Abstract
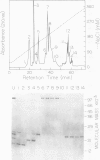
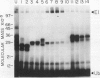
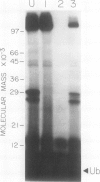
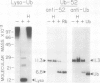
The highly conserved protein ubiquitin is involved in several cellular processes in eukaryotes as a result of its covalent ligation to a variety of target proteins. Here, we describe the purification of several enzymatic activities involved in ubiquitin-protein conjugate formation and disassembly from wheat germ (Triticum vulgare) by a combination of ubiquitin affinity chromatography and anion-exchange high performance liquid chromatography. Using this procedure, ubiquitin activating enzyme (E1), several distinct ubiquitin carrier proteins (E2s) with molecular masses of 16, 20, 23, 23.5, and 25 kilodaltons, and a ubiquitin-protein hydrolase (isopeptidase) were isolated. Purified E1 formed a thiol ester linkage with 125I-ubiquitin in an ATP-dependent manner and transferred bound ubiquitin to the various purified E2s. The ubiquitin protein hydrolase fraction was sensitive to hemin, and in an ATP-independent reaction, was capable of removing the ubiquitin moiety from both ubiquitin 125I-lysozyme conjugates (ε-amino or isopeptide linkage) and the ubiquitin 52-amino acid extension protein fusion (α-amino or peptide linkage). Using this procedure, wheat germ represents an inexpensive source from which enzymes involved in the ubiquitin pathway may be isolated.
Full text
PDF


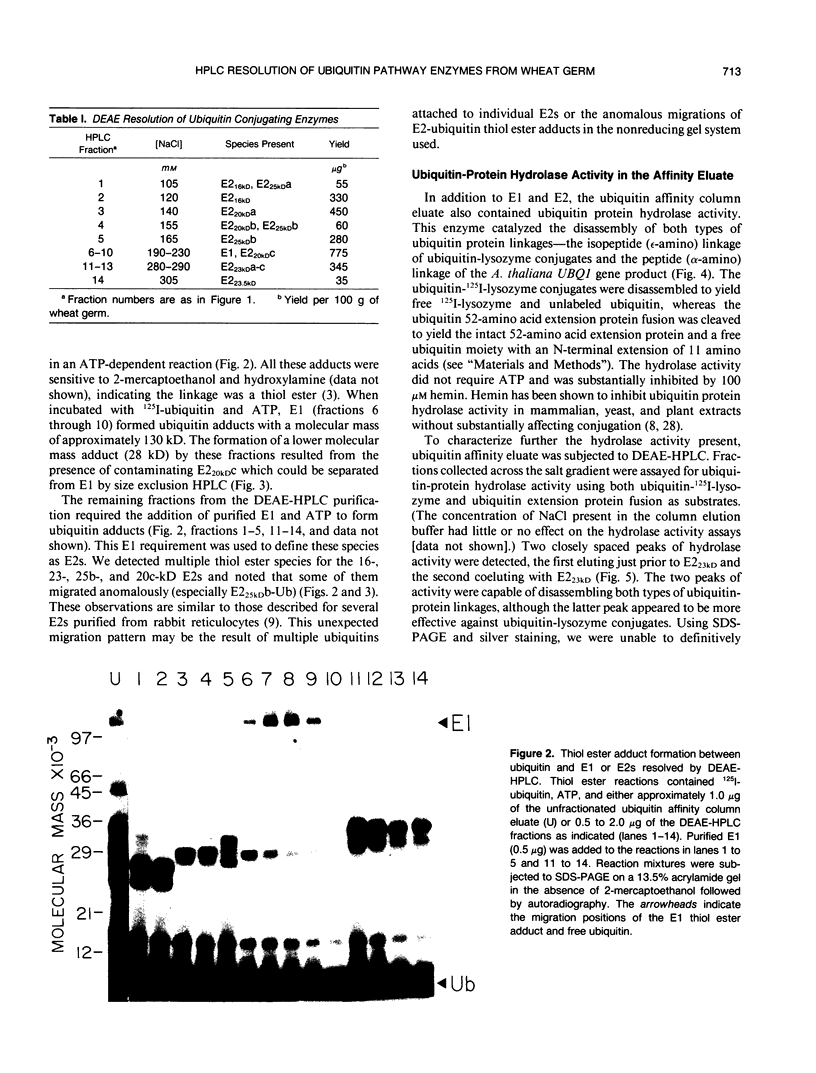
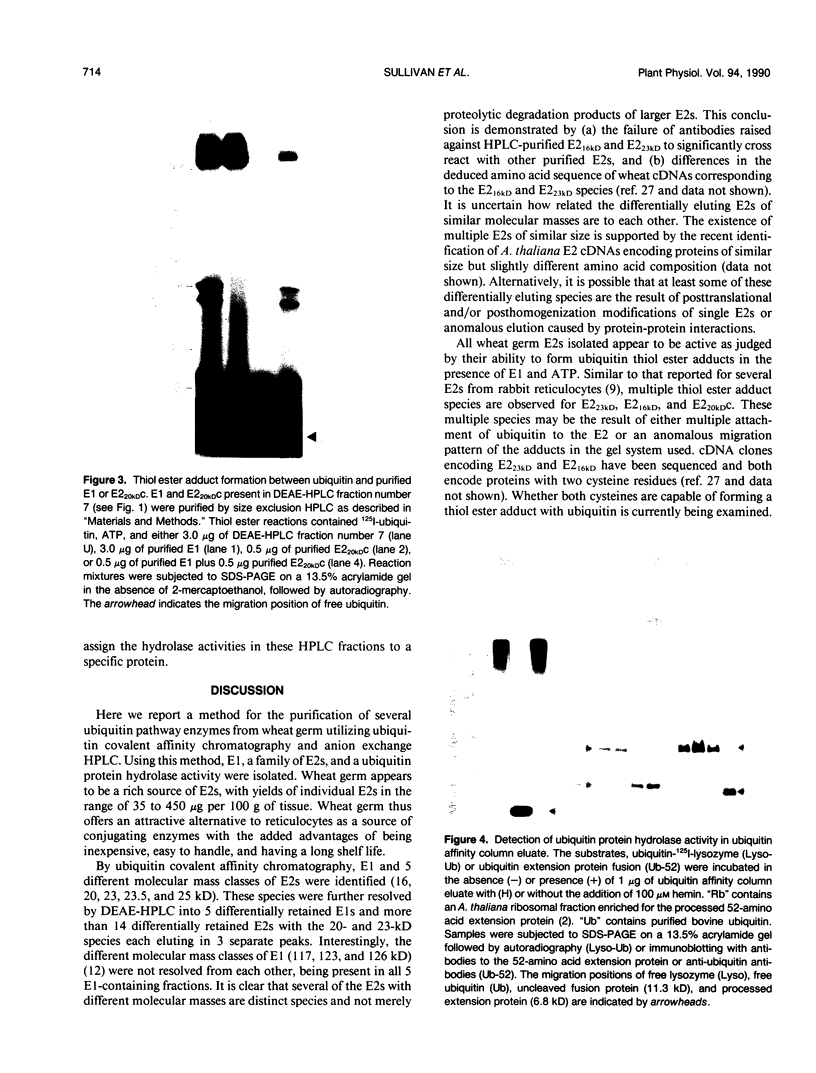
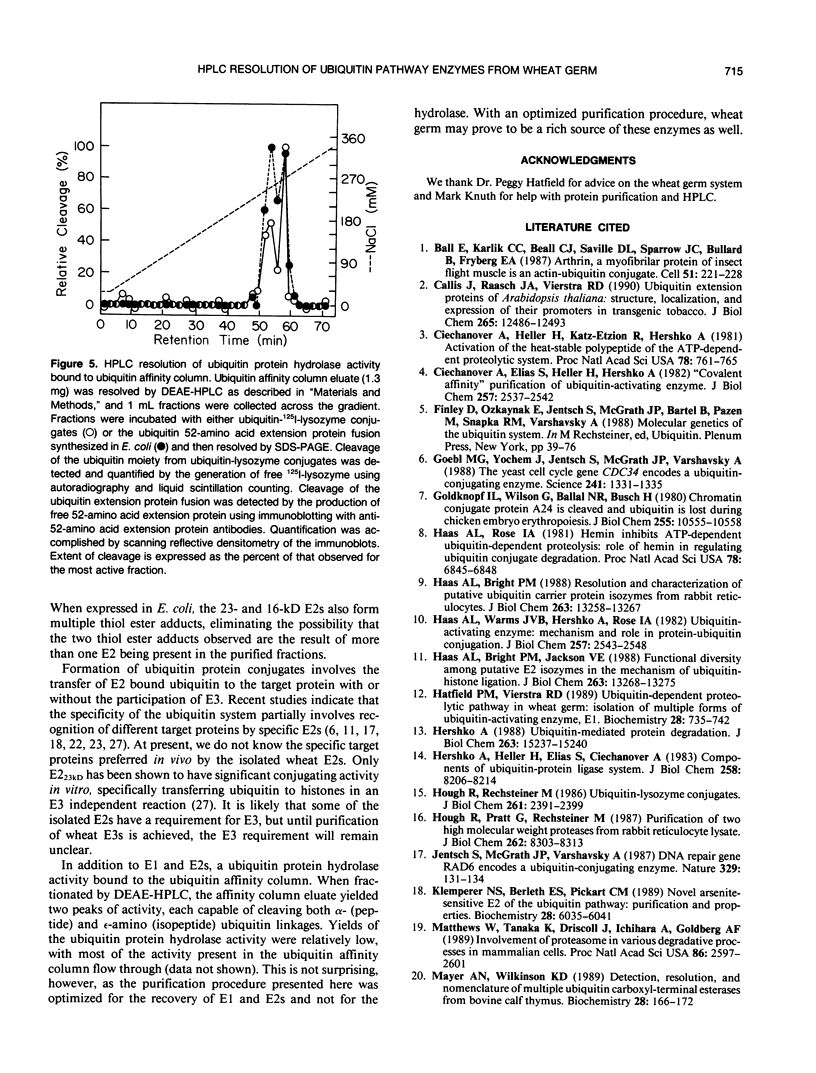

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball E., Karlik C. C., Beall C. J., Saville D. L., Sparrow J. C., Bullard B., Fyrberg E. A. Arthrin, a myofibrillar protein of insect flight muscle, is an actin-ubiquitin conjugate. Cell. 1987 Oct 23;51(2):221–228. doi: 10.1016/0092-8674(87)90149-8. [DOI] [PubMed] [Google Scholar]
