Abstract
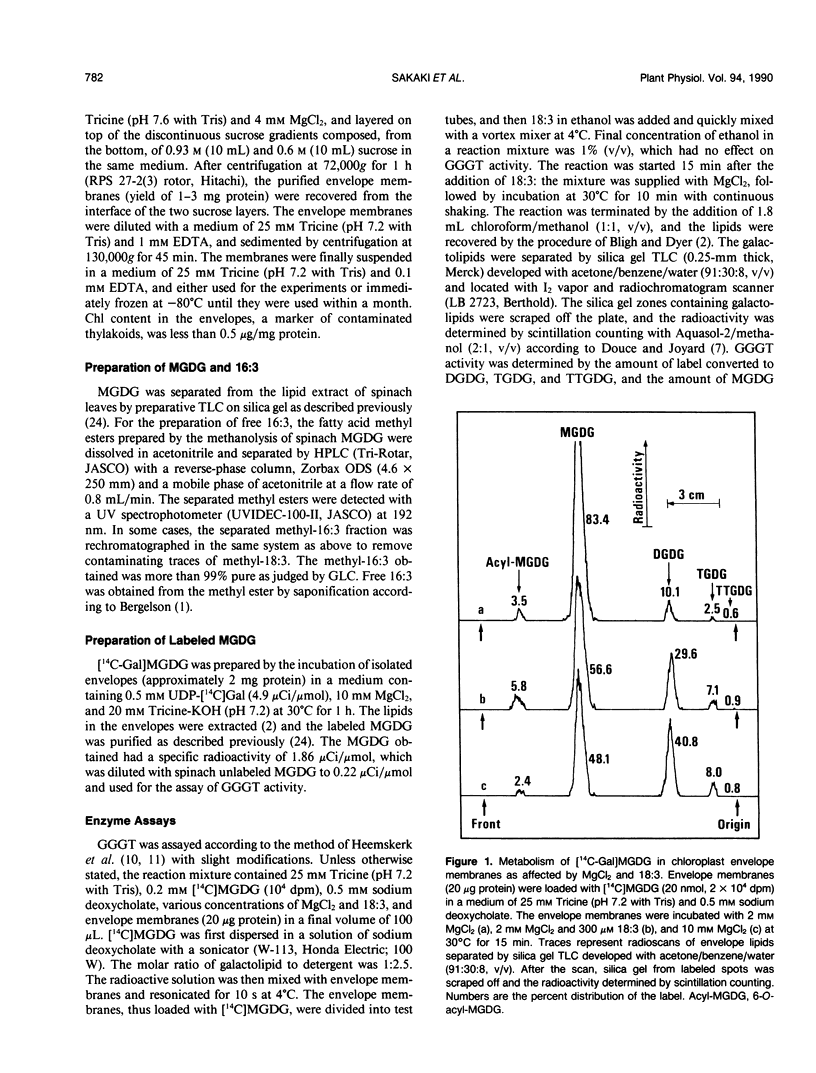
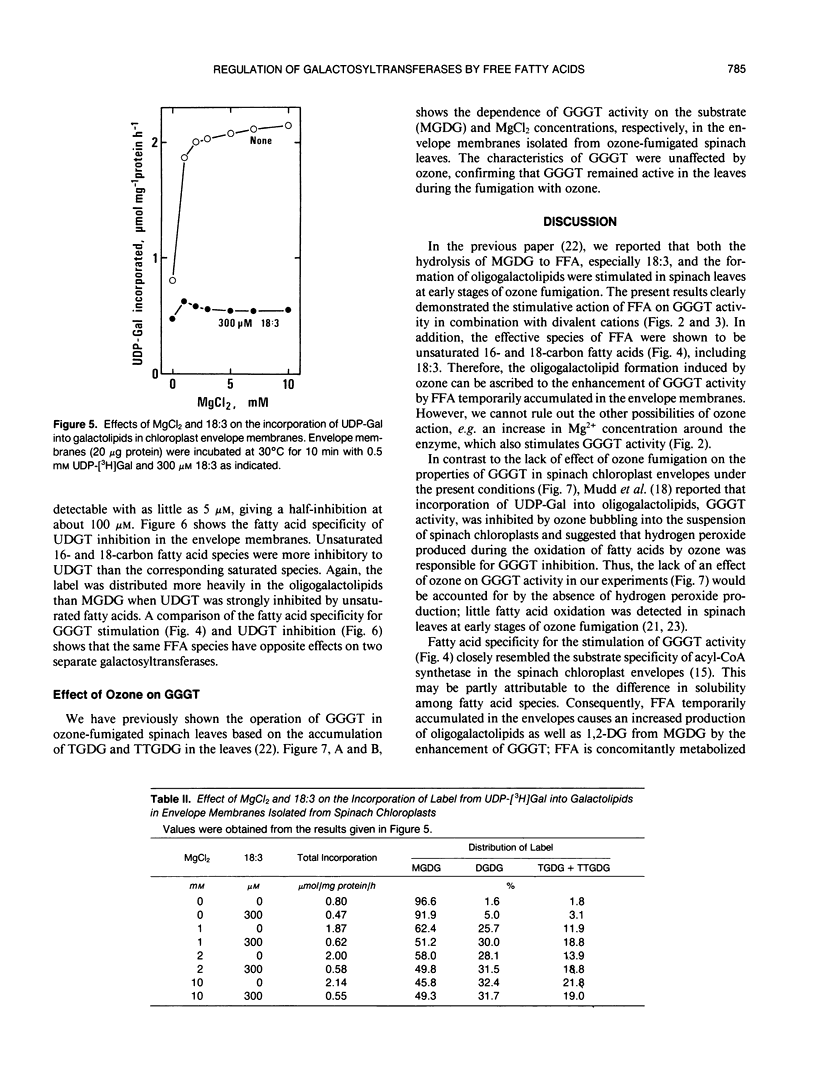
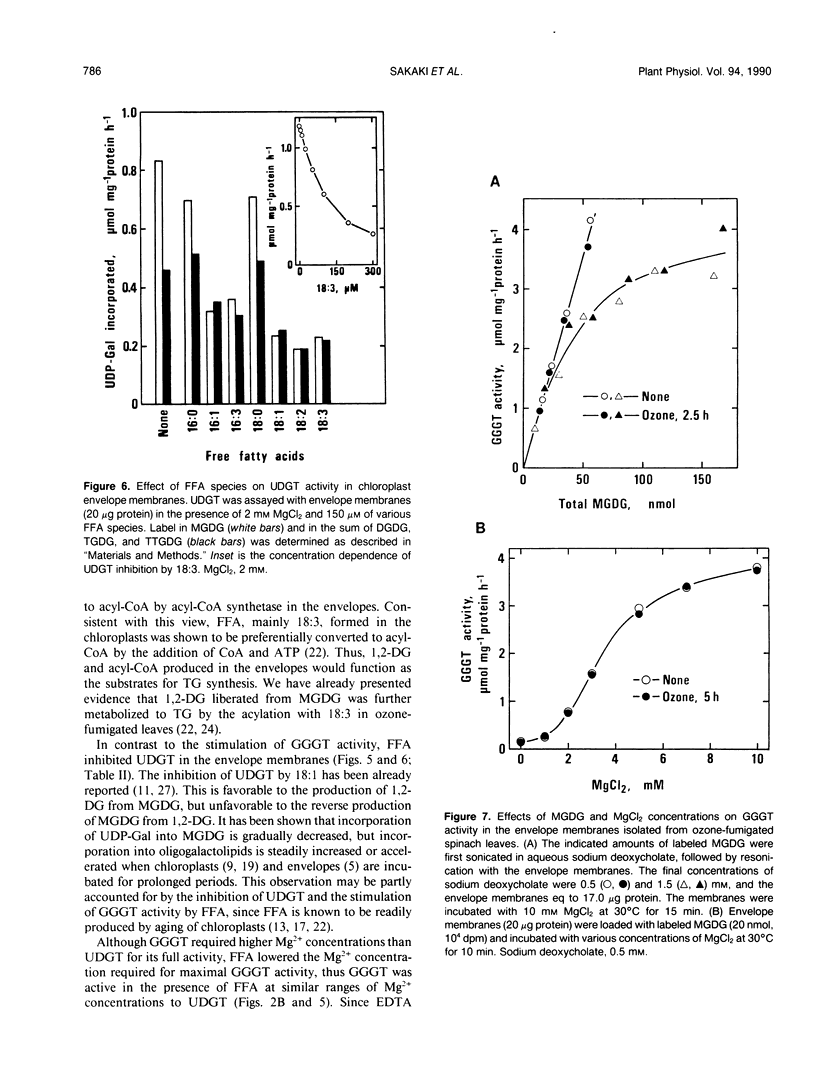
Effects of MgCl2 and free fatty acids (FFA) on galactolipid:galactolipid galactosyltransferase (GGGT) and UDP-galactose: 1,2-diacylglycerol galactosyltransferase (UDGT) in chloroplast envelope membranes isolated from spinach (Spinacia oleracea L.) leaves were examined. GGGT activity was sigmoidally stimulated by MgCl2 with a saturated concentration of more than 5 millimolar. Free α-linolenic acid (18:3) caused a drastic increase in GGGT activity under limiting concentrations of MgCl2, without affecting its maximum activity at higher MgCl2 concentrations. Free 18:3 alone did not affect the GGGT activity. The effective species of FFA for the stimulation of GGGT activity in the presence of MgCl2 were unsaturated 16- and 18-carbon fatty acids. GGGT activity was also stimulated by 18:3 in the presence of MnCl2, CaCl2 and a high concentration of KCl in place of MgCl2. UDGT activity was hyperbolically enhanced by MgCl2 with a saturated concentration of 1 to 2 millimolar. In contrast to GGGT, UDGT was severely inhibited by 18:3, and MgCl2-induced stimulation was completely abolished by 18:3. Unsaturated 16- and 18-carbon fatty acids were more inhibitory to UDGT than the saturated acids. The dependence of GGGT activity on monogalactosyldiacylglycerol (MGDG) and MgCl2 concentrations was identical in the envelope membranes isolated from