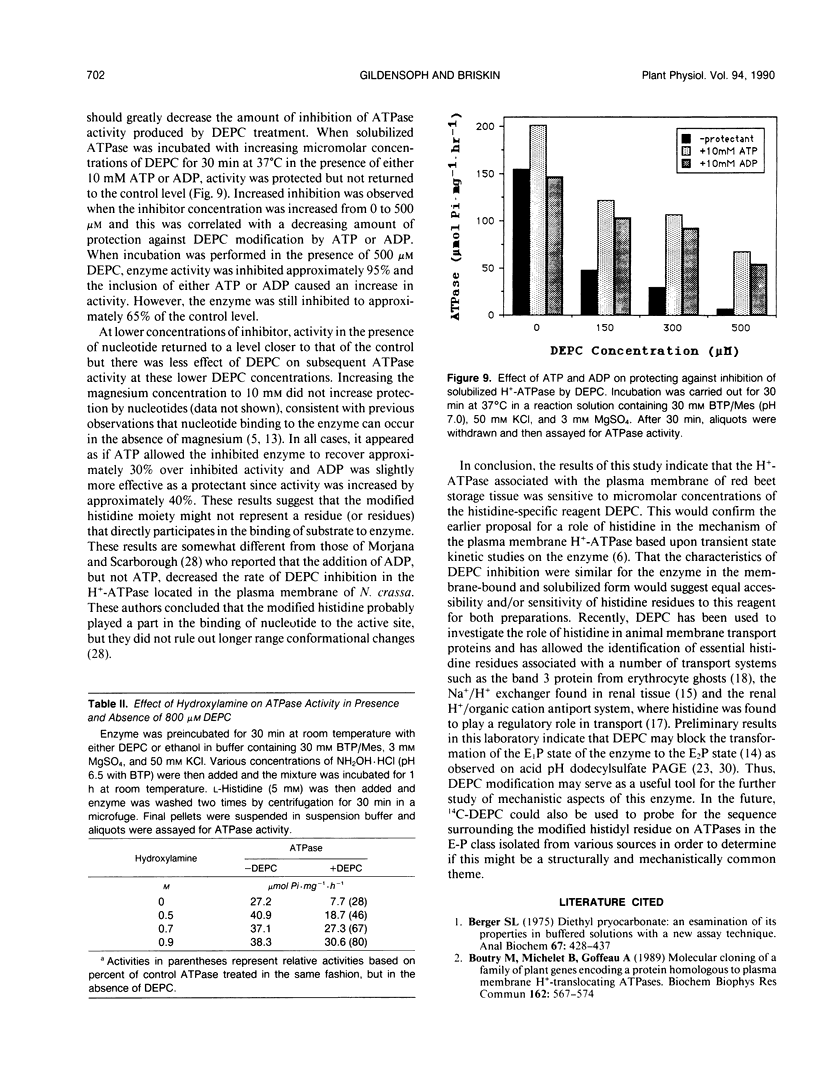
Incubation of the red beet (Beta vulgaris L.) plasma membrane H+-ATPase with micromolar concentrations of diethylpyrocarbonate (DEPC) resulted in inhibition of both ATP hydrolytic and proton pumping activity. Enzyme activity was restored when DEPC-modified protein was incubated with hydroxylamine, suggesting specific modification of histidine residues. Kinetic analyses of DEPC inhibition performed on both membrane-bound and solubilized enzyme preparations suggested the presence of at least one essential histidine moiety per active site. Inclusion of either ATP (substrate) or ADP (product and competitive inhibitor) in the modification medium reduced the amount of inhibition observed in the presence of DEPC. However, protection was not entirely effective in returning activity to noninhibited control values. These results suggest that the modified histidine does not reside directly in the ATP binding region of the enzyme, but is more likely involved in enzyme regulation through subtle conformational effects.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger S. L. Diethyl pyrocarbonate: an examination of its properties in buffered solutions with a new assay technique. Anal Biochem. 1975 Aug;67(2):428–437. doi: 10.1016/0003-2697(75)90315-2. [DOI] [PubMed] [Google Scholar]
- Boutry M., Michelet B., Goffeau A. Molecular cloning of a family of plant genes encoding a protein homologous to plasma membrane H+-translocating ATPases. Biochem Biophys Res Commun. 1989 Jul 31;162(2):567–574. doi: 10.1016/0006-291x(89)92348-6. [DOI] [PubMed] [Google Scholar]
- Briskin D. P. Intermediate reaction states of the red beet plasma membrane ATPase. Arch Biochem Biophys. 1986 Jul;248(1):106–115. doi: 10.1016/0003-9861(86)90406-6. [DOI] [PubMed] [Google Scholar]
- Briskin D. P. Phosphorylation and dephosphorylation reactions of the red beet plasma membrane ATPase studied in the transient state. Plant Physiol. 1988 Sep;88(1):84–91. doi: 10.1104/pp.88.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin D. P., Poole R. J. Characterization of the solubilized plasma membrane ATPase of red beet. Plant Physiol. 1984 Sep;76(1):26–30. doi: 10.1104/pp.76.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin D. P., Poole R. J. Evidence for a beta-Aspartyl Phosphate Residue in the Phosphorylated Intermediate of the Red Beet Plasma Membrane ATPase. Plant Physiol. 1983 Aug;72(4):1133–1135. doi: 10.1104/pp.72.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L., Carilli C. T., Farley R. A., Perlman D. M. Location of binding sites on the (Na,K)-ATPase for fluorescein-5'-isothiocyanate and ouabain. Ann N Y Acad Sci. 1982;402:289–291. doi: 10.1111/j.1749-6632.1982.tb25749.x. [DOI] [PubMed] [Google Scholar]
- Dominici P., Tancini B., Borri Voltattorni C. Chemical modification of pig kidney 3,4-dihydroxyphenylalanine decarboxylase with diethyl pyrocarbonate. Evidence for an essential histidyl residue. J Biol Chem. 1985 Sep 5;260(19):10583–10589. [PubMed] [Google Scholar]
- Gacheru S. N., Trackman P. C., Kagan H. M. Evidence for a functional role for histidine in lysyl oxidase catalysis. J Biol Chem. 1988 Nov 15;263(32):16704–16708. [PubMed] [Google Scholar]
- Giannini J. L., Gildensoph L. H., Briskin D. P. Selective production of sealed plasma membrane vesicles from red beet (Beta vulgaris L.) storage tissue. Arch Biochem Biophys. 1987 May 1;254(2):621–630. doi: 10.1016/0003-9861(87)90145-7. [DOI] [PubMed] [Google Scholar]
- Giannini J. L., Holt J. S., Briskin D. P. Isolation of sealed plasma membrane vesicles from Phytophthora megasperma f. sp. glycinea. I. Characterization of proton pumping and ATPase activity. Arch Biochem Biophys. 1988 Sep;265(2):337–345. doi: 10.1016/0003-9861(88)90136-1. [DOI] [PubMed] [Google Scholar]