- Callis J., Raasch J. A., Vierstra R. D. Ubiquitin extension proteins of Arabidopsis thaliana. Structure, localization, and expression of their promoters in transgenic tobacco. J Biol Chem. 1990 Jul 25;265(21):12486–12493. [PubMed] [Google Scholar]
- Ciechanover A., Elias S., Heller H., Hershko A. "Covalent affinity" purification of ubiquitin-activating enzyme. J Biol Chem. 1982 Mar 10;257(5):2537–2542. [PubMed] [Google Scholar]
- Ciechanover A., Heller H., Katz-Etzion R., Hershko A. Activation of the heat-stable polypeptide of the ATP-dependent proteolytic system. Proc Natl Acad Sci U S A. 1981 Feb;78(2):761–765. doi: 10.1073/pnas.78.2.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebl M. G., Yochem J., Jentsch S., McGrath J. P., Varshavsky A., Byers B. The yeast cell cycle gene CDC34 encodes a ubiquitin-conjugating enzyme. Science. 1988 Sep 9;241(4871):1331–1335. doi: 10.1126/science.2842867. [DOI] [PubMed] [Google Scholar]
- Goldknopf I. L., Wilson G., Ballal N. R., Busch H. Chromatin conjugate protein A24 is cleaved and ubiquitin is lost during chicken erythropoiesis. J Biol Chem. 1980 Nov 25;255(22):10555–10558. [PubMed] [Google Scholar]
- Haas A. L., Bright P. M., Jackson V. E. Functional diversity among putative E2 isozymes in the mechanism of ubiquitin-histone ligation. J Biol Chem. 1988 Sep 15;263(26):13268–13275. [PubMed] [Google Scholar]
- Haas A. L., Bright P. M. The resolution and characterization of putative ubiquitin carrier protein isozymes from rabbit reticulocytes. J Biol Chem. 1988 Sep 15;263(26):13258–13267. [PubMed] [Google Scholar]