non- and ozone (0.5 microliter/liter)-fumigated spinach leaves, indicating that GGGT remained active in the leaves during ozone fumigation. The results are discussed in relation to the regulation of galactolipid biosynthesis by the endogenous FFA in the envelopes and to the involvement of GGGT in the triacylglycerol synthesis from MGDG in ozone-fumigated leaves.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Cline K., Keegstra K. Galactosyltransferases involved in galactolipid biosynthesis are located in the outer membrane of pea chloroplast envelopes. Plant Physiol. 1983 Feb;71(2):366–372. doi: 10.1104/pp.71.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk J. W., Bögemann G., Helsper J. P., Wintermans J. F. Synthesis of mono- and digalactosyldiacylglycerol in isolated spinach chloroplasts. Plant Physiol. 1988 Mar;86(3):971–977. doi: 10.1104/pp.86.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry L. E., Strasser R. J., Siegenthaler P. A. Alteration in the acyl lipid composition of thylakoids induced by aging and its effect on thylakoid structure. Plant Physiol. 1982 Feb;69(2):531–536. doi: 10.1104/pp.69.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyard J., Stumpf P. K. Synthesis of Long-Chain Acyl-CoA in Chloroplast Envelope Membranes. Plant Physiol. 1981 Feb;67(2):250–256. doi: 10.1104/pp.67.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCarty R. E., Jagendorf A. T. Chloroplast damage due to enzymatic hydrolysis of endogenous lipids. Plant Physiol. 