- Gildensoph L. H., Briskin D. P. Modification of an essential arginine residue associated with the plasma membrane ATPase of red beet (Beta vulgaris L.) storage tissue. Arch Biochem Biophys. 1989 May 15;271(1):254–259. doi: 10.1016/0003-9861(89)90276-2. [DOI] [PubMed] [Google Scholar]
- Grillo F. G., Aronson P. S. Inactivation of the renal microvillus membrane Na+-H+ exchanger by histidine-specific reagents. J Biol Chem. 1986 Jan 25;261(3):1120–1125. [PubMed] [Google Scholar]
- Harper J. F., Surowy T. K., Sussman M. R. Molecular cloning and sequence of cDNA encoding the plasma membrane proton pump (H+-ATPase) of Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1234–1238. doi: 10.1073/pnas.86.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori R., Maegawa H., Kato M., Katsura T., Inui K. Inhibitory effect of diethyl pyrocarbonate on the H+/organic cation antiport system in rat renal brush-border membranes. J Biol Chem. 1989 Jul 25;264(21):12232–12237. [PubMed] [Google Scholar]
- Izuhara K., Okubo K., Hamasaki N. Conformational change of band 3 protein induced by diethyl pyrocarbonate modification in human erythrocyte ghosts. Biochemistry. 1989 May 30;28(11):4725–4728. doi: 10.1021/bi00437a032. [DOI] [PubMed] [Google Scholar]
- Kasamo K. Essential Arginyl Residues in the Plasma Membrane H-ATPase from Vigna radiata L. (Mung Bean) Roots. Plant Physiol. 1988 May;87(1):126–129. doi: 10.1104/pp.87.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai H., Utagawa T., Yamada H. Studies on tyrosine phenol lyase. Modification of essential histidyl residues by diethylpyrocarbonate. J Biol Chem. 1975 Mar 10;250(5):1661–1667. [PubMed] [Google Scholar]
- LEVY H. M., LEBER P. D., RYAN E. M. INACTIVATION OF MYOSIN BY 2,4-DINITROPHENOL AND PROTECTION BY ADENOSINE TRIPHOSPHATE AND OTHER PHOSPHATE COMPOUNDS. J Biol Chem. 1963 Nov;238:3654–3659. [PubMed] [Google Scholar]
- Lichtner R., Wolf H. U. Dodecyl sulphate/polyacrylamide-gel electrophoresis at low pH values and low temperatures. Biochem J. 1979 Sep 1;181(3):759–761. doi: 10.1042/bj1810759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior W. B., Jr, Fahrney D. Ethoxyformylation of proteins. Reaction of ethoxyformic anhydride with alpha-chymotrypsin, pepsin, and pancreatic ribonuclease at pH 4. Biochemistry. 1970 Jan 20;9(2):251–258. doi: 10.1021/bi00804a010. [DOI] [PubMed] [Google Scholar]
- Miles E. W. Modification of histidyl residues in proteins by diethylpyrocarbonate. Methods Enzymol. 1977;47:431–442. doi: 10.1016/0076-6879(77)47043-5. [DOI] [PubMed] [Google Scholar]
- Morjana N. A., Scarborough G. A. Evidence for an essential histidine residue in the Neurospora crassa plasma membrane H+-ATPase. Biochim Biophys Acta. 1989 Oct 2;985(1):19–25. doi: 10.1016/0005-2736(89)90097-7. [DOI] [PubMed] [Google Scholar]
- Mühlrad A., Hegyi G., Horányi M. Studies on the properties of chemically modified actin. 3. Carbethoxylation. Biochim Biophys Acta. 1969 May;181(1):184–190. doi: 10.1016/0005-2795(69)90240-2. [DOI] [PubMed] [Google Scholar]
- Riordan J. F., McElvany K. D., Borders C. L., Jr Arginyl residues: anion recognition sites in enzymes. Science. 1977 Mar 4;195(4281):884–886. doi: 10.1126/science.190679. [DOI] [PubMed] [Google Scholar]
- Sokol P. P., Holohan P. D., Ross C. R. Arginyl and histidyl groups are essential for organic anion exchange in renal brush-border membrane vesicles. J Biol Chem. 1988 May 25;263(15):7118–7123. [PubMed] [Google Scholar]
- Xu K. Y. Any of several lysines can react with 5'-isothiocyanatofluorescein to inactivate sodium and potassium ion activated adenosinetriphosphatase. Biochemistry. 1989 Jul 11;28(14):5764–5772. doi: 10.1021/bi00440a010. [DOI] [PubMed] [Google Scholar]