- Haas A. L., Rose I. A. Hemin inhibits ATP-dependent ubiquitin-dependent proteolysis: role of hemin in regulating ubiquitin conjugate degradation. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6845–6848. doi: 10.1073/pnas.78.11.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A. L., Warms J. V., Hershko A., Rose I. A. Ubiquitin-activating enzyme. Mechanism and role in protein-ubiquitin conjugation. J Biol Chem. 1982 Mar 10;257(5):2543–2548. [PubMed] [Google Scholar]
- Hershko A., Heller H., Elias S., Ciechanover A. Components of ubiquitin-protein ligase system. Resolution, affinity purification, and role in protein breakdown. J Biol Chem. 1983 Jul 10;258(13):8206–8214. [PubMed] [Google Scholar]
- Hershko A. Ubiquitin-mediated protein degradation. J Biol Chem. 1988 Oct 25;263(30):15237–15240. [PubMed] [Google Scholar]
- Hough R., Pratt G., Rechsteiner M. Purification of two high molecular weight proteases from rabbit reticulocyte lysate. J Biol Chem. 1987 Jun 15;262(17):8303–8313. [PubMed] [Google Scholar]
- Hough R., Rechsteiner M. Ubiquitin-lysozyme conjugates. Purification and susceptibility to proteolysis. J Biol Chem. 1986 Feb 15;261(5):2391–2399. [PubMed] [Google Scholar]
- Jentsch S., McGrath J. P., Varshavsky A. The yeast DNA repair gene RAD6 encodes a ubiquitin-conjugating enzyme. Nature. 1987 Sep 10;329(6135):131–134. doi: 10.1038/329131a0. [DOI] [PubMed] [Google Scholar]
- Klemperer N. S., Berleth E. S., Pickart C. M. A novel, arsenite-sensitive E2 of the ubiquitin pathway: purification and properties. Biochemistry. 1989 Jul 11;28(14):6035–6041. doi: 10.1021/bi00440a047. [DOI] [PubMed] [Google Scholar]