1965 Jul;40(4):725–735. doi: 10.1104/pp.40.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd J. B., McManus T. T., Ongun A., McCullogh T. E. Inhibition of glycolipid biosynthesis in chloroplasts by ozone and sulfhydryl reagents. Plant Physiol. 1971 Sep;48(3):335–339. doi: 10.1104/pp.48.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongun A., Mudd J. B. Biosynthesis of galactolipids in plants. J Biol Chem. 1968 Apr 10;243(7):1558–1566. [PubMed] [Google Scholar]
- Sakaki T., Kondo N., Yamada M. Pathway for the synthesis of triacylglycerols from monogalactosyldiacylglycerols in ozone-fumigated spinach leaves. Plant Physiol. 1990 Oct;94(2):773–780. doi: 10.1104/pp.94.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki T., Saito K., Kawaguchi A., Kondo N., Yamada M. Conversion of monogalactosyldiacylglycerols to triacylglycerols in ozone-fumigated spinach leaves. Plant Physiol. 1990 Oct;94(2):766–772. doi: 10.1104/pp.94.2.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Besouw A., Wintermans J. F. Galactolipid formation in chloroplast envelopes. I. Evidence for two mechanisms in galactosylation. Biochim Biophys Acta. 1978 Apr 28;529(1):44–53. doi: 10.1016/0005-2760(78)90102-9. [DOI] [PubMed] [Google Scholar]
- van Besouw A., Wintermans J. F. The synthesis of galactosydiacylglycerols by chloroplast envelopes. FEBS Lett. 1979 Jun 1;102(1):33–37. doi: 10.1016/0014-5793(79)80922-9. [DOI] [PubMed] [Google Scholar]