- Matthews W., Driscoll J., Tanaka K., Ichihara A., Goldberg A. L. Involvement of the proteasome in various degradative processes in mammalian cells. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2597–2601. doi: 10.1073/pnas.86.8.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A. N., Wilkinson K. D. Detection, resolution, and nomenclature of multiple ubiquitin carboxyl-terminal esterases from bovine calf thymus. Biochemistry. 1989 Jan 10;28(1):166–172. doi: 10.1021/bi00427a024. [DOI] [PubMed] [Google Scholar]
- Pickart C. M., Rose I. A. Functional heterogeneity of ubiquitin carrier proteins. J Biol Chem. 1985 Feb 10;260(3):1573–1581. [PubMed] [Google Scholar]
- Pickart C. M., Vella A. T. Ubiquitin carrier protein-catalyzed ubiquitin transfer to histones. Mechanism and specificity. J Biol Chem. 1988 Oct 15;263(29):15076–15082. [PubMed] [Google Scholar]
- Rechsteiner M. Ubiquitin-mediated pathways for intracellular proteolysis. Annu Rev Cell Biol. 1987;3:1–30. doi: 10.1146/annurev.cb.03.110187.000245. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Sullivan M. L., Vierstra R. D. A ubiquitin carrier protein from wheat germ is structurally and functionally similar to the yeast DNA repair enzyme encoded by RAD6. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9861–9865. doi: 10.1073/pnas.86.24.9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra R. D., Langan S. M., Haas A. L. Purification and initial characterization of ubiquitin from the higher plant, Avena sativa. J Biol Chem. 1985 Oct 5;260(22):12015–12021. [PubMed] [Google Scholar]
- Vierstra R. D., Sullivan M. L. Hemin inhibits ubiquitin-dependent proteolysis in both a higher plant and yeast. Biochemistry. 1988 May 3;27(9):3290–3295. doi: 10.1021/bi00409a025. [DOI] [PubMed] [Google Scholar]
- Wilkinson K. D., Lee K. M., Deshpande S., Duerksen-Hughes P., Boss J. M., Pohl J. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science. 1989 Nov 3;246(4930):670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]